Abstract
Apoptosis is critical to homeostasis of multicellular organisms. In immune privileged sites such as the eye, CD95 ligand (FasL)-induced apoptosis controls dangerous inflammatory reactions that can cause blindness. Recently, we demonstrated that apoptotic cell death of inflammatory cells was a prerequisite for the induction of immune deviation after antigen presentation in the eye. In this report, we examine the mechanism by which this takes place. Our results show that Fas- mediated apoptosis of lymphoid cells leads to rapid production of interleukin (IL)-10 in these cells. The apoptotic cells containing IL-10 are responsible for the activation of immune deviation through interaction with antigen-presenting cells (APC). In support of this, we found that apoptotic cells from IL-10+/+ animals fed to APC in vitro promote Th2 cell differentiation, whereas apoptotic IL-10−/− cells, as well as nonapoptotic cells, favor Th1 induction. Thus, apoptotic cell death and tolerance are linked through the production of an antiinflammatory cytokine to prevent dangerous and unwanted immune responses that might compromise organ integrity.
Keywords: apoptosis, inflammation, immune privilege, FasL, interleukin-10
ike cell proliferation, apoptosis is a continuous physiological process associated with embryonic development, metamorphosis, immune system maturation, and cell turnover (1). This type of cell death differs from necrotic death in that apoptosis is a highly regulated process induced by a specific stimulus, whereas necrosis results from the failure to control cellular homeostasis after injury. Apoptosis is an indispensable part of adaptive immunity since the immune system must deal with pathogens, as well as its own potentially damaging cells, to prevent the formation of self-reactive responses and autoimmunity (2). One hallmark of apoptotic death is that dead cells are recognized, phagocytized (3), and removed to minimize inflammatory or immune reactions that could result from the release of cellular components. However, it has been noted that apoptosis can actually prevent or inhibit inflammatory responses, suggesting that immune regulatory processes might result from its induction. Indeed, the well-documented antiinflammatory effects of UV irradiation are an excellent example, in which exposure of skin to UV radiation of wavelength 280–320 nm (UVB)1 leads to the direct inhibition of cell mediated immunity through the induction of inhibitory cytokines (4). Similarly, recent studies have shown that apoptosis of lymphoid cells in an immune privileged site leads to the induction of immune deviation (5).
In immunology there is considerable interest in a cell surface receptor protein that induces apoptotic death when ligated by specific antibody or its natural ligand. This protein, Fas (APO-1, CD95), is related to members of the TNF receptor family with broad distribution on both lymphoid and nonlymphoid cells. The natural ligand for Fas (CD95 ligand, Fas ligand, FasL) is a member of the TNF family of cytokines that has been found in numerous sites throughout the body (6). Fas–FasL interactions have been implicated as the mechanism behind clonal deletion after in vivo exposure to superantigen and peptide (7), controlling T cell expansion during immune responses (6), and killing by cytotoxic T cells (8). It has also been shown that FasL plays an important role in tumor biology, since a growing number of tumors have been found to express functional FasL as a protective mechanism against the Fas+ cells of the immune system (9, 10).
Fas–FasL interactions are also important in the maintenance of immune privilege in organs such as the eye (11), the placenta (12), and the testis (13). Inflammatory reactions are inhibited in immune privileged sites since even minor episodes can threaten organ integrity and function. In the eye constitutive FasL expression controls the proliferation of Fas+ lymphoid cells that enter this organ in response to viral infection (11), and it is a significant factor in the remarkable success of corneal transplants (14). FasL-induced cell death of lymphoid cells is also critical to the induction of immune deviation to antigens placed in the eye (5). This form of immune deviation requires apoptotic cell death of Fas+ lymphoid cells induced by FasL+ ocular cells. Interestingly, it is death by apoptosis that is the critical factor, rather than cell death restricted to the Fas–FasL pathway.
In current paradigms, immune deviation is the preferential activation of one Th cell subset over another, and in the case of the eye a Th2-type response is typically induced. This is manifested by a dominant antibody response, a diminished delayed-type hypersensitivity (DTH) response (15–19), and the loss of IFN-γ production (5). The production of Th2 cytokines IL-4 and/or IL-10 in the spleen has also been noted in animals with immune deviation induced in this manner (20). The link between cell death and immune deviation suggests that there may be an antiinflammatory component to apoptosis in the eye that is manifest through the inhibition of systemic immune responses. Here we examine the mechanisms linking apoptosis and immune regulation using a combination of in vivo and in vitro methods. Our results show that apoptosis of immune effector cells can redirect the final outcome of an immune response through the production of an antiinflammatory cytokine that regulates immunity through interaction with APC.
Materials and Methods
Mice
C57BL/6 (B6) and BALB/c were purchased from the National Cancer Institute. C57BL/6-lpr (B6-lpr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6-bcl-xL transgenic mice (21) were provided by Dr. Stan Korsmeyer (Washington University). DO11.10 transgenic mice (22, 23) were provided by Dr. Ken Murphy (Washington University). B6-IL-10−/− and B6-IL-4−/− mice were provided by Dr. Osami Kanagawa (Washington University). In all in vivo experiments, groups consisted of five or more animals. Experiments were repeated at least three times with similar results before they were reported.
Reagents
2,4,6-trinitro-1-chlorobenzene (TNCB) was purchased from Eastern Chemical Co. (Smithtown, NY). 2,4,6 trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma Chemical Co. (St. Louis, MO). Recombinant murine IL-10, IL-4, and IFN-γ, as well as the rat anti–mouse mAbs to IL-10 (SXC-1 and JES5.2A5) were purchased from Genzyme Inc. (Cambridge, MA).
Medium for all in vitro cultures was DME media (Sigma Chemical Co., St. Louis, MO) supplemented with 10% FCS, l-glutamine, sodium pyruvate, penicillin/streptomycin/fungizone, Hepes, and 2 × 10−5 M 2-mercaptoethanol.
Isolation of T Cells
T cells were isolated from spleen cell suspensions as previously described (15, 16). In brief, 5-ml econocolumns (Bio-Rad Laboratories, Richmond, CA) were filled with glass beads (mean diameter 200 μm) coated with normal mouse Ig. The columns were washed 3× with HBSS, followed by the addition of a 1/6 dilution of rabbit anti–mouse Ig. After a 1-h incubation, the column was washed 3× with HBSS. Spleen cells (108 cells/2.5-ml beads) were added to the column with a flow rate of one drop per 6–10 s. Cells were collected as they emerged from the column. Yields were typically 25–30% of the applied cells that consisted of 95–98% CD3+ cells.
TNP Coupling of Cells
RBC-free single-cell suspensions of purified T cells were coupled with TNP as previously described (15, 16). In brief, 108 cells were incubated in 0.5 ml HBSS and 0.5 ml 10 mM TNBS for 7–10 min at room temperature. After incubation, cells were washed 3× with HBSS before use.
Induction of Immune Deviation
Immune deviation was induced as described (15, 16, 24). In brief, TNP-coupled T cells (5 × 105) were injected into the anterior chamber (AC) in 0.005-ml vol using a 0.25-ml Hamilton Microliter syringe (Hamilton Co., Reno, NV) fitted with a 33-gauge needle. Mice were anesthetized with Metofane (methoxyflurane) and injections were done under a dissecting microscope. In some experiments, irradiated TNP-T cells were used for injection. These cells were used within 1 h of treatment with 3,000 R γ-irradiation. Mice were immunized 48 h after AC injection with 0.050 ml of 1% TNCB in acetone/olive oil (3:1) applied to shaved abdominal skin. 5 d later mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right footpad and 0.033 ml PBS in the left footpad. Measurements were made 24 h later using an engineer's micrometer. Values are expressed in μm (± SE) and represent the difference between the right footpad (antigen challenge) and the left footpad (PBS challenge). Background values represent the difference between the challenged and unchallenged foot in unimmunized mice.
Generation of Peritoneal Exudate Cells
Peritoneal exudate cells (PEC) used for transfer to naive mice after incubation with apoptotic cells were harvested by lavage from adult mice injected intraperitoneally 3 d earlier with 0.1 ml IFA (Sigma Chemical Co.). PEC used in the DO11.10 cultures were prepared by injecting 1.0 ml i.p. of freshly prepared ConA in saline (60 μg/ml; Sigma Chemical Co.), followed 4 d later with 0.5 ml of 3% proteose peptone (Difco, Detroit, MI). Cells were harvested 3 d later by peritoneal lavage. By FACS® analysis the adherent cells were MHC class II+ (66%), Mac-1+ (88%), F4/ 80+ (66%), B7-1+ (56%), B7-2+ (73%), LFA-1+ (95%), and B220−.
Adoptive Transfer of Adherent Cells
After harvest from the peritoneum of IFA-treated mice, 5 × 106 cells in 1 ml of DME/10% FCS were placed in the wells of a 24-well plate and incubated for 2 h at 37°C in a 5% CO2 incubator. Wells were then washed 3× with HBSS and 1 × 106 spleen cells (irradiated or untreated) were added per well. After two additional hours of incubation at 37°C, wells were washed 3× with HBSS and the plates placed at 4°C for 30 min. Cells were dislodged by vigorous pipetting, washed again in HBSS, counted, and 1 × 104 viable cells were transferred to naive mice. Recipient mice were immunized with TNCB to determine if immune deviation was established as described above.
DO11.10 Cultures
PEC (1.5 × 106 in 2 ml DME/10% FCS) were placed in wells of a 24-well plate. After 2 h incubation at 37°C, nonadherent cells were removed by washing with HBSS. OVA protein (100 μg/ml) along with irradiated (3,000 R) or untreated TNP-T cells (1 × 106) were added. Recombinant murine IL-10 (40 ng/ ml) was added to some wells as a control. After 2–4 h incubation at 37°C, the medium was removed and PEC were extensively washed to remove any nonassociated cells. OVA (100 μg/ml) and 3 × 105 purified DO11.10 T cells were then added to the well and the cultures were maintained for 5 d at 37°C in a 5% CO2 atmosphere. Live cells were then collected, washed with HBSS, and restimulated with 0.3 μM OVA323–339 peptide plus fresh irradiated syngeneic splenocytes as APC. 48 h later supernatants were collected and the amount of IL-4 and IFN-γ was determined as described below. Cytokine profiles of the resulting cell populations are displayed graphically as described (22, 23), where the amount of IL-4 (y axis) is plotted versus the amount of IFN-γ (x axis).
Cytokine Production in Spleen of Mice with Immune Deviation
4 d after immunization of mice with TNCB, the splenocytes were collected and coupled with TNP as described above. 106 cells per well in a 24-well plate were cultured in DME and 10% FCS and supernatants were collected 48 h later. Cytokine content was measured as described below.
IL-10 Production In Vitro
Splenocytes from mice were harvested and RBC-free single-cell suspensions prepared. Cells were exposed to 3,000 R irradiation and 1 × 107 cells/2 ml were cultured in DME and 10% FCS 24 h. Supernatants were then collected. Splenocytes from B6, B6-lpr, and bcl-xL transgenic mice were exposed to FasL by incubating 1 × 107 spleen cells with 1.5 × 106 murine FasL- expressing L cells (obtained from Dr. John Russell, Washington University) in DME and 10% FCS. Supernatants were harvested 24 h later. T hybridoma A1.1 (28; 106/well in a 24-well plate) was activated with 10 ng/ml PMA (Sigma Chemical Co.) and 500 ng/ml Ionomycin (Sigma Chemical Co.) overnight and supernatants were collected. Where indicated, 10 μg/ml Fas-Fc chimeric protein (obtained from Dr. Douglas Green, La Jolla Institute; reference 28) was included in the culture to block apoptosis. All supernatants generated were assayed for IL-10 by ELISA as described below.
Detection of Cytokines
IFN-γ.
Serial dilution of recombinant murine IFN-γ or supernatant was added to wells of 4 × 103 WEHI-279 cells in DME and 10% FCS in a 96-well plate. Cultures were incubated for 40 h at 37°C in 5% CO2, pulsed 8 h with 1 μCi [3H]TdR, and harvested for scintillation counting. Growth inhibition of WEHI-279 reflected the amount of IFN-γ (U/ml) that was determined by comparison to the standard curve constructed from values obtained with recombinant IFN-γ.
IL-4.
Serial dilution of recombinant murine IL-4 or supernatant was added to wells of 2 × 103 6I4 cells in DME and 10% FCS. Cultures were incubated for 32 h at 37°C in 5% CO2, pulsed 16 h with 1 μCi [3H]TdR, and harvested for scintillation counting. Growth of 6I4 reflected the amount of IL-4 (U/ml) which was determined by comparison to a known standard (recombinant murine IL-4).
IL-10.
IL-10 was quantitated using a sandwich ELISA developed with two anti–IL-10 mAbs as described (25). ELISA plates (96-well, Nunc Polysorb Immuno plates; Roskilde, Denmark) were coated with purified JES5.2A5 mAb (2 μg/ml) diluted in coating buffer (0.1 M NaHCO3 and 0.1 M Na2CO3). After overnight incubation at 4°C, wells were blocked with 1% BSA in PBS for 2 h at room temperature. Plates were then washed 3× with PBS containing 0.1% Tween-20 (PBS/Tween) and the recombinant murine IL-10 standard or test samples were added (100 μl/ well). Standard and unknowns were diluted in PBS/Tween containing 1% BSA. Plates were incubated overnight at 4°C, wells were washed 4× with PBS/Tween, and 100 μl/well of biotinylated mAb SXC-1 (1 μg/ml) was added for 45 min at room temperature. Wells were washed at least six times, followed by the addition of 100 μl of 1:500 avidin-alkaline phosphatase (Sigma Chemical Co.) for 30 min at room temperature. Wells were washed at least eight times with PBS/Tween and 100 μl of p-nitrophenyl phosphate (Zymed Labs, South San Francisco, CA) was added. Upon color development, the plate was analyzed on an ELISA plate reader at 405 nm OD. The ELISA did not react with IL-2, IL-4, TNF-α, IL-1α, or IFN-γ.
Statistical Analysis.
Significant differences between groups were evaluated using a two-tailed Student's t test (P < 0.01).
Results
Apoptotic Cells Make IL-10.
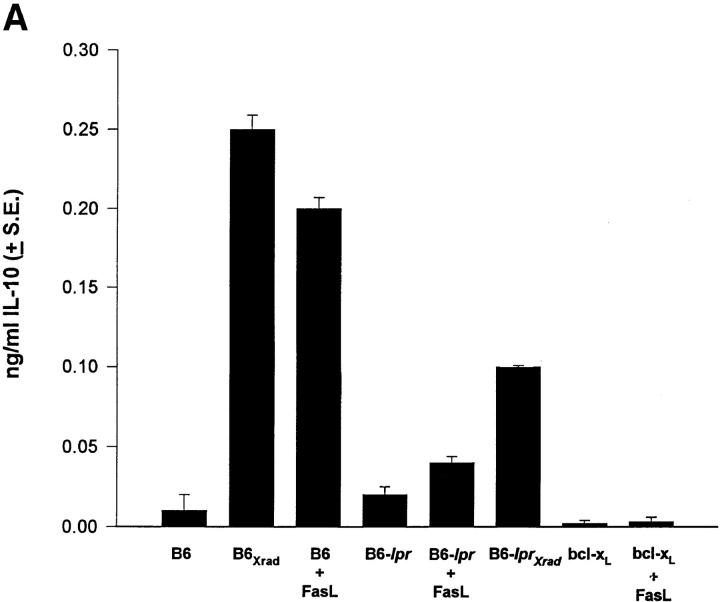
The direction of the response elicited by AC injection of antigen is established very early (within the first two days) by apoptotic cell death of T cells in the eye (5). Since production of IL-4 and IL-10 can direct Th cell differentiation toward a Th2 response, we asked whether a potent apoptotic signal delivered to Fas+ cells could induce the production of these cytokines. Although we could not detect IL-4 (not shown), results in Fig. 1 A show that cells induced to undergo Fas-mediated apoptosis produce a significant amount of IL-10 protein. Supernatants from normal spleen cells had undetectable quantities of IL-10, but spleen cells exposed to FasL (FasL+ L cells) had significant levels. Exposure of B6-lpr splenocytes to FasL induced low levels of IL-10 demonstrating that the induction was via a Fas–FasL interaction. Interestingly, irradiation of B6 or B6-lpr spleen cells also leads to IL-10 production suggesting that IL-10 production may be linked to the apoptosis rather than solely to the Fas–FasL pathway.
Figure 1.
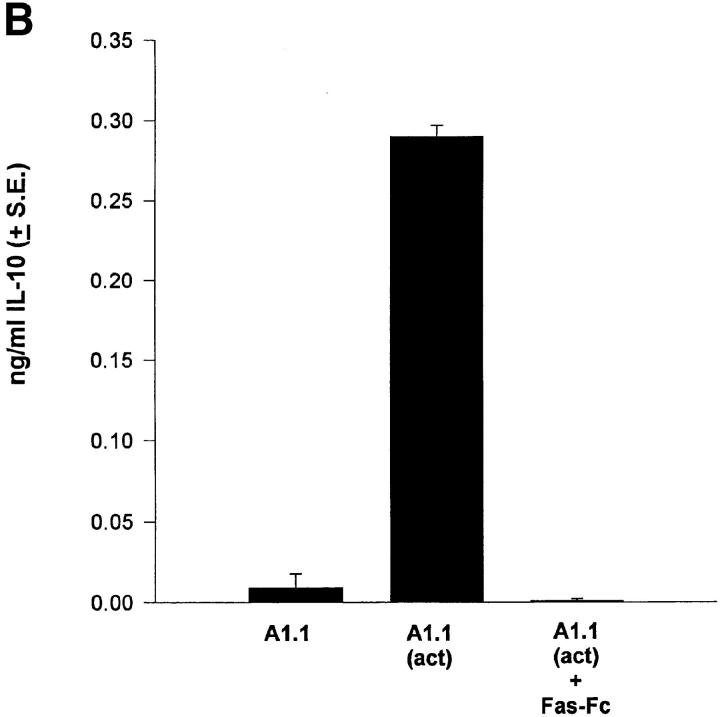
Apoptotic cells make IL-10. (A) Splenocytes from B6, B6-lpr, or B6-bcl-xL transgenic mice were cultured alone or with FasL+ L cells (FasL). B6 or B6-lpr splenocytes were given 3,000 R irradiation (Xrad). After 24 h supernatants were harvested and the amount of IL-10 determined by ELISA. (B) The T hybridoma cell A1.1 was cultured alone, or with PMA/Ionomycin (act) for 18 h and the supernatants collected. The chimera protein Fas-Fc (10 μg/ml) was included in some wells to block apoptosis. The amount of IL-10 was determined by ELISA. These experiments were repeated at least three times with similar results.
To further explore the role of apoptosis in IL-10 production, we examined spleen cells from bcl-xL transgenic mice whose T cells overexpress bcl-xL (21). Bcl-xL has been shown to protect cells from apoptotic cell death, and it has recently been shown that it can block Fas–FasL- induced cell death (5, 27). In our system, TNP-T cells overexpressing bcl-xL did not undergo apoptosis in the eye, nor could they activate immune deviation (5). As shown in Fig. 1 A, bcl-xL transgenic cells did not produce IL-10 when given a potent FasL signal. This is consistent with the idea that it is apoptotic T cells that are producing IL-10 since blocking Fas-mediated apoptosis prevents the IL-10 production.
To further examine whether T cells induced to undergo apoptosis produce IL-10 we examined a T cell hybridoma that undergoes Fas–FasL-mediated activation-induced cell death (AICD) after exposure to PMA and ionomycin (28). These results (Fig. 1 B) show that activation of A1.1 induced significant IL-10 production that was absent when apoptosis was prevented by the addition of the chimeric protein Fas-Fc. We conclude from data in Fig. 1 that cells induced to undergo apoptosis by Fas or irradiation produce IL-10. When cell death is prevented, either by blocking the Fas–FasL interaction, or overexpression of bcl-xL, IL-10 is not produced. IL-10 production in our studies was not the result of the release of preformed IL-10 for two reasons. First, induction of apoptosis by either irradiation or Fas resulted in rapid IL-10 mRNA production as detected by RT-PCR (Fig. 1 A). Second, IL-10 protein was not detected in unstimulated T cells when total cell extracts were analyzed (Fig. 1 A) by ELISA.
Immune Deviation and IL-10.
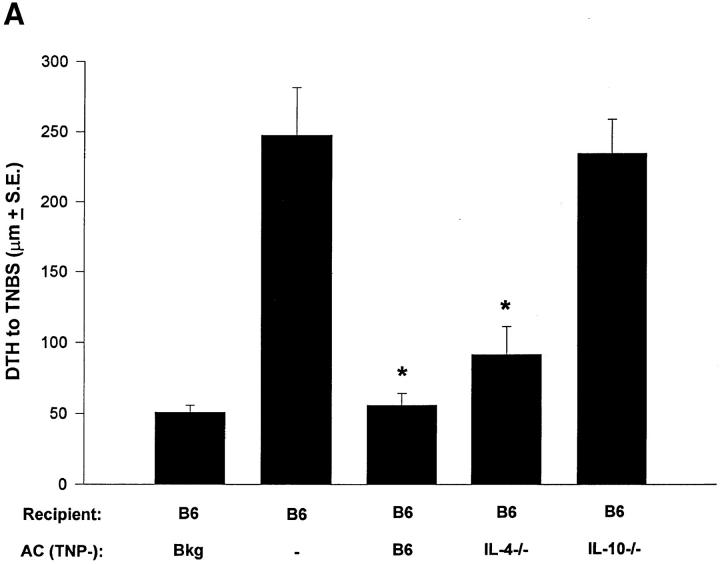
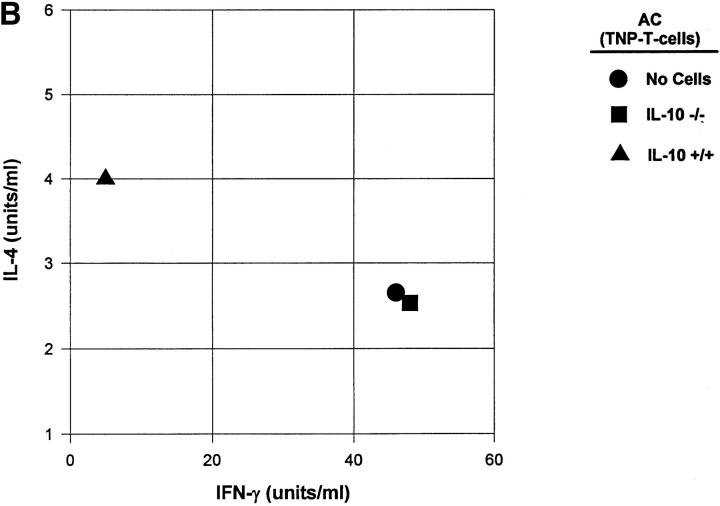
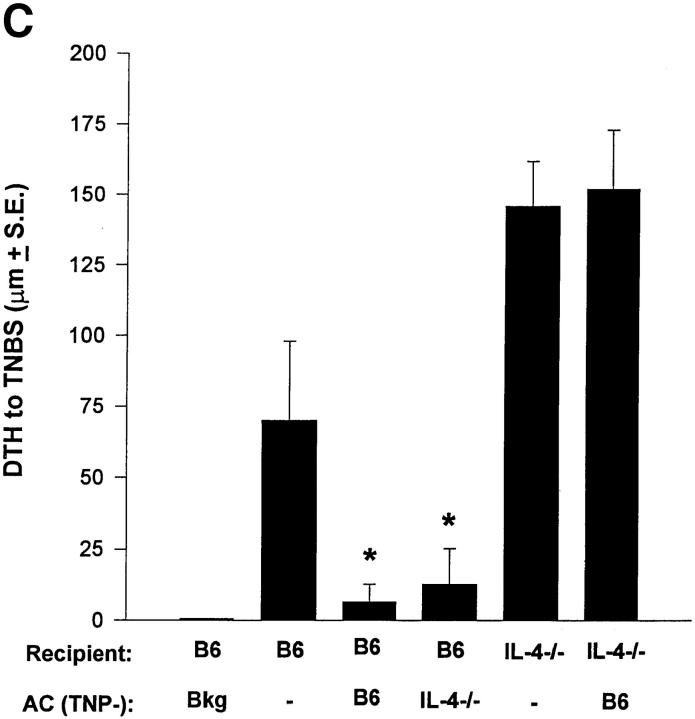
We further examined the role of Th2 cytokine production in apoptotic lymphoid cells using a well-established model for the induction of immune deviation (5, 15, 16, 25, 29). We compared the ability of normal, IL-4−/−, and IL-10−/− TNP–coupled T cells to induce immune deviation in normal B6 mice. As shown in Fig. 2 A, TNP-T cells from B6 and IL-4−/− mice induced immune deviation, whereas those from IL-10−/− mice did not. Fig. 2 B shows the cytokine profiles from the spleens of these animals and verifies that immune deviation was established. Mice receiving TNP-T cells from IL-10+/+ mice produced significant IL-4 and very little IFN-γ, whereas mice receiving IL-10−/− cells had significant IFN-γ production and were indistinguishable from immune animals in this respect.
Figure 2.
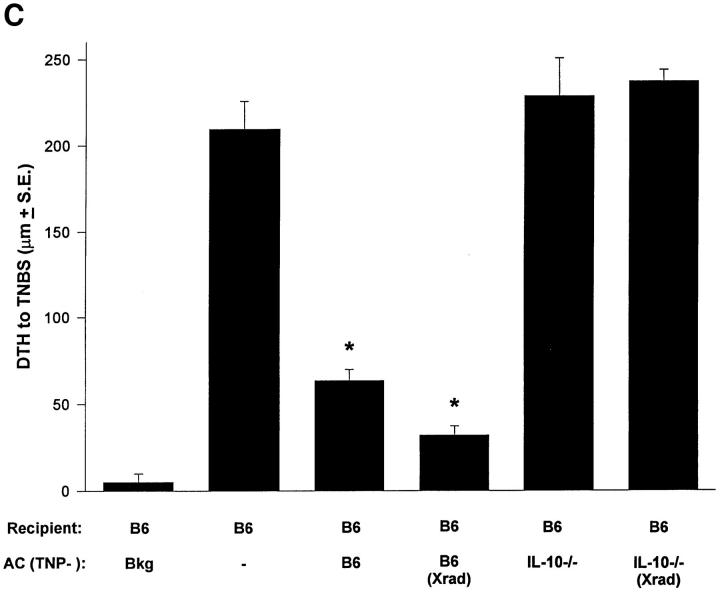
Immune deviation and IL-10. (A) Immune deviation. B6 mice were AC-injected with TNP-T cells prepared from normal B6, IL-4−/−, or IL-10−/− mice 48 h before immunization with 1% TNCB on shaved abdominal skin. 5 d later mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right footpad and 0.033 ml PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right footpad (antigen challenge) and left footpad (PBS challenge). Background (Bkg) values represent the difference between challenged and unchallenged sites in naive mice. (B) Cytokine production. B6 mice were injected AC with TNP-coupled IL-10+/+ or IL-10−/− T cells 48 h before immunization with 1% TNCB on shaved abdominal skin. 4 d later spleens were harvested, single-cell suspensions prepared, and cells were coupled with TNP. Cells were then cultured in DME/10% FCS for 48 h and supernatants were collected for cytokine analysis. (C) Immune deviation with irradiated cells. B6 mice were AC-injected with TNP-T cells or irradiated (Xrad) TNP-T cells from B6 or IL-10−/− mice. 48 h later mice were sensitized with 1% TNCB on shaved abdominal skin. 5 d later mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right footpad and 0.033 ml PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right footpad (antigen challenge) and left footpad (PBS challenge). Background (Bkg) values represent the difference between challenged and unchallenged sites in naive mice.
Because apoptosis is required for the induction of immune deviation, one possible explanation for the inability of IL-10−/− cells to induce immune deviation was that IL-10−/−cells were incapable of undergoing apoptosis in the eye. To address this, IL-10−/− TNP-T cells were killed by γ-irradiation before injection into the eye. This procedure did not allow IL-10−/− TNP-T cells to induce immune deviation (Fig. 2 C), as was the case for TNP-coupled lpr cells when apoptosis was induced in this manner (5). Even when the concentration of the TNP IL-10−/− cells was increased 10× (not shown), they were incapable of stimulating immune deviation. TUNEL stains 24 h after injection of TNP-cells confirmed that IL-10−/− cells that were not irradiated actually underwent apoptosis in the eye after injection into the AC (not shown).
Immune Deviation in IL-10−/− and IL-4−/− Mice.
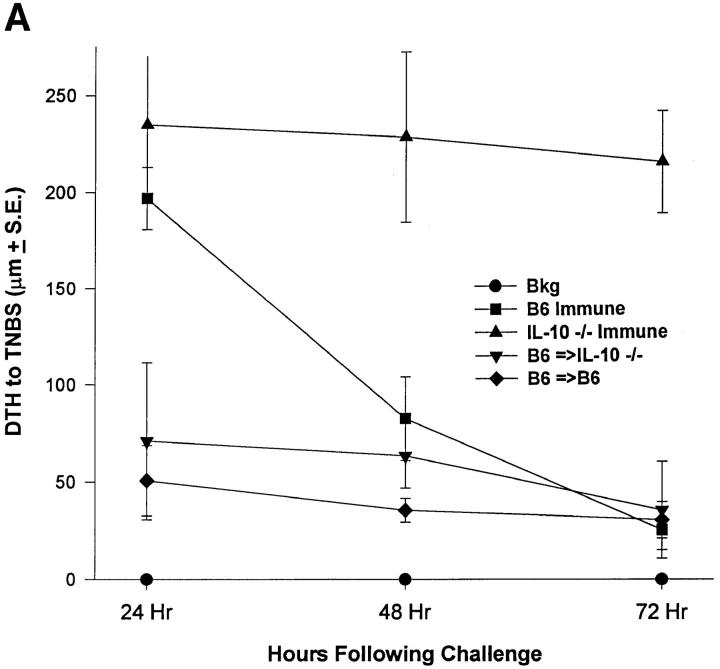
Our results suggest that IL-10 production induced by Fas-mediated apoptosis was required for the induction of immune deviation. This further emphasizes that early antiinflammatory events (i.e., apoptosis and IL-10 production) in a relatively small lymphoid cell population can dictate the outcome of a systemic immune response. The question then became whether presentation of apoptotic cells that do produce IL-10 to animals incapable of producing IL-10 would have an effect. To test this, IL-10−/− mice were injected in the AC with TNP-T cells from IL-10+/+ or IL-10−/− mice. IL-10−/− mice have been shown to have heightened and uncontrolled DTH responses due to the lack of this important antiinflammatory cytokine (30). As shown in Fig. 3 A, normal B6 mice had a typical 24 h peak response that returned to near background level by 72 h. IL-10−/− mice had a heightened response that did not diminish by 72 h after challenge. Interestingly, treatment of IL-10−/− mice with IL-10+/+ cells resulted in inhibited DTH that never developed during the 3-d measurement period. Cytokine profiles (Fig. 3 B) confirmed the DTH data by showing that IL-10−/− mice receiving IL-10+/+ TNP-cells have significantly reduced IFN-γ production compared with IL-10−/− mice and mice receiving IL-10−/− TNP-cells in the AC. In contrast to these results, IL-4−/− mice do not develop immune deviation when presented with TNP-T cells from B6 mice (Fig. 3 C). Thus, immune deviation can be established in IL-10−/− mice when apoptotic cells containing IL-10 are presented in the eye. However, IL-4 is required systemically to activate immune deviation even when IL-10 containing apoptotic cells are present in the eye.
Figure 3.
Immune deviation in IL-10−/− and IL-4−/− mice. (A) Immune deviation in IL-10−/− mice. B6 TNP-T cells were AC-injected into B6 (B6 =>B6) or IL-10−/− (B6 =>IL-10−/−) mice 48 h before immunization with 1% TNCB on shaved abdominal skin. 5 d later mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right footpad and 0.033 ml PBS in the left footpad. Measurements (μm ± SE) were taken 24, 48, and 72 h later and represent the difference between right footpad (antigen challenge) and left footpad (PBS challenge). Background (Bkg) values represent the difference between challenged and unchallenged sites in naive mice. (B) Cytokine production. IL-10−/− mice were injected AC with TNP-T cells from B6 or IL-10−/− mice 48 h before immunization with 1% TNCB on shaved abdominal skin. 4 d later spleens were harvested, single-cell suspensions prepared, and cells were coupled with TNP. Cells were then cultured in DME and 10% FCS for 48 h and supernatants were collected for cytokine analysis. (C) Immune deviation in IL-4−/− mice. B6 or IL-4−/− mice were AC-injected with TNP-T cells prepared from B6 or IL-4−/− mice 48 h before immunization with 1% TNCB on shaved abdominal skin. 5 d later mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right footpad and 0.033 ml PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right footpad (antigen challenge) and left footpad (PBS challenge). Background (Bkg) values represent the difference between challenged and unchallenged sites in naive mice. Background values for IL-4−/− mice and B6 mice were essentially identical.
Apoptotic Cells and Antigen Presentation.
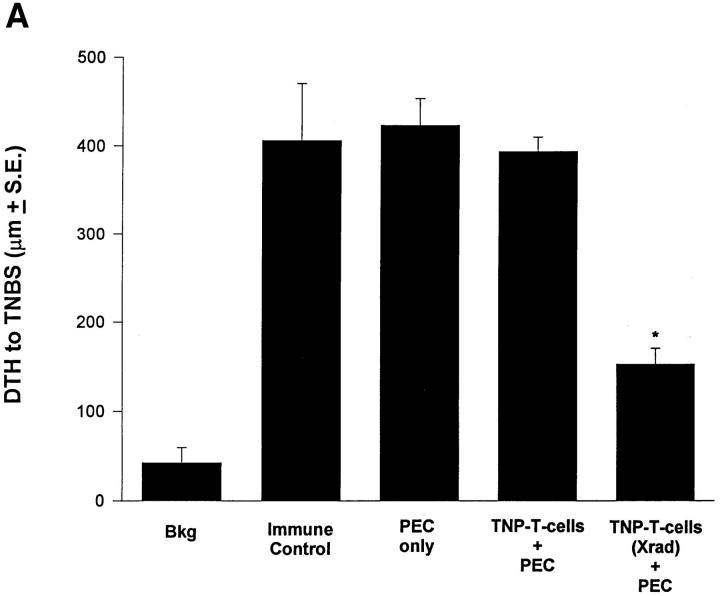
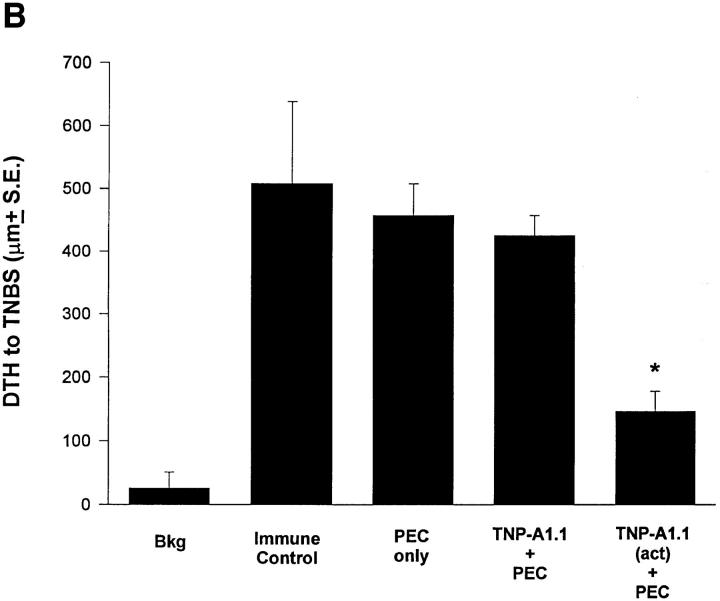
Results obtained from our in vivo experiments suggest that IL-10 production in apoptotic cells is responsible for the effect of cell death on the immune response. Even when mice are incapable of producing IL-10, IL-10+/+ apoptotic cells presented via the eye can establish immune deviation. We were then interested in how these effects could be amplified from the eye to influence the systemic immune response. One possibility is through the function of APC that are involved very early in determining the direction of an immune response by virtue of their cytokines (e.g., IL-12; references 22, 23) and costimulators (e.g., B7-1, B7-2; reference 31). APC have also been shown to be involved in immune deviation induced via the eye (19, 29). To test the effect of apoptotic cells on antigen presentation we incubated adherent PEC with TNP-T cells that were either irradiated (apoptotic; reference 5) or left untreated (nonapoptotic). 2 h later PEC were washed, dislodged from the plate, and injected intravenously into naive recipients. Results in Fig. 4 A show that PEC given apoptotic cells induced immune deviation when transferred, whereas those fed nonapoptotic cells did not. FACS® analysis for Thy-1 and CD3 revealed that excess apoptotic cells were not carried in the transferred population (not shown). A similar experiment was performed in Fig. 4 B where the T cell hybridoma A1.1 was activated to produce IL-10 (see Fig. 1 B) and coupled with TNP before incubation for 2–4 h with PEC. Adoptive transfer of these PEC intravenously to naive recipients resulted in the induction of immune deviation in recipient mice. (Note: We examined the expression of B7-1 and B7-2 on APC exposed to apoptotic and nonapoptotic IL-10+/+ cells, as well as APC fed IL-10−/− T cells. These studies found no significant influence on the expression of these costimulatory molecules [not shown].)
Figure 4.
Apoptotic cells and antigen presentation. (A) Adherent PEC were incubated with live or apoptotic (Xrad) TNP-T cells from B6 mice for 2 h. (B) The T hybridoma cell A1.1 was activated with PMA and Ionomycin before incubation with PEC for 2 h. For A and B, PEC were then washed, dislodged from the plate, and 1 × 104 viable cells injected intravenously into naive B6 mice. 48 h later recipient mice were immunized with 1% TNCB on shaved abdominal skin. 5 d later mice were challenged with 0.033 ml 10 mM TNBS in PBS in the right footpad and 0.033 ml PBS in the left footpad. Measurements (μm ± SE) were taken 24 h later and represent the difference between right footpad (antigen challenge) and left footpad (PBS challenge). Background (Bkg) values represent the difference between challenged and unchallenged sites in naive mice.
Apoptotic Cells Can Direct Th Cell Development In Vitro.
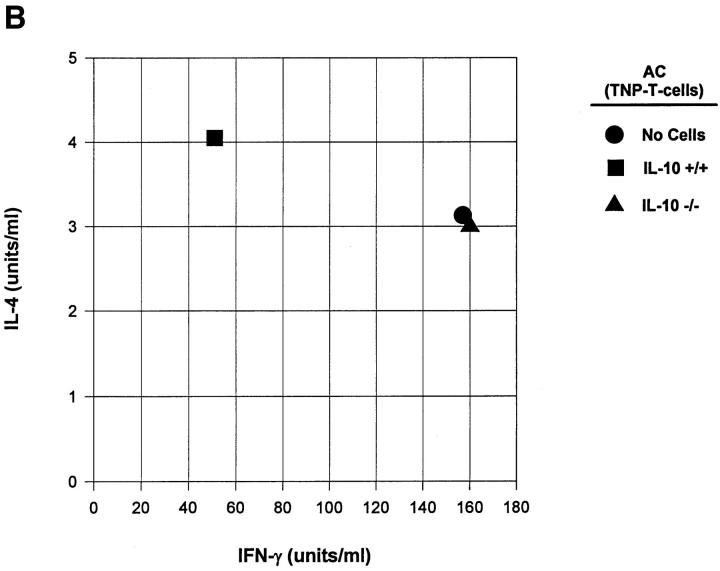
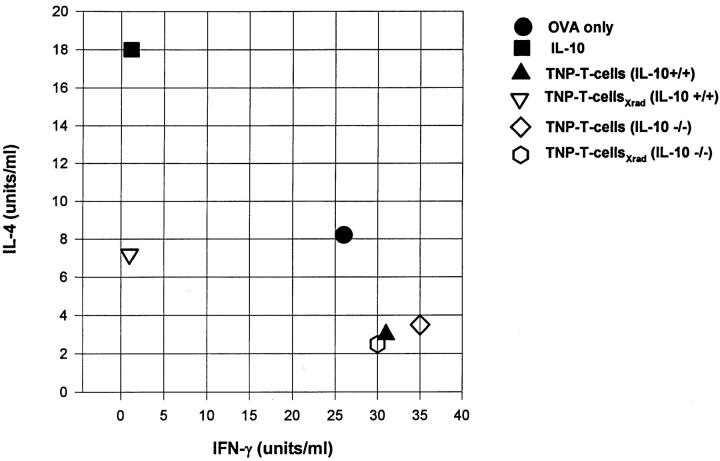
Although the data in Fig. 4 suggest that apoptotic cells induce immune deviation through processing and presentation by APC, we sought a more direct proof that apoptotic cells could influence T cell development. For these studies we employed a well established in vitro system (22, 23) where T cells from DO11.10 transgenic mice are cultured with APC in the presence of OVA. The development of Th1 or Th2 cells is assessed by examining the cytokine profiles of the resultant cell populations after restimulation with their specific peptide (OVA323–339; see Materials and Methods). Adherent PEC were incubated for 2–4 h with irradiated or untreated T cells from IL-10+/+ or IL-10−/− mice before the addition of purified DO11.10 T cells and OVA. When IL-4 and IFN-γ production were examined in the resultant cell population (Fig. 5) the presence of irradiated IL-10+/+ T cells in the preculture resulted in a Th2-like cytokine profile (high IL-4, low IFN-γ). In contrast, irradiated IL-10−/− T cells resulted in T cells that were Th1 like (low IL-4, high IFN-γ). Thus, apoptotic cells containing IL-10 were able to influence the differentiation of Th cells.
Figure 5.
Apoptotic cells can direct Th cell development in vitro. Adherent PEC were incubated with live or apoptotic (Xrad) TNP-T cells from IL-10+/+ or IL-10−/− mice for 2–4 h. Recombinant murine IL-10 was included as a control. After washing away the nonassociated cells, OVA antigen along with purified DO11.10 T cells were added and cultures were maintained for 5 d. Live cells were then harvested and restimulated with OVA323–339 peptide and splenic APC for 48 h. Supernatants were collected for cytokine analysis.
Discussion
Immune privilege is a multifaceted process that has significant biological relevance to organs and tissues key to survival. An organ's privilege relates to its ability to control inflammatory reactions, that can, while attempting to resist an invader, inflict significant damage to vital structures. No place is immune privilege better illustrated than in the eye where numerous mechanisms have been shown to protect against sight threatening bouts of inflammation (reviewed in reference 17). Two important components of immune privilege are the expression of FasL (5) and the development of immune deviation (17, 19). FasL controls the spread of inflammation by killing Fas+ lymphoid cells that enter the eye, whereas the predisposition to immune deviation after cell death restrains dangerous cell mediated responses such as DTH in favor of Th2-type reactions. The mechanisms by which apoptosis and tolerance are linked is the subject of the present report.
It has been long thought that apoptosis was a passive process that did not elicit inflammation, but created a situation where inflammatory responses were not evoked. However, the well-documented effects of UV irradiation on the immune response (4), as well as the requirement for apoptotic cell death to establish an antiinflammatory immune deviation (5), suggest that this may not be the case. Implicit in these findings is that apoptosis may involve the induction of antiinflammatory immune responses, thereby linking cell death and immune tolerance. Our studies reported here found that the production of the antiinflammatory cytokine (IL-10) in the apoptotic cells and targeting of the apoptotic cells to the APC results in a Th2-type immune deviation. Thus, apoptosis of immune effector cells (T cells) can redirect the final outcome of an immune response to minimize dangerous immune reactions (e.g., Th1 responses) that can damage important organs.
To our knowledge, this is the first demonstration of a link between Fas-mediated death and IL-10 production in lymphoid effector cells. However, the link between DNA damage and IL-10 production was recently examined by Nishigori et al. (32). These authors showed that the production of IL-10 was linked to UV-induced pyrimidine dimers in the keratinocytes of UV-exposed skin. Since IL-10 is now thought to mediate the well known inhibitory effects of UV-irradiation on cell mediated immunity (4), the link to DNA damage suggests a mechanism by which this takes place. UV-induced apoptosis also requires Fas (33), supporting our linkage of Fas-mediated death and IL-10 production. Taken together, these observations show that apoptosis has an antiinflammatory component that helps control potentially harmful immune responses.
Our results here also establish that apoptotic cells are targeted to the APC to mediate their effect on cell mediated immunity. Apoptotic cells are an efficient vehicle to target the antiinflammatory cytokines to the APC pathways. Apoptosis is a natural process resulting in membrane changes that “mark” the cells for removal by the reticuloendothelial system. The appearance of phosphatidylserine in the membrane (3) has been shown to be important. Additionally, phagocytic cells are known to use the αvβ3integrin/thrombospondin receptor (CD36) system to recognize apoptotic lymphoid cells (34). Although many particles (e.g., protein-coupled beads) can be phagocytized in vitro (35), apoptotic cells are much more efficiently taken up in vivo due to these surface markers (3, 34). This is supported by our recent studies on tolerance induction by intravenous injection of TNP-T cells. Here we found that irradiation of the TNP-coupled cells before intravenous injection reduced the amount of cells needed to achieve tolerance by 200-fold (17). Thus, apoptosis results in immune deviation and tolerance because the antigen-laden cells can be much more efficiently delivered to the phagocytes. This also provides another efficient level of control by targeting the regulatory cytokines where they can most efficiently influence the immune response (i.e., the APC).
A recent scientific correspondence in Nature (36) described the production of IL-10 in cultures of monocytes by exposure to apoptotic PBL. These results support our conclusions that apoptosis and the production of antiinflammatory cytokines are linked. In the experiment shown in that correspondence, IL-10 was found in cultures where the monocytes were exposed to UV-irradiated PBL. The authors concluded that source of the IL-10 was the monocytes stimulated to produce IL-10 by the apoptotic cells, although this was not tested. In light of this, one interpretation of our results might be that apoptotic T cells induce monocytes or macrophages to produce IL-10, leading to the in vivo and in vitro effects in our system. We think this is unlikely for several reasons. First, apoptotic T cells (5, 15–17, 24) capable of making IL-10 (Fig. 2, A and C) induce immune deviation, whereas apoptotic IL-10−/− T cells do not. This is further emphasized by our results showing apoptotic IL-10+/+ T cells injected into the AC induce immune deviation in IL-10−/− mice (Fig. 3 A). The APC (and any monocytes or macrophages) in this situation are of recipient origin (i.e., IL-10−/−) and can not synthesize IL-10. The only source of IL-10 is donor T cells injected into the AC, thus, IL-10 from apoptotic T cells is responsible. Second, apoptotic IL-10+/+ T cells are required to induce Th2 differentiation of DO11.10 T cells in culture (Fig. 5), whereas apoptotic IL-10−/− cells promote Th1 differentiation. Thus, APC in the culture (that are IL-10+/+) can not be stimulated to produce sufficient IL-10 by the apoptotic T cells to influence T cell differentiation. Third, a T cell hybridoma induced to undergo AICD produced significant IL-10, and it activates immune deviation when presented to the immune system via APC. Thus, although monocytes can produce IL-10, our results show that the IL-10 is produced by the T cells after the induction of apoptosis. Taken together, these results are consistent with our hypothesis that apoptotic T cells make IL-10 and influence the outcome of the immune response. Although it is possible that IL-10 production by monocytes may play a role in regulation of the immune response in other systems of immune deviation, the apoptotic T cells in the UV-irradiated PBL, rather than the monocytes, could be the true source of IL-10 in that system (36).
Although IL-10 has noted properties in the control of the DTH response (25) and the development of Th2 cells (22, 23), it also has other important antiinflammatory properties. IL-10 suppresses the production of proinflammatory cytokines such as IL-6, TNF-α, and IL-1, and inhibits chemotaxis by decreasing IL-8 production (37). IL-10 has also been noted to decrease class II expression (38) and down-modulate the costimulator B7-1 on macrophages (39). Thus, the production of IL-10 by apoptotic cells could regulate several aspects of the inflammatory response to control potentially damaging reactions. In the present system IL-10 influences T cell differentiation leading to Th2-type responses. Although we did not examine mechanisms by which IL-10 leads to immune deviation, we can speculate that IL-10 inhibits IL-12 production in the APC (40, 41). Since IL-12 is critical to the development of Th1 cells (42), inhibition of this cytokine may lead to Th2 development. In support of this it has been shown that IL-10 inhibits Th1 activation through the APC (43).
A previous study showed that Th1 and Th2 cells differentially used B7-1 and B7-2 (44). We did not observe any significant effect on B7 expression after interaction of apoptotic cells with APC (data not shown), even though IL-10 has been shown to prevent upregulation of B7-1 in macrophages (39). That we observed no significant changes in B7 expression could be accounted for by noting that these authors examined the effect of IL-10 on B7 expression during PEC activation with IFN-γ. We did not investigate the effect of apoptotic cells on APC activation, but used macrophages that were already activated in vivo. Thus, we were determining whether IL-10 could decrease or alter B7-1 and/or B7-2 expression in cells fully capable of inducing differentiation of T cells.
We do not know if the IL-10 is released from the apoptotic cells when they are brought into close proximity with the APC or if IL-10 functions after uptake by the phagocytes. We have attempted studies with anti-IL-10 antibody in the preculture but the results are inconclusive due to the effect of anti–IL-10 on Th cell differentiation in the culture system (22, 23). We also performed studies using Transwell™ cultures to determine if contact between the apoptotic cells and the APC was required for the effect of IL-10 in our system. In this case we saw a partical effect of IL-10 on Th cell differentiation (data not shown), suggesting that the close proximity of the apoptotic cell is necessary to concentrate IL-10 to the APC. Since the Transwell™ was in place only for 3–4 h, this suggested that sufficient IL-10 was produced in this time period to affect APC function. We are also examining the interaction of apoptotic cells with phagocytes to determine the receptors used to take up the dead cells. We are particularly interested in thrombospondin receptors (CD36) due to their involvement in apoptotic cell phagocytosis in human systems (34). Unfortunately, the lack of reagents to mouse CD36 has hampered these studies. However, we do know that apoptotic cells containing IL-10 are critical for the effect on immune deviation and T cell differentiation since apoptotic IL-10−/− cells do not perform these functions.
The site of interaction of the APC and apoptotic cells in vivo has not been precisely determined. It is well known that an intact spleen is required for the establishment of immune deviation when antigens are presented via the eye. It has been suggested that resident dendritic cells in the eye capture antigen, enter the blood, and lodge in the spleen. There they present the antigen to T cells resulting in immune deviation (19). Although it is certainly possible that apoptotic cells are engulfed by phagocytic cells in the eye, recent studies in our lab (17) suggest an alternative possibility. We can detect what appears to be apoptotic bodies in the blood of animals injected in the eye with TNP cells. This, coupled with our inability to detect phagocytized apoptotic cells in the eye suggest that the dead cells may be washed from the eye, enter the blood, and interact with the APC in the spleen. This will require further study.
An interesting result reported here is the ability of IL-10+/+ TNP-T cells injected into the AC to induce immune deviation in IL-10−/− mice. We can infer from this that IL-10 may not be necessary for the reduction of DTH responses seen in the periphery of mice with immune deviation. IL-10 has been shown to regulate the magnitude of the contact hypersensitivity response (25) and is an important regulator of IFN-γ production (45). However, our results suggest that reduced IFN-γ production in this model may not be mediated directly by IL-10, but may be reflected in vivo by a reduction in (or lack of) IFN-γ producing Th1 cells. Thus, the preferential activation of Th2 cells is the result of altered differentiation rather than a direct regulation of Th1 cells by Th2 cytokines. We also found that IL-4 was not needed in the eye for immune deviation to be established, but was required in the periphery for Th2 response development. Thus, the increase in Th2 responses requires the presence of IL-4, perhaps as the growth factor for Th2 cells and a suppressor of Th1 cells. This has been noted in other models (42, 46) and supports the idea that the induction of immune deviation in our system may be altered differentiation rather direct regulation.
We would suggest that apoptosis and the induction of antiinflammatory responses may be a more general phenomenon. Killing potentially dangerous cells by apoptosis and actively regulating inflammation provides an efficient level of protection in numerous situations. In support of our hypothesis, Bellone et al. (47) have recently shown that engulfment and processing of apoptotic bodies can yield epitopes for cytotoxic T cells. These results, along with ours, suggest that scavenging of apoptotic cells may have implications for central and peripheral tolerance. Perhaps cells other than lymphocytes killed through Fas, with TNF-α, or through other death receptors use antiinflammatory effects and antigen presentation to control inflammation. We would further suggest that the physical association of apoptotic cells and self antigens after apoptosis provides a good way to prevent potential autoimmune reactions that might ensue during normal physiological responses. Whether this is a general phenomenon or restricted to immune privileged sites, it is clear that apoptosis and antiinflammatory immune responses are linked to provide efficient protection to vital organs critical to survival.
Acknowledgments
This work is supported by National Eye Institute grants EY06765 and EY08972 and by a Department of Ophthalmology and Visual Sciences grant from Research to Prevent Blindness, Inc., New York, NY.
Abbreviations used in this paper
- AC
anterior chamber
- AICD
activation-induced cell death
- B6
C57BL/6
- B6-lpr
C57BL/6-lpr
- DTH
delayed-type hypersensitivity
- PBL
peripheral blood lymphocytes
- PEC
peritoneal exudate cells
- TNBS
2,4,6 trinitrobenzene sulfonic acid
- TNCB
2,4,6-trinitro-1-chlorobenzene
- UVB
UV radiation of wavelength 280– 320 nm
References
- 1.Kroemer G. The pharmacology of T cell apoptosis. Adv Immunol. 1995;58:211–296. doi: 10.1016/s0065-2776(08)60621-5. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK. Die and let live. Eliminating dangerous lymphocytes. Cell. 1996;84:655–657. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 3.Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie R, LaFace D, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of bcl-2 and abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes: an essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 5.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 6.Nagata S, Golstein P. Fas the death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 7.Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos A, Green DR. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int Immunol. 1995;7:1451–1458. doi: 10.1093/intimm/7.9.1451. [DOI] [PubMed] [Google Scholar]
- 8.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahne M, Rimoldi D, Schroter P, Romero M, Schreier L, French P, Schneider T, Bornand A, Fontana D, Leinard D, et al. Melanoma cell expression of Fas (Apo-1, CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 10.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counter attack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 12.Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–4128. [PubMed] [Google Scholar]
- 13.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 14.Stuart PM, Griffith TS, Usui N, Pepose JS, Yu X, Ferguson TA. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson TA, Waldrep JC, Kaplan HJ. The immune response and the eye. II. The nature of T suppressor cell induction in anterior chamber associated immune deviation ACAID. J Immunol. 1987;139:352–357. [PubMed] [Google Scholar]
- 16.Ferguson TA, Hayashi J, Kaplan HJ. The immune response and the eye. III. Anterior chamber associated immune deviation can be adoptively transferred by serum. J Immunol. 1989;143:821–826. [PubMed] [Google Scholar]
- 17.Ferguson TA, Griffith TS. A vision of cell death: insights into immune privilege. Immunol Rev. 1997;156:167–184. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye: I. F1-lymphocyte induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- 19.Wilbanks G, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation ACAID. II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146:3018–3023. [PubMed] [Google Scholar]
- 20.Li X-Y, D'Orazio T, Niederkorn JY. Role of Th1 and Th2 cell in anterior chamber associated immune deviation. Immunol. 1996;89:34–40. doi: 10.1046/j.1365-2567.1996.d01-714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-xLand bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh C-S, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh C-S, Macotonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 24.Griffith TS, Herndon JM, Lima J, Kahn M, Ferguson TA. The immune response and the eye. T-cell receptor α-chain related molecules regulate the systemic immunity to antigen presented via the anterior chamber of the eye. Int Immunol. 1995;7:1617–1625. doi: 10.1093/intimm/7.10.1617. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson TA, Dube P, Griffith TS. Regulation of contact hypersensitivity by interleukin 10. J Exp Med. 1994;179:1597–1604. doi: 10.1084/jem.179.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;4:1201–1208. [PubMed] [Google Scholar]
- 27.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 co-stimulation can promote T cell survival by enhancing the expression of bcl-xL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 28.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubl A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/ Fas ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson TA, Herndon JM, Dube P. The immune response and the eye: a role for TNF in anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 1994;35:2643–2651. [PubMed] [Google Scholar]
- 30.Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperline AI, Bluestone JA. The complexities of T-cell co-stimulation: CD28 and beyond. Immunol Rev. 1996;153:155–182. doi: 10.1111/j.1600-065x.1996.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 32.Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, Kripke ML. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc Natl Acad Sci USA. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leverkus M, Yaar M, Gilchrest BA. Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp Cell Res. 1997;232:255–262. doi: 10.1006/excr.1997.3514. [DOI] [PubMed] [Google Scholar]
- 34.Akbar AN, Savill J, Gombert W, Bofill M, Borthwick NJ, Whitelaw F, Grundy J, Janossy G, Salmon M. The specific recognition by macrophage of CD8+, CD45RO+T cells undergoing apoptosis: a mechanism for T cell clearance during resolution of viral infections. J Exp Med. 1994;180:1943–1947. doi: 10.1084/jem.180.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacsivics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 36.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 37.Van Hogezand RA, Verspaget HW. New therapies for inflammatory bowel disease: an update on chimeric anti-TNFα antibodies and IL-10 therapy. Scand J Gastroenterol Suppl. 1997;223:105–107. [PubMed] [Google Scholar]
- 38.de Waal-Malefyt R, Haanen J, Spits H, Roncarolo M-G, de Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. IL-10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down-regulation of class II MHC expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1231. [PubMed] [Google Scholar]
- 40.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 43.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore K, O'Garra A. IL-10 acts on the antigen presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3450. [PubMed] [Google Scholar]
- 44.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 45.Macatonia SE, Doherty TM, Knight SC, O'Garra A. Differential effect of IL-10 on dendritic cell-induced T-cell proliferation and IFN-gamma production. J Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- 46.Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 47.Bellone M, Lezzi G, Rovere P, Galati G, Ronchetti A, Ptotti MP, Davoust J, Rugarli C, Manfredi AA. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol. 1997;159:5391–5399. [PubMed] [Google Scholar]