Abstract
Otitis media is an extremely common pediatric inflammation of the middle ear that often causes pain and diminishes hearing. Vulnerability to otitis media is due to eustachian tube dysfunction as well as other poorly understood factors, including genetic susceptibility. As EYA4 mutations cause sensorineural hearing loss in humans, we produced and characterized Eya4-deficient (Eya4–/–) mice, which had severe hearing deficits. In addition, all Eya4–/– mice developed otitis media with effusion. Anatomic studies revealed abnormal middle ear cavity and eustachian tube dysmorphology; thus, Eya4 regulation is critical for the development and function of these structures. We suggest that some human otitis media susceptibility reflects underlying genetic predisposition in genes like EYA4 that regulate middle ear and eustachian tube anatomy.
Introduction
Otitis media, an inflammation of the middle ear, accounts for 24 million pediatric office visits annually (1), is responsible for over $4 billion in annual health care costs, and prompts more antibiotic prescriptions and surgery (ear ventilation tubes) than any other pediatric disease (2). Otitis media occurs at least once in 90% of children before age 2 years (3, 4). This extensive vulnerability during childhood reflects immature eustachian tube function (5) as well as other poorly understood susceptibility factors, including environmental triggers and genetic influence (6, 7). Additional etiologies include developmental abnormalities (cleft palate) and nasopharyngeal tumors, conditions that further indicate eustachian tube dysfunction as a primal trigger for otitis media.
Composed of a bony ostium from the middle ear and a distensible cartilaginous segment that links the tympanic cavity to the nasopharynx, the eustachian tube is responsible for regulating middle ear air pressure, removing normal ear secretions, and protecting otic tissues from nasopharyngeal secretions. A highly specialized mucociliary epithelium that lines the tube further supports these physiologic functions. Without normal venting and clearance of secretions by the eustachian tube, mucosal secretions accumulate and the tympanic cavity becomes inflamed and often develops an effusion, features that define otitis media. Otoscopic examination reveals a hypervascular and retracted tympanic membrane. Without an associated infection, pain is minimal, but with microbial invasion, acute otitis media ensues with inflammation, increased middle ear pressure, and resultant outward bulging of the tympanic membrane, producing severe otalgia. Otitis media produces a 25- to 30-decibel (dB) conductive hearing loss that typically reverses when the eustachian tube regains function and the accumulated middle ear fluids are drained. Recognition and treatment of otitis media, with or without infection, may prevent long-term sequelae of hearing loss; speech, language, and learning deficits; and behavioral issues (4).
Human mutations in 2 EYA gene family members cause sensorineural hearing loss. Human EYA1 mutations cause branchiootorenal (BOR) syndrome (OMIM 113650) (8), with branchial arch defects including lacrimal duct abnormalities, preauricular fistulae, and hearing loss (mixed conductive and sensorineural), with abnormally shaped external ears, cochlear malformations, and renal anomalies (9). EYA4 mutations cause sensorineural hearing loss (DFNA10 locus on chromosome 6) without affecting external ear or other craniofacial structures; these mutations are sometimes accompanied by cardiomyopathy (10–13). The onset of hearing loss due to EYA4 mutations is notably broad, ranging from early in childhood to adulthood (14), and hearing deficits are not stable until adulthood. Factors accounting for the range of age at onset of hearing loss among identical mutation carriers are unknown.
Eya genes encode transcriptional coactivators (15, 16) that translocate into the nucleus in association with Six molecules, where they regulate gene expression. Functional studies indicate that Eya1 is a nuclear phosphatase that promotes release of transcriptional repression by Dachshund on target promoters (17–19). Although genes regulated by Eya molecules remain largely unknown (20), the consequences of human EYA mutations implicate these nuclear phosphatases in diverse mammalian organ functions.
To elucidate the roles of Eya4 in auditory function, we generated Eya4-deficient (Eya4–/–) mice. Eya4–/– mice exhibited early-onset and profound hearing defects. In characterizing the mechanisms for deafness in Eya4–/– mice, we uncovered otitis media with effusion that occurs in the context of developmental defects in eustachian tube and middle ear. We suggest that Eya4–/– mice model human otitis media that arises from eustachian tube dysfunction and illuminate a requirement for this nuclear phosphatase in middle ear maturation. In addition, these data suggest a mechanism to account for variable hearing loss in patients with EYA4 mutations.
Results
Production of Eya4-deficient mice.
The endogenous Eya4 gene was targeted by homologous recombination in embryonic stem cells, and heterozygous 129S6/SvEv Eya4+/– mice were produced (Figure 1, A and B, and data not shown). Eya4+/– mice were viable and fertile, but homozygous 129S6/SvEv Eya4-null mice (Eya4–/–), generated by breeding, died shortly after birth. At weaning, 0 of 337 mice from Eya4+/– matings were Eya4–/– (P = 6.0E–26; Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI32899DS1). RT-PCR analyses confirmed the absence of Eya4 RNA in Eya4–/– mice (Figure 1C).
Figure 1. Eya4-targeting strategy.
(A) A Neo and Zeo cassette flanked by loxP sites (black triangles) was inserted into Eya4 exons 8–10 by homologous recombination in bacteria. The mutant allele was introduced into ES cells using standard homologous recombination techniques (see Methods). (B) PCR analyses of wild-type (+/+), homozygous Eya4–/– (–/–), and heterozygous Eya4+/– (+/–) genotypes. Primers intron 7F (I7F), exon 8R (E8R), and NeoR demonstrated the presence of wild-type (516 bp) and mutant (446 bp) alleles. (C) RT-PCR of cardiac cDNA using external forward primer 4F and internal reverse primer 9R identified a single 469-bp product in wild-type (+/+) and heterozygous Eya4+/– (+/–) but not Eya4–/– (–/–) mice.
Because of the recognized common occurrence of middle ear infections in the 129S6/SvEv strain (21), Eya4+/– mice were bred onto the CBA/J background. An inbred line was established and characterized and is reported on here. Fortuitously, from the F3 generation onward, most Eya4–/– mice in the CBA/J background survived (Supplemental Table 1), although overall viability of Eya4–/– mice was significantly reduced in comparison with heterozygous mutant or wild-type mice (P = 1.3E–3). At birth, body weights of Eya4–/– mice were comparable to wild-type or Eya4+/– littermates. However, adult Eya4–/– mice weighed 25% less than either wild-type or Eya4+/– mice, and at 10 months of age, the average weights of Eya4–/– mice (30.7 ± 4.49 g; n = 30) mice were significantly less (P < 1.0E–6) than of wild-type (41.2 ± 7.96 g; n = 25) or Eya4+/– (40.3 ± 5.23 g; n = 19) mice. Eya4–/– females reproduced normally, but male Eya4–/– mice were sterile or had significantly diminished fertility (e.g., only 2 litters were obtained from 13 male Eya4–/– mice of reproductive age; P < 9.0E–14) in comparison with normal fertility of heterozygous Eya4+/– male mice.
Hearing deficiency and otitis media in Eya4–/– mice.
Because the activities and responses to auditory stimuli by Eya4–/– mice suggested hearing deficits, distortion product otoacoustic emission (DPOAE) and auditory brain stem response (ABR) thresholds were assessed in 10-week-old mice. In comparison with wild-type mice, DPOAE and ABR studies (n = 4 each genotype) indicated significant functional loss in peripheral auditory sensitivity of Eya4–/– mice (DPOAE, P = 4.05E–12; ABR, P = 8.66E–13).
To assess potential mechanisms for hearing loss in Eya4–/– mice, we examined the anatomy and histology of auditory canals (Table 1). Unexpectedly, all 21-day-old Eya4–/– mice (n = 50) showed hypervascularity of the tympanic membrane along the manubrium of malleus, marked tympanic membrane retraction, and middle ear effusions (Figure 2A), consistent with otitis media. Tympanic cavities of age-matched wild-type or Eya4+/– mice (n = 50; P = 6.2E–18) showed no signs of otitis media (data not shown). Examinations of other regional organ systems potentially involved in otitis media, including the respiratory tract, oral cavity, and the eye showed no overt inflammatory or infectious processes in more than fifty 10-week-old Eya4–/– mice.
Table 1 .
Evidence of otitis media in Eya4–/– mice with and without antibiotic prophylaxis
Figure 2. Middle ear pathology in Eya4–/– mice.
(A) Macroscopic views of tympanic membrane from wild-type (+/+) and Eya4–/– (–/–) mice. Eya4–/– mouse ears had air bubbles (asterisk), indicative of middle ear effusion, and dilated capillaries (black arrow) as well as tympanic membrane retraction (see also Figure 3). Scale bars: 1 mm. (B and C) PAS-stained paraffin sections of mice, age 3 weeks. (B) The mesotympanum of middle ear cavity shows inflammatory cells (red arrow) in Eya4–/– mice, and the mucoperiosteum (black arrowheads) is thickened. A, stapedial artery; I, incus; M, malleus; OW, bony niche of the oval window; T, tympanic membrane. Scale bars: 200 μm. (C) The hypotympanum regions of Eya4–/– mice show effusion (asterisk), inflammatory cells (red arrow), hyperplastic ciliated epithelial cells (black arrows), and expanded lamina propria connective tissue in the mucoperiosteum (arrowhead; corresponding regions denoted by gray arrow/arrowhead in wild-type). MEC, middle ear cavity.
To exclude the possibility that CBA/J background genes accounted for otitis media with effusion, we bred the Eya4–/– allele onto BALB/c, C57BL/6, and Swiss-Webster backgrounds. Eya4-null mice on the BALB/c (n = 3), C57BL/6 (n = 8), and SW (n = 3) backgrounds developed bilateral otitis media with effusion that was indistinguishable from that observed in the CBA/J background (data not shown). Based on the phenotypes of these Eya4-null mice, we concluded that background genetic loci (21, 22) were unrelated to observed ear pathology; instead, otitis media with effusion was a direct consequence of Eya4-deficiency.
Onset of otitis media.
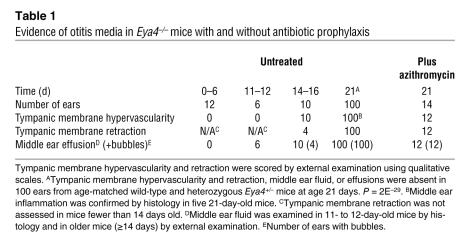
Because the mouse ear undergoes considerable postnatal maturation, we serially characterized ear development in wild-type and Eya4–/– mice (Table 1) to date the onset of otitis media. Before day 6, the external earflap (pinna) was folded in both wild-type and Eya4–/– mice, providing a protective barrier, and the middle ear cavity was filled with mesenchymal cells, as has been previously reported (23). There was no delay in the onset of middle ear cavitation (24), which became evident at day 6, and no inflammation in the newly formed small middle ear space in wild-type or Eya4–/– mice (Figure 3A). At days 11–12, when the pinna was unfolded in all mice, the middle ear cavity was largely aerated and mesenchymal cells were found only near ossicles in wild-type mice. In contrast, the middle ear cavity of Eya4–/– mice remained full of mesenchyme but showed no signs of inflammation (Figure 3B).
Figure 3. Comparison of postnatal middle ear cavitation in wild-type (+/+) and Eya4–/– (–/–) mice.
(A) Six-day-old mice (n = 6, each genotype) had no middle ear effusions. Mesenchymal cells (black arrows) are found in the middle ear cavity, but the middle ear space (red arrows) is visible. Co, cochlea. Scale bars: 200 μm. (B) At days 11 and 12 (n = 3 mice of each genotype), mesenchymal cells (black arrows) have largely disappeared from the middle ear cavity of wild-type mice, but a few cells remain around the ossicles. M, malleus. Many more mesenchymal cells and collagen (asterisks), a by-product of mesenchyme regression, are present in the MECs of Eya4–/– mice than in wild-type mice. See magnified inset (scale bars: 200 μm) showing mucoperiosteum and mesenchyme regression. The marked inward bulging of tympanic membrane in Eya4–/– mice correlated with tympanic membrane retraction that was visible through the external auditory canal (EAC). Scale bar: 40 μm. (C) At days 14–16 (n = 5 for each genotype), some collagen (black asterisks) remains in both genotypes, but Eya4–/– mice also show a hyperplastic mucosa (red asterisk) with inflammation. The opening of the eustachian tube (ET) is indicated (arrowheads). Inset shows a polyp (red arrowhead) and numerous inflammatory cells (black arrow) found in some Eya4–/– mice.
By postnatal days 14–16, tympanic membrane retraction and middle ear effusions, with or without air bubbles, were seen through the eardrum of Eya4–/– mice (Figure 3C) and the middle ear mucosa showed inflammation in all mutant ears (n = 10; Table 1). At weaning (day 21), all Eya4–/– mice, but no wild-type mice, had tympanic membrane retraction with hypervascularity and middle ear effusion with or without air bubbles (Figure 2A and Table 1; Eya4–/–, n = 50; wild-type, n = 40; P = 6.2E–18). Young Eya4–/– mice had serous effusions that contained few or no granulocytes (Figure 2, B and C); effusions in older Eya4–/– mice were more purulent and contained abundant granulocytes (data not shown).
Antibiotic prophylaxis in Eya4–/– mice.
To assess whether antibiotics could suppress otitis media and effusions in Eya4–/– mice, we administered azithromycin to pregnant females and continued prophylactic antibiotic treatment until pups were weaned. Azithromycin is efficacious in treating a variety of rodent models of human infection, including acute otitis media due to Haemophilus influenzae (25). At age 3 weeks, the tympanic cavities from both ears of 39 mice were evaluated for signs of effusion and inflammation (wild-type, n = 13; Eya4+/–, n = 19; Eya4–/–, n = 7). Visual inspection suggested no otitis media in 33 mice (all wild type; all Eya4+/–; 1 Eya4–/–), and histological studies confirmed the absence of effusion or inflammatory cells (Figure 4A and Table 1). In contrast, 6 of 7 azithromyocin-treated Eya4–/– mice had tympanic cavities with erythema, dilated capillaries, and histologic sections that demonstrated effusion and inflammation. We interpreted the persistence of otitis media with effusion in azithromyocin-treated Eya4–/– mice to indicate a predominant nonbacterial etiology.
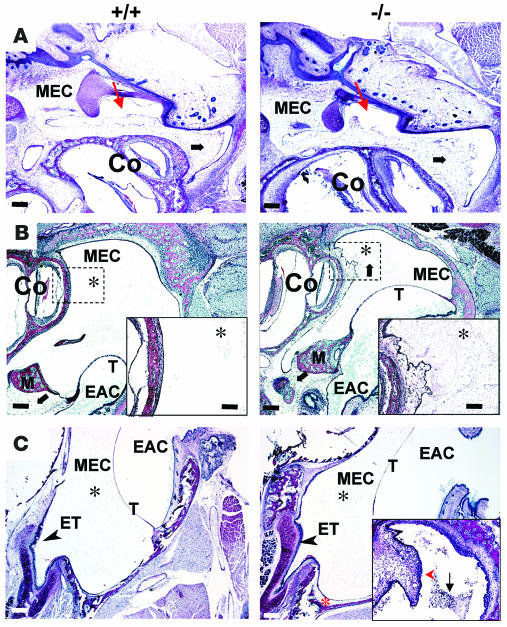
Figure 4. Morphometry of wild-type (+/+) and Eya4–/– (–/–) middle ear cavity in azithromycin-treated mice.
(A) The OpTC of Eya4–/– mice was smaller than that in wild-type. Scale bars: 200 μm. (B) A 3D reconstruction of portions of the middle ear cavity, eustachian tube, and nasopharynx (NP). The moET, isthmus, cartilaginous segment (cartET), and opening of the eustachian tube within the nasopharynx (OpNP) are indicated on the wild-type 3D reconstruction. The location of the OpTC is denoted (white asterisk). Eustachian tube dimensions (see Table 2) were obtained by measurement of the structures identified. Scale bar: 300 μm.
Eustachian tube dysfunction.
Impaired eustachian tube function is recognized as the initial mechanism that triggers otitis media in humans (5). Impairment can be transient, as occurs during an acute upper respiratory tract infection, or can be more permanent, as occurs with tumor obstruction (26) or craniofacial anomalies, such as cleft palate (5). Because Eya1-null mice have multiple craniofacial abnormalities, including palatal defects (27), we considered whether similar anatomic defects occurred in Eya4–/– mice and increased otitis media susceptibility. Skull preparations of 4-week-old wild-type and Eya4–/– mice (n = 3, each genotype) showed no overt craniofacial or palatal defects (Figure 5, A and B; data not shown). However, newborn Eya4–/– mouse skulls (n = 8) demonstrated incomplete fusion of palatal bones (Figure 5, C and D); the lack of fusion was not observed in newborn wild-type mice (n = 4; P = 0.002). We concluded that Eya4 deficiency resulted in a developmental delay rather than a fixed defect in skull bone maturation.
Figure 5. Bone and cartilaginous skull tissue anatomy of wild-type (+/+) and Eya4–/– (–/–) newborn mice.
Top (A) and lateral (B) skull views demonstrated no craniofacial abnormalities in mutant or wild-type mice (n = 3 for each genotype), but ventral view (C) showed abnormal fusion of palatal bones (dashed box) in Eya4–/– mice. (D) Higher magnification of palatal bones (C, dashed box) and schematic detailing morphologic abnormalities. Note wide separation between flanking palatal bones (incomplete fusion) in Eya4–/– skulls. Pt, pterygoid bone; m, maxillary shelves; P, palate bone. Scale bar: 1 mm.
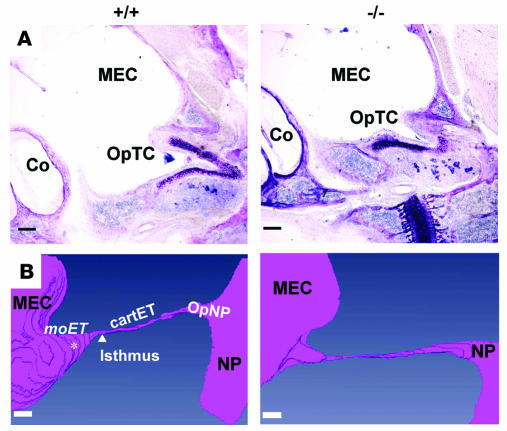
To identify other anatomical defects that might contribute to otitis media development, we analyzed the middle ear cavity and eustachian tube of Eya4–/– and wild-type mice (n = 4 each genotype) and assembled serial light micrographs into 3D reconstructions. Although the bony capsule of the middle ear was smaller in Eya4–/– mice than in wild-type mice, otic structures appeared normal (data not shown). The eustachian tube opening within the tympanic cavity (OpTC) (Figure 4A) is formed by the tube’s medial osseous segment (moET). Although eustachian tube length (measured from the ostium along the lateral cartilaginous segment to the torus tubarius of the nasopharynx; Figure 4B) was 3 mm in both wild-type and Eya4–/– mice (Table 2), both the moET and OpTC in Eya4–/– mice were abnormal and diminutive (Figure 4B and Table 2), approximating only 50% of the moET in wild-type mice. Scanning electron micrographs (n = 5 for each genotype) showed that the OpTC was narrowed and abnormally positioned at the anterior perimeter of the middle ear in Eya4–/– mice (Figure 6A). Some (20%) Eya4–/– mice had a polyp that obstructed the OpET (Figure 6A). Polyps were absent in ears of all wild-type mice.
Table 2 .
Eustachian tube dimensions of wild-type and Eya4–/– mice
Figure 6. Scanning electron micrographs of middle ear cavities from wild-type (+/+) and Eya4–/– (–/–) mice.
(A) Lateral view of the eustachian tube ostium (arrow) from 16-month-old mice. The ostium is malpositioned in the Eya4–/– ear, near to the edge of the middle ear bony capsule (BC). The junction of the bony capsule with the temporal bone (thick dashed line) and the osseous portion of eustachian tube (thin dashed line) are highlighted. A polyp (asterisk) is obstructing the Eya4–/– OpTC. Axis: A, anterior; P, posterior; L, lateral; M, medial. Scale bars: 1 mm. (B) Lateral view of eustachian tube ostia epithelia in 3-week-old mice shows increased numbers of goblet cells and swollen epithelia in Eya4–/– mice, although cilia are morphologically indistinguishable from cilia in wild-type mice. Scale bars: 5 μm. (C) The mucociliary epithelia at the eustachian tube ostia of 16-month-old mice show rarefaction of cilia in the mucosa of Eya4–/– mice. Scale bars: 5 μm.
Eustachian tube dysmorphology corresponded to developmental expression of Eya4. In situ hybridization of E10.5 and E12 embryos (Supplemental Figure 1, A and B) confirmed previously reported Eya4 expression in the otic vesicle (16) and also revealed expression in the developing semicircular canals and cochlea but not in the nascent endolymphatic duct. We found that Eya4 expression was also strong in the middle ear–forming region and surrounding the first branchial pouch, from which eustachian tube structures were derived (28).
Middle ear cilia morphology.
Cilia within the eustachian tube are critically involved in clearance of normal secretions and abnormal fluids (5), and patients with primary ciliary dyskinesia or Kartagener syndrome have increased incidences of otitis media (29). Because Eya4 was expressed in eustachian tube mucociliary epithelia of newborn mice (Supplemental Figure 1C), we considered whether ciliary abnormalities contributed to otitis media in Eya4–/– mice. Using scanning electron microscopy, we assessed the integrity of the mucociliary epithelium in wild-type and Eya4–/– mice (n = 6 each genotype). At weaning, the cilia in mutant and wild-type mice were comparable in number and morphologically indistinguishable (Figure 6B), implying that Eya4 did not have an essential developmental role for these specialized structures. Despite normal-appearing cilia, the middle ear cavity epithelium of young Eya4–/– mice was swollen and had increased numbers of goblet cells compared with wild-type littermates (Figure 6B). At 16 months, the cilia density was diminished in Eya4–/– mice (Figure 6C) and epithelial swelling was more pronounced. We interpreted the temporal progression of initially normal numbers and appearance of cilia to progressive epithelial swelling and cilia loss to indicate that otitis media caused rather than resulted from a compromised mucociliary epithelium in Eya4–/– mice.
Fbxo11, Six1, and Eya1 expression.
Middle ear inflammation and increased otitis media susceptibility have been observed in mice with mutations in Eya1, Six1, and Fbxo11 (27, 30–32). To determine whether Eya4–/– mice developed otitis media because these genes were dysregulated by the absence of Eya4, we compared expression in wild-type and mutant mice. At E12.5, Eya1 and Six1 expression was comparable in wild-type and Eya4–/– mice (Supplemental Figure 2, A–D; n = 3, each genotype). Fbxo11 expression was studied in the cochlea of newborn Eya4–/– pups and also was comparable to that found in wild-type mice (Supplemental Figure 2E; n = 2 each genotype).
Discussion
Heritability studies and familial aggregations suggest an important role for genetics in otitis media susceptibility; however, knowledge of the identity and function of genes that participate in middle ear development and their contribution to otitis media remains lacking. Here, we demonstrate a critical role for Eya4 in structuring the eustachian tube, particularly the moET, and positioning the opening of the tube within the middle ear. We found that eustachian tube maldevelopment in Eya4–/– mice impaired normal tube function and rendered mice profoundly susceptible to otitis media with effusion.
The major physiologic functions of the eustachian tube include ventilation/pressure regulation of the middle ear, protection of the middle ear from nasopharyngeal secretions, and clearance of middle ear secretions via the tube into the nasopharynx (5). Tubal physiology is predicated on appropriately sized and positioned orifices in the middle ear cavity and the nasopharynx in addition to tubal function. In Eya4–/– mice, the middle ear cavity was small, the osseous segment of the eustachian tube diminutive, and the middle ear orifice of the tube reduced and malpositioned. In the neonatal period, middle ear cavity size and malformation would impair redistribution of embryonic mesenchyme cells (33), which would account for the prolonged mesenchymal disappearance that occurred in Eya4–/– mice. In addition, the developmental delay in palate fusion found in Eya4–/– mice might superimpose dysfunction at the nasopharyngeal end of the eustachian tube, as occurs in human palate clefting. Both structural malformations and eustachian tube dysfunction would mechanically impede clearance of normal middle ear secretions in Eya4–/– mice and predispose to polyp formation and loss of cilia (Figure 6), as occurs in experimentally induced eustachian tube malfunction (34). Taken together, the primary anatomic defects produced by genetic loss of Eya4–/– would so impair ventilation and clearance functions of the eustachian tube that mice become uniformly susceptible to otitis media as manifest by secondary responses (e.g., effusion, polyp formation, and loss of cilia).
Eya4–/– mice demonstrated salient features of human otitis media that is caused by eustachian tube dysfunction, including juvenile onset erythema plus retraction of the tympanic membrane and middle ear effusion. The changes observed in the eustachian tube mucociliary epithelium of Eya4–/– mice, including diminished cilia density and increased numbers of goblet cells, also typify histopathologic findings of childhood otitis media (35). Based on the restricted developmental defects produced by Eya4 deficiency and the absence of evidence suggesting primary infection (e.g., serous effusions and absence of bacteria on histopathology, lack of overt pain or systemic manifestations, and failure of antibiotic prophylaxis), we suggest that Eya4–/– mice provide a new genetic model for pediatric otitis media susceptibility caused by eustachian tube dysfunction. Further insights into Eya4 targets that influence eustachian tube morphology and physiology may indicate new therapeutic pathways for this prevalent childhood disorder.
Eya4–/– mice expand our knowledge about genetic determinants that protect against otitis media. Increased susceptibility to infectious otitis media has been identified in other mouse strains (21, 22), in mice with spontaneous mutations (36, 37), and in mice that, like Eya4–/– mice, were engineered to carry a specific gene mutation (38–40). These mice indicate several distinct mechanisms by which otitis media vulnerability can be increased. A missense mutation in the TLR4 gene renders C3H/HeJ mice unresponsive to LPS and vulnerable to Gram-negative organisms, including H. influenzae, one of the common bacterial causes of human otitis media (41). Evi1-null mice, produced during a N-ethyl-N-nitrosourea mutagenesis screen, exhibited increased susceptibility to otitis media, possibly by affecting transcriptional regulation of neutrophil or mucin genes through a TGF-β/SMAD signaling pathway (42). Susceptibility to otitis media by Jeff mice reflects an Fbxo11 mutation; this F-box gene may influence ubiquitination pathways and impair inflammatory responses in the middle ear or increase infection because of associated craniofacial abnormalities (including cleft palate) found in Jeff mice (31, 32). Both Eya1- and Six1-deficient mice (27, 30) also have craniofacial anomalies affecting the palate and show increased middle ear inflammation, observations that underscore the importance of Eya/Six transcriptional complexes in middle ear development and function. While our studies found no evidence for direct regulation by Eya4 of Fbox11, Eya1, or Six1, further analyses of these transcriptional networks may reveal crosstalk that links developmental pathways in the middle ear.
An intriguing question raised by these studies is whether otitis media contributes to hearing loss in human patients with dominant EYA4 mutations. The broad range of age at onset of hearing deficits and the initially progressive but subsequently stable fixed deficit found later in life are atypical for a genetic form of sensorineural deafness. We speculate that some clinical variability in onset of hearing loss among mutation carriers reflects Eya4-mediated susceptibility to otitis media with or without infection in addition to dysregulation of Eya4 targets that directly participate in sensorineural hearing processes.
Methods
The Animal Care and Use Committee of Harvard Medical School approved all procedures involving mice.
Gene targeting.
An 11.6-kb NheI genomic fragment encoding Eya4 exons 7 to 11 was subcloned from a genomic 129S6/SvEv BAC library (Invitrogen) into a vector carrying thymidine kinase selection marker (gift of J. Rossant, Hospital for Sick Children, Toronto, Ontario, Canada). The neomycin (Neo) resistance gene was inserted into Eya4 sequences using homologous recombination in Escherichia coli (43). In brief, a 2481-bp segment encoding Neo and Zeocin (Zeo) resistance genes flanked by LoxP sites was PCR amplified using the following primers: forward, TGCTATTTTTCTGATATTTAGGCCCTATCCACACATTCTTTCTACACCAGCAGCTCAAACggatcctctagagtcgagg; reverse, GAAGAGCTTCCTAACTGCGTCCAATATCAAGTGATCAGCCCTGAAATAATCTATATATCCcccctcgaggacc. These primers share 5′ ends homologous to Eya4 genomic sequence (upper-case letters) and 3′ ends homologous with plasmid sequences (lower-case letters) carrying a LoxP-Neo/Zeo-LoxP cassette constructed by inserting a Zeo cassette with bacterial promoter 3′ of the Neo cassette. Amplicon and circular thymidine kinase template plasmid were coelectroporated into E. coli RecE/RecT YZ2000 competent cells (gift of Francis Stewart, University of Technology, Dresden, Germany). The targeting construct was then electroporated into embryonic stem cell line 129S6/SvEv Tc1 (gift of Philip Leder, Harvard Medical School), and desired clones were identified by Southern blot analyses using a 5′ P32-labeled probe (see Supplemental Figure 1). Recombinant embryonic stem cell clones were injected into mouse blastocysts. PCR-based genotyping of tail DNA was performed using the following primers: forward, intron 7F, GCTGGCCACGACCATTATTCCC; and reverse, wild-type primer, exon 8R, ACGGCCGGCTGCTGCATC, and Neo primer, NeoR, GCCAAGCTAGCTTGGCTGGACG.
RNA analysis.
RNA was extracted from selective tissues using TRIzol reagent (Invitrogen). About 1.5 μg of total RNA and 50 ng of random primer were used for reverse transcription. Primers to assess Eya4 exon deletions were exon 4F, cggaacctcagaattccagg; and exon 9R, GCTGGCAACATCACACCAagatcg. A 157-bp Fbxo11 amplicon was assessed in cochlea RNA using the following primers: forward, TTGGTGCAGCACCTGGCAAGGTTG; and reverse, GTGTGCGTTGTGATGCTGAGCAG.
In situ hybridization.
Sense and antisense digoxigenin-labeled probes were transcribed from a linear pCR4Blunt-TOPO (Invitrogen) plasmid carrying Eya4 exons 8 to 12 cDNA. An 855-bp probe for Six1 was amplified using the following primers: Six1, forward, ATGTCGATGCTGCCGTCGTTTGG; and Six1, reverse, TTAGGAACCCAAGTCCACCAAACTGG. A 720-bp probe for Eya1 was amplified using the following primers: Eya1, forward, ATGGAAATGCAGGATCTAACCAGC; and Eya1, reverse, CGTCATGTAGTGTGCTGGATAC. Fragments were cloned into pCR4Blunt-TOPO (Invitrogen) plasmid, transcribed as described for Eya4 probes, and hybridized to either whole mouse E12.5 embryos or to 10-μm paraffin-embedded sections (44).
DPOAE and ABR thresholds.
Hearing was studied in anesthetized (intraperitoneal xylazine, 20 mg/kg, and ketamine, 100 mg/kg) 10-week-old CBA/J wild-type and mutant mice (F3 generation). Distortion products at 2f1-f2 were measured for both ears as described previously (45), using primary-tone level with f2/f1 = 1.2, f2 level 10 decibel lower than f1, and primaries in increments of 5 dB. Threshold was defined as the f1 level required to produce a DPOAE at 0 dB sound pressure level. ABR potentials were recorded by insertion of needle electrodes at the vertex, pinna, and tail. Sound levels were increased in 5-dB steps, and responses were amplified, filtered, and averaged using an A-D board in a LabVIEW-driven data acquisition system (National Instruments). ABR thresholds were determined by visual inspection of stacked waveforms and by evaluation of the growth of peak-to-peak response amplitude with increasing sound pressure level (46).
Antibiotic prophylaxis.
Azithromycin (50 mg/kg/d) (47) was added to drinking water (final concentration of 0.25 g/l) of pregnant females and drug treatment was maintained through lactation. At 21 days, antibiotic-treated litters were sacrificed, pinna removed, and eardrums inspected through the external auditory canal using a stereomicroscope.
Histology and 3D reconstruction.
Mouse heads were fixed using 4% PFA in PBS and paraffin embedded. Longitudinal sections of 5 μm were cut and stained with PAS to examine the middle ear. Sections used for 3D reconstruction were prepared as above except that fixed tissue was embedded in Araldite resins (48). Serial longitudinal sections of 40-μm thickness were stained with epoxy stain (Electron Microscopy Sciences). One digital image of the area including the middle ear cavity to the nasopharynx was taken for each ear of each section. AMIRA software (Mercury Computer Systems) was used to align the stack for each ear, to select and contour each area of interest (middle ear cavity, eustachian tube, and nasopharynx), and to generate a 3D surface view for each ear. Measurements were made from 3D reconstructed images.
Skeletal preparations.
Skulls from newborn mice were fixed in 95% ethanol, stained with Alcian blue solution, washed in 95% ethanol, and stained in alizarin red solution (44).
Scanning electronic microscopy.
Skulls from 3-week-old and 16-month-old mice were fixed in 1.5% PFA/2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3). To ensure complete fixation, the external auditory canal was flushed with fixative. After decalcification (0.12M EDTA, pH 7), middle ear cavities were dissected and then gradually dehydrated. Samples were subject to the critical drying point process and gold coated prior to observation using scanning electron microscopy (Carl Zeiss SMT). Four ears of each genotype were evaluated.
Statistics.
Statistical significance (i.e., P values) of group sizes for dichotomous variables was assessed using Fisher’s exact test, assuming a 2-tailed distribution. The significance of differences in the means of a quantitative trait was assessed by 2-tailed Student’s t test. For all tests, P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Connie Miller, Hoabing Wang, Irfan Saadi, Anne Pizard, Claudio Punzo, and Leslie Liberman for their technical help and advice and friendship. This project was support by grants from the Howard Hughes Medical Institute (to C.E. Seidman), the NIH (to J.G. Seidman and C.E. Seidman), and NIDCD RO1 DC0188 and NIDCD P30 DC 05209 (to M.C. Liberman).
Footnotes
Nonstandard abbreviations used: ABR, auditory brain stem response; dB, decibel; DPOAE, distortion product otoacoustic emission; moET, eustachian tube medial osseous segment; Neo, neomycin; OpTC, eustachian tube opening within tympanic cavity; Zeo, Zeocin.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:651–658 (2008). doi:10.1172/JCI32899
Christine E. Seidman and J.G. Seidman contributed equally to this work.
See the related Commentary beginning on page 471.
References
- 1.Schappert S.M. Office visits for otitis media: United States, 1975-90. Adv. Data. 1992;214:1–19. [PubMed] [Google Scholar]
- 2.Bondy J., Berman S., Glazner J., Lezotte D. Direct expenditures related to otitis media diagnoses: extrapolations from a pediatric medicaid cohort. Pediatrics. 2000;105:E72. doi: 10.1542/peds.105.6.e72. [DOI] [PubMed] [Google Scholar]
- 3.Paradise J.L., et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–333. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Paradise J.L., et al. Otitis media and tympanostomy tube insertion during the first three years of life: developmental outcomes at the age of four years. Pediatrics. 2003;112:265–277. doi: 10.1542/peds.112.2.265. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone, C.D. 2005. The Eustachian tube: structure, function and role in the middle ear. B.C. Decker, Inc. Hamilton, Ontario, Canada. 240 pp. [Google Scholar]
- 6.Casselbrant M.L., et al. The heritability of otitis media: a twin and triplet study. JAMA. 1999;282:2125–2130. doi: 10.1001/jama.282.22.2125. [DOI] [PubMed] [Google Scholar]
- 7.Casselbrant M.L., Mandel E.M. Genetic susceptibility to otitis media. Curr. Opin. Allergy Clin. Immunol. 2005;5:1–4. doi: 10.1097/00130832-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Online Mendelian Inheritance in Man. http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM. [Google Scholar]
- 9.Chen A., et al. Phenotypic manifestations of branchio-oto-renal syndrome. Am. J. Med. Genet. 1995;58:365–370. doi: 10.1002/ajmg.1320580413. [DOI] [PubMed] [Google Scholar]
- 10.Pfister M., et al. A 4-bp insertion in the eya-homologous region (eyaHR) of EYA4 causes hearing impairment in a Hungarian family linked to DFNA10. Mol. Med. 2002;8:607–611. [PMC free article] [PubMed] [Google Scholar]
- 11.Wayne S., et al. Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum. Mol. Genet. 2001;10:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill M.E., et al. A gene for autosomal dominant late-onset progressive non-syndromic hearing loss, DFNA10, maps to chromosome 6. Hum. Mol. Genet. 1996;5:853–856. doi: 10.1093/hmg/5.6.853. [DOI] [PubMed] [Google Scholar]
- 13.Schonberger J., et al. Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss. Nat. Genet. 2005;37:418–422. doi: 10.1038/ng1527. [DOI] [PubMed] [Google Scholar]
- 14.De Leenheer E.M., et al. DFNA10/EYA4 — the clinical picture. Adv. Otorhinolaryngol. 2002;61:73–78. doi: 10.1159/000066807. [DOI] [PubMed] [Google Scholar]
- 15.Ohto H., et al. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell. Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borsani G., et al. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum. Mol. Genet. 1999;8:11–23. doi: 10.1093/hmg/8.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Rayapureddi J.P., et al. Eyes absent represents a class of protein tyrosine phosphatases. Nature. 2003;426:295–298. doi: 10.1038/nature02093. [DOI] [PubMed] [Google Scholar]
- 18.Tootle T.L., et al. The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature. 2003;426:299–302. doi: 10.1038/nature02097. [DOI] [PubMed] [Google Scholar]
- 19.Li X., et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- 20.Rebay I., Silver S.J., Tootle T.L. New vision from Eyes absent: transcription factors as enzymes. Trends Genet. 2005;21:163–171. doi: 10.1016/j.tig.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Rosowski J.J., Brinsko K.M., Tempel B.I., Kujawa S.G. The aging of the middle ear in 129S6/SvEvTac and CBA/CaJ mice: measurements of umbo velocity, hearing function, and the incidence of pathology. J. Assoc. Res. Otolaryngol. 2003;4:371–383. doi: 10.1007/s10162-002-3047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinn M.D., Bean-Knudsen D., Ermel R.W. Incidence of otitis media in CBA/J and CBA/CaJ mice. Hear. Res. 1992;59:1–6. doi: 10.1016/0378-5955(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 23.Park K., Lim D.J. Luminal development of the eustachian tube and middle ear: murine model. Yonsei Med. J. 1992;33:159–167. doi: 10.3349/ymj.1992.33.2.159. [DOI] [PubMed] [Google Scholar]
- 24.Roberts D.S., Miller S.A. Apoptosis in cavitation of middle ear space. Anat. Rec. 1998;251:286–289. doi: 10.1002/(SICI)1097-0185(199807)251:3<286::AID-AR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Girard D., Finegan S.M., Dunne M.W., Lame M.E. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J. Antimicrob. Chemother. 2005;56:365–371. doi: 10.1093/jac/dki241. [DOI] [PubMed] [Google Scholar]
- 26.Kubba H., Pearson J.P., Birchall J.P. The aetiology of otitis media with effusion: a review. Clin. Otolaryngol. Allied Sci. 2000;25:181–194. doi: 10.1046/j.1365-2273.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu P.X., et al. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 28.Mallo M. Formation of the outer and middle ear, molecular mechanisms. Curr Top Dev. Biol. 2003;57:85–113. doi: 10.1016/s0070-2153(03)57003-x. [DOI] [PubMed] [Google Scholar]
- 29.el-Sayed Y., al-Sarhani A., al-Essa A.R. Otological manifestations of primary ciliary dyskinesia. Clin. Otolaryngol. Allied Sci. 1997;22:266–270. doi: 10.1046/j.1365-2273.1997.00895.x. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W., et al. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardisty R.E., et al. The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media. J. Assoc. Res. Otolaryngol. 2003;4:130–138. doi: 10.1007/s10162-002-3015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardisty-Hughes R.E., et al. A mutation in the F-box gene, Fbxo11, causes otitis media in the Jeff mouse. Hum. Mol. Genet. 2006;15:3273–3279. doi: 10.1093/hmg/ddl403. [DOI] [PubMed] [Google Scholar]
- 33.Piza J., Northrop C., Eavey R.D. Embryonic middle ear mesenchyme disappears by redistribution. Laryngoscope. 1998;108:1378–1381. doi: 10.1097/00005537-199809000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Larsen P.L., Tos M., Kuijpers W., van der Beek J.M. The early stages of polyp formation. Laryngoscope. 1992;102:670–677. doi: 10.1288/00005537-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Matsune S., Sando I., Takahashi H. Distributions of eustachian tube goblet cells and glands in children with and without otitis media. Ann. Otol. Rhinol. Laryngol. 1992;101:750–754. doi: 10.1177/000348949210100906. [DOI] [PubMed] [Google Scholar]
- 36.Steel K.P., Moorjani P., Bock G.R. Mixed conductive and sensorineural hearing loss in LP/J mice. Hear. Res. 1987;28:227–236. doi: 10.1016/0378-5955(87)90051-7. [DOI] [PubMed] [Google Scholar]
- 37.Vogler C., et al. A novel model of murine mucopolysaccharidosis type VII due to an intracisternal a particle element transposition into the beta-glucuronidase gene: clinical and pathologic findings. Pediatr. Res. 2001;49:342–348. doi: 10.1203/00006450-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Liao J., et al. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt-Ullrich R., et al. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- 40.Yang A., et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 41.MacArthur C.J., Hefeneider S.H., Kempton J.B., Trune D.R. C3H/HeJ mouse model for spontaneous chronic otitis media. Laryngoscope. 2006;116:1071–1079. doi: 10.1097/01.mlg.0000224527.41288.c4. [DOI] [PubMed] [Google Scholar]
- 42.Parkinson N., et al. Mutation at the Evi1 locus in Junbo mice causes susceptibility to otitis media. PLoS Genet. 2006;2:e149. doi: 10.1371/journal.pgen.0020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Buchholz F., Muyrers J.P., Stewart A.F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 44.Pizard A., et al. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol. Cell. Biol. 2005;25:5073–5083. doi: 10.1128/MCB.25.12.5073-5083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darrow K.N., Maison S.F., Liberman M.C. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J. Neurophysiol. 2007;97:1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maison S.F., et al. Loss of alpha CGRP reduces sound-evoked activity in the cochlear nerve. J. Neurophysiol. 2003;90:2941–2949. doi: 10.1152/jn.00596.2003. [DOI] [PubMed] [Google Scholar]
- 47.Girard A.E., et al. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob. Agents. Chemother. 1987;31:1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirose K., Liberman M.C. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.