Abstract
Chlamydia trachomatis, an obligate intracellular bacterial pathogen of mucosal surfaces, is a major cause of preventable blindness and sexually transmitted diseases for which vaccines are badly needed. Despite considerable effort, antichlamydial vaccines have proven to be elusive using conventional immunization strategies. We report the use of murine bone marrow–derived dendritic cells (DC) pulsed ex vivo with killed chlamydiae as a novel approach to vaccination against chlamydial infection. Our results show that DC efficiently phagocytose chlamydiae, secrete IL-12 p40, and present chlamydial antigen(s) to infection sensitized CD4+ T cells. Mice immunized intravenously with chlamydial-pulsed DC produce protective immunity against chlamydial infection of the female genital tract equal to that obtained after infection with live organisms. Immunized mice shed ∼3 logs fewer infectious chlamydiae and are protected from genital tract inflammatory and obstructive disease. Protective immunity is correlated with a chlamydial-specific Th1-biased response that closely mimics the immune response produced after chlamydial infection. Thus, ex vivo antigen-pulsed DC represent a powerful tool for the study of protective immunity to chlamydial mucosal infection and for the identification of chlamydial protective antigens through reconstitution experiments. Moreover, these findings might impact the design of vaccine strategies against other medically important sexually transmitted diseases for which vaccines are sought but which have proven difficult to develop.
Keywords: Chlamydia, pulsed dendritic cells, immunization, CD4+ T cells, mucosal protective immunity
C hlamydia trachomatis is an obligate intracellular epitheliotropic bacterium with a unique biphasic intracellular life cycle (1). C. trachomatis infection of the conjunctival epithelium causes trachoma, the world's leading cause of preventable blindness (2). Infection of the genitourinary tract of humans with C. trachomatis is a major cause of sexually transmitted diseases (STD).1 Genital infection of women constitutes a significant risk since sequelae often lead to pelvic inflammatory disease, ectopic pregnancy, or reproductive disability (3–6). Immunotherapy is believed to be the most promising and effective strategy for controlling these medically important diseases; however, despite years of effort, an efficacious vaccine does not exist.
The lack of progress towards the development of a chlamydial vaccine has been in part due to an incomplete understanding of host immune mechanisms that mediate protective immunity against infection of the genital mucosa. The mouse model of C. trachomatis infection of the female genital tract mimics human infection (7–10) and is therefore a useful preclinical model for the study of adaptive immunity to infection and vaccine development. The availability of gene knockout mice with targeted immune deficiencies has made the mouse model very useful for the study of immunity to chlamydial infection. Collectively, infection of knockout mice (11, 12) and adoptive immunization using polyclonal T cell subsets (13), or T cell clones (14) provide compelling evidence that MHC class II–restricted CD4+ T cell responses are central to the development of adaptive protective immunity to chlamydial infection of the female genital tract. Protective immunity, defined by accelerated clearance of epithelial infection and reduction in cervicovaginal shedding of infectious organisms, is mediated by an IL-12–dependent T helper type 1 immune response (15, 16). Antibodies, either humoral or local (11, 17–19) and CD8+ T cells (11, 12) play subordinate roles in chlamydial clearance from the murine genital tract. Despite this knowledge, it has not been straightforward in practice to develop conventional vaccines that target protective antichlamydial CD4+ Th1-mediated immunity at the genital mucosa. To date, immunization that provides optimum protective immunity against chlamydial infection of the genital tract has been achieved only by using viable chlamydiae (20, 21) a finding that has led investigators to conclude that effective vaccination against chlamydiae will require the use of live-attenuated chlamydial organisms. Genetic systems for chlamydiae are not available and are difficult to develop, therefore it is unlikely that a safe and efficacious live attenuated chlamydial vaccine will be forthcoming in the near future.
Dendritic cells (DC) are potent professional APC that play a central role in the induction of T cell immunity in vivo (22, 23). Large numbers of DC with powerful in vivo antigen presenting properties can be propagated in vitro using recombinant cytokines (24). It is well documented that ex vivo antigen-pulsed DC are effective inducers of tumor-specific protective immunity (25–31). The utility of ex vivo antigen-pulsed DC as an alternative approach towards the development of new immunotherapies against infectious agents has not been extensively studied (22). In this report we show that adoptively transferred DC pulsed ex vivo with nonviable chlamydial organisms are potent inducers of chlamydial-specific CD4+ Th1 immune responses that elicit levels of protective immunity against chlamydial genital tract challenge equal to that obtained after infection with live chlamydial organisms. Our findings offer encouragement for the future development of an efficacious vaccine against C. trachomatis diseases of humans and perhaps other infectious agents for which vaccines are sought after but have not been forthcoming.
Materials and Methods
Chlamydiae and Mice.
The mouse pneumonitis strain of C. trachomatis (MoPn) was grown in HeLa 229 cells. Infectious elementary bodies (EB) were purified by density gradient centrifugation and infectious forming units (IFU) were determined as previously described (32). Chlamydial EB were heat killed (HK) by incubation at 56°C for 30 min. Heat-treated EB inoculated onto monolayers of HeLa 229 cells did not yield recoverable IFU (data not shown). After heat inactivation of chlamydiae the number of IFU in purified stock preparations was used to calculate the ratio of chlamydiae incubated with DC. Female C57BL/10 (H-2b) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and used between 8 and 12 wk of age. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility in filter top cages under standard environmental conditions and provided food and water ad libitum.
Dendritic Cell Cultures.
Bone marrow–derived dendritic cells were prepared as described (24, 33). In brief, 2 × 107 bone marrow cells were cultured in IMDM (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FCS, 50 μM 2-ME, 10 μg/ml gentamicin sulfate, 10 ng/ml murine GM-CSF, and 1 × 103 U/ml IL-4 (PharMingen, San Diego, CA). On day 3 of culture, nonadherent cells were removed and fresh medium containing GM-CSF and IL-4 was added. On day 5 of culture, DC were isolated by transferring nonadherent cells to new culture plates and incubating at 37°C for at least 2 h. This was repeated once to remove contaminating macrophages. The purity of DC was assessed by FACS® analysis after staining with anti-I-Ab, N418 and B220 mAbs. Consistent with other reports (24, 33), between 80 and 90% of the cells showed uniformly high levels of MHC class II expression, low level CD11C αxβ2-integrin (N418) staining, and negative staining for B220.
Fluorescent Antibody Staining and Electron Microscopy.
DC were resuspended in IMDM and mixed with HK chlamydial EB at a ratio of 1:50, respectively. The mixture was incubated at 37°C for 1.5 h with periodic mixing. DC were washed, resuspended in IMDM-10, added to 24-well plates containing coverslips and incubated at 37°C for 24 h. The cells were then fixed with absolute methanol and stained with mAb EVI-H1 specific to chlamydial LPS followed by FITC-labeled goat anti–mouse IgG. For electron microscopy, chlamydial inoculated DC were harvested by gentle scraping and fixed in 2.5% glutaraldehyde/4% paraformaldehyde in 0.1 M sodium cacodylate buffer and 0.05 M sucrose. Samples were then post-fixed in Karnovsky's 0.5% OsO4/0.8% K3Fe(CN)6, followed by 1% tannic acid. Cells were stained en bloc in 1% uranyl acetate, dehydrated with ethanol, and embedded in Spurr's resin. Thin sections were cut with an RMC MT-7000 ultramicrotome, stained with 1% uranyl acetate and Reynold's lead citrate and observed at 80 kV on a transmission electron microscope (CM-10; Philips Electron Optics, Mahwah, NJ).
CD4+ T Cell Proliferation and Cytokine Assays.
CD4+ T cells were isolated from the spleens of 3–5 chlamydial-infected mice or DC-immunized mice using anti–L3T4-microbeads (Miltenyi Biotec Inc., Auburn, CA) following the manufacturer's instructions. Pooled CD4+ cells were cultured in triplicate in 96-well plates (3 × 105 cells/well) in DME (GIBCO BRL) supplemented with 10% FCS, 50 μM 2-ME, 100 U/ml penicillin, 10 μg/ml gentamicin, or in AIM V serum-free medium (GIBCO BRL) supplemented with 2-ME and antibiotics. CD4+ cells isolated from mice immunized with DC were cultured in serum-free media to avoid immunological recognition of serum-derived proteins. DC or syngeneic splenocytes were used as APC. DC APC were incubated with HK EB (ratio of 1:0.1–100) at 37°C for 1.5 h, washed and incubated in IMDM-10 containing GM-CSF for 40 h as described by Inaba et al. (34). Chlamydial-pulsed DC were irradiated (3,000 Rad) and 3 × 104 cells/well were added to CD4+ cells. Splenocyte APC were incubated with HK EB (ratio of 1:5), irradiated, washed, and then 5 × 105 cells/well were added to CD4+ cells. After 48 h incubation, supernatants (100 μl) were collected for cytokine assays and cell proliferation was measured by incorporation of [3H]thymidine (1 μCi/well, 90 Ci/mmol; DuPont-NEN, Boston, MA). Plates were pulsed overnight and radioactivity determined using a TopCount NXT microplate scintillation counter (Packard Instrument Company, Meriden, CT). In some experiments CD4+ cells and APC were cultured in 1-ml aliquots at the cell concentrations described above and supernatants were collected at 72 h for cytokine determination.
Flow Cytometry.
DC alone or DC that were inoculated with HK EB and incubated at 37°C in IMDM-10 containing GM-CSF for 40 h were stained with FITC-conjugated AF6-120.1 (anti-I-Ab), GL1 (anti-CD86, B7-2), 3E2 (anti-CD54, ICAM-1), or rat anti-CD80 (B7-1) followed by FITC-conjugated mouse anti–rat antibodies (PharMingen, San Diego, CA). Staining was done in PBS containing 5% FCS on ice for 20 min. Flow cytometry analysis was performed with a FACStar® instrument (Becton Dickinson, San Jose, CA). Data were collected on 10,000 cells and dead cells were excluded from analysis by propidium iodide staining.
Adoptive Immunization and Chlamydial Challenge.
DC were incubated with medium or HK EB (ratio of 1:25) at 37°C for 1.5 h, washed and incubated in IMDM-10 containing GM-CSF for 40 h. The culture supernatants were collected at 24 h after inoculation for cytokine assays. Chlamydial-pulsed DC were collected and injected intravenously by infusion into the retro-orbital sinus of mice at a concentration of 1 × 106 cells/mouse in 0.2 ml PBS. Mice were injected three times with DC at weekly intervals. 7 d before chlamydial challenge mice were treated with Depo-Provera (The Upjohn Company, Kalamazoo, MI) to synchronize estrous. Mice were challenged intravaginally with chlamydiae (1,500 IFU) 14 d after the third immunization. Protection was assessed by quantifying the number of IFU recovered from cervicovaginal swabs taken at different times after infection (13). Entire genital tracts were removed from mice at 7 and 70 d after infection for histopathology (Histo-Path of America, Millersville, MD) and gross pathological evaluation for the presence of hydrosalpinx, respectively.
Antibody and Cytokine ELISA.
Serum antibody titers of chlamydial-specific IgG1 and IgG2a were assayed by ELISA using formalin fixed MoPn EB and alkaline phosphatase-conjugated goat anti–mouse IgG antibodies (Southern Biotechnology Associates, Birmingham, AL) as described previously (12). All cytokines were measured by ELISA using corresponding specific capture and detection antibodies and cytokine levels were calculated using standard curves constructed using recombinant murine cytokines (PharMingen).
Results
Phagocytosis of Killed Chlamydiae by DC.
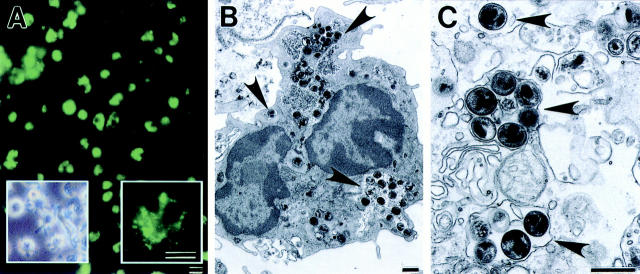
Chlamydial EB were heat killed and their interactions with DC were studied by fluorescent antibody staining and electron microscopy. DC exposed to HK EB and stained with an antichlamydial LPS mAb 24 h later exhibited intense fluorescent staining. The majority of DC were positively stained and fluorescence appeared as a fine punctate pattern distributed throughout the cytoplasm mixed with less frequent numbers of larger particulate staining structures (Fig. 1 A). Control uninoculated DC did not exhibit fluorescent staining (data not shown). Examination of DC by electron microscopy 4 h after addition of HK EB showed intracellular organisms present in cytoplasmic vacuoles. Chlamydial EB were visualized as 200–400-nm electron dense particles that were present in the majority of DC examined. Chlamydial EB were localized within tight membrane bound vacuoles containing either single or multiple organisms (Fig. 1, B and C). These results demonstrate that DC phagocytose killed chlamydiae, a finding recently described by Ojcius et al. (35).
Figure 1.
Dendritic cells ingest HK chlamydiae. (A) Fluorescent antibody staining of DC pulsed with HK EB. The majority of DC stain positive for chlamydial antigen indicating that DC uniformly ingested HK chlamydial organisms. Fluorescence was visible as fine punctate and large aggregate particles in the same DC (A, right inset). Inset on the left shows a phase contrast photomicrograph of cultured DC. Note the characteristic large processes or veils extending in many directions from the DC. (B and C) Electron micrographs of DC inoculated with HK chlamydial organisms for 4 h. Vacuoles containing EB (arrows) were evident throughout the DC cytoplasm. EB were present in vesicles either individually or as aggregates. Bars: (A) 5 μm; (B and C) 0.5 μm.
Cytokine Secretion by DC.
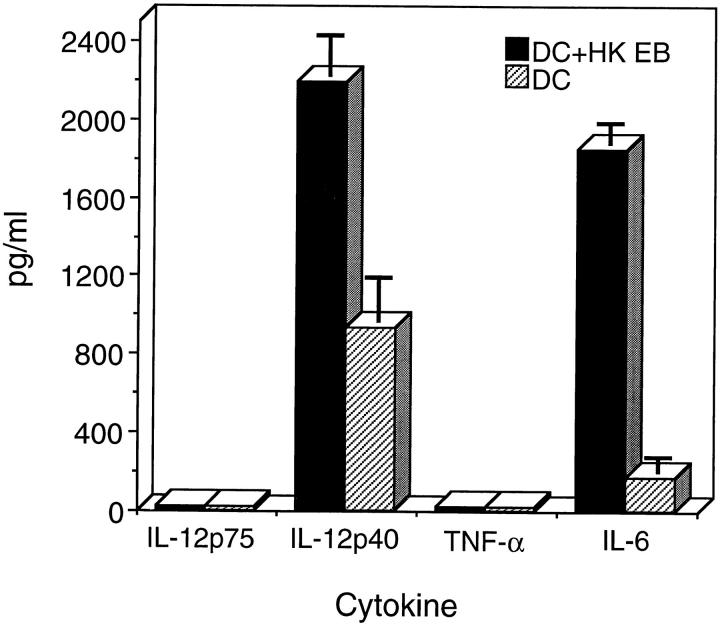
IL-12 is an important cytokine in driving the differentiation of precursor Th cells to a Th1 phenotype (36, 37). Recent studies have shown that DC, not macrophages, produce IL-12 in vivo upon microbial stimulation (38). Therefore, it is important to determine if ingestion of chlamydial EB stimulated DC to produce IL-12. The supernatants from chlamydial-pulsed and unpulsed DC were collected 24 h after inoculation and assayed by ELISA for IL-12 p75, IL-12 p40, TNF-α, and IL-6. Chlamydial-pulsed DC were found to secrete elevated levels of the IL-12 p40 subunit and IL-6 (Fig. 2). Neither the IL-12 p75 heterodimer nor TNF-α was detectable by ELISA in culture supernatants of chlamydial-pulsed DC. Bioactive IL-12 p75 is composed of a constitutively expressed p35 subunit and an induced p40 subunit. The p40 subunit is expressed at levels 10–50 times greater than the p75 heterodimer (39); this factor likely explains our inability to detect p75. The inability to detect IL-12 p75 heterodimer has also been reported by others, and elevated levels of p40 expression has been translated to reflect sufficient levels of IL-12 p75 for in vivo bioactivity (38). The expression of the adhesion molecule ICAM-1 and the costimulatory molecules B7-1 and B7-2 on chlamydial-pulsed DC was analyzed by FACS®. There was no upregulation of these cell surface molecules after chlamydial ingestion by DC (data not shown).
Figure 2.
Chlamydial-pulsed DC produce IL-12. DC were inoculated with HK EB (filled bars), ratio 1:25, or medium alone (striped bars) at 37°C for 1.5 h, washed and incubated in IMDM-10 containing GM-CSF. The culture supernatants were collected 24 h after inoculation with HK EB and analyzed by ELISA for IL-12 p75, IL-12 p40, IL-6, and TNF-α. Chlamydial-pulsed DC secreted elevated levels of IL-12 p40 and IL-6. Neither IL-12 p75 nor TNF-α was detectable in the supernatants of chlamydial-pulsed DC. Results are expressed as pg/ml ± SD.
Presentation of Chlamydial Antigen(s) to Infection Sensitized CD4+ T Cells by Chlamydial-pulsed DC.
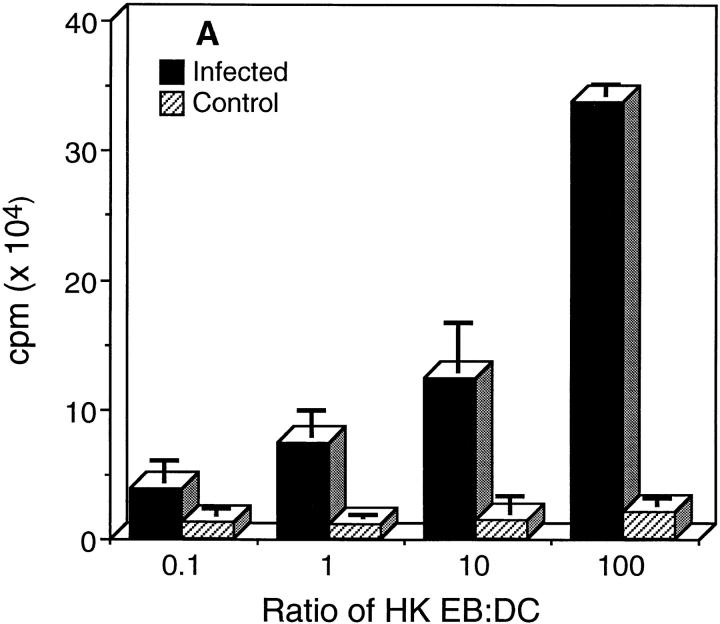
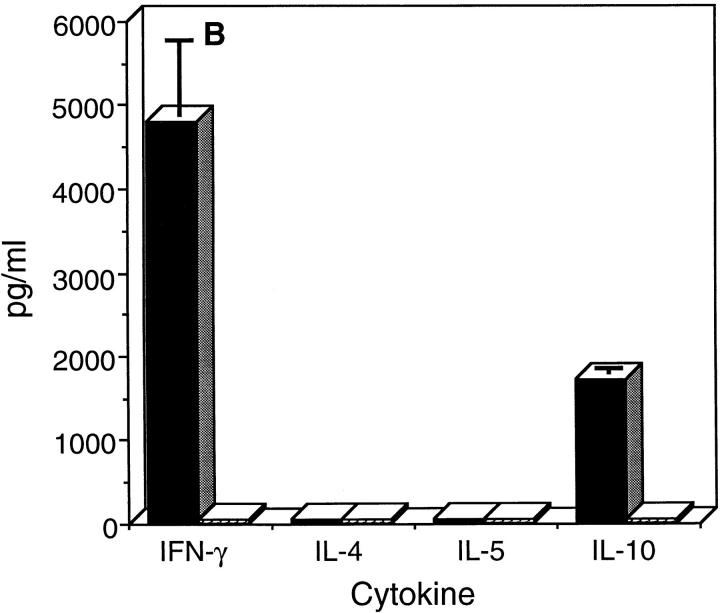
We next asked whether chlamydial-pulsed DC could present processed chlamydial antigen(s) to CD4+ T cells recovered from the spleens of mice that had been previously infected and resolved chlamydial genital infection similar to those results described by Ojcius et al. (35). These mice exhibit optimum protective immunity to vaginal rechallenge and immune CD4+ T cells recovered from their spleens are protective after adoptive transfer to naive mice (13). The proliferative response and cytokine secretion profiles of immune CD4+ T cells after incubation with chlamydial-pulsed DC are shown in Fig. 3. Immune CD4+ T cells proliferated strongly in an antigen specific, dose-dependent manner against chlamydial-pulsed DC (Fig. 3 A). Analysis of cytokines secreted by immune CD4+ T cells revealed the expression of IFN-γ and IL-10 with no detectable expression of IL-4 or IL-5 (Fig. 3 B). Splenic CD4+ T cells isolated from naive mice did not proliferate or secrete detectable levels of cytokines after incubation with DC pulsed with HK EB. These findings indicate that in vitro pulsed DC present chlamydial antigen(s) in common with those recognized during natural infection with chlamydiae, and imply that chlamydial-pulsed DC might induce a similar protective CD4+ Th1 immune response in vivo.
Figure 3.
Chlamydial-pulsed DC present antigen(s) to CD4+ T cells isolated from chlamydial-infected mice. (A) Proliferation assay. (B) Cytokine assay. Splenic CD4+ T cells were isolated from chlamydial-infected (filled bars) or naive control mice (striped bars) and incubated with irradiated chlamydial-pulsed DC at 37°C for 48 h. CD4+ T cell proliferation was measured by incorporation of [3H]thymidine and cytokines secreted by CD4+ T cells assayed by ELISA. Data are presented as the mean of triplicate samples ± SD.
Immune Response of Mice after Adoptive Transfer of Chlamydial-pulsed DC.
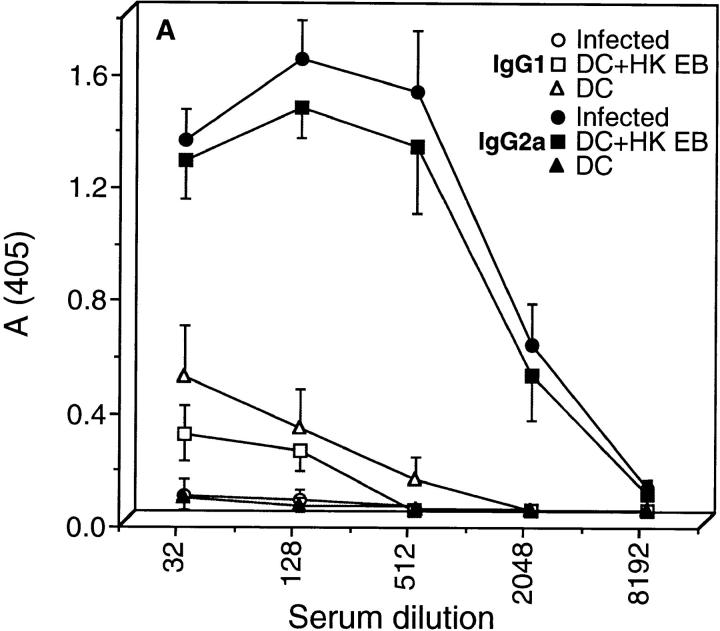
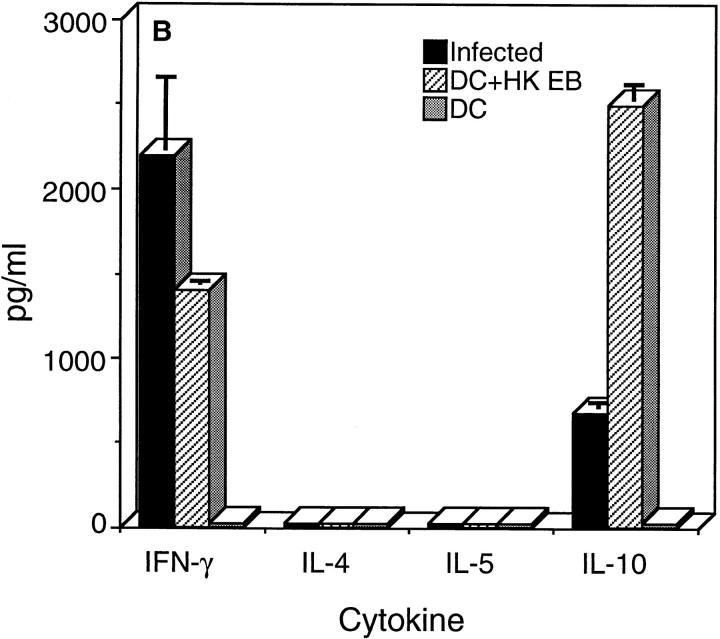
To investigate whether chlamydial-pulsed DC were capable of eliciting a chlamydial-specific CD4+ Th1 immune response, chlamydial-pulsed DC or DC alone were adoptively transferred to mice by intravenous injection. After immunization, mouse sera were analyzed by ELISA for anti-chlamydial IgG1 and IgG2a-specific antibodies, and splenic CD4+ T cells isolated from immunized mice were assessed for antigen-specific cytokine production (Fig. 4). The serum antibody and splenic CD4+ T cell responses of chlamydial-infected mice were assayed in parallel and compared with mice immunized with chlamydial-pulsed DC. Chlamydial-specific serum antibodies of infected and DC-immunized mice were predominately of the IgG2a isotype with little to no detectable IgG1, an Ig profile consistent with stimulation of type 1 immune responses (Fig. 4 A). Splenic CD4+ T cells isolated from chlamydial-infected and antigen-pulsed DC-immunized mice secreted IFN-γ and IL-10 but not IL-4 or IL-5 (Fig. 4 B). Thus, chlamydial-infected mice and mice immunized with chlamydial-pulsed DC produced a strikingly similar Th1-biased immune response as shown by the predominance of IgG2a serum antibodies, and elevated levels of IFN-γ production by CD4+ T cells.
Figure 4.
Mice immunized with chlamydial-pulsed DC produce a CD4+ Th1 immune response. (A) IgG1 and IgG2a chlamydial-specific antibody responses in the sera of mice immunized with chlamydial-pulsed DC or infected with chlamydiae. Mice were bled 7 d after the third immunization with DC or 35 d after intravaginal infection and serum antibody responses were assayed by ELISA against formalin fixed C. trachomatis MoPn EB. Five mice per group were analyzed and sera were tested individually. Results are expressed as the mean OD ± SD. (B) Antigen-specific CD4+ T cell cytokine secretion profiles of mice immunized with chlamydial-pulsed DC or infected with chlamydiae. CD4+ T cells were obtained from the spleens of mice 6 d after a primary immunization with DC (dotted bars), DC pulsed with HK EB (striped bars), or 35 d after infection (filled bars), and then cultured in serum-free medium with syngeneic chlamydial-pulsed splenocytes as APC. Culture supernatants were collected 72 h after incubation for cytokine assays. Cytokine results are expressed as pg/ml ± SD.
Mice Adoptively Immunized with Chlamydial-pulsed DC Are Protected against Chlamydial Genital Tract Infection.
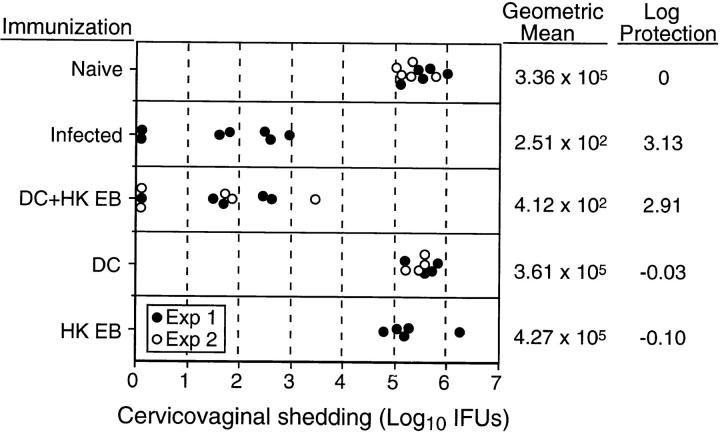
To ascertain if the immune response produced by mice immunized with chlamydial-pulsed DC was protective, vaccinated animals were challenged intravaginally with chlamydiae and protection was assessed by (a) quantifying the numbers of infectious chlamydiae recovered from the vaginas of mice, (b) evaluating the inflammatory response present in genital tissue by routine histopathological examination, and (c) monitoring the development of hydrosalpinx by gross examination. Naive mice and mice immunized with equivalent numbers of DC or HK EB alone were similarly challenged and evaluated. The magnitude of protection conferred to mice by adoptively transferred chlamydial-pulsed DC was compared with the level of immunity exhibited by previously infected mice. Immunity after infection with live organisms is highly protective in this model. Mice adoptively immunized with chlamydial-pulsed DC shed 2.91 logs less infectious organisms than naive control mice after intravaginal challenge (Fig. 5). This level of protection was equivalent to that observed in the previously infected group that shed 3.13 logs less organisms than controls. Immunization with equivalent numbers of control DC or HK EB was not protective.
Figure 5.
Mice immunized with chlamydial-pulsed DC are protected against chlamydial infection of the genital tract. Naive mice, mice immunized with DC pulsed with HK EB, DC alone, or HK EB alone, or mice that had previously been infected with chlamydiae were challenged intravaginally with 1,500 IFU of C. trachomatis MoPn EB. The results shown are from two different experiments and the circles represent culture results from individual mice. The solid circles are results from experiment 1, and the open circles from experiment 2. All mice were cultured at 5 d after challenge and protective immunity was monitored by quantifying the number of chlamydial IFU recovered from cervicovaginal swabs. Naive mice and mice immunized with equivalent numbers of DC or HK EB alone served as negative controls. Mice that had been previously infected genitally and had resolved their primary infection (45 d after infection) served as positive controls. Note that mice immunized with DC pulsed with HK EB were equally as immune as mice previously infected genitally, both groups shedding ∼3 logs less chlamydiae than the naive control group. In contrast, mice immunized with equivalent numbers of DC or HK EB alone were not protected. Infected mice and mice immunized with DC pulsed with HK EB cleared infection by day 10 after challenge whereas the remaining groups cleared infection between 21 and 28 d after challenge.
Disease was assessed by histopathological evaluation of genital tract tissue and scoring for the development of hydrosalpinx by gross examination in chlamydial challenged mice. Fig. 6 shows a representative example of hematoxylin- and eosin-stained uterine tissue from mice immunized with DC alone or DC pulsed with HK EB. Sections of the reproductive tract of the DC-immunized mice revealed intense inflammatory infiltrates from the ectocervix to the oviducts. The infiltrates, consisting primarily of PMN with scattered mononuclear cells, were observed from serosal to mucosal surfaces, but were most concentrated at the mucosa. The lumen of the uterine horns were engorged with purulent exudate (Fig. 6 A) and more distally, intraepithelial PMN were abundant (Fig. 6 C), often associated with destruction of the endometrial lining. The stroma of the endometrium exhibited vascular congestion, was hypercellular and contained scattered PMN. In contrast, the reproductive tracts of mice immunized with DC pulsed with HK EB were normal or showed mild inflammatory reactions. Fig. 6 B shows the stained uterine tissue of a culture negative mouse immunized with DC pulsed with HK EB. The folds of endometrium were readily recognized, the lumen of the uterus clear, and the endometrial stroma arranged in loose networks of cells. Under high magnification the epithelium of the endometrium exhibited a uniform arrangement of nuclei at the basal aspect of the cells (Fig. 6 D) which contrasts with the disordered arrangement seen in the DC mice (Fig. 6 C). In the case of the mice that shed low numbers of infectious chlamydiae the inflammatory infiltrates were mild and similar in character to those observed in unprotected mice.
Figure 6.
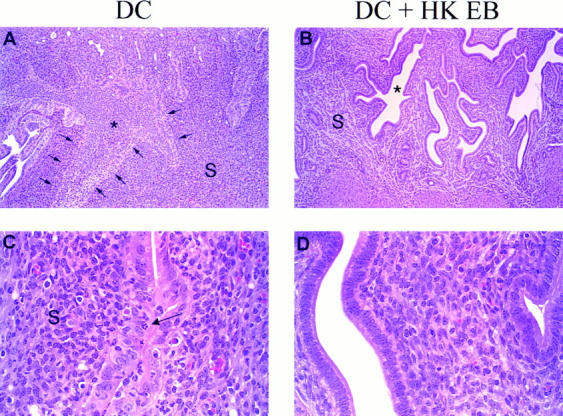
Histopathology of genital tract tissues of mice immunized with chlamydial-pulsed DC. A comparison of hematoxylin- and eosin-stained sections of the wall of the uterus from a DC-immunized mouse (A and C) and a mouse immunized with DC pulsed with HK EB 7 d after challenge (B and D). Note the difficulty distinguishing the border of the endometrial folds (A, arrows point to the epithelial layer). This is due to the lumen (*) of the uterus being engorged with inflammatory exudate. PMN can be seen within the stroma (S) of the endometrium as well as within the epithelial layer (C, arrow) and the nuclei of the epithelial cells are disordered. This picture contrasts dramatically with the mouse immunized with chlamydial-pulsed DC in which the endometrial folds are distinct, the lumen (*) empty, the stroma (S) arranged in a loose network (B) and the nuclei of the epithelial layer oriented at the basal aspect of the cells (D). The results shown are representative of stained sections from three mice of each experimental group. Magnification: (A and B) 30×; (C and D) 125×.
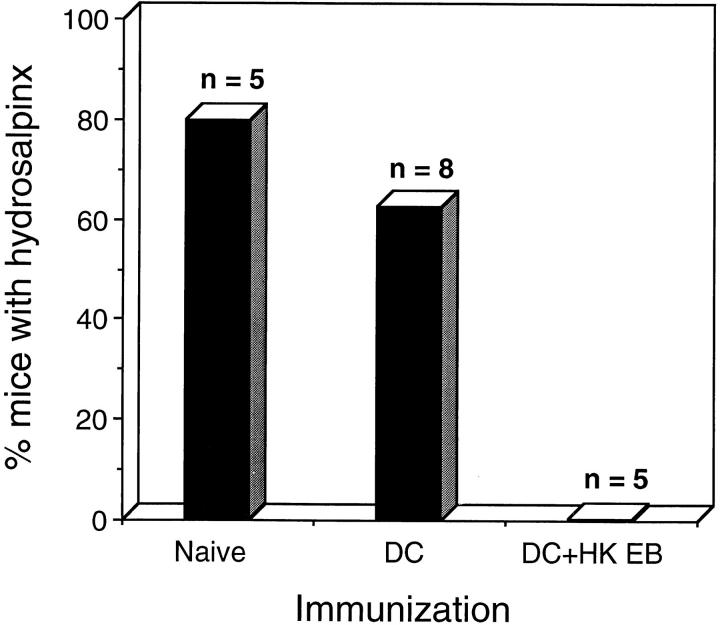
Hydrosalpinx is a common sequelae of chlamydial genital tract infection in the mouse model that develops due to a consequence of inflammatory infiltrates in the walls of the oviducts leading to scarring and obstruction of fluid flow (8–10). This is the most important sequelae of chlamydial STD because it leads to infertility, which is generally not amenable to surgical correction (10). Therefore, it was important to ascertain whether immunization with chlamydial-pulsed DC prevented the development of hydrosalpinx. The incidence of hydrosalpinx in DC-immunized and control mice is shown in Fig. 7. 80% (4/5) of naive control mice and 62% (5/8) of mice immunized with DC alone exhibited hydrosalpinx after chlamydial infection. In contrast, none (0/5) of the mice immunized with chlamydial-pulsed DC developed hydrosalpinx. Collectively, these results demonstrate that immunization with chlamydial-pulsed DC induced a highly significant level of protective immunity against chlamydial infection. Immunization reduced the infectious burden of chlamydiae shed from genital tissue to levels equivalent to that obtained from previously infected mice, and prevented genital tract inflammatory disease and its obstructive sequelae.
Figure 7.
Incidence of hydrosalpinx in mice immunized with chlamydial-pulsed DC. Naive and DC-immunized groups of mice were killed 70 d after intravaginal chlamydial challenge and scored for the presence or absence of hydrosalpinx. Hydrosalpinx is a common sequelae of infection in this model and results from occlusion of the oviduct. Mice suffering hydrosalpinx are infertile. The numbers above the columns correspond to the number of mice used for each of the experimental groups. The DC pulsed with HK EB immunized group is significantly different than the naive control group (P < 0.05, two-tailed Fisher's exact test).
Discussion
We have shown that adoptively transferred DC pulsed ex vivo with HK chlamydiae elicit a chlamydial-specific CD4+ Th1-biased immune response in vivo that confers a level of protective immunity against infection of the female genital tract equivalent to that produced by infection. Protection was demonstrated by ∼3 logs fewer infectious chlamydiae shed from the cervicovagina of vaccinated mice, minimal to no inflammatory response in genital tract tissue, and prevention of hydrosalpinx. These studies are the first to describe the development of a highly efficacious vaccine against chlamydial infection of the genitourinary tract despite nearly a decade of effort. The observation that ex vivo antigen-pulsed DC are effective mediators of protective tumor immunity is well documented (25–31); however, we are aware of only one report using pulsed DC to adoptively immunize against infectious diseases (40), and no reports demonstrating the use of this approach to protect against mucosal pathogens that infect the genitourinary tract. Inaba et al. (34) described that DC pulsed with Mycobacteria in vitro induced a strong T cell response in vivo to mycobacterial antigens; but infectious challenge experiments were not performed by these investigators. More recently, investigators have suggested ex vivo antigen-pulsed DC could be potentially useful for adoptive transfer immunotherapies to vaccinate against infectious agents (22, 33, 41), including chlamydiae (35), but direct evidence using in vivo infection models were not described. The findings reported in this work have experimentally tested and proven this hypothesis in a preclinical animal model of immunity and infection.
Chlamydia (15) and other intracellular parasites, such as Mycobacteria (42), Listeria (43), Leishmania (44), and Toxoplasma (45) elicit IL-12–dependent CD4+ Th1 responses after infection that have been shown to be important in the generation of protective immunity to infection. A common goal and continuous challenge for investigators studying these pathogens has been the development of immunization strategies capable of producing CD4+ Th1 protective immunity. Successful results towards this end have been recently described after coimmunization with Leishmania (46) or Listeria (47, 48) antigens and exogenous IL-12. In those studies potent antiparasite Th1 immunity was elicited and high levels of protective efficacy achieved. We have used this same strategy in attempts to generate protective Th1 antichlamydial immunity. Mice immunized intraperitoneally with HK EB plus IL-12 generated a strong chlamydial-specific Th1 immune response; however, they were not protected after chlamydial genital tract challenge (unpublished observations). The reason this approach was unsuccessful for preventing chlamydial infection is likely due to differences in the host cells infected by Chlamydia and these other intracellular parasites. Chlamydial infection is restricted to epithelial cells of the genital tract whereas Listeria and Leishmania organisms infect macrophages in the spleen and subcutaneous tissue of the foot pad, respectively. Interestingly, immunization with chlamydial-pulsed DC or HK EB plus exogenous IL-12 both induced Th1 immune responses, but importantly, only chlamydial-pulsed DC elicited immunity capable of protecting against infection of the genital tract. DC possess multiple properties that promote Th1 immune responses including their high level of MHC class II expression, secretion of the Th1-polarizing cytokine IL-12, and homing properties that direct migration to the parafollicular areas of lymphatic tissue where they interdigitate between T cells (22, 23, 37, 49–51). The capabilities of antigen-pulsed DC to migrate to regional or mucosal lymphoid tissue may be the key to their ability to activate and drive differentiation of chlamydial-specific Th1 cells that are capable of homing to the genital mucosa. These T cell populations may not be sufficiently sensitized after parenteral immunization with HK EB and exogenous IL-12, which could explain the ineffectiveness of this immunizing strategy for the prevention of chlamydial infection of the genital tract. It will be important to use this model to define the migration patterns of adoptively transferred DC that allow them to induce mucosal protective immunity and to identify components that mediate DC homing. These studies may provide important clues for the design of conventional vaccines capable of targeting protective T cell immunity at the genital mucosa.
At present it would be impractical to suggest the use of autologous ex vivo antigen-pulsed DC as immunotherapies for the prevention of chlamydial infections of humans. Nevertheless, the results described herein clearly demonstrate the feasibility of producing a highly efficacious vaccine against chlamydial infection of the genital tract using nonviable organisms. This is an important finding since previous immunization studies using this model have shown that optimum protective immunity against chlamydial genital infection could only be produced using viable organisms (20, 21) although significant levels of protection against infertility have been described after immunization of mice with chlamydial outer membrane complexes (52). These findings have led to the conclusion that a vaccine effective against chlamydial genital tract infection can only be attained using live attenuated organisms. The work reported here argues strongly against this hypothesis and demonstrates that antigens capable of eliciting protective cellular immunity are present on intact nonreplicating chlamydiae. Thus, it should be possible to develop an efficacious chlamydial vaccine using conventional approaches. To reach this goal it will be important to understand the immunizing property(s) of chlamydial-pulsed DC that confer protective Th1-mediated immunity mucosally, and to identify chlamydial protective antigens that can be used for immunological targeting and vaccine development. The DC system described here represents a powerful means to identify chlamydial protective antigens through reconstitution experiments. For example, DC pulsed ex vivo with acellular or recombinant antigens, or transfected with DNA encoding antigen(s) can be used to efficiently target antichlamydial immunity at the genital mucosa.
DC pulsed ex vivo with inactivated pathogens are theoretically capable of independently inducing multi-faceted immune responses that include antibodies, CD4+ and CD8+ T cell–mediated cellular immunity against a broad range of antigens expressed by a pathogen. This powerful targeted approach to immunization, coupled with the ability of DC to elicit functional mucosal immunity after parenteral immunization, has obvious advantages for the development of vaccines to control other infectious agents. Adoptive immunization with ex vivo antigen-pulsed DC may be particularly applicable in vaccine development against infectious diseases that constitute more severe health risks to humans. An example where such an approach might be considered is in the design of immunotherapeutic strategies against HIV, a disease that would justify both an unconventional and aggressive approach to vaccination. In support of this idea is the recent observation that HIV-specific CD4+ responses are strongly associated with the control of viremia (53). Thus, the use of HIV or antigen-pulsed autologous DC might be a means of efficiently stimulating vigorous anti-HIV CD4+ immunity.
Acknowledgments
We are grateful to Gary Hettrick and Robert Evans for expert assistance in the preparation of graphic illustrations and Dr. Mike Parnell for veterinary assistance. We are also grateful to Drs. Kim Hasenkrug and Linda Perry for their critical review of this manuscript.
Abbreviations used in this paper
- DC
dendritic cells
- EB
elementary body
- HK
heat killed
- IFU
inclusion forming unit
- MoPn
mouse pneumonitis
- STD
sexually transmitted disease
References
- 1.Schachter J. The intracellular life of Chlamydia. . Curr Top Microbiol Immunol. 1988;138:109–139. [PubMed] [Google Scholar]
- 2.Jones BR. The prevention of blindness from trachoma. Trans Ophthalmol Soc UK. 1975;95:16–33. [PubMed] [Google Scholar]
- 3.Brunham RC, Binns B, McDowell J, Paraskevas M. Chlamydia trachomatisinfection in women with ectopic pregnancy. Obstet Gynecol. 1986;67:722–726. doi: 10.1097/00006250-198605000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Brunham RC, Maclean IW, Binns B, Peeling RW. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985;152:1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- 5.Chow JM, Yonekura ML, Richwald GA, Greenland S, Sweet RL, Schachter J. The association between Chlamydia trachomatisand ectopic pregnancy. JAMA (J Am Med Assoc) 1990;263:3164–3167. [PubMed] [Google Scholar]
- 6.Jones RB, Ardery BR, Hui SL, Cleary RE. Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril. 1982;38:553–558. doi: 10.1016/s0015-0282(16)46634-3. [DOI] [PubMed] [Google Scholar]
- 7.Barron AL, White HJ, Rank RG, Soloff BL, Moses EB. A new animal model for the study of Chlamydia trachomatisgenital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 8.Patton DL, Landers DV, Schachter J. Experimental Chlamydia trachomatissalpingitis in mice: initial studies on the characterization of the leukocyte response to chlamydial infection. J Infect Dis. 1989;159:1105–1110. doi: 10.1093/infdis/159.6.1105. [DOI] [PubMed] [Google Scholar]
- 9.Swenson CE, Donegan E, Schachter J. Chlamydia trachomatis-induced salpingitis in mice. J Infect Dis. 1983;148:1101–1107. doi: 10.1093/infdis/148.6.1101. [DOI] [PubMed] [Google Scholar]
- 10.de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatismouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DM, Grubbs BG, Pack E, Kelly K, Rank RG. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. . Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II–restricted responses in Chlamydia trachomatisgenital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatisinfection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, TH1lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 15.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatisis mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 16.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatischronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatisgenital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson M, Ward M, Lycke N. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. . Immunology. 1997;92:422–428. doi: 10.1046/j.1365-2567.1997.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatisfails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly KA, Robinson EA, Rank RG. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatisfollowing genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal S, Peterson EM, de la Maza LM. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatisgenital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 24.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte-macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, Lotze MT. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 27.Gyure LA, Barfoot R, Denham S, Hall JG. Immunity to a syngeneic sarcoma induced in rats by dendritic lymph cells exposed to the tumour either in vivo or in vitro. . Br J Cancer. 1987;55:17–20. doi: 10.1038/bjc.1987.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific, CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celluzzi CM, Falo LD., Jr Cutting edge: physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J Immunol. 1998;160:3081–3085. [PubMed] [Google Scholar]
- 30.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 31.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Garcia-Prats MD, DeLeo AB, Lotze MT. Bone marrow-derived dendritic cells serve as potent adjuvants for peptide-based antitumor vaccines. Stem Cells. 1997;15:94–103. doi: 10.1002/stem.150094. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. . Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson M, Stockinger B, Wick MJ. Bone marrow-derived dendritic cells can process bacteria for MHC-I and MHC-II presentation to T cells. J Immunol. 1997;158:4229–4236. [PubMed] [Google Scholar]
- 34.Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojcius DM, Bravo de Alba Y, Kanellopoulos JM, Hawkins RA, Kelly KA, Rank RG, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 36.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 37.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh C-S, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 38.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 40.Mbow ML, Zeidner N, Panella N, Titus RG, Piesman J. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect Immun. 1997;65:3386–3390. doi: 10.1128/iai.65.8.3386-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brossart P, Goldrath AW, Butz EA, Martin S, Bevan MJ. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–3276. [PubMed] [Google Scholar]
- 42.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosisinfection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 43.Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeriain SCID and C.B-17 mice. Reversal by IFN-γ. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 44.Mattner F, Di Padova K, Alber G. Interleukin-12 is indispensable for protective immunity against Leishmania major. . Infect Immun. 1997;65:4378–4383. doi: 10.1128/iai.65.11.4378-4383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. . J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 46.Afonso LCC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. . Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 47.Miller MA, Skeen MJ, Ziegler HK. Nonviable bacterial antigens administered with IL-12 generate antigen-specific T cell responses and protective immunity against Listeria monocytogenes. . J Immunol. 1995;155:4817–4828. [PubMed] [Google Scholar]
- 48.Miller MA, Skeen MJ, Ziegler HK. A synthetic peptide administered with IL-12 elicits immunity to Listeria monocytogenes. . J Immunol. 1997;159:3675–3679. [PubMed] [Google Scholar]
- 49.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 50.Barratt-Boyes SM, Watkins SC, Finn OJ. In vivo migration of dendritic cells differentiated in vitro. A chimpanzee model. J Immunol. 1997;158:4543–4547. [PubMed] [Google Scholar]
- 51.Grouard G, Durand I, Filgueira L, Banchereau J, Liu Y-J. Dendritic cells capable of stimulating T cells in germinal centres. Nature. 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 52.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatisinduces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg E, Billingsley J, Caliendo A, Boswell S, Sax P, Kalams S, Walker B. Vigorous HIV-1-specific CD4+T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]