Abstract
Most T cells develop through the thymus, where they undergo positive and negative selection. Some peripheral T cells are known to develop in the absence of thymus, but there is insufficient information about their selection. To analyze the selection of extrathymically developed T cells, we reconstituted thymectomized male or female recipient mice with bone marrow cells of mice transgenic for male H-Y antigen–specific T cell receptor (TCR). It was revealed that the T cells bearing self-antigen–specific TCR were not deleted in thymectomized male recipients. More importantly, the absence of H-Y antigen–specific T cells in thymectomized female recipients suggests positive selection of extrathymically developed T cells by the self-antigen. The extrathymically developed T cells in male mice expressed interleukin (IL)-2 receptor β chain (IL-2Rβ) and intermediate levels of CD3 (CD3int) but were natural killer cell (NK)1.1−. They rapidly produced interferon γ but not IL-4 after TCR cross-linking. Furthermore, a similar pattern of cytokine production was observed in CD3intIL-2Rβ+NK1.1− cells in normal mice which have been shown to develop extrathymically. These results suggest that extrathymically developed CD3intIL-2Rβ+NK1.1− cells in normal mice are also positively selected by self-antigens.
Keywords: extrathymic T cells, H-Y transgenic mice, bone marrow transfer, interleukin 2 receptor β chain, inteferon γ
Most peripheral T cells require the thymus for their development. Immature T cells undergo positive and negative selection in the thymus, and consequently only T cells whose antigen receptors are specific for foreign antigen peptides in the context of self-MHC molecules can mature and appear in the periphery. It is also known that some peripheral T cells do not require the thymus for their development. These extrathymically developed T cells are abundant in the gut (1–3) and liver (4–6). Among the intestinal intraepithelial lymphocytes (iIELs), those that express homodimeric α/α CD8 chains have been shown to develop extrathymically (2, 3). Recently, it was revealed that all of the extrathymically developed T cells in both spleen and liver express IL-2Rβ and intermediate levels of CD3 (CD3int; reference 6). Many reports have demonstrated the incomplete negative selection of these extrathymically developed T cells by self-antigens as assessed by anti-Vβ mAbs in conjunction with the endogenous superantigen Mls system (7–11). However, the conditions that induce positive selection of extrathymically developed T cells are still unclear. Negative selection of extrathymically developed T cells by self-antigen peptides has not been fully addressed.
In male mice expressing a transgenic TCR specific for male H-Y antigen peptides in the context of H-2 Db molecules (H-Y transgenic mice), deletion of immature thymocytes was observed (12). This clearly demonstrates negative selection of intrathymically developed self-reactive T cells. However, it has also been shown that there are many male antigen-specific T cells with or without CD8 molecules in the periphery of male H-Y transgenic mice, although the origin of these T cells is still unclear (13, 14). Rocha et al. reported that among the iIELs of male H-Y transgenic mice, those that express heterodimeric α/β CD8 chains are deleted, whereas those with CD8 α/α are not deleted. Moreover, iIELs expressing transgenic TCR and CD8 are not detected in female mice (15). This suggests positive selection of extrathymically developed T cells, especially iIELs, by self-antigens. Similar results were also reported by Poussier et al. (16).
In this study, we reconstituted thymectomized, lethally irradiated male or female recipient mice with bone marrow cells from female H-Y transgenic mice to analyze the selection of extrathymically developed T cells in spleen and liver. We also examined the phenotypes and functions of the T cells developed in thymectomized recipients.
Materials and Methods
Mice.
The mice transgenic for male H-Y antigen–specific TCR (H-Y transgenic mice) were provided by Dr. T.W. Mak, The Amgen Institute (Toronto, Ontario, Canada). C57BL/6 mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and maintained in specific pathogen–free conditions.
Bone Marrow Transfer.
The recipient male or female C57BL/6 mice (H-2b) were thymectomized or sham operated. After 2 wk, they were lethally irradiated (11 Gy), then injected intravenously with 5 × 106 T cell–depleted bone marrow cells from female H-Y transgenic mice (H-2b) on the same day. 7 wk after bone marrow transfer, spleen and liver lymphocytes were collected and used for flow cytometric analysis or in vitro culture assays.
Preparation of Liver Mononuclear Cells.
The liver mononuclear cells (MNC) were harvested as reported previously, with minor modifications (17). In brief, the livers were pressed through 100-gauge stainless steel mesh, then suspended in 5 ml 45% Percoll and layered on 5 ml 67.5% Percoll solution. The gradient was centrifuged at 2,500 rpm at 20°C for 25 min. The cells at the interface were harvested and washed with complete culture medium, then used as liver MNC.
Flow Cytometric Analysis and Antibodies.
The spleen cells and MNC were stained with FITC-conjugated T3.70 mAb (a gift of T.W. Mak), PE-conjugated anti-CD8 mAb (2.43; PharMingen, San Diego, CA), and allophycocyanin (APC)-conjugated anti-CD3 mAb (2C11; PharMingen), in combination with biotin-conjugated anti–IL-2Rβ mAb (TM-β1; Seikagaku Kogyo Co., Tokyo, Japan), anti-NK1.1 mAb (PK136; PharMingen), or anti-CD44 mAb (1M7; PharMingen), followed by staining with streptavidin-perCP (Becton Dickinson, San Jose, CA). They were then analyzed using a FACSCalibur® flow cytometer with the CELLQuest program (Becton Dickinson).
In Vitro Proliferation and Cytokine Production Assay.
5 × 105 spleen cells of male thymectomized or female nonthymectomized mice reconstituted with bone marrow cells from female H-Y transgenic mice were cultured with 5 × 105 irradiated (30 Gy) spleen cells of female or male C57BL/6 mice in 96-well plates. 20 U/ml of recombinant human IL-2 were added to some cultures. The cultures were pulsed with [3H]thymidine on day 4, followed by harvesting 6 h later. To examine the proliferative response to TCR cross-linking, 5 × 105 responder cells were cultured in 96-well plates precoated with or without 5 μg/ml T3.70 mAb. After 2 d, the cultures were pulsed with [3H]thymidine, followed by harvesting 6 h later. To examine the cytokine production, each culture was also performed in 24-well plates at 2.5 × 106 responder cells/ml, and supernatants were collected 24 or 96 h later. IFN-γ or IL-4 in the culture supernatants were measured by an ELISA method.
Analysis of Intracellular Cytokine Synthesis.
C57BL/6 mice were injected intravenously with 4 μg of anti-CD3 mAb (2C11). After 90 min, the spleen cells were harvested, washed, and suspended at 106 cells/ml in complete culture medium, then incubated for 3 h at 37°C in the presence of 10 μg/ml Brefeldin A (Wako Pure Chemical Industries, Ltd., Osaka, Japan). These cells were harvested, washed, and incubated for 30 min at 4°C with PE-conjugated anti-TCR αβ mAb, biotin-conjugated anti–IL-2Rβ mAb, and APC-conjugated anti-CD8 mAb, followed by PerCP-conjugated streptavidin. The cells were washed and fixed in 100 μl of solution A (Cell Perm & Fix; Caltag Laboratories, Inc., South San Francisco, CA) for 30 min at room temperature, and then washed again and resuspended in 50 μl of solution B (permeabilization buffer) containing 1 μg of FITC-conjugated anti–IL-4 mAb or anti–IFN-γ mAb. Negative control samples were incubated with FITC-conjugated isotype-matched mAbs. The cells were incubated for 30 min at 4°C and washed, and cell fluorescence was analyzed by a flow cytometer.
Results
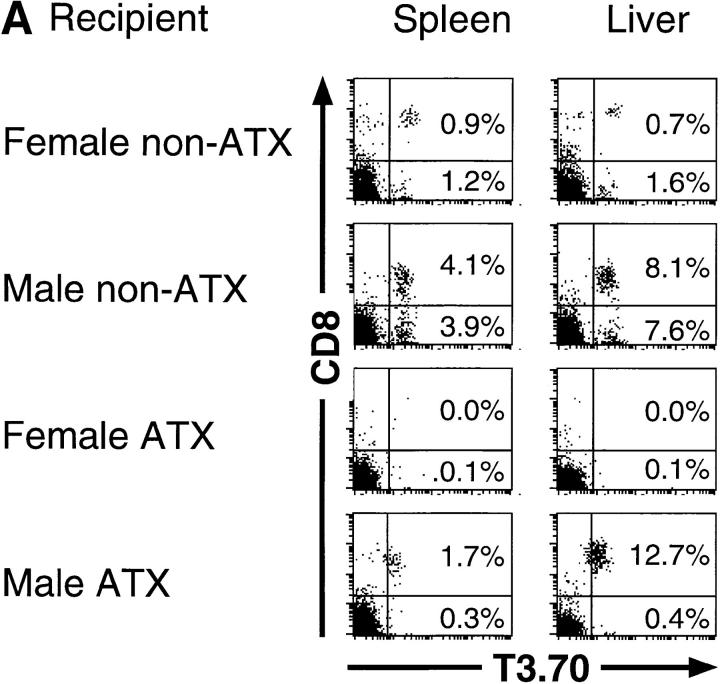
Development of T3.70+ Cells in Thymectomized Male but not Female Recipient Mice after Bone Marrow Transfer.
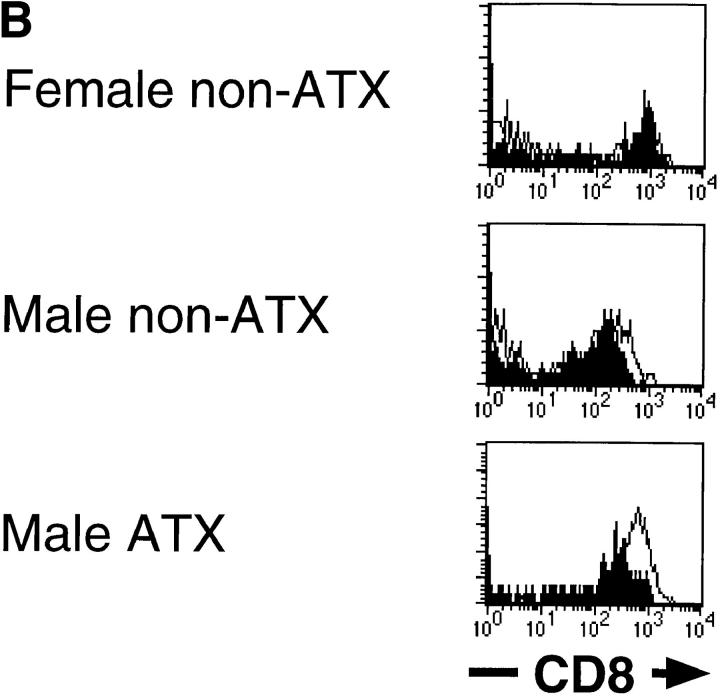
The syngeneic male or female C57BL/6 mice with or without adult thymectomy were lethally irradiated and transferred with T cell–depleted bone marrow cells from female H-Y transgenic mice. 7 wk later, spleen and liver lymphocytes were analyzed using a flow cytometer (Fig. 1 A). In female nonthymectomized recipient mice, T cells bearing transgenic TCR α chain (T3.70+) appeared in both spleens and livers. It has been observed in male H-Y transgenic mice that in spite of negative selection in the thymus, many T3.70+ cells with or without CD8 were present in the periphery, although the levels of CD8 expression are relatively low compared with female mice (13). These two populations of T cells developed in the male nonthymectomized recipients. T3.70+ cells were also observed in both spleens and livers of male thymectomized recipients, but most of them were CD8+. Thus, it is suggested that T3.70+CD8+ cells in the periphery of male H-Y transgenic mice may develop extrathymically, whereas T3.70+CD8− cells may develop through the thymus. Surprisingly, T3.70+ cells were scarcely detected in either spleens or livers of the female thymectomized recipient mice. Therefore, T3.70+ cells in female H-Y transgenic mice may be thymus derived, and none of them can develop extrathymically. These results suggest not that the extrathymically developed self-antigen–specific T cells are not deleted, but rather that the extrathymic development of T cells requires expression of TCR specific for self-antigens. Rocha et al. reported the positive selection of T3.70+ cells in extrathymically developed iIELs in male H-Y transgenic mice (15). They also showed that T3.70+ cells in iIELs express higher levels of CD8 than T3.70+ cells in lymph nodes. Similarly, we found that extrathymically developed T3.70+ cells in liver of male mice express higher levels of CD8 than those in spleen (Fig. 1 B).
Figure 1.
(A) Development of H-Y antigen–specific T cells in male or female thymectomized mice. C57BL/6 mice (H-2b) with or without adult thymectomy (ATX ) were lethally irradiated (11 Gy) and injected intravenously with 5 × 106 T cell– depleted bone marrow cells from female H-Y transgenic mice (H-2b). 7 wk after bone marrow transfer, development of T cells with transgenic TCR α chain (T3.70+) in the spleen and liver was examined by flow cytometric analysis. Four mice are examined, and representative data are shown. The same experiments were repeated, and similar results were obtained. (B) Expression levels of CD8 on T3.70+ cells developed in the bone marrow chimera mice. Expression of CD8 on T3.70+ cells in the spleen ( filled histograms) and liver (open histograms) were analyzed by overlaying histograms.
In Vitro Proliferative Response of T3.70+ T Cells Developed in Male Thymectomized Recipients.
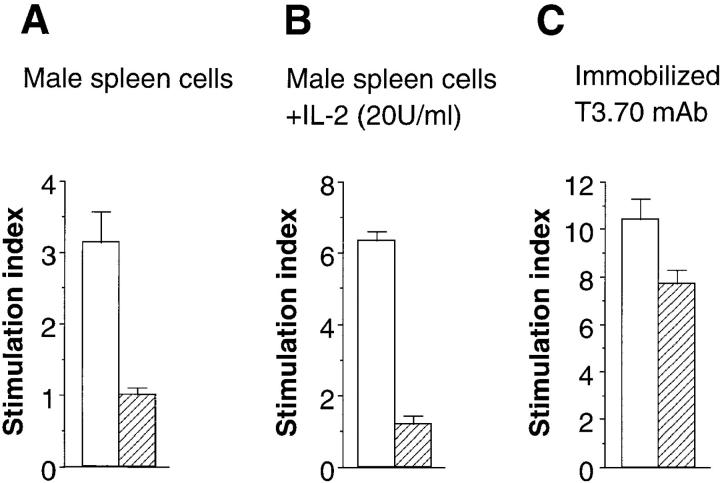
Similar to unmanipulated male H-Y transgenic mice, the thymectomized male recipient mice reconstituted with bone marrow cells of H-Y transgenic mice showed no signs of autoimmunity. It has been reported that T3.70+ T cells in male H-Y transgenic mice are unresponsive to male antigen but can respond to anti-TCR mAbs in vitro (13). Therefore, we next examined the proliferative response of the T cells developed in male thymectomized mice to male antigens in vitro. T cells in the female nonthymectomized recipient mice proliferated in response to male stimulator cells, whereas those in male thymectomized mice showed no proliferative response (Fig. 2 A). The unresponsiveness of T cells in male thymectomized mice could not be reversed by the addition of IL-2 (Fig. 2 B). Nevertheless, T cells in male thymectomized mice showed significant proliferative response to anti-TCR antibodies (Fig. 2 C ). Therefore, self-antigen– specific T cells, which developed in the absence of thymus, seem to be unresponsive to the self-antigen but are not completely anergic to TCR stimulation. This discrepancy may be explained by their low avidity to H-Y antigen, possibly due to somewhat lower levels of CD8 than T3.70+ cells developed in female recipients (Fig. 1). However, the absence of T3.70+ T cells in female thymectomized mice strongly argued that the T3.70+ T cells in male thymectomized mice should functionally recognize H-Y antigens to develop.
Figure 2.
Proliferative response of extrathymically developed H-Y antigen–specific T cells. Proliferative response of the spleen cells, which developed in female nonthymectomized mice (white bars) or male thymectomized mice (hatched bars), in response to male stimulator cells in the absence (A) or presence of 20 U/ml of IL-2 (B) or to immobilized T3.70 antibodies (C ) were examined. Data are represented as stimulation index.
Cytokine Production of Extrathymically Developed T Cells.
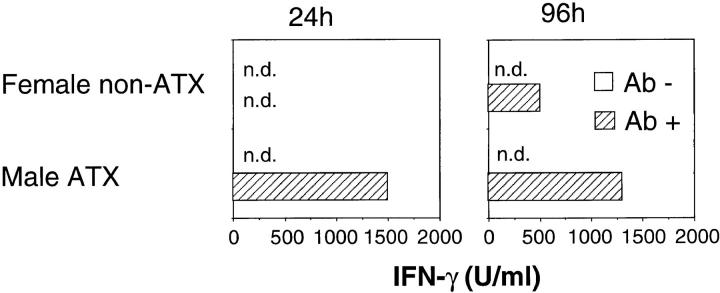
Interestingly, after stimulation with anti-TCR antibodies, IFN-γ was rapidly produced by T cells in male thymectomized mice, within 24 h, while T cells in female nonthymectomized mice produced IFN-γ only 4 d, but not until 24 h, after stimulation (Fig. 3). No IL-4 was detected in the culture supernatants (data not shown). Neither IFN-γ nor IL-4 were detected in the supernatants of the cultures stimulated with male stimulator cells (data not shown).
Figure 3.
IFN-γ production of extrathymically developed H-Y antigen–specific T cells. Spleen cells of female nonthymectomized or male thymectomized recipient mice were cultured in the presence (hatched bars) or absence (white bars) of immobilized T3.70 antibodies. 24 h (left) or 96 h (right) after incubation, culture supernatants were harvested, and IFN-γ was measured by an ELISA assay. ATX, Adult thymectomy. n.d., Not detectable.
Expression of Surface Molecules on Extrathymically Developed T Cells.
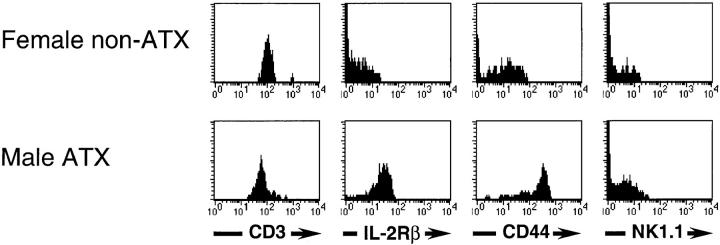
Rapid production of effector cytokines after TCR stimulation has been shown to be a unique feature of NK1.1+ T cells (NK-T cells; references 18 and 19). Although NK-T cells are found in the thymus, extrathymic development of liver and spleen NK-T cells has also been demonstrated (6, 20, 21). Therefore, the question remained whether T cells developed in male thymectomized mice have similar phenotypes of surface molecules as NK-T cells. Interestingly, similar to NK-T cells in normal mice, T3.70+ cells in male thymectomized mice expressed IL-2Rβ, a high level of CD44, and an intermediate level of CD3 (CD3int), but were NK1.1− (Fig. 4). Thus, T3.70+ cells in male thymectomized mice are phenotypically similar to CD3intIL-2Rβ+NK1.1− T cells in normal mice which have also been shown to develop extrathymically (22, 23). On the other hand, CD8+ T cells in female nonthymectomized mice were CD3highIL-2Rβ−CD44low, similar to conventional intrathymically developed naive T cells.
Figure 4.
Expression of surface molecules on H-Y antigen–specific T cells. Expression of various surface molecules on T3.70+CD8+ cells developed in female nonthymectomized (top) or male thymectomized recipient mice (bottom) were analyzed using a flow cytometer. ATX, Adult thymectomy.
Cytokine Production of CD3intNK1.1−CD8+ T Cells in Normal Mice after Intravenous Injection of Anti-CD3 Antibodies.
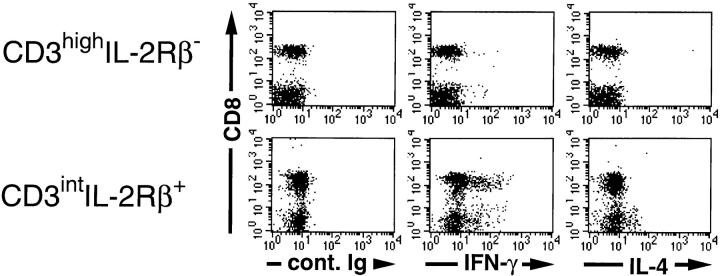
Although CD3intNK1.1+ T cells, most of which are CD4−CD8− or CD4+CD8−, have been shown to produce IL-4 in addition to IFN-γ rapidly after intravenous injection of anti-TCR antibodies (18), production of these cytokines by CD3intNK1.1− T cells, most of which are CD4−CD8+, has not been fully addressed. Therefore, we examined the cytokine production of CD3intNK1.1−CD8+ T cells in normal mice after in vivo administration of anti-TCR antibodies. As shown in Fig 5, IFN-γ–producing cells were observed in both CD8+ and CD8− populations of CD3intIL-2Rβ+ cells, but not in those of CD3highIL-2Rβ− cells. IL-4–producing cells were only detected in the CD8− population of CD3int cells, which contains NK-T cells. This implies that, like T3.70+CD8+ T cells in male H-Y transgenic mice, CD3intIL-2Rβ+CD8+ cells in normal mice produce IFN-γ but not IL-4 rapidly after TCR stimulation. These results indicate that extrathymically developed T3.70+CD8+ T cells in the periphery of male H-Y transgenic mice are phenotypically and functionally indistinguishable from CD3intNK1.1−CD8+ T cells in normal mice. It can also be suggested that extrathymically developed CD3intNK1.1−CD8+ T cells in normal mice are positively selected by some self-antigens.
Figure 5.
Production of IFN-γ or IL-4 by various subsets of T cells after intravenous injection of anti-TCR mAb in normal mice. C57BL/6 mice were injected intravenously with 3 μg of anti-CD3 mAb (2C11). After 90 min, spleen cells were harvested and cultured for 3 h at 37°C in the presence of 10 μg/ml Brefeldin A. Intracellular cytokine-producing cells were examined by flow cytometry and analyzed by gating on either CD3highIL-2Rβ− (top) or CD3intIL-2Rβ+ (bottom) cells. Note that all of the CD3int cells but none of the CD3high cells express IL-2Rβ.
Discussion
In this study, we have shown that extrathymically developed T cells expressing self-antigen–specific TCR are not deleted, but rather cannot develop in the absence of the self-antigen. This feature is in striking contrast to the positive and negative selection events in the thymus. There have been many reports showing the incomplete negative selection of extrathymically developed T cells (7–11). Moreover, using H-Y transgenic mice, it was also reported that among iIELs, those with male antigen–specific TCR and CD8 α/α were positively selected independently of the thymus only in male mice (15). These studies are consistent with our results showing positive selection of extrathymically developed splenic or liver T cells by self-antigens.
It has been shown that many T cells expressing male antigen–specific TCR with or without CD8 are present in the periphery of male H-Y transgenic mice, despite the absence of mature CD8 single positive thymocytes (13). Our results suggest that those with CD8 may develop extrathymically and those without CD8 may develop intrathymically. This was confirmed by analyzing the phenotypes of T cells in unmanipulated male H-Y transgenic mice. Of the T cells in male transgenic mice, those with and without CD8 show phenotypes of extrathymically and intrathymically developed T cells, respectively (our unpublished observations). It is possible that because of the absence of CD8 molecules and the resulting low avidity to H-Y antigen, CD8− cells may escape the negative selection in the thymus and appear in the periphery of male H-Y transgenic mice. A previous report showing the absence of T cells in male H-Y transgenic mice crossed with nude mice suggests thymic origin of all peripheral T cells in male H-Y transgenic mice (14). We have no precise explanation for the discrepancy between those results and ours. We could not detect any remnant thymus in our male thymectomized recipients macroscopically. The striking difference in the proportion of CD8+ to CD8− cells between nonthymectomized and thymectomized recipients also argues against remanence of thymus. Moreover, studies showing extrathymic development of iIELs in H-Y transgenic mice support our results (15, 16). Unfortunately, liver and iIELs of H-Y transgenic mice crossed with nude mice have not been studied. Because extrathymically developed T cells are abundant in the gut (1–3) and liver (4–6), T3.70+ cells may be detected only in these locations. It is well known that the number of extrathymically developed T cells in adult thymectomized radiation chimera is close to that in euthymic mice and is much larger than that in nude mice (24, 25). This could also account for the discrepancy. Another possibility is that T3.70+CD8+ T cells found in male thymectomized mice were the progeny of a few T cell contaminants in the donor bone marrow cells from female H-Y transgenic mice and expanded only in male recipients. However, it has been shown that T3.70+ cells from female H-Y transgenic mice became anergic to TCR stimulation and downmodulated CD8 expression after transfer into male recipient mice (14), in contrast to T3.70+ cells developed in our male recipient mice, which showed proliferative response to T3.70+ mAbs (Fig. 2 C) and expressed high levels of CD8, especially those in the liver (Fig. 1 B).
It has been shown that CD3intIL-2Rβ+ cells are the only splenic or liver T cells that develop extrathymically (6, 22). Among CD3intIL-2Rβ+ cells, those with NK1.1 (NK-T cells) have been shown to produce IL-4 and IFN-γ rapidly after TCR stimulation without prior priming (18, 19). Interestingly, Yoshimoto and Paul observed the expression of IFN-γ mRNA in splenic CD8 T cells after intravenous injection of anti-CD3 mAb, although it was not addressed whether they were also CD3intIL-2Rβ+ cells or CD3high conventional T cells (18). In addition, rapid IFN-γ production after administration of anti-TCR antibodies was also observed in CD1 knockout mice, which selectively lack NK-T cells (26–28). We have shown in this study that the CD8+ T cells that produce IFN-γ rapidly after TCR stimulation are CD3intIL-2Rβ+ cells (Fig. 4). We also showed that extrathymically developed male antigen–specific T cells in male recipient mice rapidly produce IFN-γ but not IL-4 after TCR cross-linking. In addition, they are CD3intIL-2Rβ+ but NK1.1−. Therefore, extrathymically developed CD3intIL-2Rβ+ T cells in normal mice, especially those of the CD8+NK1.1− population, are phenotypically and functionally indistinguishable from extrathymically developed T3.70+CD8+ T cells in male transgenic mice. These results imply that extrathymically developed T cells in normal mice may be also positively selected by self-antigens.
We could not detect any response of the extrathymically developed, potentially self-reactive T cells to the self-antigens in vitro. However, a previous study showing slow expansion of T3.70+CD8+ T cells in male transgenic mice upon transfer into syngeneic male recipients (14) suggests responsiveness of these T cells to male antigen in vivo which may be too weak to be detected in vitro. Expansion of extrathymically developed T cells with age has also been observed in normal mice (4, 29, 30). Similar to male antigen–specific T cells in male H-Y transgenic mice, extrathymically developed, potentially self-reactive T cells in normal mice can respond to TCR cross-linking by antibodies (6). Nevertheless, neither male H-Y transgenic mice nor athymic nontransgenic mice develop autoimmune disease. It will be important to further clarify the in vivo function of extrathymically developed self-antigen–specific T cells.
Acknowledgments
We are grateful to T.W. Mak for providing us with H-Y transgenic mice and T3.70 hybridomas.
This work was supported in part by a grant from the Ministry of Education, Science, and Culture of Japan.
References
- 1.Mosley RL, Styre D, Klein JR. Differentiation and functional maturation of bone marrow-derived intestinal epithelial T cells expressing membrane T cell receptor in athymic radiation chimeras. J Immunol. 1990;145:1369–1375. [PubMed] [Google Scholar]
- 2.Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandeira A, Itohara S, Bonneville M, Burlen-Defranoux O, Mota-Santos T, Coutinho A, Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor γδ. Proc Natl Acad Sci USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iiai T, Watanabe H, Seki S, Sugiura K, Hirokawa K, Utsuyama M, Takahashi-Iwanaga H, Iwanaga T, Ohteki T, Abo T. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992;77:556–563. [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtsuka K, Iiai T, Watanabe H, Tanaka T, Miyasaka M, Sato K, Asakura H, Abo T. Similarities and differences between extrathymic T cells residing in mouse liver and intestine. Cell Immunol. 1994;153:52–66. doi: 10.1006/cimm.1994.1005. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodes RJ, Sharrow SO, Solomon A. Failure of T cell receptor Vβ negative selection in an athymic environment. Science. 1989;246:1041–1044. doi: 10.1126/science.2587987. [DOI] [PubMed] [Google Scholar]
- 8.Fry AM, Jones LA, Kruisbeek AM, Matis LA. Thymic requirement for clonal deletion during T cell development. Science. 1989;246:1044–1046. doi: 10.1126/science.2511630. [DOI] [PubMed] [Google Scholar]
- 9.Murosaki S, Yoshikai Y, Ishida A, Nakamura T, Matsuzaki G, Takimoto H, Yuuki H, Nomoto K. Failure of T cell receptor Vβ negative selection in murine intestinal intra-epithelial lymphocytes. Int Immunol. 1991;3:1005–1013. doi: 10.1093/intimm/3.10.1005. [DOI] [PubMed] [Google Scholar]
- 10.Rocha B, Vassalli P, Guy-Grand D. The Vβ repertoire of mouse gut homodimeric αCD8+ intraepithelial T cell receptor α/β+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawachi H, Watanabe H, Haga M, Iiai T, Hatakeyama K, Abo T. Self-reactive T cell clones in a restricted population of interleukin-2 receptor β+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995;25:2272–2278. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- 12.Kisielow P, Blüthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 13.Teh H-S, Kishi H, Scott B, von Boehmer H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989;169:795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Boehmer H, Kirberg J, Rocha B. An unusual lineage of α/β T cells that contains autoreactive cells. J Exp Med. 1991;174:1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 α/α co- receptors by self-antigen in the murine gut. Proc Natl Acad Sci USA. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poussier P, Teh HS, Julius M. Thymus-independent positive and negative selection of T cells expressing a major histocompatibility complex class I restricted transgenic T cell receptor α/β in the intestinal epithelium. J Exp Med. 1993;178:1947–1957. doi: 10.1084/jem.178.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiromatsu K, Matsuzaki G, Tauchi Y, Yoshikai Y, Nomoto K. Sequential analysis of T cells in the liver during murine listerial infection. J Immunol. 1992;149:568–573. [PubMed] [Google Scholar]
- 18.Yoshimoto T, Paul WE. CD4pos, NK1.1posT cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Paul WE. Cultured NK1.1+CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 20.Sykes M. Unusual T cell populations in adult bone marrow. Prevalence of CD3+CD4−CD8− and αβTCR+ NK1.1+ cells. J Immunol. 1990;145:3209–3215. [PubMed] [Google Scholar]
- 21.Kikly K, Dennert G. Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice. J Immunol. 1992;149:403–412. [PubMed] [Google Scholar]
- 22.MacDonald HR. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanage T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–2983. [PubMed] [Google Scholar]
- 24.Poussier P, Edouard P, Lee C, Binnie M, Julius M. Thymus-independent development and negative selection of T cells expressing T cell receptor α/β in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J Exp Med. 1992;176:187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin T, Matsuzaki G, Kenai H, Nakamura T, Nomoto K. Thymus influences the development of extrathymically developed intestinal intraepithelial lymphocytes. Eur J Immunol. 1993;23:1968–1974. doi: 10.1002/eji.1830230836. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y-H, Chiu NM, Mandal M, Wang N, Wang C-R. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 27.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Kaer LV. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 28.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4- secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 29.Takimoto H, Nakamura T, Takeuchi M, Sumi Y, Tanaka T, Nomoto K, Yoshikai Y. Age-associated increase in number of CD4+CD8+ intestinal intraepithelial lymphocytes in rats. Eur J Immunol. 1992;22:159–167. doi: 10.1002/eji.1830220124. [DOI] [PubMed] [Google Scholar]
- 30.Ohteki T, Okuyama R, Seki S, Abo T, Sugiura K, Kusumi A, Ohmori T, Watanabe H, Kumagai K. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992;149:1562–1570. [PubMed] [Google Scholar]