Abstract
Respiratory syncytial virus (RSV) remains a major cause of morbidity and mortality in infants and the elderly and is a continuing challenge for vaccine development. A murine T helper cell (Th) type 2 response associates with enhanced lung pathology, which has been observed in past infant trials using formalin-inactivated RSV vaccine. In this study, we have engineered an optimized plasmid DNA vector expressing the RSV fusion (F) protein (DNA-F). DNA-F was as effective as live RSV in mice at inducing neutralizing antibody and cytotoxic T lymphocyte responses, protection against infection, and high mRNA expression of lung interferon γ after viral challenge. Furthermore, a DNA-F boost could switch a preestablished anti-RSV Th2 response towards a Th1 response. Critical elements for the optimization of the plasmid constructs included expression of a secretory form of the F protein and the presence of the rabbit β-globin intron II sequence upstream of the F-encoding sequence. In addition, anti-F systemic immune response profile could be modulated by the route of DNA-F delivery: intramuscular immunization resulted in balanced responses, whereas intradermal immunization resulted in a Th2 type of response. Thus, DNA-F immunization may provide a novel and promising RSV vaccination strategy.
Keywords: respiratory syncytial virus, DNA vaccine, vector design, F protein, immune modulation
Respiratory syncytial virus (RSV)1 is the principal etiological agent of bronchiolitis and pneumonia in infants and young children worldwide, causing in the USA alone an estimated 4,500 deaths and 91,000 hospitalizations annually (1). RSV is also responsible for an estimated 3.3 million cases of respiratory tract diseases in the elderly annually in the USA (2). Thus, there is an urgent need for a safe and effective RSV vaccine. Protective immunity against RSV is provided by virus-neutralizing antibodies against the surface fusion (F) and attachment (G) proteins (3). The conserved F protein is a cross-protective antigen against both RSV A and B subtypes (4, 5) and a target for human CTLs shown to reduce disease severity (6, 7).
RSV has been a difficult vaccination target. Immunity elicited by primary infection declines rapidly and cannot prevent reinfection although the severity of the disease decreases (3). The development of an RSV vaccine that confers better protection than natural immunity remains a challenge. Furthermore, vaccination of infants with a formalin-inactivated (FI)-RSV preparation in the 1960's did not prevent RSV disease despite the induction of strong anti-F antibody responses, and led to enhanced pulmonary disease, even death, in some vaccinees upon RSV infection (8, 9).
Although the mechanism remains unknown, vaccine-augmented lung disease may be caused by structurally altered antigens and has been attributed to an imbalanced cell-mediated immune response of the Th2 type (10). More recent views of immune effector mechanisms emphasize the importance of functionally polarized T helper cell subsets characterized by distinct cytokine repertoires (Th1 and Th2; reference 11). In the mouse model of RSV infection, the pattern of pulmonary cytokine response depends on the nature of the priming immunogen. Enhanced lung pathology observed in mice vaccinated with FI-RSV and challenged with live virus is mediated by CD4+ T cells and is associated with Th2 cytokines (IL-4, IL-5, and IL-10). In contrast, immunization with live RSV that does not cause enhanced lung pathology elicits a mixed Th1/Th2 cytokine response dominated by IFN-γ, a Th1 cytokine (12–15).
Candidate live attenuated RSV vaccines currently under evaluation in humans have shown promise in chimpanzees (16). However, it will be important to assess their risk of genetic reversion to the wild-type virus. Subunit vaccines require purification of viral proteins that can lead to denaturation, and deleterious immune responses in particular (3) when formulated in alum, the only adjuvant for human use, known to induce Th2 responses. The development of a DNA-based RSV vaccine offers several advantages, including its simplicity, the de novo synthesis of properly folded and glycosylated protein antigens, the ability to induce both neutralizing antibody and cell-mediated responses, including CTLs, and the possibility to modulate the pattern of immune responses by the route of DNA administration (17, 18). Furthermore, DNA immunization can elicit lifelong immunity against viruses (19), a highly desirable property for an RSV vaccine.
The objective of this study was to determine whether protective immunity against RSV could be elicited by DNA immunization and to identify elements critical to the success of this strategy. We found that vaccination of mice with an optimized plasmid DNA vector expressing the RSV F protein (DNA-F) induced virus-neutralizing antibodies, CTLs, protection against viral challenge, and high mRNA expression of lung IFN-γ after viral challenge. A DNA-F boost could also switch a preestablished anti-RSV Th2 response towards a Th1 response. Critical elements for the optimization of the plasmid constructs included expression of a secretory form of the protein and the presence of rabbit β-globin intron II sequence upstream of the F protein–encoding sequence. In addition, anti-F systemic immune responses could be modulated by the route of DNA-F delivery.
Materials and Methods
Construction of Plasmids Expressing the RSV F Protein.
Four plasmid vectors (pXL1–pXL4) were constructed to assess the respective protective abilities of full-length and truncated RSV F proteins in the presence or absence of the rabbit β-globin intron II sequence. The 1.6-kb SspI–PstI fragment containing the promoter and intron A sequences of human CMV Towne strain derived from plasmid pRL43a was provided by Dr. G.S. Hayward (Johns Hopkins University, Baltimore, MD; reference 20) and was used in all vectors. For the construction of pXL1 and pXL2, a 1.6-kb EcoRI–BamHI fragment containing the truncated form of the F cDNA originally cloned from a clinical subgroup A RSV isolate was excised from plasmid pRSVF (21). For the construction of pXL3 and pXL4, the full-length F cDNA was excised as a 1.9-kb EcoRI fragment from a recombinant pBluescript M13-SK containing the F gene insert. The rabbit β-globin intron II sequence derived from pSG5 (Stratagene, La Jolla, CA) was inserted upstream of the F protein–encoding sequence in pXL2 and pXL4. With the exception of the CMV promoter and intron A sequences, the other vector components (including the bovine growth hormone poly-A sequence) in pXL1–pXL4, were derived from plasmid pRc/CMV (Invitrogen, San Diego, CA). The integrity of the F gene and intron II sequences was confirmed by DNA sequencing.
Immunization and RSV Challenge Protocols.
Plasmid DNA was purified using plasmid Mega kits from Qiagen (Chatsworth, CA) according to the manufacturer's instructions. For intramuscular immunization, the tibialis anterior muscles of BALB/c mice (male, 6–8-wk-old, 8–12/group, from The Jackson Laboratory, Bar Harbor, ME) were injected bilaterally with 2 × 50 μg (1 μg/μl in PBS) of a given plasmid. 5 d before DNA injection, the muscles were treated with 2 × 50 μl (10 mM in PBS) of cardiotoxin (Latoxan, Rosans, France) to increase DNA uptake and enhance immune responses (22). Animals were boosted 6 wk later with the same dose of plasmid DNA. For intradermal immunization, 100 μg of pXL2 (2 μg/μl in PBS) was injected near the base of the tail and boosted 6 wk later with an equivalent dose of plasmid DNA. Mice in the control groups were immunized either intramuscularly with the vector backbone (pXL0), intranasally with 106 PFU of a clinical RSV strain of the A2 subtype provided by Dr. B. Graham (Vanderbilt University, Nashville, TN; reference 23), or intramuscularly with an FI-RSV vaccine (100 μl) prepared according to the procedures used for the 1960's trials (8, 9). 4 wk after the second immunization, mice were challenged intranasally with 106 PFU of the RSV A2 strain. Lungs were aseptically removed 4 d later, weighed, and homogenized in 2 ml of DMEM containing 10% FCS and high glucose. The number of PFU in lung homogenates was determined in duplicate as previously described (24).
Immunogenicity Studies.
Immune sera were analyzed for anti-RSV F antibody titers (IgG, IgG1, and IgG2a, respectively) using ELISAs and for RSV-specific plaque reduction titers. ELISAs were performed with an immunoaffinity-purified full-length RSV F protein (50 ng/ml) using twofold serial dilutions of immune sera. Goat anti–mouse IgG antibody conjugated to alkaline phosphatase (Jackson ImmunoResearch, Mississauga, Ontario, Canada) was used as secondary antibody. For the measurement of IgG1 and IgG2a antibody titers, the secondary antibodies used were, respectively, monospecific sheep anti–mouse IgG1 (Serotec, Toronto, Ontario, Canada) and rat anti–mouse IgG2a (Zymed, San Francisco, CA) antibodies conjugated to alkaline phosphatase. Plaque reduction titers were determined according to Prince et al. (24). The RSV-specific plaque reduction titer was defined as the serum dilution yielding 60% reduction in plaque number. Both ELISAs and plaque reduction assays were performed in duplicate and data are expressed as the means of two determinations.
CTL Studies.
Spleens from immunized mice were removed to prepare single cell suspensions, which were then pooled. Splenocytes were incubated at 2.5 × 106 cells/ml in complete RPMI medium containing 10 U/ml of murine IL-2 with γ-irradiated (3,000 rads) syngeneic splenocytes (2.5 × 106 cells/ml) infected with 1 PFU/cell RSV for 2 h. CTL activity was assessed in a standard 4-h Cr-release assay 5 d after in vitro restimulation. Target cells were 51Cr-labeled uninfected BALB/c fibroblasts (BC cells) and persistently RSV-infected BCH4 fibroblasts (25), respectively. Effector cells were incubated with 2 × 103 target cells at varying E/T ratios (200 μl, 96-well V-bottomed plates, 4 h at 37°C). Spontaneous and total Cr releases were determined by incubating target cells either with medium or with 2.5% Triton X-100 in the absence of effector splenocytes. The percentage of specific Cr release was calculated as (counts − spontaneous counts)/(total counts − spontaneous counts) × 100. Tests were performed in triplicate and data are expressed as the means of three determinations. The experiment was performed three times. To determine the phenotype of CTLs induced by DNA immunization, effector cells were incubated for 1 h with 10 μg/ml of either a pool of anti-CD4 mAbs (GK1.5 and YTS 177.9; references 26, 27) or a pool of anti-CD8 mAbs (53-6.7, YTS 169 and YTS 105.18; references 26, 28) before adding the target cells. To determine the effect of anti-MHC class I and class II antibodies on CTL killing, 51Cr-labeled BC or BCH4 cells were incubated either with 20 μl of culture supernatant from a hybridoma secreting an anti-H2 class I mAb (34-1-2S) that recognizes both Kd and Dd antigens (29) or with 50 μl of an anti-H2 class II mAb (MK-D6) that recognizes I-Ad (30) before the addition of the effector cells.
Analysis of Cytokine Expression in Lung Tissues.
4 d after RSV challenge, lungs were removed from mice and immediately frozen in liquid nitrogen. Total RNA was prepared from lungs homogenized in TRIzol/β-mercaptoethanol by chloroform extraction and isopropanol precipitation. Reverse transcriptase PCR was then carried out on the RNA samples using IL-4, IL-5, or IFN-γ–specific primers (CloneTech, Mississauga, Ontario, Canada). IL-4 and IL-5 messages were amplified for 25 cycles, whereas IFN-γ mRNA was amplified for 30 cycles. The amplified products were then liquid-hybridized to cytokine-specific 32P-labeled probes (CloneTech), resolved on 5% polyacrylamide gels, and quantitated by scanning of the radioactive signals in the gels. At least three mouse lungs were removed from each treatment group and analyzed for lung cytokine expression a minimum of two times.
Statistical Analyses.
Data were not distributed normally and therefore were analyzed using the nonparametric Mann-Whitney test (SigmaStat software; Jandel Scientific Software, Guelph, Ontario, Canada). Comparisons were made at a significance level of 0.05 (P <0.05).
Lung Histopathology Studies.
4 d after viral challenge, lungs from immunized mice were asceptically removed and fixed by airway perfusion with PBS-buffered formalin. Two hematoxylin and eosin–stained paraffin-embedded sections were prepared for each mouse lung. Lungs were sectioned to the largest cross-sectional area. One slide contained the left lung lobes and the other slide the right lung lobes. Individual slides were then read blindly in random order and scored using a modification of the procedure described by Murphy et al. (31). In brief, inflammatory infiltrates of individual bronchioles and pulmonary vessels were scored from 1 to 6: 1, surrounding space free of infiltrating cells; 2, surrounding space contains few infiltrating cells; 3, surrounding space contains focal aggregates of infiltrating cells; 4, surrounding space contains single uninterrupted layer of infiltrating cells; 5, surrounding space contains two uninterrupted layers of infiltrating cells; and 6, surrounding space contains three or more uninterrupted layers of infiltrating cells.
Slides were uncoded after being scored and values were entered into a computer-based statistics program (SuperANOVA; Abacus Concepts, Berkeley, CA). Main effects on mean scores for bronchioles and blood vessels were tested by one-way analyses of variance. Pairwise comparisons were made using Duncan's new multirange test at a significance level of 0.05 (P <0.05).
Results and Discussion
Construction of Plasmid Vectors (pXL1–pXL4) Encoding the RSV F Protein.
To determine if protective immunity against RSV could be elicited by DNA immunization and to optimize the DNA vectors, we engineered four plasmid constructs expressing the RSV F gene under the control of the immediate-early promoter and intron A sequences of human CMV and the bovine growth hormone poly-A sequence. Plasmids pXL1 and pXL2 were designed to express a truncated F gene to produce the soluble extracellular domain of the F protein. Plasmids pXL3 and pXL4 encode the full-length, membrane-anchored F protein. To circumvent the tendency of RSV F RNA to undergo aberrant splicing, we inserted the rabbit β-globin intron II sequence upstream of the F coding region in pXL2 and pXL4. These plasmids were then tested in mice for their immunoprotective ability against RSV.
Mouse Anti-F Antibody Responses Elicited by pXL1–pXL4.
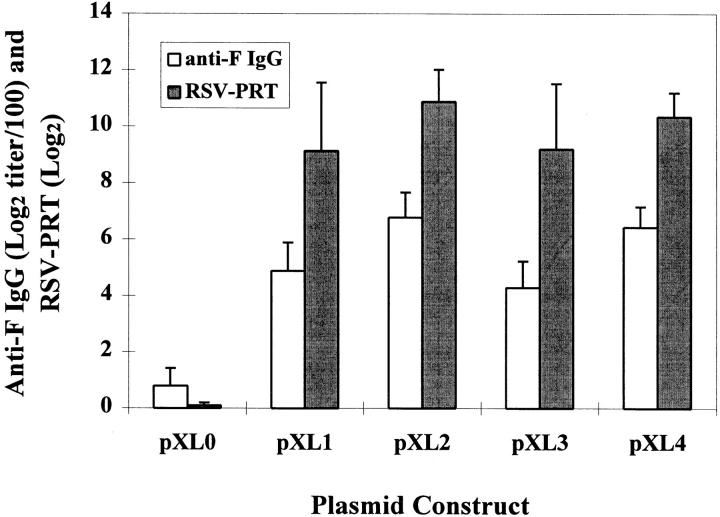
Groups of nine BALB/c mice received bilateral intramuscular injections of 100 μg of each individual plasmid, followed by a boost 6 wk later. Blood samples were obtained at 10 wk for antibody measurements. All four F protein– expressing plasmids (pXL1–pXL4), but not the vector backbone control (pXL0), induced strong humoral responses (Fig. 1). Levels of anti-F IgG antibodies reached endpoint ELISA titers of 5–7 (Log2 titer/100). There was no significant difference among anti-F IgG antibody levels elicited by the four F plasmids. Substantial in vitro RSV-specific plaque reduction titers (Log2 titers, 9.14–10.88) indicated that the anti-F antibodies induced by pXL1–pXL4 had strong virus-neutralizing activity.
Figure 1.
Serum antibody responses to pXL1–pXL4 immunization. Mice were immunized with 100 μg i.m. of pXL1–pXL4 and the vector control pXL0, respectively, at 0 and 6 wk. Immune sera obtained at 10 wk were analyzed for both anti-F IgG ELISA and RSV-specific plaque reduction titers (RSV-PRT).
Protective Ability of pXL1–pXL4 against RSV in Mice.
To assess the protective ability of these vectors, DNA- immunized mice were challenged intranasally with RSV 4 wk after the boost and lungs were recovered 4 d later for measurements of virus replication. All plasmids generated protective immunity, reducing RSV titers by at least 10-fold compared with pXL0 (Table 1). However, pXL1 encoding a secretory F protein was 100-fold more effective than pXL3 encoding the full-length protein. Addition of the β-globin intron II in pXL2 and pXL4 resulted in an increase of only 1.5-fold in protein expression in vitro (data not shown). However, the presence of the intron dramatically enhanced the protective ability of the vectors. No virus could be detected after RSV challenge in mice immunized with pXL2 encoding the secreted F protein. Addition of the intron II also improved the protective immunity induced by the plasmid coding for the membrane-bound F protein by two orders of magnitude (pXL4 versus pXL3). Thus, the F gene encoding a secreted protein and the presence of the rabbit β-globin intron II sequence upstream of the F gene critically contribute to the protective ability of the plasmids with only pXL2 conferring full protection. Our more recent results with pXL2 showed that this vector could also confer full protection against RSV infection in mice at a higher infectious virus titer of 5.2 (Log10 PFU/g lung; data not shown). These findings are consistent with the emerging evidence that bone marrow–derived APCs are responsible for immune responses in distal tissues after intramuscular DNA immunization (32, 33). If this pathway involves the release of the antigen from myocytes and its uptake by APCs, it may be advantageous to use secretory forms of (viral) antigens for DNA immunization.
Table 1.
Immunoprotective Abilities of pXL1, -2, -3, and -4 Plasmids Administered via the Intramuscular Route
| Immunogen | S* | M* | Intron II* | After RSV challenge | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean virus lung titer‡ (Log10 PFU/g) | No. fully protected mice§/ No. immunized mice | |||||||||
| pXL1 | + | − | − | 0.72 ± 0.99 | 5/8 | |||||
| pXL2 | + | − | + | 0.00 ± 0.00 | 9/9 | |||||
| pXL3 | − | + | − | 2.77 ± 0.72 | 0/8 | |||||
| pXL4 | − | + | + | 0.66 ± 1.00 | 6/9 | |||||
| pXL0 | − | − | − | 3.93 ± 0.27 | 0/12 | |||||
Mice were immunized with 100 μg i.m. of either pXL1–pXL4 or pXL0 at 0 and 6 wk and challenged with 106 PFU i.n. of a clinical RSV strain of the A2 subtype 4 wk after the boost. Lungs were removed 4 d later to determine the RSV titer.
S, M, and intron II indicate that the F cDNA construct encodes either a secretory (S) or a membrane-anchored (M) form of the protein or contains the β-globin intron II sequence.
Detection sensitivity: 101.96 PFU/g lung.
Fully protected mice refers to animals with no detectable RSV in lungs after RSV challenge.
Although similar levels of neutralizing antibodies were elicited by pXL1–pXL4, their protective ability differed by two to three orders of magnitude. Protection against RSV infection can be adoptively transferred by virus-neutralizing antibodies (34). However, our results indicate that high levels of neutralizing antibodies are not always sufficient for full protection. Vaccination with pXL3 resulted in a strong neutralizing antibody response but only mediocre reduction in virus lung titers after challenge. Therefore, other protective immune effector mechanisms seem to be important. To investigate the role of CTLs, pXL2 and pXL3 were compared in mice for the induction of RSV-specific CTLs. Both vectors were found to elicit comparable CTL responses at 100-μg doses (data not shown), indicating that the difference in their protective ability against RSV infection of the lungs could not be attributed to any difference in CTL activity. The only difference observed was in the production of IFN-γ by immune splenocytes. A significantly higher IFN-γ response was induced by pXL2 than by pXL3 (3,327 ± 331 pg/ml vs. 1,474 ± 591 pg/ml 72 h after restimulation in vitro). Treatment of muscle tissue with cardiotoxin before immunization with DNA-F vectors was found to improve homogeneity of the anti-F antibody responses among different mice of the same group. However, our more recent results indicate that the pretreatment step can be eliminated after further modification of the vector using a more effective signal peptide for enhanced F protein expression/secretion (data not shown).
Influence of the Route of pXL2 Administration.
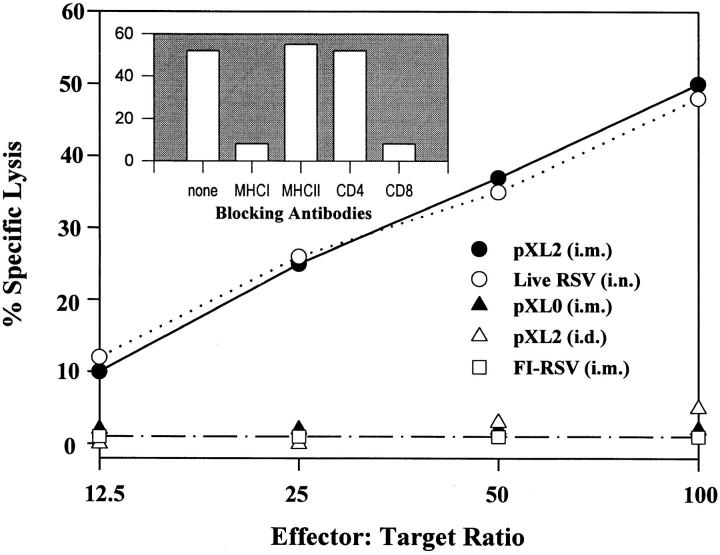
We next determined whether the route of pXL2 administration influenced the immune responses. Intramuscular and intradermal injections of pXL2 led to similar titers of serum anti-F IgG antibodies and RSV-specific plaque reduction titers (Table 2, A). However, intramuscular immunization induced a mixed systemic Th1/Th2 response characterized by high levels of anti-F IgG1 and IgG2a antibodies and the generation of MHC class I–restricted, CD8+ CTLs (Fig. 2), similar to that observed after RSV infection (Table 2, B, and Fig. 2). In contrast, intradermal delivery of pXL2 predominantly elicited IgG1 antibodies but no CTLs, reminiscent of the Th2 response induced by FI-RSV immunization (Table 2, B, and Fig. 2). Although the serum RSV-specific plaque reduction titer observed after pXL2 immunization by either route (mean Log2 titer, 8.60–10.49) was significantly lower than that observed after RSV infection (mean Log2 titer, 12.88), similar lung protection against live RSV challenge was conferred by all immunizations tested (Table 2). Thus, immune response profiles against RSV can be modulated by the route of plasmid delivery.
Table 2.
Influence of the Route of Administration on pXL2 Immunogenicity and Protective Ability
| Immunogen | Route | Mean anti-RSV F ELISA titer |
IgG1/IgG2a | Mean plaque reduction titer | Mean virus lung titer* | No. fully protected mice‡/ No. immunized mice | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | ||||||||||||||||
| Log2 titer/100 + SD | Log2± SD | Log10 PFU/g ± SD | ||||||||||||||||
| A | pXL2 | i.m. | 7.63 ± 0.92 | 4.25 ± 1.91 | 4.00 ± 1.71 | 1.06 | 10.49 ± 0.76 | 0.00 ± 0.00 | 9/9 | |||||||||
| pXL2 | i.d. | 7.35 ± 1.00 | 5.00 ± 1.00 | 0.50 ± 1.27 | 10.00 | 8.60 ± 2.00 | 0.43 ± 1.13 | 8/9 | ||||||||||
| pXL0 | i.m. | 0.50 ± 0.51 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.36 ± 0.50 | 4.30 ± 0.22 | 0/9 | |||||||||||
| B | RSV | i.n. | 9.00 ± 0.67 | 5.00 ± 0.82 | 6.90 ± 0.57 | 0.73 | 12.88 ± 0.34 | 0.00 ± 0.00 | 9/9 | |||||||||
| FI-RSV | i.m. | 7.10 ± 0.99 | 4.60 ± 0.97 | 0.00 ± 0.00 | infinite | 9.20 ± 1.10 | 0.00 ± 0.00 | 9/9 | ||||||||||
Mice were immunized with pXL2 (100 μg), pXL0 (100 μg), RSV (106 PFU), or FI-RSV (100 μl) at 0 and 6 wk. Immune sera were analyzed at wk 9, 1 wk before the RSV challenge (106 PFU, i.n.). Lung RSV titers were determined 4 d after challenge.
Detection sensitivity: 101.69 PFU/g lung.
“Fully protected mice” refers to animals with no detectable RSV in lungs after RSV challenge.
Figure 2.
Characterization of RSV-specific CTLs induced by pXL2. Mice were immunized with pXL2 (100 μg, i.m. or i.d.), pXL0 (100 μg, i.m.), RSV (106 PFU, i.n.), or FI-RSV (100 μl, i.m.) at 0 and 6 wk. 4 wk after the boost, immune splenocytes were stimulated with γ-irradiated syngeneic splenocytes infected with RSV. CTL activity was assessed in a standard 4-h 51Cr-release assay 5 d later using uninfected BC cells and persistently RSV-infected BCH4 fibroblasts as targets. To determine the phenotype of CTLs induced by intramuscular pXL2-immunization, the effector cells were incubated with pools of either anti-CD4 or anti-CD8 mAbs and the targets with either anti-MHC class I or class II antibodies (blocking antibodies), respectively, before the CTL assay performed at 100:1 E/T ratio.
Lung Cytokine Response after Viral Challenge.
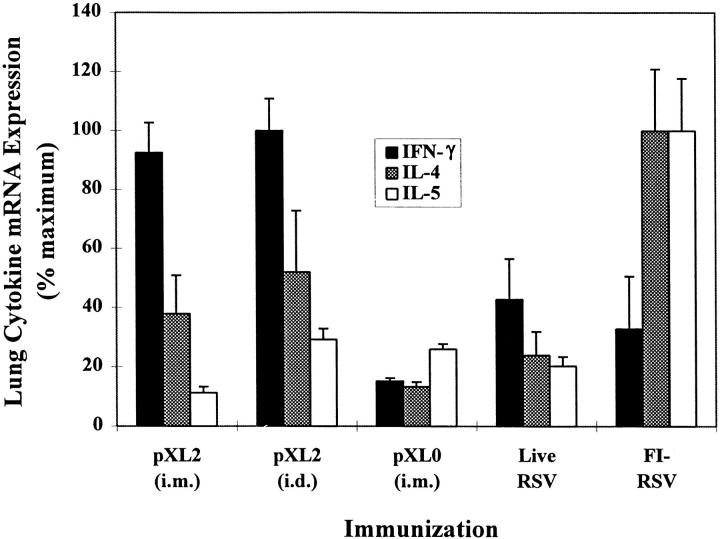
We expected that the local immune response to RSV challenge would mirror the systemic response. To test this, lungs recovered 4 d after the challenge were analyzed for the expression (mRNA) of IFN-γ, IL-4, and IL-5, the latter contributing to the eosinophil accumulation associated with enhanced lung pathology (10, 14, 35). Mice immunized with FI-RSV produced by far the highest IL-4 and IL-5 levels and the lowest IFN-γ mRNA levels among all immunized animals (Fig. 3), consistent with the systemic and local Th2 responses previously observed by us and others (14, 35, 36). In contrast, preimmunization with live RSV generated a balanced Th1/Th2 cytokine profile, whereas injection with pXL0 resulted in an overall low cytokine response after primary RSV infection. Mice immunized with pXL2 by either the intramuscular or intradermal route generated comparable balanced Th1/Th2 cytokine patterns characterized by a significantly higher IFN-γ response than that induced by live RSV and significantly lower IL-4 and IL-5 responses than those observed with FI-RSV immunization (P <0.05, Mann-Whitney test). Thus, the predominant systemic Th2 response observed in mice primed by intradermal pXL2 injection was not predictive of the pulmonary cytokine mRNA profile.
Figure 3.
Mouse lung cytokine mRNA expression profile after pXL2 immunization. Mice were immunized as in Fig. 2 and challenged intranasally with RSV (106 PFU) at 10 wk. Total RNA was prepared from lung homogenates 4 d after challenge and subjected to reverse transcriptase PCR using IL-4, IL-5, or IFN-γ-specific primers. The amplified products were then liquid-hybridized to cytokine-specific 32P-labeled probes, resolved on 5% polyacrylamide gels, and quantitated. Data expressed as percentage of maximum signal were normalized for each cytokine. Three lungs were removed from each immunization group and analyzed for cytokine mRNA levels at least twice.
Lung Histopathology after Viral Challenge.
To assess the degree of lung histopathology induced by pXL2, the intensity of pulmonary inflammatory reactions to live RSV challenge was analyzed 4 d after the challenge using a standard scoring system for bronchiolar and vascular cellular infiltration (31). In mice immunized with pXL0, scores indicative of very mild cellular infiltrates were significantly lower than those in the other immunization groups (P <0.05, Duncan's new multirange test, SuperANOVA; Table 3). Comparable focal peribronchiolar and perivascular cellular aggregates were found in protected animals primed with RSV, FI-RSV, or pXL2, regardless of the route of delivery. Eosinophils were not present in any significant number. Thus, we could not identify animals with enhanced lung pathology. Our data also indicate that a balanced lung cytokine response to RSV challenge in mice preimmunized with RSV is associated with the absence of enhanced histopathology as previously reported by others (14, 31). In contrast, FI-RSV priming does not necessarily lead to enhanced pulmonary inflammatory reactions to live RSV challenge in mice despite the induction of a strong Th2 response in the lungs. This finding is in agreement with prior observations that it is difficult to document consistent enhancement of lung histopathology in mice primed with FI-RSV and challenged with live RSV (12, 37), and with the recent finding that other factors such as the phenotype of effector cells may be important determinants of lung disease (38). The F protein encoded by the DNA-F vaccine should be expressed, glycosylated, and folded in the native conformation, yielding an F glycoprotein with its protective epitopes intact. It was demonstrated that DNA-F immunization of mice led to balanced lung cytokine expression after RSV challenge with a stronger dominance of IFN-γ than was observed after vaccination with RSV. Thus, our data suggest that DNA-F immunization would not lead to enhanced lung pathology after RSV challenge.
Table 3.
Evaluation of Pulmonary Inflammation of Immunized Mice after RSV Challenge
| Immunogen | Bronchioles | Blood Vessels | ||||||
|---|---|---|---|---|---|---|---|---|
| No. scored | Mean ± SD | No. scored | Mean ± SD | |||||
| pXL2 (i.m.) | 262 | 3.10 ± 1.15 | 390 | 3.53 ± 1.30 | ||||
| pXL2 (i.d.) | 287 | 2.42 ± 1.25 | 330 | 2.54 ± 1.40 | ||||
| RSV (i.n.) | 160 | 3.16 ± 0.96 | 263 | 4.39 ± 1.09 | ||||
| FI-RSV (i.m.) | 193 | 2.36 ± 1.08 | 257 | 2.62 ± 1.26 | ||||
| pXL0 (i.m.) | 157 | 1.35 ± 0.60 | 242 | 1.45 ± 0.87 | ||||
Switch of a Th2 Response towards Th1 by pXL2 Immunization.
To test whether pXL2 immunization could switch a preestablished Th2 response towards Th1, we intramuscularly primed and boosted mice with either pXL2 or a vaccine preparation containing the native F protein formulated in alum (Table 4). As expected, pXL2 elicited a balanced Th1/Th2 response, typified by significant IgG1 and IgG2a antibody titers and high levels of splenic IFN-γ and CTLs. This pattern was maintained when pXL2-primed mice were boosted with the alum-adjuvanted subunit vaccine that by itself induced a Th2 response. Furthermore, a pXL2 boost could switch the Th2 response established in mice primed with the subunit vaccine towards Th1. These results are consistent with those obtained with plasmid vectors encoding ovalbumin and β-galactosidase (39).
Table 4.
Switch of Immune Responses From Th2 Towards Th1 by pXL2
| Immunogens | IFN-γ | Mean anti-RSV F ELISA titer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prime | Boost | Total IgG | IgG1 | IgG2a | IgG1/IgG2a | |||||||
| pg/ml | Log2 titer/100 | |||||||||||
| pXL2 | pXL2 | 3,887 | 6.0 ± 1.2 | 2.0 ± 0.4 | 2.6 ± 0.6 | 0.8 | ||||||
| pXL2 | Subunit-alum | 3,238 | 12.4 ± 1.9 | 9.2 ± 0.4 | 7.2 ± 1.2 | 1.2 | ||||||
| Subunit-alum | pXL2 | 1,249 | 13.0 ± 0.8 | 9.8 ± 0.4 | 7.2 ± 1.1 | 1.4 | ||||||
| Subunit-alum | Subunit-alum | 93 | 11.2 ± 0.5 | 8.8 ± 0.8 | 0.8 ± 1.8 | 11.0 | ||||||
| PBS | PBS | ND* | 0 | 0 | 0 | |||||||
BALB/c mice were primed and boosted intramuscularly with either pXL2 (100 μg) or an alum-adjuvanted RSV subunit preparation containing 0.5 μg of the F protein at 0 and 6 wk. This subunit vaccine was prepared by detergent extraction of a viral concentrate followed by ion-exchange chromatography. Immune sera were analyzed at 8 wk for anti-F IgG, IgG1, and IgG2a ELISA titers. The levels of IFN-γ production by immune spleen cells were quantitated by ELISA (PharMingen, Mississauga, Ontario, Canada) after restimulation of these cells with an equal number of γ-irradiated, RSV-infected syngeneic splenocytes for 96 h.
ND, not detectable.
Collectively, our results reveal distinct differences in murine immune responses to human RSV, FI-RSV, and plasmid DNA-F vaccination. We have demonstrated for the first time that an optimized RSV F protein–encoding vector induced high neutralizing antibody titers, CTL responses, protection against live RSV challenge, and high expression of lung IFN-γ after viral challenge. The DNA-F vaccine could also switch a preestablished Th2 response towards Th1, a potential advantage in prime-boost immunization strategies. DNA-F vaccination mimics the immunity elicited by natural RSV infection and thus provides a novel and promising approach for the development of an efficacious human vaccine. However, rational improvement of the DNA immunization technology is essential for its eventual success in humans. This is illustrated by the necessity of a rather large dose of DNA vector for full protection in the mouse model. Improvement of DNA immunization may be expected by further optimization of expression vectors and enhanced efficiency of vector delivery, perhaps in conjunction with targeting of professional APCs (40). It will also be important to transfer the technology from small animal models to natural hosts. We are in the process of establishing an infection model for RSV in monkeys in order to evaluate the immunogenic and protective properties of candidate DNA-based vaccines in non-human primates. Preliminary results to date show significant virus-neutralizing antibody responses to DNA-F vaccination.
Abbreviations used in this paper
- BC cells
Balb/c fibroblasts
- DNA-F
optimized plasmid DNA vector expressing the RSV F protein
- FI-RSV
formalin-inactivated RSV
- F protein
fusion protein
- RSV
respiratory syncytial virus
Footnotes
We wish to express our sincere appreciation to Nancy Scollard and Anjna Kurichh for their expertise in viral and CTL assays, and to Bill Bradley and Diane England for oligonucleotide synthesis and DNA sequencing. We also wish to thank Dr. Raymond Oomer and Shahneela Usman for the purification of the RSV F protein. We are grateful to Dr. David Brownstein (Yale University, New Haven, CT), who evaluated the lung histopathology.
References
- 1.Hall CB. Prospects for a respiratory syncytial virus vaccine. Science. 1994;265:1393–1394. doi: 10.1126/science.7915433. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Walsh EE. Humoral immunity to respiratory syncytial virus infection in the elderly. J Med Virol. 1992;36:39–43. doi: 10.1002/jmv.1890360108. [DOI] [PubMed] [Google Scholar]
- 3.Murphy BR, Hall SL, Kulkarni AB, Crowe JE, Jr, Collins PL, Connors M, Karron RA, Chanock RM. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 1994;32:13–36. doi: 10.1016/0168-1702(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PR, Jr, Olmsted RA, Prince GA, Murphy BR, Alling DW, Walsh EE, Collins PL. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: evaluation of the contributions of F and G glycoproteins to immunity. J Virol. 1987;61:3163–3166. doi: 10.1128/jvi.61.10.3163-3166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson PR, Collins PL. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol. 1988;69:2623–2628. doi: 10.1099/0022-1317-69-10-2623. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs D, Bangham CR, McMichael AJ. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987;2:769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 7.Cherrie AH, Anderson K, Wertz GW, Openshaw PJ. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66:2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 9.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 10.Openshaw PJ. Immunopathological mechanisms in respiratory syncytial virus disease. Springer Semin Immunopathol. 1995;17:187–201. doi: 10.1007/BF00196165. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 12.Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC, III, Sotnikov AV, Murphy BR. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DC. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 14.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srikiatkhachorn A, Braciale TJ. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe JE, Jr, Bui PT, Siber GR, Elkins WR, Chanock RM, Murphy BR. Cold-passaged, temperature-sensitive mutants of human respiratory syncytial virus (RSV) are highly attenuated, immunogenic, and protective in seronegative chimpanzees, even when RSV antibodies are infused shortly before immunization. Vaccine. 1995;13:847–855. doi: 10.1016/0264-410x(94)00074-w. [DOI] [PubMed] [Google Scholar]
- 17.Ulmer JB, Sadoff JC, Liu MA. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 18.Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justewicz DM, Webster RG. Long-term maintenance of B cell immunity to influenza virus hemagglutinin in mice following DNA-based immunization. Virology. 1996;224:10–17. doi: 10.1006/viro.1996.0501. [DOI] [PubMed] [Google Scholar]
- 20.Pizzorno MC, O'Hare P, Sha L, LaFemina RL, Hayward GS. trans-Activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988;62:1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du RP, Jackson GE, Wyde PR, Yan WY, Wang Q, Gisonni L, Sanhueza SE, Klein MH, Ewasyshyn ME. A prototype recombinant vaccine against respiratory syncytial virus and parainfluenza virus type 3. Biotechnology (NY) 1994;12:813–818. doi: 10.1038/nbt0894-813. [DOI] [PubMed] [Google Scholar]
- 22.Davis HI, Michel M-L, Mancini M, Schleel M, Whalen RG. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994;12:1503–1509. doi: 10.1016/0264-410x(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 23.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 24.Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 25.Fernie BF, Ford EC, Gerin JL. The development of Balb/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981;167:83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- 26.Qin S, Wise M, Cobbold SP, Leong L, Kong Y-C, Parnes JR, Waldmann H. Induction of tolerance in peripheral T cells with monoclonal antibodies. Eur J Immunol. 1990;20:2737–2745. doi: 10.1002/eji.1830201231. [DOI] [PubMed] [Google Scholar]
- 27.Wilde DB, Marrack P, Kappler J, Dialynas DP, Fitch FW. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983;131:2178–2183. [PubMed] [Google Scholar]
- 28.Ledbetter JA, Rouse RH, Micklem HS, Herzenberg LA. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. J Exp Med. 1980;152:280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozato K, Mayer NM, Sachs DH. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982;34:113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2–restricted, interleukin-2–producing T cell hybridomas. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy BR, Sotnikov A, Paradiso PR, Hildreth SW, Jenson AB, Baggs RB, Lawrence L, Zubak JJ, Chanock RM, Beeler JA, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–540. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- 32.Doe B, Selby M, Barnett S, Baenziger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow–derived cells. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki A, Torres CA, Ohashi PS, Robinson HL, Barber BH. The dominant role of bone marrow– derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 34.Connors M, Collins PL, Firestone CY, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia—RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 35.Graham BS. Pathogenesis of respiratory syncytial virus vaccine–augmented pathology. Am J Respir Crit Care Med. 1995;152:S63–66. doi: 10.1164/ajrccm/152.4_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 36.Doherty PC. Vaccines and cytokine-mediated pathology in RSV infection. Trends Microbiol. 1994;2:148–150. doi: 10.1016/0966-842x(94)90662-9. [DOI] [PubMed] [Google Scholar]
- 37.Hancock GE, Speelman DJ, Frenchick PJ, Mineo-Kuhn MM, Baggs RB, Hahn DJ. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in Balb/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13:391–400. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]
- 38.Tang YW, Graham BS. T cell source of Type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J Clin Invest. 1997;99:2183–2191. doi: 10.1172/JCI119391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raz E, Tighe H, Sato Y, Corr M, Dudler JA, Roman M, Swain SL, Spiegelberg HL, Carson DA. Preferential induction of a Th1 immune response and inhibition of specific IgG antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]