Abstract
The results of recent research indicate that agonist replacement may be a viable option in the treatment of cocaine dependence. For example, d-amphetamine and modafinil have shown promise in managing cocaine dependence in preliminary clinical trials. The aim of this study was to determine the physiological and subject-rated effects of acute intranasal cocaine doses during chronic atomoxetine treatment. Atomoxetine was chosen because it produces pharmacological and subject-rated effects similar to those of prototypical stimulants and thus may also be a viable agonist replacement therapy. To this end, seven cocaine dependent subjects were maintained on doses of atomoxetine (0 [lead in], 5, 10, 20 and 0 [washout] mg, four times daily) for 3-5 days prior to completing experimental sessions in which ascending doses of intranasal cocaine (4, 20, 40 and 60 mg) were administered. Cocaine produced prototypical cardiovascular and subject-rated effects. Atomoxetine attenuated the systolic pressure increasing effects and enhanced the heart rate increasing effects of cocaine, but was otherwise devoid of effects. These results indicate that cocaine is well tolerated during atomoxetine maintenance. Further research is needed to better determine the effects of atomoxetine and cocaine combinations.
Keywords: Humans, Physiological Effects, Pharmacotherapy, Atomoxetine, Cocaine
1. Introduction
Results from preliminary clinical trials indicate that d-amphetamine, methylphenidate and modafinil may be effective as agonist replacement pharmacotherapies in the management of stimulant dependence (Dackis et al., 2005; Grabowski et al., 2001, 2004; Tiihonen et al., 2007). Identifying other novel agonist replacement therapies is important because it is unlikely that all stimulant-dependent individuals will respond favorably to d-amphetamine, methylphenidate or modafinil. Moreover, d-amphetamine and methylphenidate have significant abuse potential which might limit their clinical usage.
Atomoxetine (Strattera®), indicated for Adult Attention Deficit Hyperactivity Disorder (ADHD), produces pharmacological effects that are similar to those observed with prototypical stimulants (Bymaster et al., 2002; Fleckenstein et al., 2000; Johanson and Fischman, 1989; Kuczenski and Segal, 1997) and thus may have potential as a replacement medication for cocaine dependence. Atomoxetine blocks reuptake at the norepinephrine transporter and increases extracellular dopamine levels in the prefrontal cortex (Bymaster et al., 2002). The behavioral effects of atomoxetine also overlap to some extent with those of prototypical stimulants (e.g., Johanson and Barrett, 1993; Kleven et al., 1990; Lile et al., 2006; Spealman, 1995), but atomoxetine appears to have less abuse potential (Gasior et al., 2005; Heil et al., 2002; Lile et al., 2006; Wee and Woolverton, 2004). Whether atomoxetine is safe to administer with cocaine, and whether chronic atomoxetine dosing can alter the abuse-related effects of cocaine in cocaine dependent subjects is unknown. The purpose of the present experiment was to determine the safety, tolerability and subject-rated effects of acutely administered cocaine during atomoxetine maintenance in cocaine dependent subjects.
2. Method
2.1. Subjects
Seven subjects (5 males, 2 females; 5 African American, 2 Caucasian) meeting criteria for cocaine dependence as determined by a computerized version of the Structured Clinical Interview for the DSM-IV completed this within-subjects placebo-controlled study. All subjects were current crack cocaine users and reported using 424 ± 83 mg cocaine in the week prior to screening. All subjects completed comprehensive medical and psychological screens.
2.2. General Procedures
Subjects were enrolled as inpatients at the University of Kentucky Chandler Medical Center General Clinical Research Center (GCRC) for up to 28 days and participated in one practice and five experimental sessions. Urine and expired breath samples were collected prior to each session to confirm drug and alcohol abstinence, respectively. Females received urine pregnancy tests prior to each session.
The Medical Institutional Review Board of the University of Kentucky approved this study, and all subjects gave written informed consent. Subjects were paid for their participation.
2.2.1. Practice Session
Subjects completed one practice session to familiarize them with experimental measures, but did not receive medications.
2.2.2. Drug Maintenance Days
Drug maintenance began on the day immediately following the practice session and continued throughout the protocol. Placebo or atomoxetine was administered orally at 0700, 1200, 1700 and 2200 h. After three to five days of maintenance on placebo, subjects completed the first experimental session (described below), and began maintenance on the subsequent condition the following day. Every three to five days thereafter, subjects completed an experimental session and then proceeded to the next maintenance condition until study completion. The order of drug maintenance conditions was constant across volunteers: placebo (lead in), 5 mg atomoxetine, 10 mg atomoxetine, 20 mg atomoxetine and placebo (washout) administered four times per day. The total daily dose for each condition was 0, 20, 40, 80 and 0 mg atomoxetine. The three to five day maintenance window was used to avoid conducting experimental sessions on weekends, when medical coverage at the GCRC was limited.
2.2.3. Experimental Sessions
Subjects received the appropriate maintenance dose at 0700 on the morning of all experimental sessions. Experimental sessions started at 0800 h and lasted 6.5 h. Four intranasal cocaine doses were given each session in ascending order 1.5 h apart (4 [placebo], 20, 40 and 60 mg). Subjects completed all experimental measures prior to drug administration and every 15 min for 1 h after each cocaine dose. If heart rate exceeded 130 beats per minute, systolic blood pressure exceeded 180 mmHg, diastolic blood pressure exceeded 120 mmHg or clinically significant ECG changes occurred following administration of cocaine at any point during the experiment, participation was terminated. No subject was excluded from participation for exceeding these parameters.
2.3. Physiological and Subject-Rated Measures
Blood pressure and heart rate were recorded using a Dinamap Pro 1000 Vital Signs monitor (Critikon, Tampa, FL). Subject-rated measures included an Adjective Rating Scale (Oliveto et al., 1992), an adapted Cocaine Craving Questionnaire (Dudish-Poulsen and Hatsukami, 1997), a Stimulant Sensitive Adjectives Scale (Di Marino et al., 1998) and a locally developed Drug-Effect Questionnaire (Rush et al., 2003). GCRC nursing staff also completed the UKU Side Effects Scale with the subjects daily (Lingjaerde et al., 1987). Although not analyzed statistically, visual inspection of this scale by unit physicians revealed no significant changes throughout study participation. Moreover, to determine that atomoxetine maintenance did not alter liver function, comprehensive liver panels were conducted before and after study participation. Liver function was normal before and after study participation.
2.4. Drug Administration
All drugs were administered in a double-blind fashion. Atomoxetine (5, 10 and 20 mg q.i.d.; Eli Lilly and Co., Indianapolis, IN) doses were prepared from commercially available drug in a size-0 capsule with cornstarch. Placebo capsules contained only cornstarch.
Cocaine doses (4 mg [placebo], 20, 40 and 60 mg) were prepared by combining the appropriate amount of cocaine HCl (Mallinckrodt, St. Louis, MO) with lactose to equal a total of 80 mg powder. During each administration, a nurse presented the subject with the powder, a mirror and a standard razor blade. The subject was instructed to divide the powder into two even “lines” and insufflate one line of powder through each nostril using a 65-mm plastic straw within 2 minutes.
2.5. Data Analysis
Data from the experimental sessions were analyzed as peak effect (i.e., the maximum score observed following each cocaine administration) using a two factor repeated-measures ANOVA with Atomoxetine maintenance condition (0 [lead in], 20, 40, 80 and 0 [washout] mg) and Cocaine dose (4 [placebo], 20, 40 and 60 mg) as the factors (StatView, Cary, NC). F values from the ANOVA were used to interpret the results. Effects were considered significant for p ≤ 0.05.
3. Results
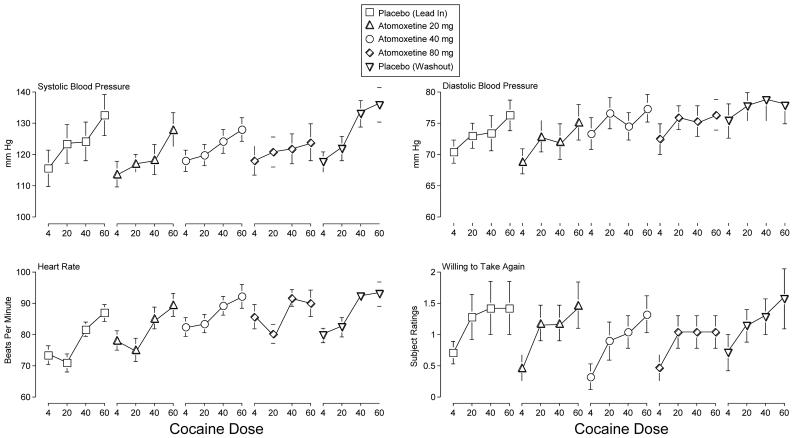
Cocaine dose-dependently increased Systolic Blood Pressure, and atomoxetine attenuated this effect across maintenance conditions relative to both placebo maintenance conditions (i.e., interaction of Atomoxetine and Cocaine; F12,72 = 2.4, p = 0.01)(Figure 1). Cocaine also dose-dependently increased Diastolic Blood Pressure and Heart Rate (i.e., main effects of Cocaine; F3,18 values > 6.8, p's < 0.01) (Figure 1). Atomoxetine doses enhanced this effect for Heart Rate relative to the first placebo maintenance (i.e., main effect of Atomoxetine; F4,24 = 17.1, p < 0.01) (Figure 1).
Figure 1.
Peak effect dose-response functions for cocaine following maintenance on placebo (lead in; squares), 20 mg atomoxetine (flat-bottomed triangles), 40 mg atomoxetine (circles), 80 mg atomoxetine (diamonds), and placebo (washout; flat-topped triangles) for Systolic Blood Pressure, Diastolic Blood Pressure, Heart Rate and subject ratings of Willing to Take Again. The maximum subject rating is 4. X-axis: Intranasal cocaine dose. Brackets indicate 1 S.E.M.
Cocaine dose-dependently increased subject ratings on the Stimulant Subscale of the Adjective Rating Scale and the Total Score on the Stimulant Sensitive Adjectives (i.e., main effects of Cocaine; F3,18 values > 6.9, p < 0.01), but atomoxetine did not modulate these effects (data not shown).
Cocaine increased subject ratings on ten items from the Drug-Effect Questionnaire: Active, Alert, Energetic; Any Effect; Good Effects; High; Irregular or Racing Heartbeat; Like Drug; Rush; Stimulated; Willing to Pay For; and Willing to Take Again (i.e., main effects of Cocaine; F3,18 values > 3.2, p's < 0.05). Atomoxetine did not alter these ratings. Figure 1 shows subject ratings for Willing to Take Again.
4. Discussion
The results of the present experiment indicate that acute administration of intranasal cocaine is safe and tolerable during oral atomoxetine maintenance. Intranasal cocaine produced prototypical stimulant-like effects (e.g., increased blood pressure and heart rate, increased subject ratings of Like Drug and Stimulated) and atomoxetine generally did not alter these effects. For example, while it appears that 80 mg atomoxetine maintenance attenuated subject ratings of Willing to Take Again (Figure 1), this effect did not attain statistical significance. In fact, there were no statistically significant effects of atomoxetine on subject ratings in this study. As discussed below, it is possible that longer maintenance on atomoxetine or maintenance on a higher dose, would have resulted in more robust attenuation of the subject-rated effects of cocaine.
Although atomoxetine failed to alter most of most of the effects of cocaine and was generally devoid of behavioral effects on its own (i.e., when comparing response to placebo cocaine across maintenance conditions, no differences were generally observed), it attenuated the systolic pressure increasing effects of cocaine under all maintenance conditions and enhanced the heart rate increasing effects of the lower cocaine doses. Importantly, the increases in heart rate were not clinically significant. The reason for the divergent effects of atomoxetine on systolic blood pressure and heart rate is not known but could be related to previous findings that both acute and short-term treatment with atomoxetine produces greater increases in heart rate than in blood pressure (Heil et al., 2002; Wernicke et al., 2003).
There are some limitations to the present experiment that should be acknowledged. The doses of cocaine produced modest peak effects. The use of higher cocaine doses, which would produce greater effects, may have resulted in an observation of more consistent interactions between atomoxetine and cocaine. Moreover, the duration of atomoxetine maintenance may not have been long enough to produce maximal interactions with cocaine. The three to five day dosing regimen was selected based on the half-life of atomoxetine, which follows first order kinetics and has a half-life of approximately four hours (Farid et al., 1985). By dosing four times daily over three to five days (i.e., 18-30 half lives of atomoxetine) steady-state conditions were achieved before assessing the behavioral effects of intranasal cocaine. However, clinical response to atomoxetine when used in the treatment of ADHD may not occur until one week of treatment has elapsed (Spencer et al., 2001). More robust and consistent interactions of atomoxetine and cocaine may have been observed had the dosing regimen been longer or the atomoxetine dose higher. Although 80-100 mg daily is the maximum recommended dosing level for managing ADHD, the dose necessary for management of cocaine dependence may be higher. Lastly, the present results are confounded by the ascending nature of dose administration for both cocaine and atomoxetine. The use of ascending dose administration is a necessity when conducting initial safety and tolerability trials, but may result in effects of order or acute tolerance to the effects of cocaine. Future studies should examine combinations of cocaine and atomoxetine when administered in randomized order.
The utility of atomoxetine for stimulant use disorders remains unclear, although the use of other agonist-type medications (e.g., modafinil, d-amphetamine, methylphenidate) has proven effective in managing stimulant dependence (Dackis et al., 2005; Grabowski et al., 2001 2004; Tiihonen et al., 2007). The results of the present study indicate that cocaine can safely be administered to atomoxetine-maintained volunteers. Moreover, this experiment provides the basis for further laboratory research and clinical trials that will be necessary to evaluate the efficacy of atomoxetine as an agonist replacement in treating cocaine dependence. Only through the conduct of additional laboratory-based and clinical research can the utility of atomoxetine for cocaine dependence be determined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Di Marino ME, Haberny KA, Felch LJ, Walsh SL, Preston KL, Bigelow GE. Development of a subject- rated rating scale sensitive to acute cocaine administration. In: Harris L, editor. Problems of Drug Dependence, 1997: Proceedings of the 59th Annual Scientific Meeting; Rockville, MD. 1998. p. 139. National Institute on Drug Abuse Research Monograph Series. [Google Scholar]
- Dudish-Poulsen SA, Hatsukami DK. Dissociation between subjective and behavioral responses after cocaine stimuli presentations. Drug Alcohol Depend. 1997;47:1–9. doi: 10.1016/s0376-8716(97)00054-9. [DOI] [PubMed] [Google Scholar]
- Farid NA, Bergstrom RF, Ziege EA, Parli CJ, Lemberger L. Single-dose and steady-state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25:296–301. doi: 10.1002/j.1552-4604.1985.tb02842.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: Pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Gasior M, Bergman J, Kallman MJ, Paronis CA. Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a concurrent schedule of food and i.v. drug delivery in rhesus monkeys. Neuropsychopharmacology. 2005;30:758–764. doi: 10.1038/sj.npp.1300593. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: A double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: Two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Heil SH, Holmes HW, Bickel WK, Higgins ST, Badger GJ, Laws HF, Faries DE. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend. 2002;67:149–156. doi: 10.1016/s0376-8716(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Barrett JE. The discriminative stimulus effects of cocaine in pigeons. J Pharmacol Exp Ther. 1993;267:1–8. [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Durell TM, Glaser PE, Rush CR. Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2006;14:136–147. doi: 10.1037/1064-1297.14.2.136. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Spencer T, Biederman J, Heiligenstein J, Wilens T, Faries D, Prince J, Faraone SV, Rea J, Witcher J, Zervas S. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:251–265. doi: 10.1089/10445460152595577. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Evaluation of the reinforcing effects of atomoxetine in monkeys: Comparison to methylphenidate and desipramine. Drug Alcohol Depend. 2004;75:271–276. doi: 10.1016/j.drugalcdep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Wernicke JF, Faries D, Girod D, Brown JW, Gao H, Kelsey D, Quinatna H, Lipetz R, Michelson D, Heiligenstein J. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003;26:729–740. doi: 10.2165/00002018-200326100-00006. [DOI] [PubMed] [Google Scholar]