Abstract
Rationale
It has been proposed that dopamine (DA) sustains up states in striatal medium spiny neurons (MSN). Testing this hypothesis requires an in vitro preparation, but up states are typically only observed in vivo.
Objectives
In this study, we used corticostriatal organotypic cocultures, a preparation in which up states have been previously observed, to test the DA control of cortically-driven plateau depolarizations.
Results
After 7–21 days in vitro in serum-free conditions, plateau depolarizations resembling up states were only observed in cultures with a critical extent of striatal DA innervation. These plateaus were completely blocked by the non-NMDA antagonist CNQX and significantly shortened by the NMDA antagonist APV or the D1 antagonist SCH23390. Intracellular interruption of Ca++ or protein-kinase A (PKA) signaling also eliminated the plateaus. The D2 antagonist eticlopride failed to disrupt the plateaus, but significantly increased MSN excitability.
Conclusions
These results suggest that coincident activation of corticostriatal glutamatergic and mesostriatal DA transmission may set ensembles of MSN into prolonged depolarizations through a D1 enhancement of striatal NMDA function in a Ca++ and PKA-dependent manner.
Keywords: Dopamine, Striatum, Electrophysiology, Persistent activity, PKA, D1
Introduction
In vivo intracellular recordings of striatal medium spiny neurons (MSN) reveal a membrane potential alternating between two steady-state values. A very negative resting membrane potential (“down state”) is interrupted by periods of sustained depolarization (“up states”) lasting a few hundred milliseconds and dependent on synchronous glutamatergic excitation (Wilson 1993; O’Donnell and Grace 1995). Electrical stimulation of the fimbria–fornix, which carries hippocampal afferents, evokes plateau depolarizations resembling up states in ventral striatal neurons (O’Donnell and Grace 1995), and up–down transitions in MSN are synchronized with cortical electrical activity (Goto and O’Donnell 2001b; Mahon et al. 2001; Tseng et al. 2001). Although there is consensus that strong glutamatergic inputs are required for up state onset, there is still some debate as to whether intrinsic voltage-gated currents are necessary to sustain the depolarization. Because many transmitters (particularly monoamines) can control glutamatergic responses and voltage-gated currents (Cepeda et al. 1993; Hernandez-Lopez et al. 1997), it is possible that they also affect striatal up states. Some data actually suggest that dopamine (DA) may sustain these depolarizations; for example, electrical stimulation of the ventral tegmental area (VTA) elicits plateau depolarizations resembling spontaneous up states in striatal and prefrontal cortical neurons in vivo, which are shortened by DA antagonists (Lewis and O’Donnell 2000; Goto and O’Donnell 2001a). Complete DA receptor blockade, however, fails to eliminate the onset of the evoked response, suggesting that DA does not mediate the transition to the up state, but contributes to sustain the depolarization. A major obstacle to study this action of DA is the need to use in vivo intracellular recordings to obtain up states, a preparation not amenable to cellular pharmacology. An in vitro preparation in which striatal up states have been recorded is the corticostriatal organotypic coculture (Plenz and Kitai 1998; Kerr and Plenz 2002). Up states in organotypic cocultures have been attributed to strong cortical activity impinging on striatal MSN. However, a role of DA on striatal up states could not be conclusively established because the cocultures had been incubated in serum-containing media for several weeks. As serum can phosphorylate PKA targets (Snyder et al. 2004) and PKA is involved in cellular responses to serum (Edin et al. 2001), it is conceivable that intracellular processes that would otherwise depend on DA receptors were activated. Thus, to assess the role of DA on striatal up states, we conducted whole-cell recordings of striatal MSN in corticostriatal (Cx–Str) organotypic cocultures with or without a midbrain piece containing the substantia nigra (SN) and VTA that had been incubated in serum-free conditions.
Materials and methods
All experimental procedures were performed according to the USPHS Guide for care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee of the Wadsworth Center.
Organotypic culture preparation
We obtained fetal tissue from timed-pregnant Sprague–Dawley rats (sperm presence considered gestation day E0). The brains were rapidly stripped of meninges in Ham’s F12 media and kept on ice for further dissection. The ventral mesencephalon was dissected out of E14–15 fetal brains, and forebrain slices (300 μm) were cut from brains of rat pups ranging from E20 to E22 using a vibratome. Coronal sections were dissected further, separating striatum (Str) and cortex (Cx). The Cx, Str, and substantia nigra–ventral tegmental area (SN/VTA) were stored in separate small petri dishes containing ice-cold F12 media. Six-well culture trays containing Costar clear membrane inserts (Costar, Albany, NY, USA) were prepared by preincubating for 1 h with 1.2 ml/well of Neurobasal medium (Gibco) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), bicarbonate (1.2 mg/ml), glutamine (2 mM), and HEPES (4.5 mg/ml). This initial medium also contained 20% horse serum. Just before slice placement, polylysine drops were added directly to the membrane inserts to promote attachment and restrict movement of the pieces during incubation. The sections were placed on the membranes using a sterile pipette with a cut and flame-polished tip. Cx and/or SN/ VTA were then placed about 1 mm from selected striatal pieces, with the SN/VTA placed close to the ventral aspect of the striatum, and Cx adjacent to the dorsal aspect. The cultures were incubated at 37°C in 5% CO2 from 5 to 26 days, with the medium changed three times per week. After 3 days in vitro (DIV), cultures were switched to serum-free Neurobasal medium containing B27 supplement (4%, Gibco).
Electrophysiology
Cocultures were placed in a submersion chamber and perfused at 2 ml/min with artificial cerebrospinal fluid (CSF) (in mM: 125 NaCl, 25 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2; pH 7.45, and osmolarity 295±5 mOsm), oxygenated with 95% O2/5% CO2, and maintained at 33–35°C. Striatal neurons were identified under visual guidance using infrared-differential interference contrast (IR-DIC) video microscopy with a 40× water-immersion objective mounted on an upright microscope (Olympus BX51). The image was detected with an IR-sensitive CCD camera (Dage-MTI) and displayed on a monitor. Patch pipettes (5–8 MΩ) were filled with (in mM): 115 K-gluconate, 10 HEPES, 2 MgCl2, 20 KCl, 2 MgATP, 2 Na2-ATP, and 0.3 GTP (pH 7.3, 280±5 mOsm). Whole-cell current-clamp recordings were performed using a computer-controlled amplifier (MultiClamp 700A; Axon Instruments), and acquired with Axoscope 8.1 (Axon Instruments) at a sampling rate of 10 KHz. Electrode potentials were adjusted to zero before recording without correcting the liquid junction potential (estimated at 10–12 mV). All drugs were mixed into oxygenated aCSF and applied into the recording solution at known concentrations. Both control and drug-containing aCSF were continuously oxygenated throughout the experiments. The D1 antagonist SCH23390, the D2 antagonist eticlopride, and both AMPA and NMDA receptor antagonists (CNQX and DL-APV, respectively) were obtained from Sigma. For intracellular Ca++ chelation, BAPTA (Sigma) was included in the recording micropipette in some experiments at a concentration of 2 mM. To block PKA activity, 20 μM of the peptide PKI [5–24] (Calbiochem, La Jolla, CA, USA) were included in the recording electrode.
Corticostriatal synaptic responses were evoked with a bipolar stimulating electrode placed in the center of the cortical piece at around 2–3 mm from the recording sites. A pair of twisted teflon-coated nichrome wires (75 μm) was used to stimulate, with the tips separated by 200 μm. During baseline recordings, two series of 10 to 15 single pulses (300 μs) were delivered with a stimulus isolation unit (ISO-Flex, AMPI, Jerusalem, Israel) controlled by a pulse generator (Master-8; AMPI) every 20 s. The stimulation intensity was first adjusted to evoke a synaptic response of around 15 to 20 mV in amplitude (typically 0.3–0.5 mA) at the beginning of the baseline recordings. Additional series of stimuli were delivered using constant intensity to assess the time course of different drug effects.
Neurobiotin reaction and immunohistochemistry
After completion of recordings, cultures were transferred to 4% paraformaldehyde in phosphate-buffered saline (PBS) or to 10% formalin for 18 h. After fixation, cultures were rinsed in PBS and incubated for 3 h in 0.5% Triton/PBS, followed by 2–16 h incubation in avidin–biotin–peroxidase complex (ABC Elite kit; Vector labs) made up in 0.2% Triton/PBS. After rinsing, cultures were reacted in 0.05% diaminobenzidine (DAB) containing 0.0015% H2O2 in the presence or absence of 0.25% nickel ammonium sulfate (for black cells). The reaction was closely monitored to assess cell staining, and was typically terminated within 5 min. In some cases, immunostaining for tyrosine hydroxylase (TH) was conducted in addition to the avidin–biotin reaction. These cultures (remaining on the membrane) were first rinsed in PBS, then in 0.2% Triton X-100/PBS for 30 min, and then incubated in 5% normal goat serum (NGS)/0.2% Triton/PBS for 2 h. Each culture was then incubated in rabbit primary antibodies to TH (Chemicon; diluted at 1:600 in 2.5% NGS/0.2% Triton X-100/PBS) for 40 h at 4°C. The cultures were then rinsed in PBS for 45 min and washed for 10 min in 0.1% Triton before a 2-h incubation at room temperature in biotinylated goat anti-rabbit secondary antibodies (1:200; Vector), followed by a 45-min rinse and a 2-h incubation in avidin–biotin complex linked to peroxidase (Elite ABC Kit, Vector). Incubation in 0.05% diaminobenzidine containing 0.0015% H2O2 was used to reveal brown or black (0.25% nickel ammonium sulfate added) immunoreactive cells. The best combination was obtained with brown Neurobiotin-labeled cells and black TH-immunoreactive fibers. Stained cultures were viewed and photographed with an Olympus Vanox Photomicroscope or Olympus BX-2 microscope with Olympus C-5050 digital camera. Quantification of DA innervation was performed by capturing 350×350 3m images centered in each of the striatal pieces, thresholding so that only the fibers appear as black on a white background, and calculating the percent of the area occupied by fibers using NIH ImageJ.
Data analysis
All values are expressed as mean±SD. The Student’s t test was used for two-group comparisons involving a single continuous variable. The effects along two or more variables were compared using repeated measures ANOVA. Differences between experimental conditions were considered statistically significant when P<0.05.
Results
Striatal DA innervation in Cx–Str–SN/VTA cocultures
Cortical and striatal pieces were cocultured with a SN/VTA piece to explore the impact of DA innervation on striatal physiology. To ascertain whether a strong DA innervation of the striatal piece had been established at the time of recording, several cultures were processed for tyrosine hydroxylase (TH) immunocytochemistry. Numerous DA neurons were observed in the SN/VTA, as well as TH-immunoreactive fibers growing into the cocultured striatal piece by 8 days in vitro (DIV) the earliest age used for recording (Fig. 1). As reported previously (Snyder-Keller et al. 2001, 2004), this innervation became distinctively patchy (in all age striata) by 10 DIV (Fig. 1).
Fig. 1.
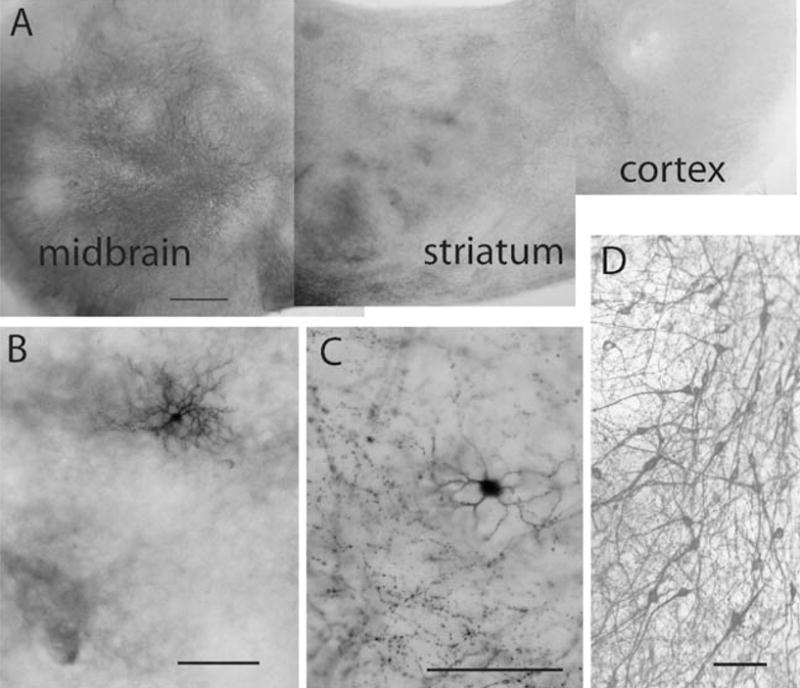
Dopamine innervation and Neurobiotin-labeled cells in Cx–Str–SN/VTA cocultures. a Photomontage of a Cx–Str–SN/VTA coculture stained for TH: E20 striatum (in middle) with E14 SN (to the left) and E20 cortex (to the right) at 22 DIV. b High-power view of the striatum illustrating a Neurobiotin-labeled cell (black) lying within a patch of TH-immunoreactive fibers (brown). c DA fibers from the SN/VTA (E14) neurons (black) innervating the striatal (E20) region containing a Neurobiotin-labeled cell (brown) at 10 DIV. d High-power view of a TH immunostaining showing DA neurons within the SN/VTA of a Cx–Str–SN/VTA coculture. Scale bars: 500 μm (a) and 100 μm (b, c, and d)
Striatal MSN recorded from corticostriatal organotypic cocultures exhibit plateau depolarizations similar to up states
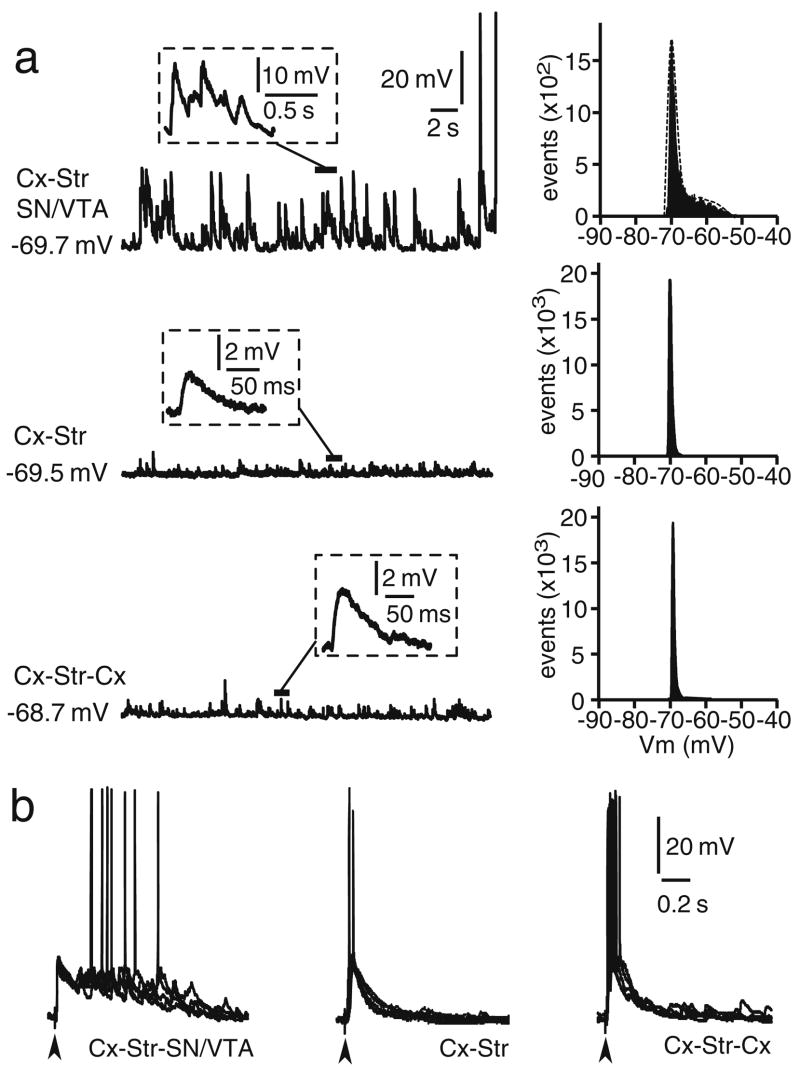
Whole-cell current clamp recordings of striatal neurons were conducted in organotypic cocultures also including a cortical piece (either somatosensory or prefrontal cortex; Cx–Str) or in triple cocultures containing Cx and the SN/ VTA (Cx–Str–SN/VTA). No apparent differences were observed between MSN recorded from Cx–Str–SN/VTA cocultures containing somatosensory or prefrontal cortices and, therefore, the data were pooled for all statistical analyses. All neurons recorded showed the characteristic ramp depolarization and delayed action potential firing in response to depolarizing current injection (Fig. 2a) and inward rectification with negative current injection (Fig. 2c). All neurons successfully filled with Neurobiotin (n=86) were identified as MSN (Fig. 2b). After 7–21 DIV in serum-free conditions, all striatal neurons recorded from Cx–Str and Cx–Str–SN/VTA cocultures exhibited similar resting membrane potential (Cx–Str: −69.7±2.2 mV; Cx–Str–SN/VTA: −69.7±2.4 mV; mean±SD) and input resistance (Cx–Str: 326.8±169.9 MΩ Cx–Str–SN/VTA: 328.1± 112.9 MΩ). Spontaneous, 10–25 mV plateau potentials resembling in vivo up states were observed only in Cx–Str–SN/VTA cocultures (Fig. 3a). These recurrent plateaus occurred irregularly at 0.5±0.3 Hz and lasted 533.4± 141.4 ms (measured as decay to half amplitude, n=33). Histograms of membrane potential distributions could be fitted to a dual Gaussian function in about 65% of MSN recorded in Cx–Str–SN/VTA cocultures. Cx–Str cocultures yielded only spontaneous brief depolarizations resembling excitatory postsynaptic potentials (EPSPs) lasting 53.6± 16.2 ms (n=18, P<0.0001 compared to Cx–Str–SN/VTA; Student’s t test; Fig. 3a). Because these comparisons were made between double and triple cocultures, it is possible that the differences observed arose from the amount of afferent innervation. To account for this factor, a set of recordings was conducted in triple cocultures containing two cortical pieces (Cx–Str–Cx). Striatal MSN recorded from Cx–Str–Cx cultures (n=12) exhibited similar resting membrane potential (−68.7±2.3 mV) and input resistance (361.3±213.88 MΩ) as the other cocultures. Doubling the cortical pieces failed to drive striatal plateau depolarizations; spontaneous short depolarizations similar to those recorded in Cx–Str cocultures (80.0±38.9 ms; P<0.0001 compared to Cx–Str–SN/VTA; Fig. 3a) were observed instead. The brief depolarizations are likely synaptic responses to active cortical afferents and the presence of DA in Cx–Str–SN/VTA cocultures may prolong these synaptic depolarizations into up states.
Fig. 2.
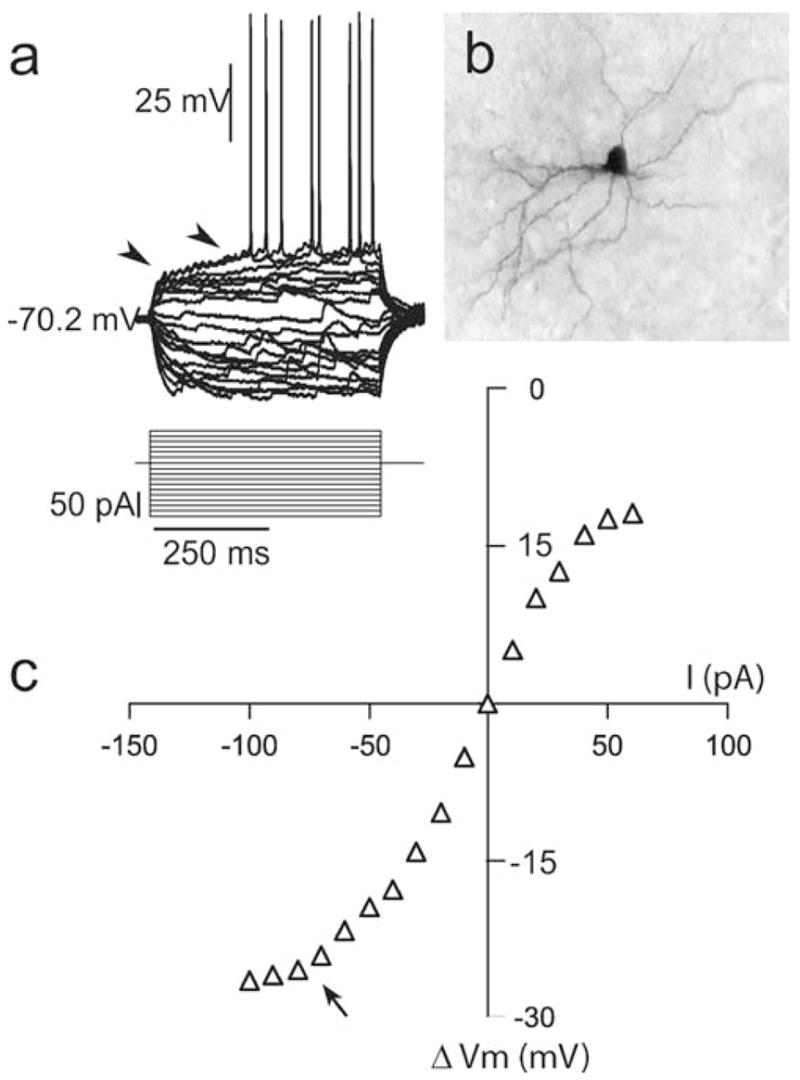
Whole-cell recording from striatal MSN in organotypic cocultures. a Overlay of voltage responses (top traces) to depolarizing and hyperpolarizing current pulses (500 ms from −100 to +60 pA, bottom traces) in a striatal neuron. The arrowheads point to the ramp depolarization and the delayed action potential firing in response to suprathreshold current pulses that are characteristic of striatal MSN. b Photograph of a representative striatal MSN filled with Neurobiotin. c Current–voltage plot obtained from the traces shown in a illustrating the typical inward rectification with negative current injection (arrow)
Fig. 3.
Spontaneous and cortically evoked synaptic activity of striatal neurons recorded from organotypic co-cultures. a Traces (left) of spontaneous depolarizations recorded in cortico–striatal–substantia nigra/ventral tegmental area (Cx–Str–SN/VTA), cortico–striatal (Cx–Str), and cortico–striatal–cortical (Cx–Str–Cx) cocultures. Only neurons recorded from Cx–Str–SN/VTA cocultures displayed recurrent plateau depolarizations resembling in vivo up states. Insets show representative spontaneous depolarizations at faster time scales. Right membrane potential distribution histogram from the traces shown on the left, revealing a bimodal distribution in the cocultures containing DA innervation. b Traces illustrating typical striatal responses to electrical cortical stimulation in Cx–Str–SN/VTA, Cx–Str, and Cx–Str–Cx cocultures. Plateau depolarizations were evoked only in cultures containing SN/VTA neurons. Only brief depolarizing post-synaptic potentials were observed in Cx–Str and Cx–Str–Cx cocultures. In this and subsequent figures showing synaptic responses, several repetitions were overlaid in each example and vertical arrowheads below the traces point to the time of electrical stimulation
Electrical stimulation of the cortical piece evoked brief EPSPs in striatal neurons of Cx–Str cocultures and plateau depolarizations in cultures containing the SN/VTA (Fig. 3b). The duration of these plateau potentials was 400.7±90.6 ms (measured as decay to half-amplitude; n=28), whereas EPSPs obtained in Cx–Str cocultures lasted 122.2±48.2 ms (P<0.0001 compared to Cx–Str–SN/VTA; n=16; Fig. 3b). In triple Cx–Str–Cx cocultures, the evoked EPSPs lasted 167.6±43.3 ms (n=10, P<0.0001 compared to Cx–Str–SN/VTA) and did not extend into a plateau depolarization (Fig. 3b). No apparent differences were observed in the amplitude or latency of evoked responses among neurons recorded from double and triple cocultures (Cx–Str–SN/VTA: 20.5±3.4 mV, 11.2±2.3 ms; Cx–Str: 22.1±4.6 mV, 11.8±1.8 ms; Cx–Str–Cx: 20.5±3.5 mV, 11.6±1.6 ms). These results suggest that activation of cortical inputs can drive striatal plateau depolarizations similar to up states in Cx–Str cocultures following 7–21 DIV in serum-free conditions, but only in the presence of SN/VTA afferents.
Striatal plateau depolarizations require activation of AMPA and NMDA receptors
Up states from striatal MSN in organotypic cocultures do require glutamatergic inputs (Plenz and Kitai 1998). The prolonged nature of evoked plateaus suggests that longer-acting NMDA receptors may be involved. To determine the role of non-NMDA and NMDA receptors in driving corticostriatal up states, some recordings were performed in the presence of the non-NMDA antagonist CNQX or the NMDA antagonist APV. Bath application of 10 μM CNQX completely blocked all evoked responses in both Cx–Str (n=9) and Cx–Str–SN/VTA (n=7) cocultures (Fig. 4). In contrast, APV (50 μM) failed to affect EPSPs in Cx–Str cocultures (data not shown), but eliminated up states in Cx–Str–SN/VTA cocultures. Bath application of APV reduced the duration of spontaneous plateau depolarizations from 507.5±209.8 ms (baseline) to 115.5±71.9 ms (n=6; P<0.01, compared to baseline; Fig. 5a), and shortened the evoked plateaus from 328.6±30.3 (baseline) to 184.8± 45.1 ms (n=5; P<0.0005, compared to baseline; Fig. 5b,c) without affecting the latency (from 11.1±1.3 to 11.1±1 ms, P=0.9) or amplitude (from 19.8±3.1 to 20.5±2.4 mV, P=0.5) of the response. These results suggest that the onset of corticostriatal synaptic responses is mediated by EPSPs driven by non-NMDA receptors, and NMDA activation extends the responses into plateau depolarizations. DA innervation seems to be required for this NMDA effect because this potentiation was observed only in Cx–Str cultures with SN/VTA.
Fig. 4.
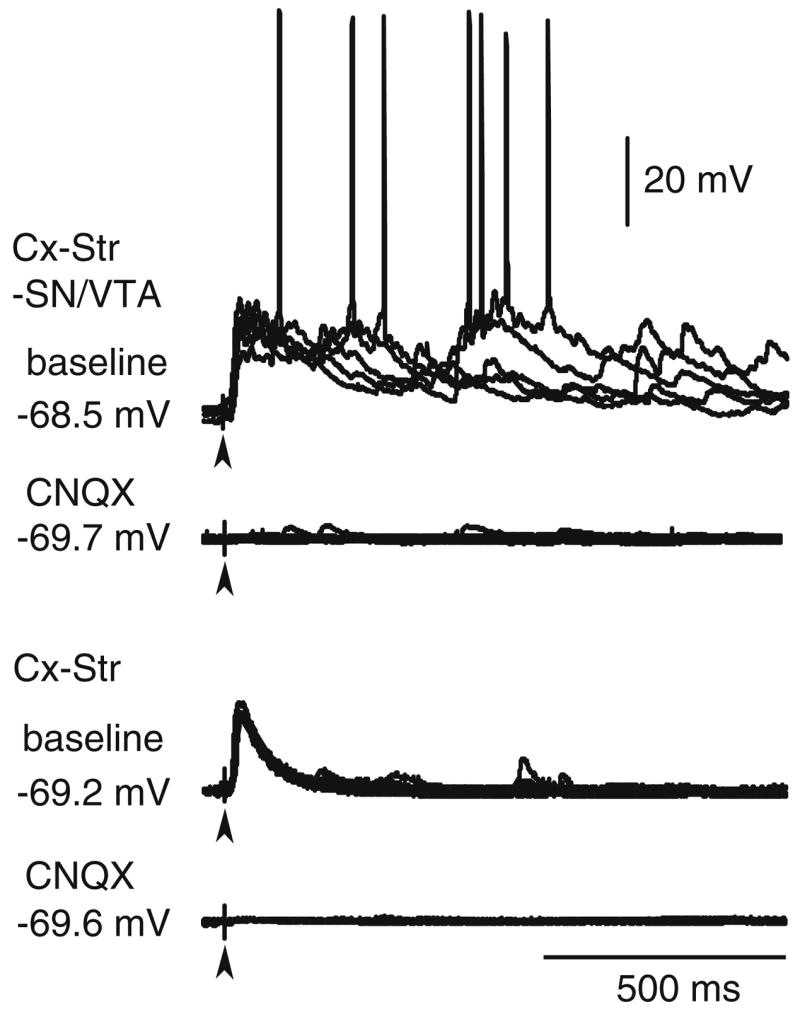
Synaptic responses and plateau depolarizations evoked in MSN by cortical stimulation require non-NMDA receptors. Traces illustrating typical responses to cortical stimulation in Cx–Str–SN/VTA (top) and Cx–Str (bottom) cocultures before and after bath application of 10 μM CNQX. Both the brief EPSPs in Cx–Str cocultures and the plateau depolarizations evoked in Cx–Str–SN/VTA cocultures were completely blocked after ~10 min of CNQX
Fig. 5.
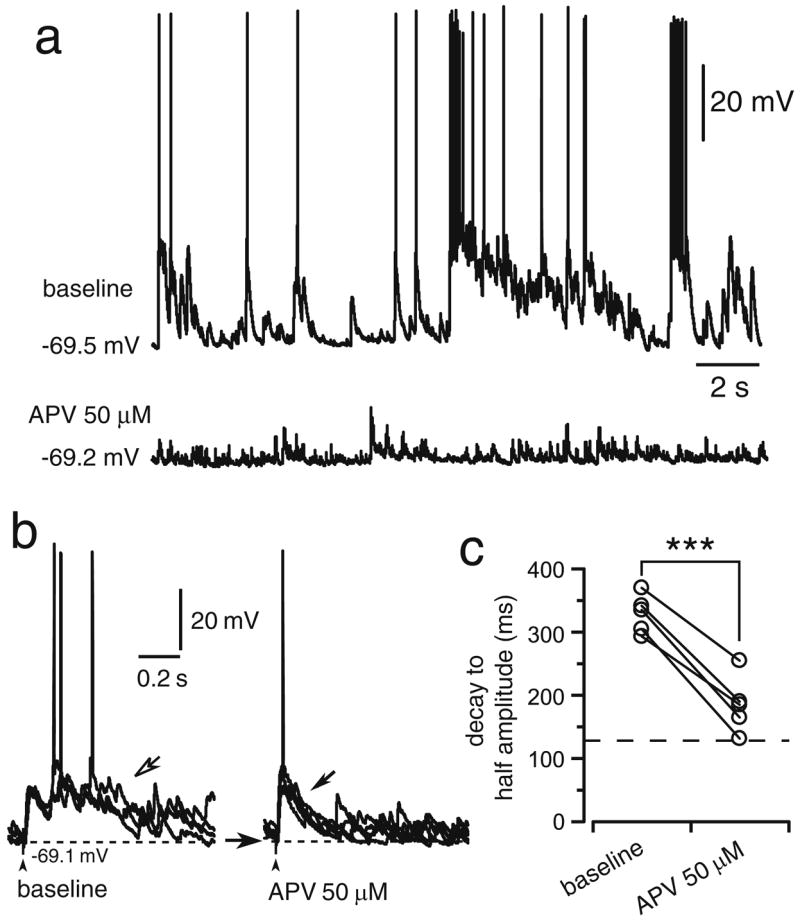
NMDA receptors sustain striatal plateau depolarizations in Cx–Str–SN/VTA cocultures. a Traces of spontaneous activity recorded from a striatal MSN before (baseline) and after bath application of the NMDA antagonist APV. After ~10 min of 50 μM APV, plateau depolarizations were completely eliminated, leaving EPSPs similar to those observed in Cx–Str or Cx–Str–Cx cocultures (see Fig. 2). b Representative traces of responses to cortical stimulation recorded from a Cx–Str–SN/VTA coculture illustrating the effect of APV. The arrows point to the evoked potential of a striatal neuron recorded during baseline (open arrow) and ~10 min after APV (black arrow). c Scatter graph summarizing the effect of APV on MSN response to cortical stimulation. The duration of the evoked potentials (measured as the decay to half amplitude) was significantly reduced in presence of 50 μM APV (***P<0.0005, compared to baseline, paired Student’s t test)
Endogenous DA facilitates striatal plateau depolarizations via D1 (not D2) receptors
Previous studies indicate a strong D1 up-regulation of NMDA responses in striatal MSN (Cepeda et al. 1993, 1998; Cepeda and Levine 1998). It is possible that this interaction is responsible for the DA-dependence of NMDA plateaus. To investigate a potential role of D1 receptors in striatal plateau depolarizations in Cx–Str–SN/VTA cocultures, some recordings were conducted in the presence of the D1 antagonist SCH23390. Bath application of SCH23390 failed to immediately affect spontaneous and evoked plateau depolarizations (not shown). Because all neurons recorded in the midbrain piece were spontaneously active (n=11; including seven putative DA neurons and four putative non-DA cells, 1.4±1.1 and 1.2±1.2 spikes/s, respectively), a D1 up-regulation of NMDA responses may have already been present in the cultures. It is conceivable that D1 blockade would impair this modulation of NMDA function only once the intracellular effects of D1-linked G protein activity wear off. To examine this possibility, we conducted some experiments incubating (not recording) Cx–Str–SN/VTA cocultures with the D1 antagonist (SCH23390, 5 μM) in the recording chamber for around 65–90 min before attempting to patch a striatal neuron (Fig. 6a). As a control, additional recordings were performed following a similar incubation but in the absence of the D1 antagonist (n=9). SCH23390 treatment reduced both spontaneous and cortically evoked plateau potentials into short depolarizations resembling those observed in Cx–Str cocultures. These effects were evident only in Cx–Str–SN/VTA cocultures that had been incubated in SCH23390 for 90–120 min (n=6). The duration and frequency of these spontaneous brief depolarizations were 83.6±46.5 ms [P<0.0001, compared to artificial CSF (aCSF) alone, which were 507.7±37 ms] and 1.1±0.4 Hz (P<0.008, compared to aCSF alone, which were 0.5± 0.1 Hz), respectively (Fig. 6b). Similarly, cortical stimulation evoked brief EPSPs, not plateaus, in cultures incubated with the D1 antagonist for at least 90 min (aCSF + SCH23390: 132.8±56.5 ms vs aCSF alone: 405.3± 65.9 ms; P<0.0001; Fig. 6c). The latency (10.9±0.5 ms) and amplitude (21.6±5.7 mV) of the responses were similar to those seen in control recordings. These results indicate that D1 receptors can sustain striatal up states, probably via an enhancement of NMDA function.
Fig. 6.
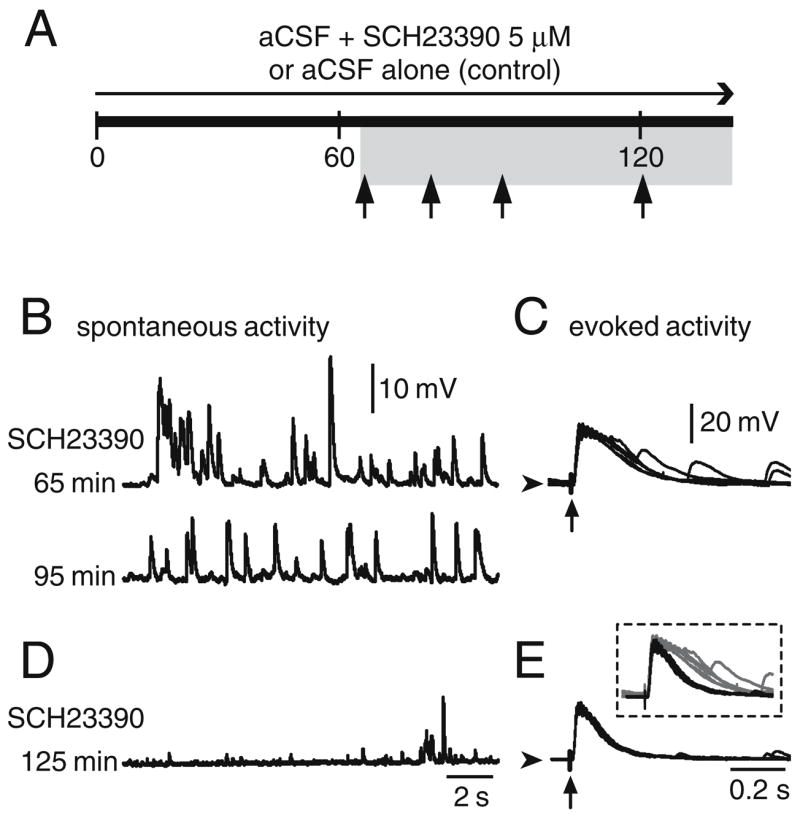
Up states recorded from Cx–Str cocultures containing SN/VTA neurons require D1 receptor activation. a Simplified diagram illustrating the time points of MSN recordings (arrows) in Cx–Str–SN/VTA cocultures that were incubated for at least 60 min with SCH23390. Time zero is the onset of incubation. b, d Representative traces from two MSN recorded in presence of the D1 antagonist SCH23390. The first neuron (b) was obtained from a Cx–Str–SN/VTA coculture that had been incubated with 5 μM SCH23390 for 60 min. Spontaneous plateau depolarizations were gradually reduced to shorter events after the subsequent 30–40 min of recording. The second neuron (d) was obtained from another Cx–Str–SN/VTA coculture preincubated with SCH23390 for 2 h. Depolarizing plateaus could not be observed; instead, short depolarizations resembling those observed in Cx–Str cocultures were recorded. The time after antagonist onset is indicated to the left of the traces. c Traces of depolarizations evoked by cortical stimulation in the same neurons are shown in b. The evoked responses still exhibited a plateau depolarization by 65 min after SCH23390 (top); at ~125 min (e); however, the evoked response consisted of EPSPs similar to those observed in Cx–Str cocultures. An overlay of the evoked traces at 65 and 125 min after the D1 antagonist is shown in the inset
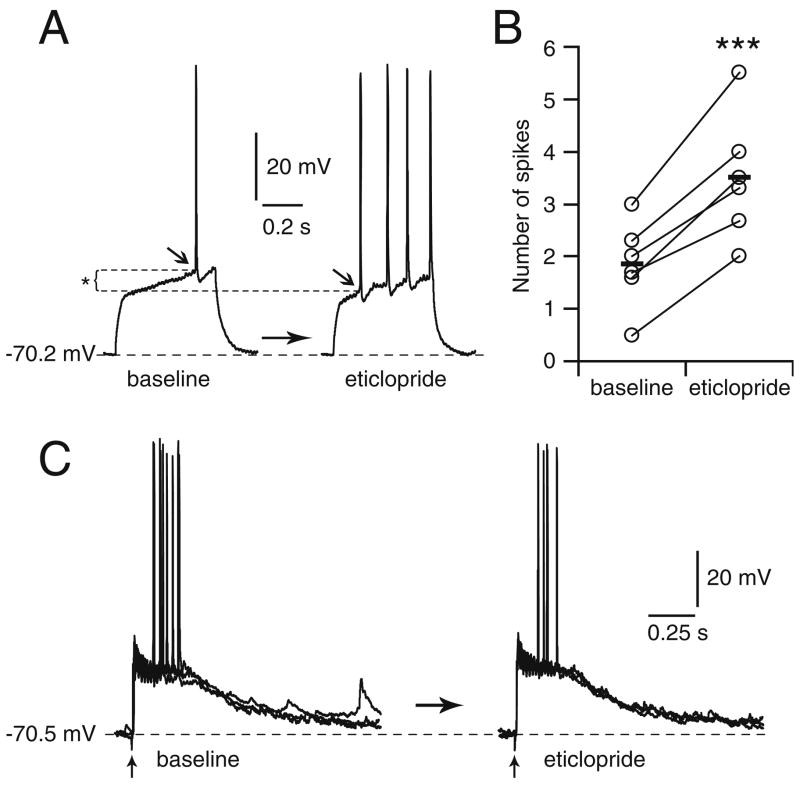
To examine the role of D2 receptors, additional experiments were conducted in Cx–Str–SN/VTA cocultures in the presence of the D2 antagonist eticlopride. Bath application of eticlopride (20 μM) significantly increased the number of spikes evoked by somatic current injection from 1.9±0.8 to 3.5±1.2 after 10–15 min of eticlopride (P<0.0005, n=6, paired Student’s t test; Fig. 7a,b), but did not cause membrane potential changes or spontaneous plateau depolarizations. Similarly, the amplitude (from 23.1±4.6 to 24.7± 4.9 mV) and duration (from 407.1±117.3 to 481.9± 193.3 ms; Fig. 7c) of cortically evoked responses were not significantly affected by eticlopride. These results suggest that D2 receptors can modulate MSN excitability but are not required to sustain DA-dependent plateau depolarizations.
Fig. 7.
MSN plateau depolarizations are not affected by bath application of the D2 antagonist eticlopride. a Representative traces of a MSN recorded from a Cx–Str–SN/VTA coculture before and after 10 min of eticlopride (20 μM) bath application. MSN excitability was significantly increased by eticlopride as revealed by the change in number of action potentials evoked by intracellular current injection (50 pA) from one to four spikes. The increased excitability was occasionally accompanied by a more negative action potential threshold (*). b Scatter graph summarizing the effect of D2 blockade on the number of action potentials evoked in response to current injection, a measurement of neuronal excitability. c Traces illustrating plateau depolarizations in response to electrical stimulation of the cortical piece before (left) and after (right) administration of the D2 antagonist
Striatal plateau depolarizations require intracellular Ca++ and protein kinase A
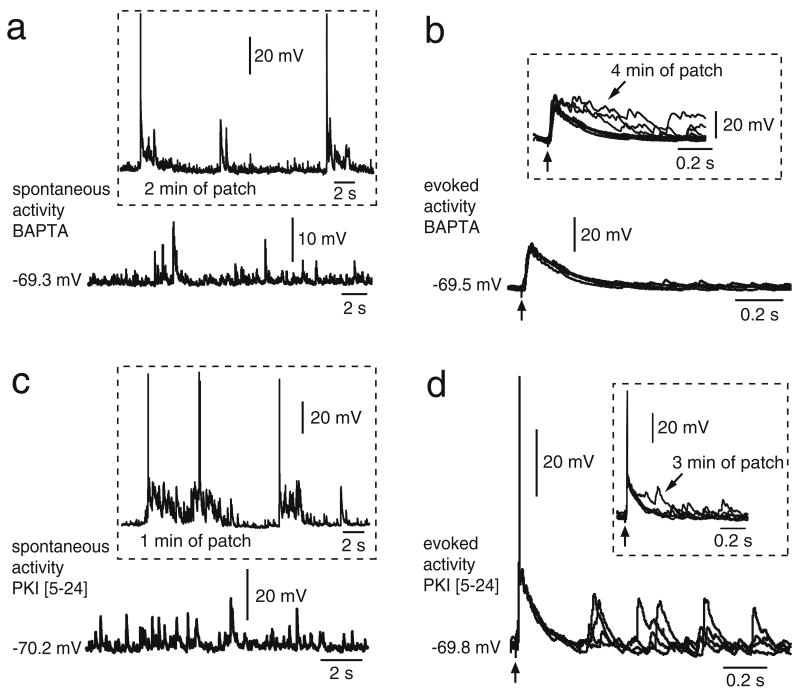
If NMDA-dependent plateau depolarizations also depend on DA, specifically on D1 receptors, they are likely to require D1-activated intracellular pathways and Ca++ (O’Donnell 2003). Indeed, we have recently shown that D1-NMDA-mediated plateau depolarizations in PFC pyramidal neurons require intracellular Ca++ and PKA (Tseng and O’Donnell 2005). To investigate whether these factors are involved in sustaining striatal up states, additional recordings were conducted in Cx–Str–SN/VTA cocultures with electrodes containing the Ca++ chelator BAPTA (2 mM) or the PKA inhibitor PKI [5–24] (20 μM). Recordings with BAPTA (n=11) yielded brief spontaneous depolarizations of duration within the range of those observed in Cx–Str cultures and significantly shorter than similar recordings without BAPTA (80.4±32.2 ms, P<0.0001; Fig. 8a), without affecting their frequency (0.5±0.3 Hz). Electrical stimulation of the cortical piece evoked EPSPs that did not extend into plateaus (n=10). These responses were shorter than in similar recordings without BAPTA (157.7±35.6 ms; P<0.0001; Fig. 8b), whereas the latency (10.2±2.2 ms) and amplitude (18.2± 4 mV) of the responses were not different. All these measures were taken at around 10 min after acquiring the whole-cell configuration. A comparable effect was observed in striatal neurons recorded in Cx–Str–SN/VTA cocultures with electrodes containing PKI [5–24] (n=10). Intracellular administration of 20 μM PKI [5–24] shifted spontaneous plateaus to brief depolarizations resembling the EPSPs observed in Cx–Str cocultures (89.2±33.2 ms duration; P<0.0001, compared to similar recordings without PKI [5–24]; Fig. 8c). PKI [5–24] also reduced cortically evoked plateaus into brief EPSPs (149.5±60.5 ms; P<0.0001, compared to similar recordings without PKI [5–24]; Student’s t test; Fig. 8d) without affecting their latency (11.0±0.5 ms) or amplitude (22.1±2.9 mV). This shortening of evoked plateaus was acquired within minutes of the whole-cell configuration; the values given correspond to measures taken at around 10 min of recording. These results indicate that intracellular Ca++ and PKA signaling are required for striatal plateau depolarizations in Cx–Str–SN/VTA cocultures.
Fig. 8.
MSN up states are Ca++ dependent and require PKA. a Representative tracing showing spontaneous activity of a striatal neuron recorded with an electrode containing the Ca++ chelator BAPTA (2 mM). A reduction in the frequency and duration of spontaneous plateau depolarizations was evident after ~10 min of recording. Inset shows the initial spontaneous activity of the same neuron 2 min after acquiring the whole-cell configuration. b Traces of the same neuron shown in a illustrating the effect of intracellular BAPTA (~10 min of recording) on the response evoked by cortical stimulation. The evoked EPSP did not extend into a plateau depolarization 10 min after acquiring the whole-cell configuration (inset shows an overlay of several traces including the initial ones recorded 4 min after patch acquisition, which did initially show an evoked plateau). EPSP duration was similar to what was observed in cocultures not containing a SN/VTA piece. c, d Striatal up states require protein kinase A. Representative traces of spontaneous (c) and cortically-evoked activity (d) of a striatal MSN obtained from a Cx–Str–SN/VTA coculture and being recorded for ~10 min with an electrode containing the protein kinase A inhibitor PKI [5–24] (20 μM). Insets show traces of the same neuron during the initial 1 to 3 min of patch recording. After ~10 min of recording, the evoked potential was shortened to values resembling those obtained from Cx–Str cocultures
Striatal plateau depolarizations recorded from Cx–Str–SN/ VTA cocultures are not dependent on cortical DA innervation
The presence of DA terminals in both cortical and striatal pieces raised the possibility that a D1 potentiation of NMDA function could occur in either of these regions. Although the density of DA terminals in the Cx was considerably sparser than that observed in the striatum (Fig. 9a,b), it is possible that striatal plateau depolarizations recorded from Cx–Str–SN/VTA cocultures resulted from an increased glutamatergic drive induced by the cortical DA innervation. To address this possibility, additional experiments were conducted in cocultures in which the SN/ VTA piece was placed next to the cortical piece, to produce a high density of DA fibers in the Cx and less DA innervation in the striatum. Indeed, a two-way ANOVA comparison revealed that the density of striatal and cortical TH fibers depends on the relative placement of the SN/VTA piece (P<0.0001, region vs configuration, F(1,40)=73.51). Thus, while a strong cortical DA innervation was obtained in all SN/VTA–Cx–Str cocultures (n=11), the striatal DA innervation was comparable to what was normally seen in the Cx of conventional triple cultures (n=9; Fig. 9a,b). Nearly half (4/9) of pyramidal neurons recorded from cortical pieces with significant DA innervation exhibited spontaneous plateau depolarizations resembling in vivo up states (Fig. 10), suggesting that DA can sustain cortical up states as previously reported (Lewis and O’Donnell 2000; Tseng and O’Donnell 2005). However, striatal MSN in those cultures did not present spontaneous or evoked plateau depolarizations. Evoked plateau depolarizations in striatal MSN were positively correlated with the degree of striatal, but not cortical, DA innervation (Fig. 9c,d). It appears that a critical level of TH fiber density (i.e., covering at least 30% of the striatal piece) is required in the striatum to observe evoked plateaus. MSN recorded from SN/VTA–Cx–Str cocultures exhibited resting membrane potential (−69.9±1.5 mV; n=13), input resistance (301.9± 50.6 MΩ), and spontaneous brief depolarizations (67.3± 15.2 ms) comparable to those recorded in Cx–Str and Cx–Str–Cx cocultures (Fig. 9e). Similarly, cortical stimulation evoked brief (93.6±23.2 ms) EPSPs (amplitude: 20.9±1.6 mV, latency: 10.9±2.6 ms, n=13) in cultures with the SN/VTA piece next to the cortical piece instead of plateau depolarizations (Fig. 9f). These results indicate that although cortical DA innervation can sustain cortical up states, this is not necessarily translated into striatal plateau depolarizations in Cx–Str–SN/VTA cocultures. Moreover, a critical level of striatal DA innervation seems to be required to potentiate MSN glutamatergic responses into sustained depolarizations. Thus, MSN up states in corticostriatal cocultures may depend on striatal DA.
Fig. 9.
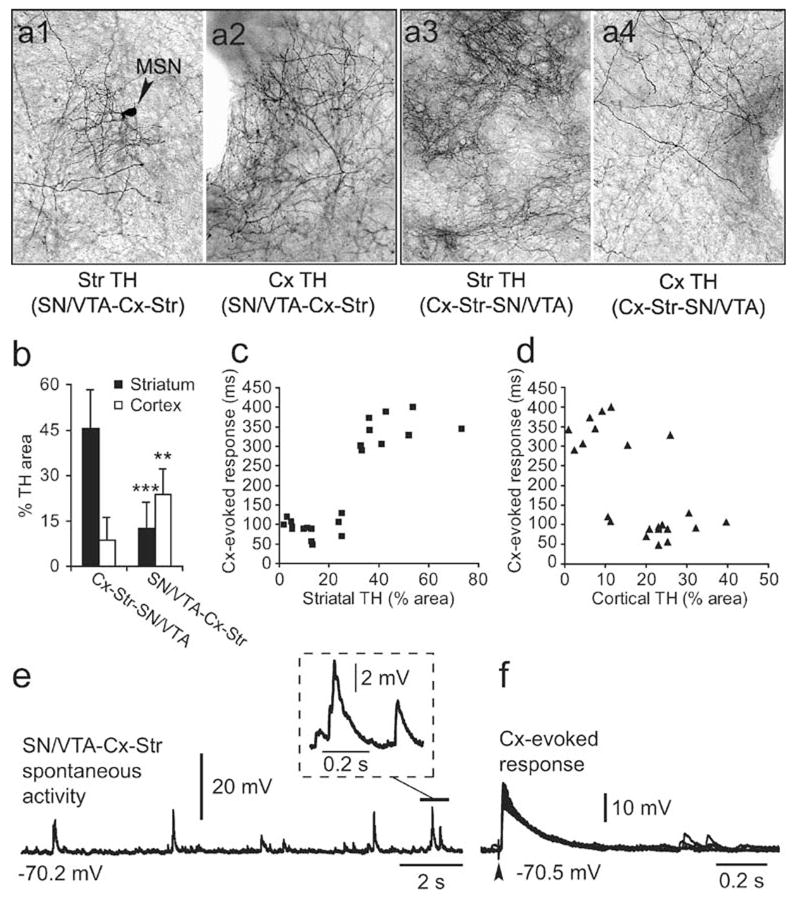
MSN up states depend on striatal, but not cortical DA innervation. a Placing the SN/VTA piece next to the Cx piece and far from the striatal piece increased the density of TH fibers in the Cx and reduced striatal DA innervation. b Bar graphs summarizing the extent of striatal and cortical DA innervation obtained from nine Cx–Str–SN/VTA and 11 SN/VTA–Cx–Str cocultures. Placing the SN/ VTA piece next to the cortical region significantly yielded higher cortical and lower striatal densities than those from the conventional configuration (***P<0.0002, **P<0.003, Tukey post hoc test after significant 2-way ANOVA). c, d Scatter-plots showing the relationship between the density of TH fibers and MSN responses to cortical stimulation. A clearly positive correlation could be observed between evoked plateau depolarizations and striatal DA innervation. As indicated in c, MSN plateau depolarizations could be observed only in presence of a critical level of striatal DA innervation. In contrast, no apparent correlation was observed between MSN-evoked plateaus and the density of cortical DA innervation. e Representative tracing showing spontaneous activity in a MSN recorded from an SN/VTA–Cx–Str coculture. In this configuration, spontaneous brief EPSPs (inset) were observed instead of plateau depolarizations. f Traces of the same neuron shown in e illustrating the response evoked by cortical stimulation. The evoked EPSP did not extend into a plateau depolarization and their duration was similar to what was observed in Cx–Str cocultures without the SN/VTA piece
Fig. 10.
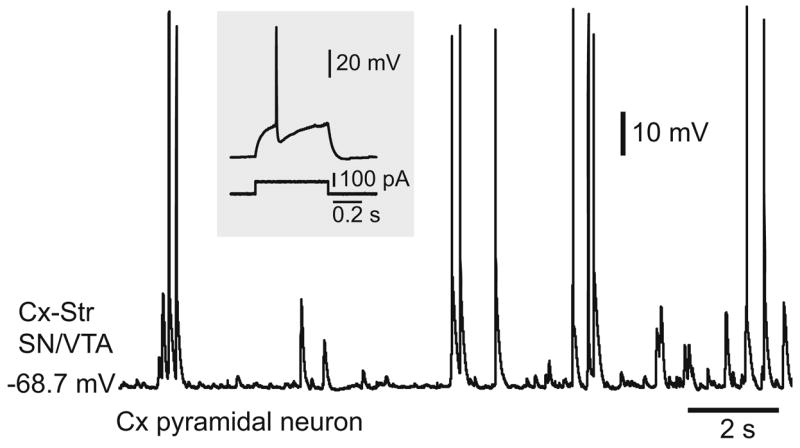
The presence of DA innervation in the cortical piece can allow spontaneous plateau depolarizations and action potential firing in some pyramidal neurons. Traces from a cortical pyramidal neuron recorded from a SN/VTA–Cx–Str coculture showing up states. Inset shows the typical voltage response to suprathreshold somatic current step injection
Can serum promote plateau depolarizations in Cs–Str cocultures?
In contrast to what we observed in the present study, MSN recorded from chronic (30 to 60 DIV) Cx–Str organotypic cocultures raised with the roller tube technique have revealed spontaneous plateau depolarizations resembling the in vivo up states, even in the absence of DA inputs (Plenz and Kitai 1998). Typically, these chronic cultures were kept in serum-containing medium to prolong the survival of neurons. To determine whether the presence of serum facilitates plateau depolarizations, additional experiments were conducted in Cx–Str cocultures kept in serum-containing medium during the entire period of culturing. After 20–30 DIV in presence of serum, 4/9 MSN recorded from Cx–Str cocultures exhibited sustained depolarizations resembling those reported above whereas the remaining five neurons only showed spontaneous EPSPs. Spontaneous depolarizations lasted 53.6±16.2 ms in MSN from Cx–Str cocultures without serum (n=15) and 324.1±250 ms in MSN from Cx–Str cocultures incubated with serum (n=9; p=0.01, Student’s t test; Fig. 11). These results suggest that chronic incubation of organotypic cultures in a serum-containing medium can modify the cellular and network properties of corticostriatal circuitry.
Fig. 11.
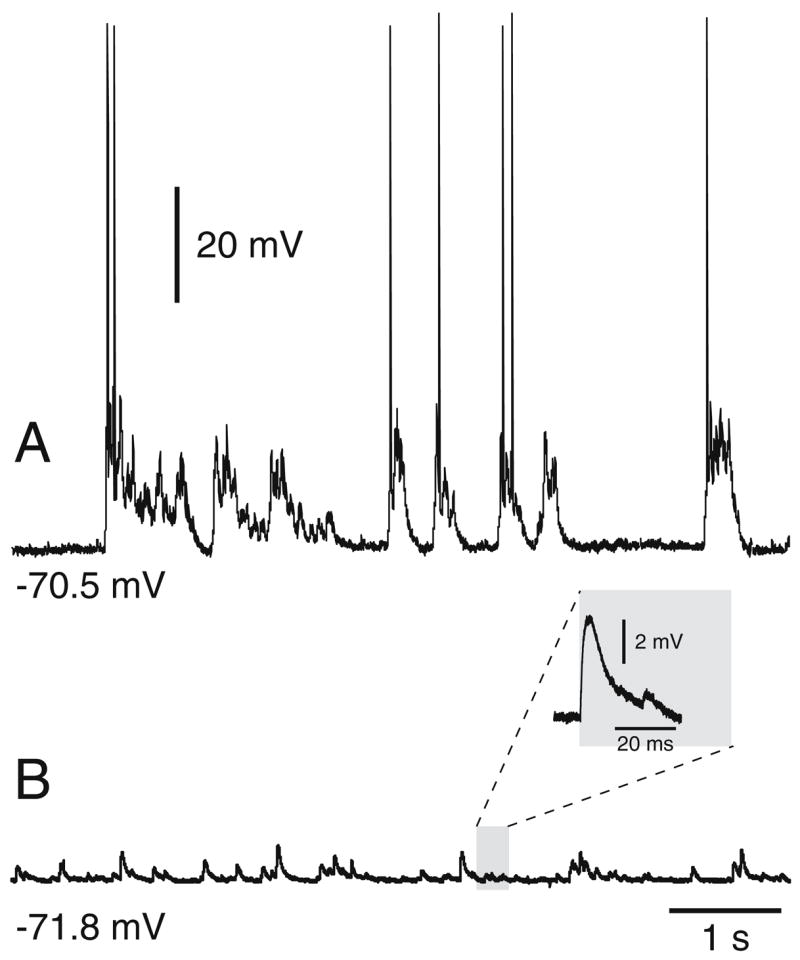
MSN in Cx–Str cocultures kept with serum-containing media can exhibit plateau depolarizations resembling in vivo up states. a Traces of a MSN recorded from Cx–Str cultures kept in serum showing spontaneous plateau depolarizations similar to those previously reported (Plenz and Kitai 1998). After 20–30 DIV, 4/9 MSN recorded exhibited plateau depolarizations. b An example of another MSN recorded from a similar serum-containing condition showing only spontaneous EPSPs
Discussion
Spontaneous and cortically-evoked plateau depolarizations resembling in vivo up states were recorded in striatal MSN from corticostriatal cocultures, but only in those also containing a SN/VTA piece providing strong striatal DA innervation or in some of those incubated in continued exposure to serum. The non-NMDA antagonist CNQX eliminated these depolarizations, confirming they are driven by glutamate. The NMDA antagonist APV and the D1 antagonist SCH23390 failed to affect the onset of evoked responses, but shortened them into depolarizations with a time-course characteristic of AMPA responses instead of plateaus. Recordings with electrodes containing the Ca++ chelator BAPTA or the PKA inhibitor PKI [5–24] did not yield striatal plateaus, but brief depolarizations. These results indicate that MSN synaptic responses to cortical activation require non-NMDA receptors. NMDA receptors can prolong these synaptic responses into plateau depolarizations, an effect that also involves PKA and intracellular Ca++, and could be facilitated by striatal D1 receptors (Fig. 12).
Fig. 12.
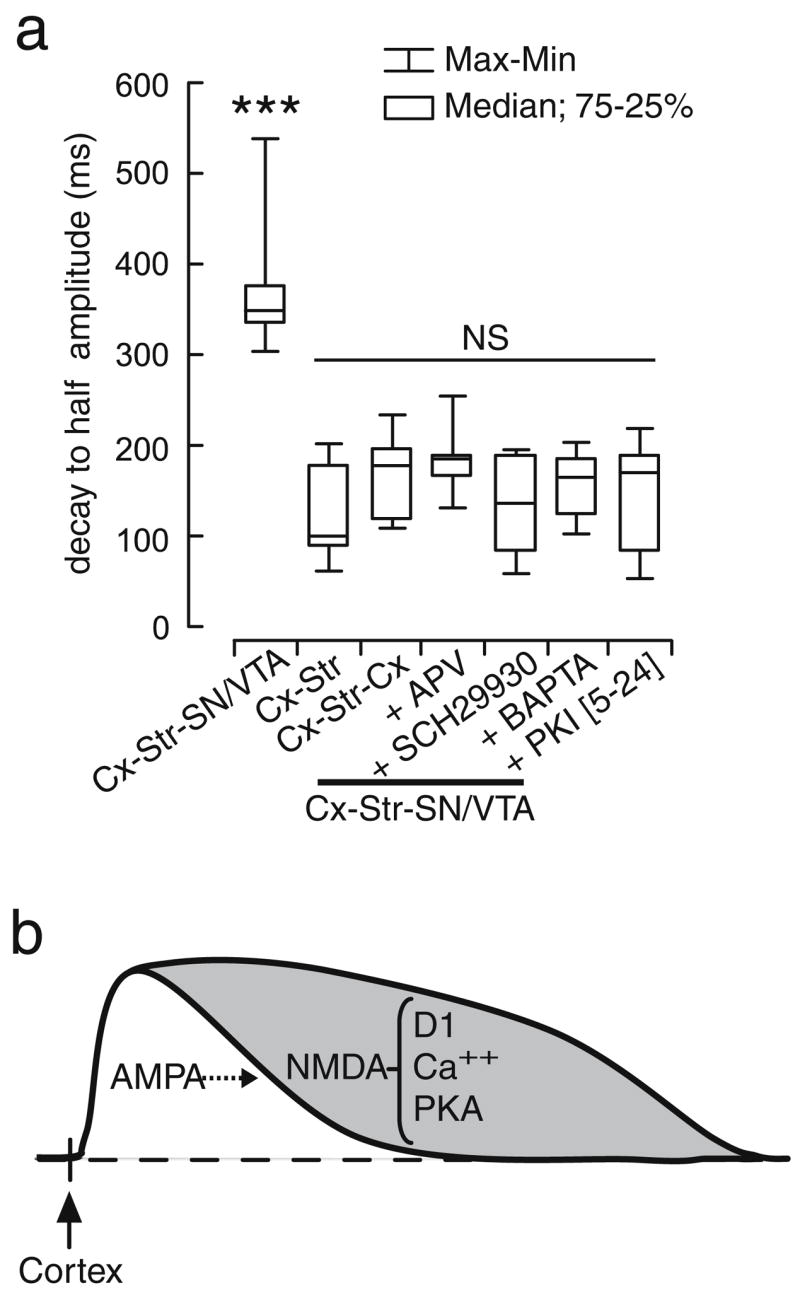
a Box plots summarizing the duration of cortically evoked striatal depolarizations observed in each experimental condition (***P<0.0002, Tukey post hoc test after significant ANOVA). b Schematic diagram illustrating the proposed sequence of events driving plateau depolarizations in striatal neurons. The transition to the up states may be mediated by AMPA receptors, with DA acting to sustain plateau depolarizations through a D1 enhancement of NMDA function in a Ca++- and PKA-dependent manner
The plateau depolarizations in organotypic cocultures may represent a phenomenon equivalent to the up states observed in vivo. The duration of MSN plateau depolarizations observed in this study matches the duration of up states observed in anesthetized animals (Wilson 1993; O’Donnell and Grace 1995; Goto and O’Donnell 2001a,b; Mahon et al. 2001; Tseng et al. 2001). Although the temporal aspect of striatal up states has been only assessed in anesthetized animals, recordings from cortical neurons in vivo have revealed slow-frequency up states in pyramidal neurons similar to those observed in the striatum (Steriade et al. 1993; Lewis and O’Donnell 2000). In awake animals, recordings from barrel cortex have revealed that pyramidal neurons also show similar duration up states during quiet wakefulness, and activation of a natural stimulus (by deflecting vibrissae) caused a transition to the up state that lasted a few hundred milliseconds if cells were in the down state (Petersen et al. 2003). Thus, persistent depolarizations such as those observed in vivo may be observed in a reduced preparation in the absence of anesthetics.
Non-NMDA glutamate receptors initiate striatal up states in organotypic cocultures, and combined DA and NMDA activation maintains them. As reported by Plenz and Kitai (1998), bath application of a non-NMDA antagonist completely eliminated striatal plateau depolarizations. NMDA antagonists, however, failed to remove the evoked synaptic response, but significantly reduced the duration of plateau depolarizations, suggesting that NMDA receptors are required to sustain up states in striatal neurons. At the negative resting membrane potential of MSN, cortical inputs are likely to activate only non-NMDA receptors. With the ensuing depolarization, NMDA receptors can become activated and sustain the depolarization. A computational model of ventral striatal MSN employing realistic ionic conductances derived from actual recordings yielded up states only when sufficient NMDA activity was included (Wolf et al. 2005). This effect of NMDA, however, can only be seen in our cultures in the presence of DA innervation and D1 receptor activation, probably reflecting the D1 potentiation of NMDA responses that has been repeatedly observed in striatal MSN (Cepeda et al. 1993, 1998). It is likely that in absence of sufficient D1 activation, NMDA responses are not strong enough to maintain the plateaus. We have recently reported that coactivation of D1 and NMDA receptors in prefrontal cortical slices can also result in pyramidal neuron sustained depolarizations resembling in vivo up states (Tseng and O’Donnell 2005). Altogether, these data imply that both striatal and cortical up states could be induced with coincident DA and NMDA activation.
Several mechanisms may account for D1 and NMDA-dependent plateau depolarizations in striatal MSN. Intra-cellular Ca++ is an important factor as revealed by the absence of plateaus in striatal recordings with electrodes containing the Ca++ chelator BAPTA. This observation is consistent with dendritic Ca++ transients being detected during up states in organotypic cocultures (Kerr and Plenz 2002). Activation of the D1–cAMP–PKA signaling pathway may also contribute to sustain plateau depolarizations. This could occur by enhancing NMDA function (Cepeda and Levine 1998; Cepeda et al. 1998; Wang and O’Donnell 2001; Tseng and O’Donnell 2004) via a PKA-dependent phosphorylation of NMDA receptors (Cepeda and Levine 1998; Cepeda et al. 1998) or by D1-induced trafficking of striatal NMDA receptor subunits to the postsynaptic membrane (Dunah and Standaert 2001). In addition to enhancing NMDA responses, D1 receptors may activate Ca++ currents that could prolong the depolarization (Surmeier et al. 1995; Hernandez-Lopez et al. 1997; Vergara et al. 2003). It is likely that the combined D1 effects on intracellular Ca++ and NMDA responses are necessary for the expression of plateau depolarizations. This is in line with recent data showing that a D1 potentiation of NMDA responses can elicit up states in prefrontal cortical neurons, an effect that also requires intracellular Ca++ and PKA (Tseng and O’Donnell 2005). Thus, striatal up states initiated by non-NMDA receptors may be sustained by a DA enhancement of postsynaptic Ca++ and NMDA function via a PKA-dependent pathway (Fig. 12).
The impact of D2 receptors on MSN excitability, plateau depolarizations and synaptic responses was also tested. The D2 antagonist eticlopride blockade failed to affect up states in MSN from Cx–Str–SN/VTA cocultures. However, it did enhance MSN excitability as revealed by the increased number of action potentials evoked by somatic current injection. These results are consistent with a report by West and Grace (2002) showing that intrastriatal administration of D2 receptor antagonists via reverse dialysis did not modify striatal up states, but enhanced MSN excitability and synaptic responses to cortical stimulation in vivo. Thus, striatal D2 receptors could regulate both MSN excitability and their response to converging corticostriatal excitatory drive, but may have little impact on up states.
The presence of MSN plateau depolarizations in Cx–Str organotypic cocultures could depend on DA-glutamate interactions in either the cortical or the striatal piece. Cortical DA innervation could be responsible for striatal plateaus by yielding enhanced glutamatergic drive to the striatal piece. This was ruled out because in cocultures with heavy cortical and sparse striatal DA innervation, cortical up states, but not striatal plateau depolarizations, were observed. Second and more importantly, the intracellular manipulations blocking MSN up states (i.e., BAPTA and PKA blockers in the pipette) strongly argue for an effect at the MSN level. Therefore, it can be concluded that MSN plateau depolarizations in this preparation are dependent on local DA actions.
Previous recordings with similar corticostriatal organo-typic cocultures have shown spontaneous up states even in the absence of DA (Plenz and Kitai 1998). Those cultures had been kept in serum-containing medium for the entire incubation period (typically 2 months), while our cultures were serum-free except for the first 3 days. Although serum may serve to prolong the survival of neurons in chronic organotypic cocultures, it also contains many active components that could have a direct effect on intracellular signaling pathways, specifically on PKA (Edin et al. 2001; Snyder et al. 2004). A subset of our data revealed that persistent incubation with serum can yield plateau depolarizations in MSN without a DA innervation. It is conceivable that in the presence of serum, NMDA currents in MSN become enhanced by serum-related factors, bypassing the required potentiation by D1 receptors. It would be of great interest to determine which element present in serum can allow this modulation.
Spontaneous up states recorded in striatal neurons in vivo are strongly dependent on synchronous cortical activity (Wilson 1993; O’Donnell and Grace 1995; Goto and O’Donnell 2001b; Mahon et al. 2001; Tseng et al. 2001) rather than DA levels. Local administration of DA antagonists (West and Grace 2002) or acute DA depletion (Reynolds and Wickens 2000) failed to eliminate striatal up states suggesting that DA is not required to maintain spontaneous up states in MSN in vivo. As other mono-amines could also activate similar second messenger cascades, it is possible that norepinephrine or acetylcholine receptors could have similar effects in vivo. On the other hand, VTA stimulation with trains of pulses mimicking DA cell burst firing resulted in prolonged plateau depolarizations resembling spontaneous up states in MSN that were shortened by blocking both D1 and D2 receptors (Goto and O’Donnell 2001a). This suggests that VTA-driven MSN up states involve DA actions that may depend on interactions between intrinsic voltage-gated conductances and the strength of local excitatory inputs (O’Donnell 2003).
Striatal D1 and NMDA-dependent plateau depolarizations may have important consequences for information processing in the basal ganglia. It has been proposed that DA regulates corticostriatal information by enhancing strong and diminishing weak glutamatergic inputs (DeFrance et al. 1985; O’Donnell and Grace 1996; Nicola et al. 2000; Schultz 2002). These diverse effects may depend on the interactions observed among specific DA and glutamate receptors (O’Donnell et al. 1999). At low DA levels, for instance, NMDA responses would not be facilitated, rendering up states less likely to be sustained. During phasic DA increase, however, a strong D1 receptor activation can occur (Gonon 1997). If this coincides with strong glutamatergic inputs, it may set the MSN into a prolonged plateau depolarization. It is possible that when a phasic DA signal reaches the striatum, the ongoing MSN activity mediated by non-NMDA receptors is enhanced by virtue of D1–NMDA coactivation resulting in prolonged up states in appropriate ensembles of MSN (O’Donnell 2003). Because coincident activation of glutamatergic corticostriatal and dopaminergic mesostriatal systems could occur in the presence of salient stimuli (Schultz 2002), the D1 modulation of corticostriatal plateau depolarizations reported in this study may serve as a mechanism by which DA strengthens the flow of relevant cortical information through the basal ganglia.
Acknowledgments
We thank Mr. Gregory Lyng for preparing some of the organotypic cocultures used in the present study. This research was supported by the Tourette Syndrome Association (ASK), USPHS grants MH57683 (PO’D), MH60131 (PO’D) and a NARSAD Independent Investigator Award (PO’D).
Contributor Information
Kuei Y. Tseng, Center for Neuropharmacology and Neuroscience, Albany Medical College, 47 New Scotland Ave (MC-136), Albany, NY 12208, USA
Abigail Snyder-Keller, Wadsworth Center, New York State Department of Health, Albany, NY 12208, USA.
Patricio O’Donnell, Center for Neuropharmacology and Neuroscience, Albany Medical College, 47 New Scotland Ave (MC-136), Albany, NY 12208, USA,e-mail: odonnep@mail.amc.edu.
References
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- DeFrance JF, Marchand JF, Sikes RW, Chronister RB, Hubbard JI. Characterization of fimbria input to nucleus accumbens. J Neurophysiol. 1985;54:1553–1567. doi: 10.1152/jn.1985.54.6.1553. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin ML, Howe AK, Juliano RL. Inhibition of PKA blocks fibroblast migration in response to growth factors. Exp Cell Res. 2001;270:214–222. doi: 10.1006/excr.2001.5345. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Network synchrony in the nucleus accumbens in vivo. J Neurosci. 2001a;21:4498–4504. doi: 10.1523/JNEUROSCI.21-12-04498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, O’Donnell P. Synchronous activity in the hippocampus and nucleus accumbens in vivo. J Neurosci. 2001b;21:RC131. doi: 10.1523/JNEUROSCI.21-04-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci. 2002;22:1499–1512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O’Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Mahon S, Deniau JM, Charpier S. Relationship between EEG potentials and intracellular activity of striatal and corticostriatal neurons: an in vivo study under different anesthetics. Cereb Cortex. 2001;11:360–373. doi: 10.1093/cercor/11.4.360. [DOI] [PubMed] [Google Scholar]
- Nicola S, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology. 1996;15:87–97. doi: 10.1016/0893-133X(95)00177-F. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann NY Acad Sci. 1999;877:157–175. doi: 10.1111/j.1749-6632.1999.tb09267.x. [DOI] [PubMed] [Google Scholar]
- Petersen CCH, Hahn TTG, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. PNAS. 2003;100:13643–13683. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex–striatum–substantia nigra organotypic cultures. J Neurosci. 1998;18:266–283. doi: 10.1523/JNEUROSCI.18-01-00266.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience. 2000;99:199–203. doi: 10.1016/s0306-4522(00)00273-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na+ channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279:45753–45758. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller A. Pattern of corticostriatal innervation in organotypic cocultures is dependent on the age of the cortical tissue. Exp Neurol. 2004;185:262–271. doi: 10.1016/j.expneurol.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller A, Costantini LC, Graber DJ. Development of striatal patch/matrix organization in organotypic co-cultures of perinatal striatum, cortex and substantia nigra. Neuroscience. 2001;103:97–109. doi: 10.1016/s0306-4522(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21:6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1–NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol. 2003;553:169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, O’Donnell P. D1 dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]