Abstract
Erythropoietin (Epo) leads to the proliferation and differentiation of erythroid precursors, but is also involved in diverse nonhematopoietic biological functions. In this issue of the JCI, Chen, Smith, and colleagues demonstrate that the temporal expression of Epo is critical for determining whether physiological or pathological repair occurs following neurovascular retinal injury in the oxygen-induced retinopathy neonatal mouse model (see the related article beginning on page 526). The pleiotrophic properties of Epo make it a likely novel therapy for treatment of neurovascular damage, but the timing of its use must be carefully considered to prevent untoward effects.
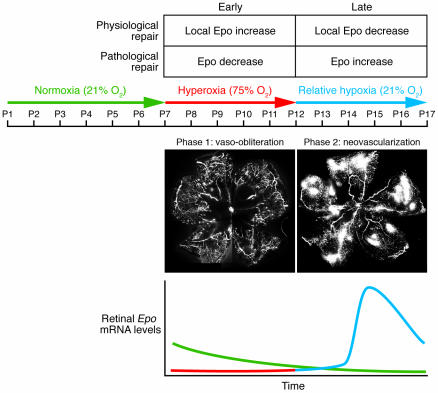
In 1994, Smith and coworkers (1) developed and refined a neonatal mouse model of proliferative oxygen-induced retinopathy (OIR; Figure 1). In this model, 7-day-old mouse pups with partially developed retinal vasculature are subjected to hyperoxia (75% oxygen) for 5 days, which stops retinal vessel growth and causes significant vaso-obliteration. On postnatal day 12, the pups are returned to room air, and by postnatal day 17, a florid compensatory retinal neovascularization occurs. This model of pathological neovascularization has been widely used as a substitute for proliferative diabetic retinopathy (DR) and in studies of various antiangiogenic compounds (2–4).
Figure 1. Mouse model of proliferative OIR.
In this model, 7-day-old mouse pups with partially developed retinal vasculature are subjected for 5 days to hyperoxia (75% oxygen), which stops retinal vessel growth and causes significant vaso-obliteration (phase 1). On postnatal day 12, pups are returned to room air, and by postnatal day 17, a florid compensatory retinal neovascularization occurs (shown in white) (phase 2). In the line graph, a representation of retinal Epo mRNA levels in the OIR model during normoxic conditions (green), phase 1 (red), and phase 2 (blue) are shown. As Chen et al. (5) show, in the OIR model during hyperoxia (phase 1), Epo levels are reduced, resulting in pathological elevations of Epo at the late stage of disease development. With Epo treatment during hyperoxia, retinal vasculature is protected and BM-derived EPCs come into the developing vasculature, promoting healthy vessels rather than pathological neovascularization as in the OIR model. Original magnification, ×5.
In this issue of the JCI, Chen, Smith, and associates highlight the importance of temporal expression of the hormone erythropoietin (Epo) in the pathogenesis of retinopathy using the OIR model (5). They report that during the hyperoxic phase of the OIR model, mRNA levels of retinal Epo were suppressed, while during the subsequent hypoxic phase Epo mRNA levels were greatly increased (Figure 1). Early administration of Epo protected against hyperoxia-induced retinal vaso-obliteration and neural apoptosis, preventing vessel dropout and hypoxia-induced neovascularization in the second phase of this disease model. However, treatment with exogenous Epo during the second phase of the OIR model actually increased pathological neovascularization. Furthermore, Chen et al. (5) also show that hyperoxia resulted in the loss of bone marrow–derived (BM-derived) endothelial progenitor cell (EPC) contribution to vascular development (2). During retinal development, the suppression of endogenous growth factors by hyperoxia with subsequent vaso-obliteration is the critical initiating event responsible for the development of pathological neovascularization. This aberrant neovascularization is responsible for the vision-threatening aspects of retinopathy of prematurity (ROP) and is also seen in DR (6, 7). Patients with these conditions are treated once pathological vessels appear. However, treating “late-stage” disease may be much less effective and, as shown in the current study by Chen et al. (5), early treatment may be a more judicious approach, at least in the case of Epo.
Epo as a vasculogenic, neuroprotective, and EPC recruitment factor
The recent identification of critical hypoxia-regulated factors and the temporal changes in their expression represents an important step toward understanding mechanisms of disease progression in retinal neovascularization. The pharmaceutical industry has aggressively identified agents to specifically target late events in disease development when neovascularization is already present and to inhibit the hypoxia-regulated factors implicated in pathological neovascularization (8, 9). Most of these factors have intrinsic neural and retinal vascular protective effects, and their elimination may eventually contribute to cell and tissue dysfunction. More importantly, their expression represents a physiological adaptive response to ischemic tissue injury.
One such factor, Epo, is a glycoprotein (molecular mass is approximately 30 kDa) produced primarily in the kidney but with localized production also reported in a range of neural and nonneural tissues (10, 11). Epo is directly activated by the transcription factors HIF-1α (12), HIF-1α–like factor (HLF, also known as EPAS1) (13), and HIF-2α (14) and thus is induced in direct response to ischemic or hypoxic conditions. The Epo receptor (EpoR) also appears to be widely expressed, and ligand binding is associated with activation of the protein tyrosine kinase JAK2 and the downstream signaling molecule STAT5 (15, 16). EpoR can also signal through PI3K-Akt, MAPK, and, as shown in the current study by Chen et al., NF-κB (5). With this broad expression of both Epo and EpoR, a pleiotrophic role for Epo is anticipated. Whereas Epo is best known for its role in hematopoiesis as a factor that leads to the proliferation and differentiation of erythroid precursors, it is also involved in diverse nonhematopoietic biological functions (10, 17, 18). These pleiotrophic effects of Epo include: (a) neuroprotection by decreasing neuronal apoptosis and inflammation in acute brain injury models, by protecting the mouse retina against light-induced degeneration (19) and by reducing retinal ganglion cell loss following axotomy (20); (b) cytoprotection by inhibiting apoptosis and promoting angiogenesis to improve cardiac function in cardiac ischemia models of heart failure (21, 22); and (c) modulation of tissue remodeling by regulating cell maturation and division, preventing intimal hyperplasia (23), and reducing pulmonary vascular remodeling (24) in a hypoxic rat model of pulmonary hypertension without affecting hemodynamics or right ventricular hypertrophy.
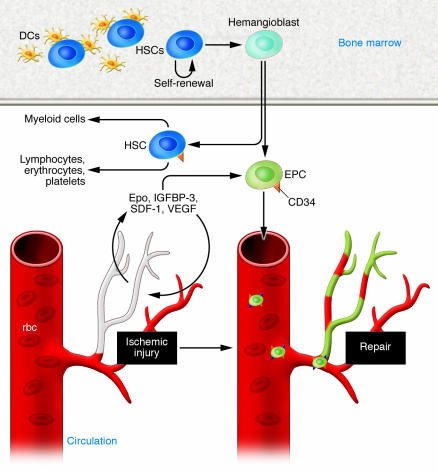
Epo clearly has direct effects on resident vasculature and is known to mobilize circulating BM-derived cell populations to mediate vascular repair in both acute and chronic injury models (25, 26). BM-derived EPCs circulate in the bloodstream and are capable of endothelial repair. They migrate to areas of ischemia, respond to hypoxia-regulated factors such as Epo, and participate in both physiological and pathological neovascularization (Figure 2). Epo stimulates EPC proliferation, adhesion, and differentiation to endothelium.
Figure 2. Epo’s angiogenic effects.
Epo has direct effects on resident vasculature, and it mobilizes circulating CD34+ BM-derived populations to stimulate their contribution to vascular repair in both acute and chronic injury models. BM-derived EPCs circulate in the bloodstream and migrate to areas of ischemia, respond to hypoxia-regulated factors such as Epo, and participate in both physiological and pathological neovascularization. Epo stimulates EPC proliferation, adhesion, and differentiation to endothelium, as do other hypoxia-regulated factors such as VEGF, stromal cell–derived factor 1 (SDF-1), and insulin-like growth factor–binding protein 3 (IGFBP-3).
Clinical aspects of Epo administration
The importance of the current study (5) is the applicability of the results to ongoing clinical therapies. Premature babies who are being treated with Epo are the same babies whose BM or peripheral blood cells are more likely to be dysfunctional. Extensive preclinical data indicate that impairment in the functional properties of these BM-derived cells leads to ineffective tissue repair (27, 28). The Epo treatment enhances the function of EPCs and may increase the benefit that these infants gain from Epo therapy. However, the timing of Epo treatment will be critical, and there is clearly a therapeutic window, which will need to be defined for each infant. Furthermore, the results could have relevance to our understanding of the pathogenetic basis of other diseases involving neovascularization (including wound healing and cancer). Recent reports reveal a possible association between elevated vitreous Epo concentrations and retinal vascular disease in DR (29), while suggesting a protective effect of Epo on the ischemic retina (30).
Studies have evaluated Epo in adult and neonatal animal models of injury and reported the prevention of hypoxic-ischemic brain injury, decreased neuronal apoptosis, decreased infarction volume, and improved functional outcomes (31, 32). However, these studies evaluated doses of Epo in the range of 1,000–5,000 U/kg, approximately 10 times the dose that generally is used to stimulate red blood cell production in infants. Clinical studies in adult stroke patients evaluated Epo doses of 33,000 U/day for 3 days, resulting in cerebrospinal fluid Epo concentrations 60- to 100-fold above baseline and improved functional outcomes (33).
Much less is known about Epo treatment for infants with anemia. The administration of human recombinant Epo to preterm infants was first reported in 1990, when Halperin et al. (34) evaluated the use of Epo to treat anemia of prematurity. Since then, doses that have been evaluated in clinical studies that involved preterm infants ranged from 100 U/kg twice per week (35) to 5,000 U/kg per week (36). Adverse effects of treatment at all doses tested have been minimal, and the decreased number of transfusions reported is thought to be beneficial. The treatment of preterm infants with Epo is considered part of clinical care in many neonatology units throughout the United States and Europe, and outcomes continue to be evaluated.
In a recent study by Bierer et al. (37), extremely low birth weight infants showed survival benefit when receiving Epo, and the frequency of transfusions was significantly decreased. Furthermore, this was the first study to report an association between serum Epo concentrations and neurodevelopmental outcomes in preterm infants who receive Epo. These observations merit additional evaluation as to the possible benefit of Epo as a neuroprotective agent in neonates. Long-term neurodevelopmental follow-up of infants who are involved in randomized studies of Epo administration is clearly needed. However, several other studies evaluating neonatal outcomes after Epo therapy have reported no differences between treatment groups (38, 39). Despite unknowns about outcomes measures, particularly with respect to ROP, Epo treatment is considered part of the pharmacological armamentarium available for premature infant care.
In conclusion, the study by Chen et al. (5) highlights the potential therapeutic advantage of using a pleiotrophic drug to treat ROP, provided that it is given early in disease progression. Epo or EpoR agonists should not only prevent retinal vessel atrophy but may also provide neuroprotection, prevent aberrant neovascularization, and enhance recruitment of BM-derived cells to the pathological area. Timing is critical since, as Chen et al. demonstrate, if Epo is given at the later hypoxic stages, the severity of retinopathy increases. However, in the case of the eye, disease progression is easy to follow without invasive investigation and allows timing of the administration of drugs to be carefully monitored, hopefully resulting in better clinical outcomes. The data reported by Chen et al. may pave the way for clinical interventions with Epo in preterm infants at the early stage of ROP in order to inhibit vaso-obliteration in the retina and block the development of retinal neovascularization seen in the late stages of this disease.
Acknowledgments
The authors would like to thank Lynn Shaw for generating the figures for this manuscript and Robert Fisher for his careful review of the manuscript.
Footnotes
Nonstandard abbreviations used: DR, diabetic retinopathy; EPC, endothelial progenitor cell; Epo, erythropoietin; EpoR, Epo receptor; OIR, oxygen-induced retinopathy; ROP, retinopathy of prematurity.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:467–470 (2008). doi:10.1172/JCI34643.
See the related article beginning on page 526.
References
- 1.Smith L.E., et al. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 2.Chen J., Smith L.E. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 3.Mino R.P., et al. Adenosine receptor antagonists and retinal neovascularization in vivo. Invest. Ophthalmol. Vis. Sci. 2001;42:3320–3324. [PubMed] [Google Scholar]
- 4.Kramerov A.A., et al. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Am. J. Pathol. 2006;168:1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J., Connor K.M., Aderman C.M., Smith L.E.H. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Invest. . 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prost M. Experimental studies on the pathogenesis of retinopathy of prematurity. Br. J. Ophthalmol. 1988;72:363–367. doi: 10.1136/bjo.72.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss S.E., Klein R., Klein B.E. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–1070. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y., et al. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest. Ophthalmol. Vis. Sci. 2007;48:5184–5190. doi: 10.1167/iovs.07-0469. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N., Damico L., Shams N., Lowman H., Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 10.Hardee M.E., Arcasoy M.O., Blackwell K.L., Kirkpatrick J.P., Dewhirst M.W. Erythropoietin biology in cancer. Clin. Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- 11.Juul S., Felderhoff-Mueser U. Epo and other hematopoietic factors. Semin. Fetal Neonatal Med. 2007;12:250–258. doi: 10.1016/j.siny.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U. S. A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ema M., et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G.L., Semenza G.L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 15.Parganas E., et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 16.Socolovsky M., Fallon A.E., Wang S., Brugnara C., Lodish H.F. Fetal anemia and apoptosis of red cell progenitors in Stat5a–/–5b–/– mice: a direct role for Stat5 in Bcl-X(L) induction. . Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 17.Milano M., Collomp R. Erythropoietin and neuroprotection: a therapeutic perspective. . J. Oncol. Pharm. Pract. 2005;11:145–149. doi: 10.1191/1078155205jp162oa. [DOI] [PubMed] [Google Scholar]
- 18.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur. J. Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 19.Grimm C., et al. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin. Cell Dev. Biol. 2005;16:531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Kilic U., et al. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J. 2005;19:249–251. doi: 10.1096/fj.04-2493fje. [DOI] [PubMed] [Google Scholar]
- 21.van der Meer P., Voors A.A., Lipsic E., van Gilst W.H., van Veldhuisen D.J. Erythropoietin in cardiovascular diseases. Eur. Heart J. 2004;25:285–291. doi: 10.1016/j.ehj.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Amann K., et al. Capillary/myocyte mismatch in the heart in renal failure — a role for erythropoietin? Nephrol. Dial. Transplant. 2000;15:964–969. doi: 10.1093/ndt/15.7.964. [DOI] [PubMed] [Google Scholar]
- 23.Urao N., et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ. Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 24.Petit R.D., Warburton R.R., Ou L.C., Hill N.S. Pulmonary vascular adaptations to augmented polycythemia during chronic hypoxia. . J. Appl. Physiol. 1995;79:229–235. doi: 10.1152/jappl.1995.79.1.229. [DOI] [PubMed] [Google Scholar]
- 25.Walker B.R., Resta T.C., Nelin L.D. Nitric oxide-dependent pulmonary vasodilation in polycythemic rats. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2382–H2389. doi: 10.1152/ajpheart.2000.279.5.H2382. [DOI] [PubMed] [Google Scholar]
- 26.Bahlmann F.H., et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 27.Napoli C., Maione C., Schiano C., Fiorito C., Ignarro L.J. Bone marrow cell-mediated cardiovascular repair: potential of combined therapies. Trends Mol. Med. 2007;13:278–286. doi: 10.1016/j.molmed.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Ward M.R., Stewart D.J., Kutryk M.J. Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc. Interv. 2007;70:983–998. doi: 10.1002/ccd.21302. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe D., et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 30.Junk A.K., et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semba R.D., Juul S.E. Erythropoietin in human milk: physiology and role in infant health. J. Hum. Lact. 2002;18:252–261. doi: 10.1177/089033440201800307. [DOI] [PubMed] [Google Scholar]
- 32.Sola A., Wen T.C., Hamrick S.E., Ferriero D.M. Potential for protection and repair following injury to the developing brain: a role for erythropoietin? Pediatr. Res. 2005;57:110R–117R. doi: 10.1203/01.PDR.0000159571.50758.39. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenreich H., et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 34.Halperin D.S., et al. Effects of recombinant human erythropoietin in infants with the anemia of prematurity: a pilot study. J. Pediatr. 1990;116:779–786. doi: 10.1016/s0022-3476(05)82671-x. [DOI] [PubMed] [Google Scholar]
- 35.Shannon K.M., et al. Recombinant human erythropoietin in the anemia of prematurity: results of a placebo-controlled pilot study. J. Pediatr. 1991;118:949–955. doi: 10.1016/s0022-3476(05)82217-6. [DOI] [PubMed] [Google Scholar]
- 36.Gumy-Pause F., et al. Stepping up versus standard doses of erythropoietin in preterm infants: a randomized controlled trial. Pediatr. Hematol. Oncol. 2005;22:667–678. doi: 10.1080/08880010500278715. [DOI] [PubMed] [Google Scholar]
- 37.Bierer R., Peceny M.C., Hartenberger C.H., Ohls R.K. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–e640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- 38.Soubasi V., et al. Follow-up of very low birth weight infants after erythropoietin treatment to prevent anemia of prematurity. J. Pediatr. 1995;127:291–297. doi: 10.1016/s0022-3476(95)70313-6. [DOI] [PubMed] [Google Scholar]
- 39.Newton N.R., Leonard C.H., Piecuch R.E., Phibbs R.H. Neurodevelopmental outcome of prematurely born children treated with recombinant human erythropoietin in infancy. J. Perinatol. 1999;19:403–406. doi: 10.1038/sj.jp.7200244. [DOI] [PubMed] [Google Scholar]