Abstract
Most tumor cells function poorly as antigen-presenting cells in part because they do not express costimulatory molecules. To provide costimulation to T lymphocytes that recognize tumor cells, we constructed a CD28-like receptor specific for GD2, a ganglioside overexpressed on the surface of neuroblastoma, small-cell lung carcinoma, melanoma, and other human tumors. Recognition of GD2 was provided by a single-chain antibody derived from the GD2-specific monoclonal antibody 3G6. We demonstrate that the chimeric receptor 3G6-CD28 provides CD28 signaling upon specific recognition of the GD2 antigen on tumor cells. Human primary T lymphocytes retrovirally transduced with 3G6-CD28 secrete interleukin 2, survive proapoptotic culture conditions, and selectively undergo clonal expansion in the presence of an antiidiotypic antibody specific for 3G6-CD28. Polyclonal CD8+ lymphocytes expressing 3G6-CD28 are selectively expanded when cultured with cells expressing allogeneic major histocompatibility complex class I together with GD2. Primary T cells given such an antigen-dependent survival advantage should be very useful to augment immune responses against tumor cells.
Keywords: adoptive cell therapy, chimeric receptors, costimulation, ganglioside, gene transfer
The challenge for cancer immunotherapy is to elicit and amplify vigorous immune responses against poorly immunogenic tumor cells that mostly express self-antigens (1– 3). Until now, most forms of immunotherapy have aimed to prime and expand tumor-reactive lymphocytes in vivo (4, 5). Alternatively, adoptive antibody and cell therapies are based on the infusion of preformed immune effectors, whether antibodies (e.g., antibodies against the GD2 antigen in neuroblastoma [6]) or lymphocytes (7–10). In principle, adoptive cell therapies are attractive in that they bypass many of the obstacles to the selective expansion of tumor-reactive lymphocytes in vivo by enabling their expansion in vitro. However, the generation of tumor-specific lymphocytes on one hand, and the sustained activity of cultured lymphocytes upon infusion in the recipient on the other, pose numerous problems (8). Adoptively transferred lymphocytes are indeed prone to cell death secondary to cytokine withdrawal, anergy secondary to inappropriate antigen presentation or downregulation of the TCR or other critical coreceptors. The engagement of the TCR is necessary to activate T cells, but not sufficient to achieve full activation. A second signal, or costimulation, is also required (11–13). The interaction of the T cell surface receptor CD28 with the costimulatory ligands B7.1 and B7.2 expressed by professional APCs can induce a strong costimulatory signal resulting in the clonal expansion of activated T cells (14–17). Thus, the costimulatory molecules expressed by the APC play a major role in determining whether survival, activation, or cell death will result from the interaction between the T lymphocyte and the APC (12–14). CD28 signaling in T cells can prevent cell death secondary to either IL-2 withdrawal or TCR-mediated activation (15–17).
The absence of natural costimulatory molecules on tumor cells creates unfavorable conditions for the survival and activation of tumor-reactive T cells (13, 18–23). Different approaches can be taken to provide costimulation to tumor-specific T lymphocytes. One is to express B7 in tumor cells (5, 24). However, the provision of costimulation in vivo by genetically modified tumor cells is confined to a small fraction of the total tumor burden. The success of this approach critically depends upon the ability to specifically target B7 expression to a sizable fraction of the tumor cells. It also depends on the sustained expression of CD28 by the activated T lymphocytes. On the other hand, the introduction in tumor-reactive T lymphocytes of a CD28-like receptor that is engaged by a tumor-associated antigen and expressed under the control of the retroviral LTR represents an alternative approach. The high efficiency gene transfer that is now achieved in human primary PBLs (25, 26) makes this approach technically feasible.
To enhance the survival and expansion of T lymphocytes reacting against tumor cells that lack costimulatory molecules but express a well-defined cell-surface antigen, we engineered a chimeric molecule that combines an antigen-specific single chain variable region fragment (scFv)1 with the signal transduction domain of CD28. We reasoned that an scFv-CD28 fusion molecule could specifically recognize the cell-surface epitope and mediate CD28 signaling functions, and thus mimic CD28/B7 interactions between T lymphocytes and tumor cells. The expression of an antigen-dependent CD28 receptor by adoptively transferred lymphocytes is expected to increase the probability that a T cell will survive and perhaps continue to proliferate when its TCR is engaged by target cells expressing the cell-surface antigen. The antigen-binding moiety we used was derived from the mAb 3G6 specific for the disialoganglioside GD2 (27), an antigen abundantly expressed by neuroblastoma, small-cell lung carcinoma, melanoma, and some other tumors (6). Highly efficient retroviral-mediated gene transfer (25, 26) enabled us to study the 3G6-CD28 fusion molecule in primary T cells rather than in cell lines or leukemic cells. We demonstrate that this receptor engages tumor-associated GD2 antigen and mediates effective CD28 signaling in primary T lymphocytes. Polyclonal CD8+ PBLs expressing 3G6-CD28 are selectively expanded when cultured with cells expressing MHC class I together with GD2. Thus, the transduced lymphocytes carry out a functional costimulatory response that enhances their survival and leads to their selective expansion in the absence of natural costimulatory molecules.
Materials and Methods
Recombinant DNA Constructs.
The chimeric cDNA 3G6-CD28 comprises sequences encoding an scFv derived from the GD2-specific antibody 3G6 (27, 28) and the portion of the CD28 comprising part of the extracellular, the transmembrane, and the cytoplasmic domains. The VL and VH cDNAs were cloned in that order separated by a glycine-rich linker and downstream of the human CD8α leader (G. Rothschild, N.K. Cheung, and M. Sadelain, unpublished observations). The CD28 coding sequence extending from nucleotide 336 to 663 was amplified by reverse transcription PCR from RNA isolated from the human leukemic T cell line Jurkat using the forward primer 5′-GCGGCCGCAATTGAAGTTATGTATCCT and the reverse primer 5′-TCGAGGATCTTGTCAGGAGCGATAGGCTGC. The primers contain NotI and BamHI sites for the insertion of the PCR product 3′ to the VL and VH domains of 3G6 in the retroviral vector SFG (29). The truncated form 3G6-CD28TR was engineered by PCR, preserving only the three first cytoplasmic amino acids after the transmembrane domain (reverse primer 5′-CCCACTCCTCATTCATTGCCCTAGGAGCTCGCC). The cDNAs encoding GD3-synthase (C. Tan and M. Sadelain, unpublished observations), B7.1 (J.-B. Latouche and M. Sadelain, unpublished observations), and HLA A2.1 (our unpublished observations; the HLA A2.1 cDNA was provided by Dr. S.Y. Yang, Sloan-Kettering Institute for Cancer Research) were cloned in the SFG vector as well.
Cells.
Producer cells derived from the packaging cell line PG13 (American Tissue Culture Collection, Rockville, MD [30]) were maintained in DMEM supplemented with glutamine, penicillin, streptomycin (GIBCO BRL, Gaithersburg, MD), and 10% fetal bovine serum (Sigma Chemical Co., St. Louis, MO). The Jurkat T cell line E.6 (American Type Culture Collection) as well as the GD2 + tumor cell line EL4 (American Type Culture Collection) and its GD2 − variant (31) were grown in RPMI 1640 (GIBCO BRL) supplemented with glutamine, penicillin, streptomycin, and 10% fetal bovine serum. NIH 3T3 fibroblasts (American Type Culture Collection) were cultured in DMEM supplemented with glutamine, penicillin, streptomycin (GIBCO BRL), and 10% donor calf serum (Sigma Chemical Co.). The coculture experiments of T cells and irradiated fibroblasts were carried out in AIMV medium (GIBCO BRL) supplemented with 5% donor calf serum.
Retroviral Gene Transfer.
High-titer producer cells were generated from the PG13 packaging cell line as described elsewhere (32). Individual PG13/3G6-CD28 clones were expanded and titrated on A549 cells by FACS® analysis as described (25). The clone PG13/3G6-CD28.12 had a titer of 7 × 105 infectious particles/ml, and the clone PG13/3G6-CD28TR.55 had a titer of 5 × 105 infectious particles/ml. The vector SFG-NTP, encoding an inactive mutant of the human low-affinity growth factor receptor (LNGFR), and the PG13/NTP.6 producer cell line are described elsewhere (25). Retroviral infection of Jurkat cells and human PBLs was carried out as described (26). In brief, PBMCs from healthy donors were separated on Ficoll activated with PHA, and exposed to retroviral particles for 18 h in the presence of polybrene (4 μg/ml). After retroviral infection, the cells were cultured in the presence of 10 U/ml IL-2 (Cetus Corp., Berkeley, CA), and the medium was changed every 3 d. Gene-modified fibroblasts were transduced as described elsewhere (32) and selected for expression of HLA A2.1, B7.1, and/or GD2 (J.-B. Latouche and M. Sadelain, unpublished observations).
Antibodies.
The purified rat antiidiotypic mAb A1G4 directed at the antigen-binding site of 3G6, the purified mouse anti-GD2 antibody 3F8, and the corresponding F(ab′)2 fragment were described earlier (31, 33). Other antibody reagents included 20.4 hybridoma supernatant (mouse anti-LNGFR; American Type Culture Collection); 9.3 ascites (mouse anti–human CD28; provided by Dr. B. Dupont, Sloan-Kettering Institute for Cancer Research); OKT3 (mouse anti–human CD3; Ortho Diagnostic Systems, Inc., Raritan, NJ); unconjugated, PE–conjugated goat anti–mouse IgG (GAM-PE) or anti–rat IgG (GAR-PE), FITC-conjugated goat anti–mouse IgG (GAM-FITC) or anti–rat IgG (GAR-FITC) antibodies (Caltag Laboratories, Inc., San Francisco, CA); conjugated mouse anti–human CD8 antibody (CD8-FITC and CD8-PE) and conjugated mouse anti–human CD25 (CD25-PE) antibody (Becton Dickinson, San Jose, CA).
FACS® Analysis.
Cell surface expression of transduced cells was determined by incubating 106 cells with the antiidiotypic mAb A1G4 or the anti-LNGFR mAb 20.4 on ice for 30 min, followed by washing and incubation with the conjugated secondary antibodies. For staining the T lymphocytes, a 20-min incubation with CD8-FITC, CD8-PE, or CD25-PE antibody was followed after blocking with unconjugated mouse serum. GD2 was detected by incubation with mouse anti-GD2 mAb 3F8 and GAM-PE antibody. Samples were acquired and analyzed with a FACScan® (Becton Dickinson).
IL-2 Secretion and IL-2R Expression.
Duplicate samples of transduced Jurkat (5 × 104/well) were stimulated in a 96-well plate in 200 μl RPMI medium with soluble antiidiotype mAb A1G4 (2 μg/ml), mAb 9.3 (1:1,000), or with EL4 or EL4GD2− cells in the presence of OKT3 (10 μg/ml), cross-linked by sheep anti–mouse beads (3 beads/cell; Dynal Inc., Lake Success, NY). Blocking of cell-surface GD2 was achieved by preincubating cells with the GD2-specific 3F8 F(ab′)2 fragment (10 μg/ml). After 24 h, cell supernatants were harvested and tested for IL-2 content by solid-phase ELISA (Genzyme Corp., Cambridge, MA). In experiments with human T lymphocytes, 105 T cells transduced with 3G6-CD28, 3G6-CD28TR, or NTP were grown overnight in 1 ml medium without IL-2, and incubated the next day with or without saturating amounts of mAbs in 24-well plates. After 24 h half of the cells were analyzed for IL-2Rα expression (CD25) by FACS®, and after 48 h the supernatants were tested for IL-2 concentration by ELISA.
Induction of Apoptosis and Assessment of Viability.
24-well-cluster tissue culture plates were coated with GAM IgG and GAR IgG (10 μg/ml) in 300 μl/well 0.05 M carbonate buffer (pH 9.6) overnight at 4°C. Plates were washed three times with PBS and overlaid with OKT3 (2 μg/ml PBS, 300 μl/well) for 1 h at 37°C. Plates were washed three times with PBS, and T lymphocytes were added at a concentration of 6 × 105/300 μl RPMI/ 10% FCS in the presence of IL-2 (10 U/ml) and mAb 9.3 (1: 1,000) or mAb A1G4 (1 μg/ml). After 3 d of incubation in a humified atmosphere of 5% CO2 at 37°C, T cells were released from the cross-linking conditions and kept for 2 d in the presence of 10 U/ml IL-2. Percentage of live cells relative to total cell number (percent viability) was determined on day 3 by counting cells stained in trypan blue (GIBCO BRL) and quantitated by FACS® analysis using forward and side scatter parameters.
Expansion of Transduced T Cells.
106 T cells were first plated in the presence of OKT3 (10 ng/ml) and 9.3 (1:1,000) or A1G4 (1 μg/ml). After 3 d of culture, the T cells were washed and replated for 3 d in T cell medium containing 10 U/ml of IL-2. This cycle was repeated once more on days 7–12. In the coculture experiments, 106 T cells were cultured for 3 d in medium supplemented with IL-2 in 24-well plates on an irradiated (10 Gy) monolayer of genetically modified NIH 3T3 fibroblasts seeded at a density of 5 × 104/well. T lymphocytes were added at a concentration of 106/ml. After 3 d of coculture, the T cells were kept for 3 d in fresh T cell medium containing 10 U/ml of IL-2. This cycle was repeated once more on days 7–12. On day 6 and 12, the percentage of transduced T cells was determined by FACS® analysis.
Results and Discussion
Antiidiotypic Antibody and Tumor-associated GD2 Antigen Specifically Induce IL-2 Secretion in Jurkat Cells that Express 3G6-CD28.
We constructed a chimeric receptor encompassing most of the human CD28 receptor fused to the scFv derived from the GD2-specific mAb 3G6 (see Materials and Methods). The fusion cDNA was cloned into the SFG vector (29) as described (32), thus placing transcription under the control of the Moloney murine leukemia virus LTR. The full-length protein (36 kD in NIH 3T3 fibroblasts and ∼40 kD in Jurkat cells) was expressed as a dimer as demonstrated by Western blot analysis using a polyclonal antibody directed against the cytoplasmic tail of CD28 (data not shown). Jurkat cells were transduced using recombinant retrovirus pseudotyped with the GaLV envelope as described previously (26). The transduced cells stained positive by FACS® analysis with the antiidiotypic antibody A1G4, one of five different antiidiotypic antibodies that specifically recognize the mAb 3G6 as well as the 3G6-CD28 fusion protein (data not shown).
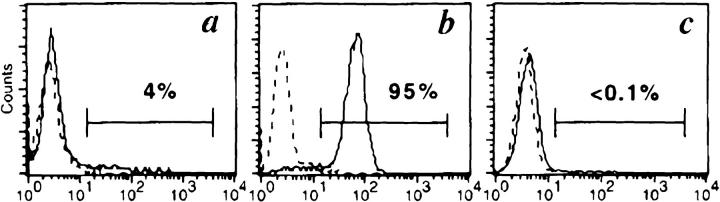
The CD28 function of 3G6-CD28 was first evaluated in a transduced Jurkat clone (obtained by limiting dilution) that harbored one intact copy of the vector as shown by Southern blot analysis (data not shown). The engagement of the 3G6-CD28 receptor by the antiidiotypic mAb A1G4 in transduced Jurkat cells appropriately triggered the earliest events of the CD28 signaling pathway (34), including the association of the 85-kD regulatory subunit of the phosphatidylinositol 3-kinase with the phosphorylated form of the fusion molecule (data not shown). We next investigated the function of 3G6-CD28 in the potentiation of IL-2 secretion, a later event of T cell activation (35, 36). Jurkat cells transduced with 3G6-CD28, 3G6-CD28TR (derived from 3G6-CD28 by truncation of the cytoplasmic tail to disable CD28 signaling [37, 38]), or NTP (25) were stimulated by immobilized anti-CD3 mAb to provide the first signal for T cell activation. Costimulation was provided by the addition of anti-CD28 mAb 9.3, by antiidiotypic mAb A1G4, or by short-term cocultivation (39) with wild-type EL4 cells which express GD2 (Fig. 1 b). As shown in Table 1, coincubation of EL4 with 3G6-CD28/Jurkat cells led to IL-2 secretion in a cell dose–dependent manner. EL4 cells had no stimulatory effect on 3G6-CD28TR/Jurkat and NTP/Jurkat. Coincubation with GD2 − EL4 (Fig. 1 c) had no effect on any of the cell lines. Furthermore, the costimulatory effect of wild-type EL4 was completely abolished by the addition of GD2-specific F(ab′)2. Anti-CD3 antibody alone had very little effect on Jurkat cells except those expressing 3G6-CD28 (Table 1). This modest IL-2 secretion could be explained by the low level of GD2 expression by Jurkat cells (Fig. 1 a) that leads to CD28 signaling by the cell–cell interaction through the fusion molecule. Indeed, the addition of GD2-specific F(ab′)2 to CD3-stimulated 3G6-CD28/Jurkat cells reduced IL-2 secretion below the level elicited by anti-CD3 mAb alone (Table 1). These results indicate, first, that 3G6-CD28 specifically recognizes cell-surface GD2 antigen and, second, that recognition of tumor-associated GD2 leads to effective CD28-dependent signaling, including IL-2 secretion.
Figure 1.
GD2 expression in Jurkat and EL4 cells. Cells were stained with mAb 3F8 (solid line) or isotype-matched mAb 20.4 (broken line), directed against GD2 and human LNGFR, respectively. (a) Jurkat cells; (b) wild-type EL4; (c) EL4GD2−. The three cell lines are, respectively, GD2 low, GD2 +, and GD2 −. GD2 expression by these cells is closely correlated with the costimulatory activity described in Table 1.
Table 1.
Tumor-associated Antigen GD2 Specifically Potentiates IL-2 Production by 3G6-CD28–transduced Jurkat T Cells Activated with Immobilized OKT3
| IL-2 production (pg/ml) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| — — | 9.3 — | A1G4 — | EL4GD2− 5:1 — | EL4GD2− 1:1 — | EL4 5:1 — | EL4 1:1 — | EL4 5:1 F(ab′)2 * | EL4 1:1 F(ab′)2 * | ||||||||||
| 3G6-CD28 | 47 ± 15 | 577 ± 79 | 189 ± 57 | 39 ± 6 | 49 ± 4 | 317 ± 33 | 138 ± 22 | 15 ± 5 | 19 ± 2 | |||||||||
| 3G6-CD28TR | 11 ± 9 | 490 ± 22 | 4 ± 5 | 2 ± 1 | 7 ± 5 | 2 ± 4 | 4 ± 2 | 4 ± 2 | 4 ± 2 | |||||||||
| NTP | 5 ± 5 | 499 ± 32 | 11 ± 2 | <1 | 7 ± 5 | <1 | <1 | <1 | 8 ± 3 | |||||||||
Results are the means ± SD obtained from duplicates. The NTP marker was used as an irrelevant control that could be monitored for cell-suface expression in parallel to 3G6-CD28 and 3G6-CD28TR. T cells (2.5 × 105/ml) were incubated with OKT3 (10 μg/ml, immobilized on sheep anti– mouse beads [3 beads/cell]) and stimulated with soluble 9.3 (ascites 1:1,000), A1G4 (2 μg/ml), EL4, or the GD2 − variant EL4GD2−.
In anti-GD2 blocking conditions, EL4 cells were preincubated with anti-GD2 F(ab′)2 fragment 3F8 (10 μg/ml). After 24 h, supernatants were collected, and IL-2 was measured by ELISA.
Stimulation of Human Primary CD3+ T Lymphocytes Expressing the Fusion Molecule 3G6-CD28 with Anti-CD3 mAb and A1G4 Increases IL-2Rα Expression and IL-2 Production.
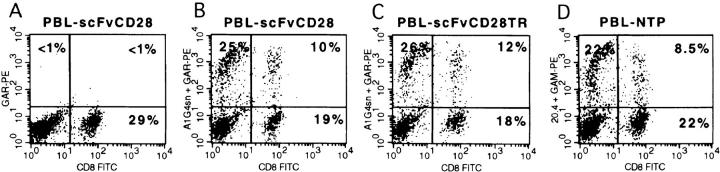
The stimulation of T cells with anti-CD3 and anti-CD28 mAbs leads to enhanced T cell proliferation associated with increased IL-2Rα (CD25) expression and IL-2 secretion (40–42). Therefore, we examined the effect of A1G4 on IL-2R expression and IL-2 secretion in transduced primary T lymphocytes. Peripheral blood T lymphocytes were transduced with GaLV-pseudotyped recombinant retrovirus after mitogen activation, as described (26). 3 d after cocultivation with the producers PG13/3G6-CD28, PG13/3G6-CD28TR, and PG13/NTP, 28–40% of the CD3+ cells stained positively for the vector-encoded receptor in both CD4+ and CD8+ subsets, as shown in Fig. 2. CD3+ lymphocytes represent 94–98% of the cells after 7 d of culture under these conditions (reference 25, and data not shown). The three groups of cells maintained in 10 U/ml of IL-2 expanded at the same rate (data not shown). Gene transfer was confirmed by Southern blot analysis, which indicated that A1G4+ cells harbored on average one integrated vector copy per cell (data not shown). As shown in Table 2, the stimulation of primary T cells with OKT3 and A1G4 increased IL-2R expression as effectively as OKT3 and anti-CD28 mAb in the 3G6-CD28–transduced population. A1G4 had no effect on the NTP-transduced T cells and a very modest effect on CD28TR+ cells. We next examined IL-2 production by T cells treated with A1G4/ OKT3, 9.3/OKT3, or OKT3 alone. Stimulation with OKT3 plus A1G4 increased IL-2 production only in 3G6-CD28–transduced T cells. The stimulation with A1G4 led to an almost equal level of IL-2 secretion as with the mAb 9.3, although the 3G6-CD28+ cells only represented 20% of the total cell population. Therefore, we concluded that the A1G4 mAb provided a progression signal leading to enhanced IL-2R expression and IL-2 production in human primary T lymphocytes transduced with the 3G6-CD28 receptor.
Figure 2.
Retroviral gene transfer of 3G6-CD28, 3G6-CD28TR, and NTP in human primary lymphocytes. PBLs were stained 72 h after retroviral infection with antibodies to CD8 (x-axis) and either A1G4 mAb to stain 3G6-CD28 and 3G6-CD28TR or 20.4 mAb to stain NTP (y-axis). Staining with secondary antibody alone (GAR-PE; A) indicates the background staining. Staining with A1G4 (antiidiotype; B and C) and with 20.4 (anti– NTP; D), followed by the corresponding PE conjugates.
Table 2.
A1G4 Potentiates IL-2 Production and IL-2Rα Expression by 3G6-CD28–transduced T Cells Stimulated with Immobilized OKT3
| IL-2Rα expression* | IL-2 secretion | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | OKT3 | OKT3/9.3 | OKT3/A1G4 | Medium | OKT3 | OKT3/9.3 | OKT3/A1G4 | |||||||||
| % | pg/ml | |||||||||||||||
| 3G6-CD28 | 5 | 20 | 65 | 70 | <0.1 | 3.2 | 16 | 13 | ||||||||
| 3G6-CD28TR | 5.5 | 25 | 60 | 35 | <0.1 | 3.5 | 25 | 2.6 | ||||||||
| NTP | 2 | 15 | 75 | 13 | <0.2 | 2.7 | 8.8 | 4.5 | ||||||||
Primary T cells (105/ml) transduced 4–12 d earlier were stimulated for 24 h with medium or plate-bound OKT3, with or without soluble anti-CD28 or A1G4 mAb on anti–mouse/anti–rat IgG–coated plates. Cells were stimulated with OKT3 (2 μg/ml) and 9.3 (ascites 1:1,000) or A1G4 (2 μg/ml). After 24 h, the cells were dual-stained with A1G4 to identify the transduced subpopulations and with anti-CD25 mAb to measure the expression of the IL-2R by FACS®. After 48 h, supernatants were collected, and IL-2 activity was measured by ELISA. Data represent one of two independent experiments.
Expressed as the percentage of CD25+ cells in the A1G4+ or NTP+ fraction.
3G6-CD28–transduced Primary T Lymphocytes Selectively Survive CD3-dependent Cell Death in the Presence of A1G4.
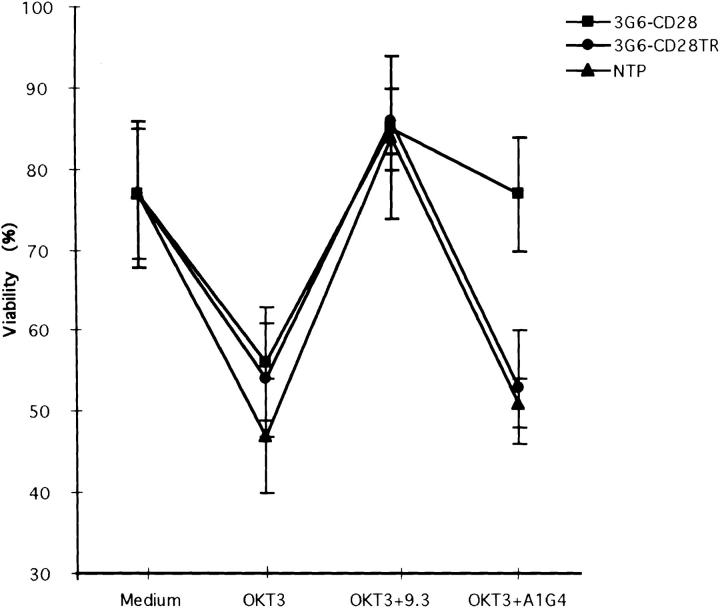
It has been previously reported that activated T cells undergo apoptosis after cross-linking their TCR if they are activated in a nonresting stage (19–23). This effect can be prevented by balanced CD3 and CD28 signaling. To examine whether cross-linking 3G6-CD28 with A1G4 mAb could mimic CD28-mediated survival, we took advantage of a model system in which T cells stimulated with anti-CD3 mAb undergo apoptosis (43–45). Primary T lymphocytes transduced with NTP, 3G6-CD28, or 3G6-CD28TR were kept for 3 d under the following conditions: without antibody, with OKT3 (to trigger apoptosis), with OKT3 and 9.3 (to prevent apoptotic cell death through endogenous CD28 signaling), or with OKT3 and A1G4 (to prevent apoptotic cell death in cells expressing the 3G6-CD28 chimeric molecule). The medium included IL-2 at 10 U/ ml to prevent cell death due to cytokine deprivation. On day 3, the T cells were released from the cross-linking conditions, and cell viability was measured as shown in Fig. 3; cell viability decreased in the presence of the plate-bound OKT3, but was preserved in all groups by the addition of anti-CD28 antibody. A1G4 maintained viability only in the 3G6-CD28–transduced T cell population (which were ∼30% A1G4+). Thus, cross-linking 3G6-CD28 with the mAb A1G4 increased the survival of these lymphocytes cultured under proapoptotic conditions as effectively as the addition of anti-CD28 antibody.
Figure 3.
3G6-CD28–transduced primary T lymphocytes selectively survive CD3-dependent cell death in the presence of A1G4. Primary T lymphocytes transduced with NTP, 3G6-CD28, or 3G6-CD28TR were kept for 3 d under one of the following conditions: medium without antibody, with OKT3, with OKT3 plus 9.3, or with OKT3 plus A1G4. On day 3, the T cells were released from the cross-linking conditions, and cell viability was measured by trypan blue exclusion and FACS® analysis. The data represent the mean ± SD of four experiments. Cell viability decreased in the presence of the plate-bound OKT3 from 77 ± 9% to 47 ± 7% in the NTP-transduced T cells, and from 77 ± 8% to 56 ± 4 and 54 ± 7% in the 3G6-CD28 and 3G6-CD28TR–transduced groups, respectively. Viability was unchanged by the addition of A1G4 to NTP- and 3G6-CD28TR– transduced T cell groups (51 ± 3 and 53 ± 7%, respectively), but increased to 77 ± 7% in the 3G6-CD28–transduced T cell populations (which were ∼30% A1G4+). Survival in all three groups was comparable in the presence of anti-CD3 and anti-CD28 antibodies (85 ± 8%).
A1G4 mAb Induces Preferential Expansion of 3G6-CD28– transduced Primary T Lymphocytes.
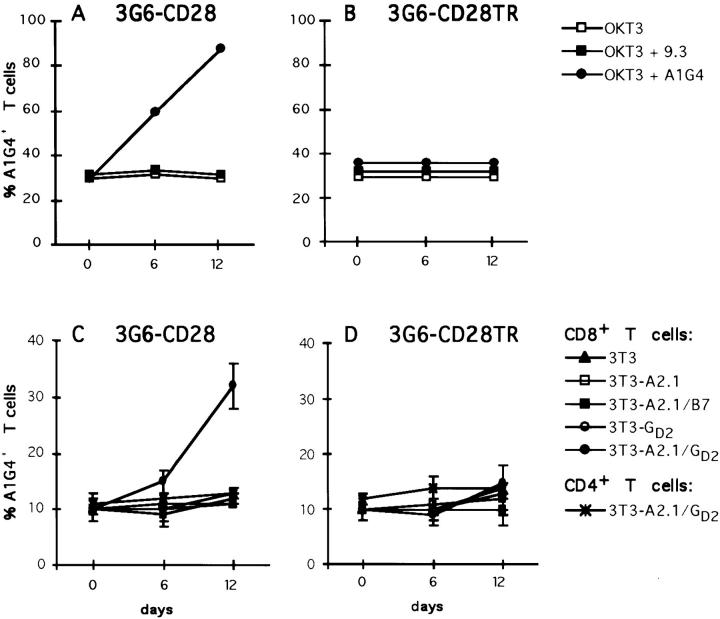
The PBLs transduced with either 3G6-CD28 or 3G6-CD28TR were plated 5 or 6 d after gene transfer in the presence of soluble OKT3 (10 ng/ml) alone or in addition soluble A1G4 or 9.3 mAb. The fraction of CD3+A1G4+ cells was monitored over 12 d. As shown in Fig. 4 B, neither of these three culture conditions increased the percentage of cells expressing 3G6-CD28TR in 12 d. The 3G6-CD28–transduced T cells also remained ∼30% A1G4+ when cultured in either OKT3 or OKT3 plus anti-CD28 antibodies, but steadily increased over time in the presence of OKT3 and A1G4 antibodies (Fig. 4 A). These data strongly suggest that the engagement of 3G6-CD28 with the antiidiotypic antibody A1G4 provides a powerful costimulatory signal dependent on the cytoplasmic domain of CD28, resulting in the selective stimulation of the transduced T cells.
Figure 4.
Antiidiotypic mAb A1G4 and cell-bound GD2 provide costimulatory signals to 3G6-CD28–transduced human primary T lymphocytes. (A and B) Primary T lymphocytes transduced with 3G6-CD28 or 3G6-CD28TR were cultured with soluble OKT3 (10 ng/ml) alone or with the addition of soluble A1G4 or 9.3 mAb as described in Materials and Methods. The percentage of transduced cells was measured by FACS® analysis on days 6 and 12. When cultured in the presence of OKT3 and A1G4 antibodies, T lymphocytes transduced with 3G6-CD28 increased from 30% A1G4+ on day 0 to 60% on day 6 and to 88% on day 12, but remained at ∼30% when cultured otherwise (data represent one of three independent experiments). (C and D) HLA A2.1− primary T lymphocytes transduced with 3G6-CD28 (C) or 3G6-CD28TR (D) were cocultured in triplicate wells with irradiated monolayers of fibroblasts as described in Materials and Methods. The fraction of A1G4+ cells was measured by FACS® analysis in both CD4+ and CD8+ subsets on days 6 and 12. In cultures with 3T3-A2.1/GD2 the percentage of CD8+A1G4+ T cells in the 3G6-CD28–transduced population increased from 10 ± 2% on day 0 to 15 ± 2% on day 6 and to 32 ± 4% on day 12 (C). The percentage of CD4+A1G4+ cells in the CD4+ T cell population remained unchanged under all of these coculture conditions (data not shown). Data represent one of three independent experiments.
Corecognition of MHC–Peptide Complexes and GD2 Selectively Activates PBLs Expressing 3G6-CD28.
To examine whether engagement of 3G6-CD28 by cell-bound GD2 antigen could activate primary T cells recognizing target cells through their TCR, we established an allogeneic coculture system. The genetically modified primary T cells were cultured with fibroblasts expressing an allogeneic MHC class I molecule (HLA A2.1), either alone or together with human B7.1 or GD2. Peripheral blood T lymphocytes from HLA A2.1− donors were transduced with 3G6-CD28, 3G6-CD28TR, or NTP, and cocultured for 12 d as described in Materials and Methods with irradiated fibroblasts. CD4+ and CD8+ lymphocytes were analyzed for transgene expression by FACS® analysis on days 6 and 12. As shown in Fig. 4, CD8+ T cells transduced with 3G6-CD28 remained a constant fraction of all T cells (10 ± 2%) when cocultured either with 3T3 cells alone, 3T3-A2.1, 3T3-A2.1/B7, or 3T3-GD2, but steadily increased to 32 ± 4% by day 12 if exposed to 3T3-A2.1/GD2 (Fig. 4 C). Under the same conditions, the fraction of cells expressing 3G6-CD28TR (Fig. 4 D) or NTP (data not shown) remained unchanged. Thus, the increase in the fraction of 3G6-CD28+ CD8+ T cells required both HLA A2.1 and GD2, which, on the other hand, had no effect on the transduced CD4+ subset (Fig. 4 C). The same result was achieved in cultures of transduced CD8+ T cells in the absence of CD4+ cells (data not shown). These data establish that the engagement of 3G6-CD28 with cell-surface GD2 antigen provides a powerful costimulatory signal to T cells specific for GD2 + target cells.
To provide the CD28 costimulatory signal to T lymphocytes that recognize target cells which lack B7 but express the GD2 antigen, we generated a GD2-specific, CD28-like fusion receptor. The fusion receptor 3G6-CD28 comprises the scFv derived from the GD2-specific mAb 3G6 as its extracellular domain and most of the human CD28 molecule. Our studies focused on human polyclonal primary T cells that were initially mitogen-activated to enable retroviral-mediated gene transfer. We demonstrated that 3G6-CD28 acts as an antigen-specific CD28-like receptor, augmenting IL-2 secretion upon contact with GD2 + tumor cells and selectively conferring increased survival to PBLs cultured under proapoptotic conditions. Antigen-dependent costimulation could be useful in several ways. One is to sustain the survival and function of T cell clones specific for defined target cells that express the appropriate MHC and peptide as well as GD2 antigen. Antigen-dependent costimulation could also be useful in polyclonal lymphocytes, acting to select from a heterogeneous population of T cells those that are able to recognize target cells through their TCR. As shown in Fig. 4, the expression of 3G6-CD28 does provide for the preferred expansion of transduced T cells engaging their TCR on GD2 + target cells. Antigen-dependent CD28 signaling may also be useful to activate an expanded repertoire of tumor-reactive T cells by lowering the threshold antigen density necessary for appropriate T cell activation (14, 46). Furthermore, 3G6-CD28 may be useful to target and sustain the activity of natural killer cells (47) against GD2 + tumor cells. Our data suggest that the concept of antigen-dependent costimulation could be extended to other cell-surface antigens.
Acknowledgments
We thank H.F. Gallardo and J. Greenberg for excellent technical assistance, and Drs. I. Rivière and D. Unutmaz for reviewing the manuscript.
This work was funded in part by Deutsche Forschungsgemeinschaft KR1580 (to A. Krause), by grant ROI-DE-FG02-93ER61658 from the United States Department of Energy (to N.-K.V. Cheung), by grant CA-08748 from the National Institutes of Health (to M. Sadelain), and by the Scholars Award of the James S. McDonnell Foundation for Molecular Medicine in Cancer Research (to M. Sadelain).
Abbreviations used in this paper
- GAM
goat anti–mouse
- GAR
goat anti– rat
- LNGFR
low-affinity nerve growth factor receptor
- NTP
inactive mutant form of the human LNGFR
- scFv
single chain variable region fragment
References
- 1.Houghton AN. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanda NK, Sercarz EE. Induction of anti-self-immunity to cure cancer. Cell. 1995;82:13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 3.Boon T, Coulie PD, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. New strategies for enhancing the immunogenicity of tumors. Curr Opin Immunol. 1993;5:719–725. doi: 10.1016/0952-7915(93)90127-e. [DOI] [PubMed] [Google Scholar]
- 5.Baskar S. Gene-modified tumor cells as cellular vaccine. Cancer Immunol Immunother. 1996;43:165–173. doi: 10.1007/s002620050318. [DOI] [PubMed] [Google Scholar]
- 6.Larson, S.M., G. Segouros, and N.K.C. Cheung. 1995. Antibodies in cancer therapy: basic principles of monoclonal antibodies-isotope conjugates. In Biological Therapy of Cancer—Principle and Practice. V.T. DeVita, S. Hellmann, and S.A. Rosenberg, editors. J.B. Lippincott Company, Philadelphia. 543–552.
- 7.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA. Karnofsky Memorial Lecture. The immunotherapy and gene therapy of cancer. J Clin Oncol. 1992;10:180–199. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- 9.Melief CJM. Tumor eradication by adoptive transfer of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 11.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz RH, Mueller DL, Jenkins MK, Quill H. T cell clonal anergy. Cold Spring Harbor Symp Quant Biol. 1989;54:605–610. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins M. The ups and downs of T cell costimulation. Immunity. 1994;1:443–448. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 14.Lenschow DJ, Walunas T, Bluestone J. CD28/ B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 15.Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM. Human T cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperling AI, Bluestone JA. The complexities of T-cell co-stimulation: CD28 and beyond. Immunol Rev. 1996;153:155–182. doi: 10.1111/j.1600-065x.1996.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 17.Linsley PS, Ledbetter JA. The role of CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 18.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 19.Boehme SA, Leonardo M. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993;23:1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 20.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338–4345. [PubMed] [Google Scholar]
- 21.Russel JH, White C, Loh DY, Meleedy-Rey P. Receptor-stimulated death pathway is opened by an antigen in mature T cells. Proc Natl Acad Sci USA. 1991;8:2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radvanyi LG, Mills GB, Miller RG. Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J Immunol. 1993;156:5704–5715. [PubMed] [Google Scholar]
- 23.Groux H, Monte D, Plouvier B, Capron A, Ameisen JC. CD3-mediated apoptosis of human medullary thymocytes and activated peripheral T cells: respective roles of interleukin-1, interleukin-2, interferon-γ and accessory cells. Eur J Immunol. 1993;23:1623–1629. doi: 10.1002/eji.1830230734. [DOI] [PubMed] [Google Scholar]
- 24.Allison JP, Hurwitz AA, Leach DR. Manipulation of costimulatory signals to enhance antitumor T-cell responses. Curr Opin Immunol. 1995;7:682–686. doi: 10.1016/0952-7915(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 25.Gallardo HF, Tan C, Ory D, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 26.Sadelain, M. 1997. Methods for retrovirus-mediated gene transfer into primary T-lymphocytes. In Methods in Molecular Medicine, Gene Therapy Protocols. P. Robbins, editor. Humana Press Inc., Totowa, NJ. 241–248. [DOI] [PubMed]
- 27.Rivlin K, Guo HF, Larson SM, Cheung NKV. Recombinant anti-ganglioside GD2scFv derived from murine IgM antibodies. Proc Am Soc Clin Oncol. 1996;15:445. . (Abstr.) [Google Scholar]
- 28.Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 29.Riviere I, Brose K, Mulligan RC. Effects of retroviral design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AD, Garcia JV, von Suhr N, Lynch CM, Wilson C, Eiden MV. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2226. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung NK, Canete A, Cheung I, Ye JN, Chongyuan L. Disialoganglioside GD2anti-idiotypic monoclonal antibodies. Int J Cancer. 1993;54:499–505. doi: 10.1002/ijc.2910540324. [DOI] [PubMed] [Google Scholar]
- 32.Riviere, I., and M. Sadelain. 1997. Methods for the construction of retroviral vectors and the generation of high titer producers. In Methods in Molecular Medicine, Gene Therapy Protocols. P. Robbins, editor. Humana Press Inc., Totowa, NJ. 59–78. [DOI] [PubMed]
- 33.Zhao XJ, Cheung NKV. GD2oligosaccharide: target for cytotoxic T lymphocytes. J Exp Med. 1995;182:67–74. doi: 10.1084/jem.182.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudd CE. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 35.June CH, Ledbetter JA, Lindsten T, Thompson CB. Evidence for the involvement of three distinct signals in the induction of IL-2 gene expression in human T lymphocytes. J Immunol. 1989;143:153–161. [PubMed] [Google Scholar]
- 36.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 37.Stein PH, Fraser JD, Weiss A. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol Cell Biol. 1994;14:3392–3402. doi: 10.1128/mcb.14.5.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng JMC, King PD, Sadra A, Liu X, Han A, Selvakumar A, August A, Dupont B. Phosphorylation of each of the distal three tyrosines of the CD28 cytoplasmic tail is required for the CD28-induced T cell IL-2 secretion. Tissue Antigens. 1996;48:1–10. doi: 10.1111/j.1399-0039.1996.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 39.Ueda Y, Levine BL, Huang ML, Freeman GJ, Nadler LM, June CH, Ward SG. Both CD28 ligands CD80 (B7-1) and CD86 (B7-2) activate phosphatidylinositol 3-kinase, and wortmannin reveals heterogeneity in the regulation of T cell IL-2 secretion. Int Immunol. 1995;7:957–966. doi: 10.1093/intimm/7.6.957. [DOI] [PubMed] [Google Scholar]
- 40.Hara T, Fu SM, Hansen JA. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen) J Exp Med. 1985;161:1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baroja ML, Lorre K, Van Vaeck F, Ceuppens JL. The anti-T cell monoclonal antibody 9.3 (anti-CD28) provides a helper signal and bypasses the need for accessory cells in T cell activation with immobilized anti-CD3 and mitogens. Cell Immunol. 1989;120:205–217. doi: 10.1016/0008-8749(89)90188-3. [DOI] [PubMed] [Google Scholar]
- 42.Cerdan C, Martin Y, Courcoul M, Mawar C, Birg F, Olive D. CD28 costimulation regulates long-term expression of the three genes (α, β, γ) encoding the high affinity IL-2 receptor. Res Immunol. 1995;146:164–168. doi: 10.1016/0923-2494(96)80251-3. [DOI] [PubMed] [Google Scholar]
- 43.Radvanyi LG, Shi Y, Vaziri H, Sharma A, Dhala R, Mills GB, Miller RG. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 44.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 45.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 and apoptosis. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 46.Russel JH. Activation-induced cell death of mature T cells in the regulation of immune responses. Curr Opin Immunol. 1995;7:382–388. doi: 10.1016/0952-7915(95)80114-6. [DOI] [PubMed] [Google Scholar]
- 47.Roger R, Breard J, Comisso M, Bohuon C, Pallardy M, Bertoglio J. CD28-mediated cytotoxicity of YT natural killer cells on B7-positive targets induces rapid necrotic death independent of granule exocytosis. Cell Immunol. 1996;168:24–32. doi: 10.1006/cimm.1996.0045. [DOI] [PubMed] [Google Scholar]