Abstract
Paired immunoglobulin-like receptor (PIR)-A and PIR-B possess similar ectodomains with six immunoglobulin-like loops, but have distinct transmembrane and cytoplasmic domains. PIR-B bears immunoreceptor tyrosine-based inhibitory motif (ITIM) sequences in its cytoplasmic domain that recruit Src homology (SH)2 domain–containing tyrosine phosphatases SHP-1 and SHP-2, leading to inhibition of B and mast cell activation. In contrast, the PIR-A protein has a charged Arg residue in its transmembrane region and a short cytoplasmic domain that lacks ITIM sequences. Here we show that Fc receptor γ chain, containing an immunoreceptor tyrosine-based activation motif (ITAM), associates with PIR-A. Cross-linking of this PIR-A complex results in mast cell activation such as calcium mobilization in an ITAM-dependent manner. Thus, our data provide evidence for the existence of two opposite signaling pathways upon PIR aggregation. PIR-A induces the stimulatory signal by using ITAM in the associated γ chain, whereas PIR-B mediates the inhibitory signal through its ITIMs.
Keywords: activation signal, Fc receptor γ chain, immunoreceptor tyrosine-based activation motif, mast cell, paired immunoglobulin-like receptor A
Cross-linking of immune receptors such as BCR, TCR, or FcR on a variety of cells leads to their activation through the sequential activation of protein tyrosine kinases (PTKs) (1–3). Several features have emerged that are common to these activating receptors. They are all oligomeric complexes in which ligand binding and signal transduction are compartmentalized into distinct receptor subunits. Hence, these receptors comprise one or more immunoreceptor tyrosine-based activation motif (ITAM)- containing subunits. When the ligand-binding subunit(s) of the receptor is engaged, the cytoplasmic ITAMs are tyrosine phosphorylated by src-family PTKs. This leads to the recruitment of syk-family PTKs, which trigger a cascade of intracellular phosphorylations that result in cellular activation.
Balancing these activation responses are the inhibitory receptors and their associated signaling molecules, which are responsible for setting threshold levels for activation signals as well as terminating activation responses. These inhibitory receptors also share several common features. They have one or more immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic domains, tyrosine phosphorylation of which leads to the recruitment of signaling molecules capable of inhibiting cell activation (4– 7). Indeed, NK inhibitory receptors recruit tyrosine phosphatase SHP-1 to inhibit NK cell activation (8–10). Certain isoforms of NK receptors that lack ITIM sequences have been proposed to function as activation receptors rather than inhibitory receptors (11). A conspicuous feature of these noninhibitory NK receptors is the presence of a basic amino acid in the transmembrane domain, which may allow their association with signal-transducing proteins such as DAP12, as demonstrated recently for killer-activating receptors (12, 13).
The paired Ig-like receptors (PIRs) recently identified on B cells and myeloid lineage cells include PIR-A molecule, which has a short cytoplasmic domain, and PIR-B molecule, which bears four potential ITIMs in its cytoplasmic domain (14, 15). In contrast to the unique transmembrane and cytoplasmic domains between PIR-A and PIR-B, extracellular regions of these molecules are very homologous, suggesting that both molecules bind the putative common ligand. We and others have recently demonstrated that PIR-B functions as an inhibitory receptor in B and mast cells (16, 17). Here, we show that PIR-A functions as an activation receptor as a consequence of its association with the ITAM-bearing FcRγ chain in mast cells.
Materials and Methods
Cells, Expression Constructs, and Abs.
Chicken DT40 cells and mouse A20 IIA1.6 cells (FcγRII-negative A20 derivative) were maintained in RPMI 1640 supplemented with 10% FCS, penicillin, streptomycin, glutamine, and 50 μM 2-ME. RBL-2H3 cells (provided by N. Yamamoto and T. Yasuda, Bayer Yakuhin, Ltd., Kyoto, Japan) and 293 T cells were cultured in MEM and DMEM, respectively, instead of RPMI 1640. FcγRIII–PIR-A chimera, mutated FcRγ chain (18), and hemagglutinin (HA)- tagged PIR-A were created by the PCR method and subcloned into the pApuro (19), pAneo (20), and pApuro (19) vectors, respectively. FcγRIII–PIR-A chimera contains the extracellular domain of human FcγRIIIAα (21) attached directly to the transmembrane and cytoplasmic regions of PIR-A (15). Resulting constructs were confirmed by DNA sequencing. These expression constructs were transfected into DT40, A20 IIA1.6, and RBL-2H3 cells by electroporation, and selected in the presence of puromycin (0.5 μg/ml) or G418 (2 mg/ml). Cell surface expression levels of FcγRIII–PIR-A were checked by flow cytometric analysis using anti–human FcγRIII mAb, 3G8 (22), and expression level of FcRγ chain was analyzed by Western blotting using anti-γ chain Ab (23). The calcium phosphate precipitation method was used for transfection into 293 T cells. Biotin-tagged 3G8 mAb, streptavidin, antiphosphotyrosine mAb (4G10), F(ab′)2 rabbit anti–mouse IgG, and anti-HA epitope mAb were purchased from PharMingen (San Diego, CA), Vector Labs. (Burlingame, CA), Upstate Biotechnology, Inc. (Lake Placid, NY), Chemicon International, Inc. (Temecula, CA), and Boehringer Mannheim (Tokyo, Japan), respectively.
Immunoprecipitation and Western Blotting Analysis.
To detect association of γ chain with FcγRIII–PIR-A, cells were solubilized in 1% digitonin buffer (1% digitonin, 0.12% Triton X-100, 150 mM NaCl, and 20 mM triethanolamine, pH 7.8). Cell lysates were sequentially incubated with mAb 3G8 or anti-HA mAb, and then with anti–mouse IgG agarose. For detection of tyrosine phosphorylation of γ chain, stimulated cells were solubilized in 1% NP-40 buffer (1% NP-40, 150 mM NaCl, 1 mM EDTA, and 20 mM Tris, pH 7.5). Cell lysates were sequentially incubated with anti–γ chain Ab and protein A agarose. Protease inhibitors and phosphatase inhibitors were added to both digitonin and NP-40 buffer as previously described (20). Immunoprecipitates were separated by SDS-PAGE gel, transferred to nitrocellulose membrane, and detected by appropriate Abs and ECL system (Amersham Pharmacia Biotech, Piscataway, NJ).
Northern Analysis.
RNA was prepared from A20 IIA1.6 and RBL-2H3 cells using the guanidium thiocyanate method. Total RNA (20 μg) was separated in a 1.2% formaldehyde gel, transferred to Hybond-N+ nylon membrane (Amersham Pharmacia Biotech), and probed with 32P-labeled FcRγ chain cDNA.
Calcium Measurements.
Cells (5 × 106) were suspended in PBS containing 20 mM Hepes (pH 7.2), 5 mM glucose, 0.025% BSA, and 1 mM CaCl2, and loaded with 3 μM Fura-2/AM at 37°C for 45 min. Cells were washed twice and adjusted to 106 cells/ml. Continuous monitoring of fluorescence from the cell suspension was performed using Hitachi F-2000 fluorescence spectrophotometer (Hitachi Limited, Tokyo, Japan) at an excitation wavelength of 340 nm and an emission wavelength of 510 nm. Calibration and calculation of calcium levels were done as previously described (24). FcγRIII–PIR-A on RBL-2H3 and DT40 cells was stimulated with anti-FcγRIII mAb (3G8, 10 μg/ml) and was subsequently cross-linked with F(ab′)2 anti–mouse IgG (25 μg/ml). Biotin-tagged 3G8 (10 μg/ml) and streptavidin (25 μg/ml) were used for stimulation of FcγRIII–PIR-A on A20 IIA1.6 cells.
Results and Discussion
PIR-A Is Capable of Activating Mast Cells.
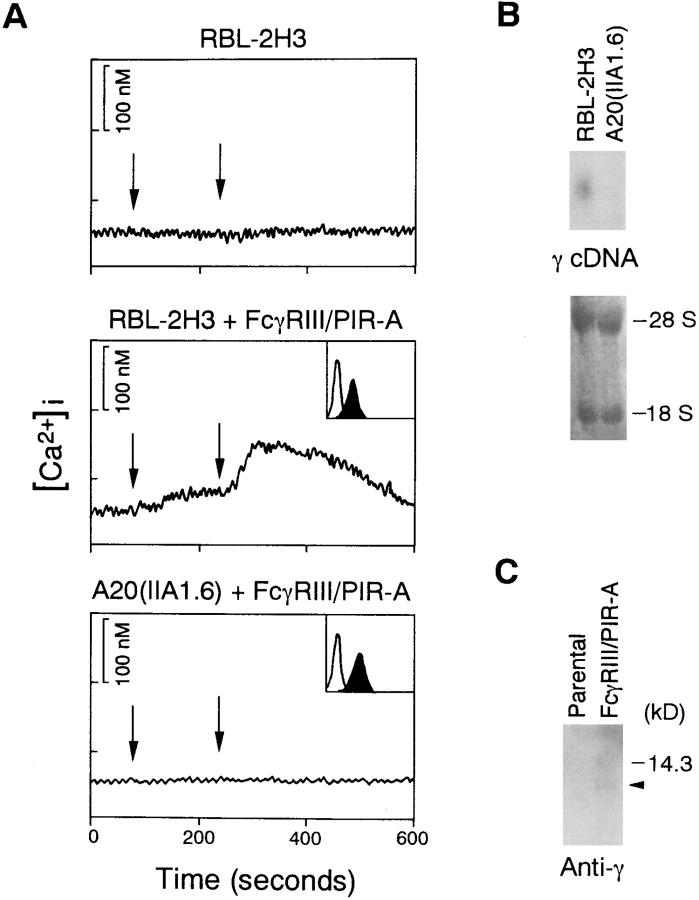
Structural features of PIR-A have prompted us to test whether PIR-A is able to transmit activation signals. For this purpose, a chimeric molecule with the transmembrane and cytoplasmic domains of PIR-A and the extracellular domain of human FcγRIII was constructed (FcγRIII–PIR-A), and transfected into mouse A20 IIA1.6 B cells and rat RBL-2H3 basophilic leukemia cells in order to obtain transformants. Expression level of this chimeric receptor was assessed by flow cytometric analysis using anti–human FcγRIII mAb, 3G8 (Fig. 1 A, insets). As shown in Fig. 1 A, stimulation of FcγRIII– PIR-A chimeric receptor on RBL-2H3 cells with 3G8 and subsequent cross-linking resulted in a transient rise in [Ca2+]i, whereas this [Ca2+]i increase could not be detected in the A20 IIA1.6 transformant expressing FcγRIII–PIR-A, demonstrating that FcγRIII–PIR-A is able to evoke the activation signal only in RBL-2H3 cells. Thus, these data suggest that FcγRIII–PIR-A is not capable of transmitting the activation signal by itself and that the responsible molecule(s) for the activation signal is expressed in RBL-2H3 cell but not in A20 IIA1.6 cells. One such candidate is the FcRγ chain, which is one component of the FcγRIII and FcεRI complexes in mast cells (25). Indeed, the γ chain is expressed in RBL-2H3 cells but not A20 IIA1.6 cells, as revealed by Northern (Fig. 1 B) and Western blotting analysis (data not shown).
Figure 1.
FcγRIII–PIR-A induces calcium mobilization and associates with FcRγ chain in RBL-2H3 cells. (A) Calcium mobilization by cross-linking of FcγRIII–PIR-A. Left arrows indicate application of 3G8 or biotin-tagged 3G8, and right arrows indicate application of F(ab′)2 anti– mouse IgG or streptavidin in RBL-2H3 or A20 IIA1.6 cells, respectively. Surface expression levels of FcγRIII–PIR-A are indicated in inset boxes. The x and y axes for the histograms indicate fluorescence intensity (log scales) and relative cell number, respectively. (B) Northern blot analysis using mouse γ cDNA as a probe. Bottom panel shows 28S and 18S ribosomal RNA pattern. (C) Association of FcγRIII–PIR-A with γ in RBL-2H3 cells. Immunoprecipitates with 3G8 were separated by 15% SDS-PAGE gel and detected by anti-γ Ab.
FcRγ Chain Associates with FcγRIII–PIR-A.
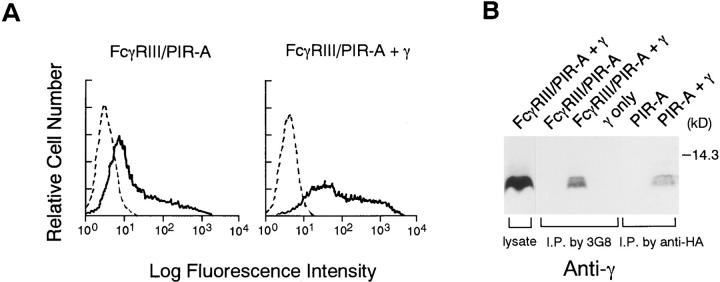
To demonstrate that γ chain is associated with FcγRIII–PIR-A, RBL-2H3 cells expressing this chimeric receptor were lysed by digitonin buffer, followed by immunoprecipitation with 3G8. The immune complexes were separated by SDS-PAGE gel and blotted with anti-γ chain Ab, demonstrating the association of γ chain with FcγRIII–PIR-A in RBL-2H3 cells (Fig. 1 C). This association was also shown by transfection experiments using 293 T cells. FcγRIII–PIR-A by itself can be expressed on the cell surface of 293 T cells to some extent, whereas cotransfection of γ with FcγRIII– PIR-A enhanced cell surface expression of this chimeric receptor (Fig. 2 A), suggesting that γ chain is involved in transport of FcγRIII–PIR-A to cell surface in 293 T cells. Moreover, the interaction of γ chain with FcγRIII–PIR-A was reconstituted in 293 T cells, as demonstrated by coimmunoprecipitation experiments using digitonin buffer (Fig. 2 B). This association was also observed by cotransfection of γ with HA-tagged PIR-A (Fig. 2 B), indicating that native PIR-A can be associated with FcRγ chain.
Figure 2.
Association of FcγRIII–PIR-A with γ chain in 293 T cells. (A) FACS® (Becton Dickinson, San Jose, CA) analysis of 293 T cells transiently expressing FcγRIII– PIR-A in the presence or absence of γ. Cells were labeled with FITC-conjugated 3G8. Nontransfected 293 T cells were used as a negative control. (B) Association was detected as described in legend to Fig. 1. In the case of association of HA-tagged PIR-A with γ, anti-HA mAb was used for immunoprecipitation instead of 3G8.
FcRγ Chain Is Involved in Transmitting Activation Signals through FcγRIII–PIR-A Complex by ITAM-dependent Mechanism.
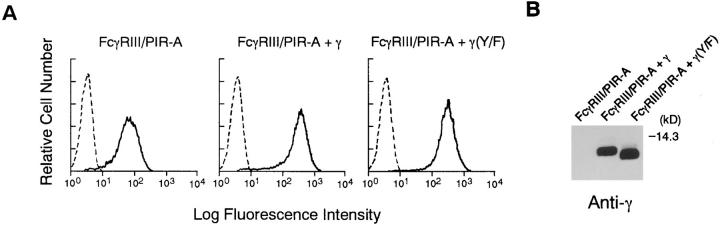
To show that the association of γ chain with FcγRIII– PIR-A is critical for transmitting activation signals, we used the DT40 B cell system, since this cell line does not express γ chain like A20 cells. After obtaining DT40 transformant expressing FcγRIII–PIR-A (Fig. 3 A), we transfected wild-type γ chain or mutated γ chain into this DT40 transformant using another drug selection marker to isolate DT40 cells expressing both FcγRIII–PIR-A and γ chain. Mutation of the γ chain (Tyr65 and Tyr76 to Phe) was designed to test whether the activation signal is dependent on the ITAM sequence located in the cytoplasmic domain of γ chain. Expression level of γ chain was assessed by Western blotting analysis with anti–γ chain Ab, and clones expressing comparable levels of wild-type γ and mutated γ were selected (Fig. 3 B) and analyzed. Although FcγRIII– PIR-A alone was expressed on the cell surface of DT40 B cells, introduction of either wild-type γ chain or mutated γ chain enhanced the level of cell surface expression of the chimeric receptor about fivefold, as demonstrated by flow cytometric analysis (Fig. 3 A). These observations strengthen our previous conclusion that γ chain contributes to cell surface expression of FcγRIII–PIR-A. However, this γ chain function does not require phosphorylation of tyrosine residues in its ITAM sequence.
Figure 3.
Expression of FcγRIII–PIR-A and γ in DT40 transformants. (A) FACS® analysis of DT40 cells by using FITC-conjugated 3G8. Parental DT40 cells were used as a negative control. (B) Expression of γ by Western blot analysis using anti-γ Ab. Transformant cells were directly dissolved in SDS-sample buffer.
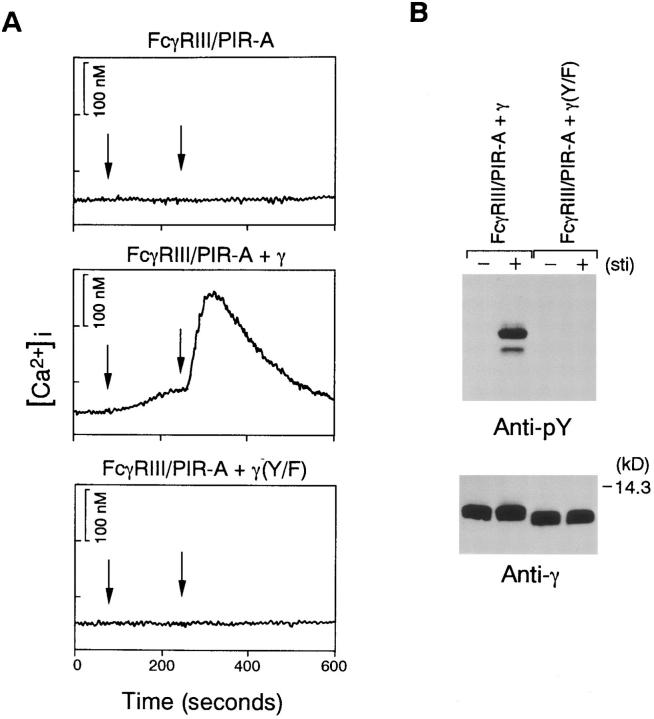
Stimulation of FcγRIII–PIR-A complex with wild-type γ chain evoked calcium mobilization upon receptor engagement, whereas this [Ca2+]i increase could not be detected in DT40 cells expressing FcγRIII–PIR-A alone nor in the receptor with mutated γ chain (Fig. 4 A), indicating that ITAM of γ chain is essential for transmitting the activation signal. This conclusion was supported further by the observation that γ chain, but not mutated γ chain, is tyrosine phosphorylated by FcγRIII–PIR-A stimulation (Fig. 4 B). Taken together, our results demonstrate that FcγRIII– PIR-A is complexed with FcRγ chain, the presence of which is essential for transmitting activation signals.
Figure 4.
The FcγRIII–PIR-A complex transmits activation signals in the presence of γ. (A) Calcium mobilization by cross-linking of FcγRIII– PIR-A. Transformants shown in Fig. 3 were stimulated by 3G8 (left arrows) and F(ab′)2 anti–mouse IgG (right arrows). (B) Tyrosine phosphorylation of γ by cross-linking of FcγRIII–PIR-A. Transformant cells were stimulated with 3G8 (3 min), and then F(ab′)2 anti–mouse IgG (3 min). Cells were dissolved by NP-40 buffer, immunoprecipitated by anti-γ Ab, separated by 15% SDS-PAGE gel, and probed with antiphosphotyrosine mAb 4G10. Membrane was reprobed with anti-γ Ab (bottom).
Coexistence of both activation and inhibitory pathways upon receptor aggregation allows immune cells to generate graded responses under different ligand conditions. Assuming that PIR-A and PIR-B recognize the same ligand, the data presented here together with previous observations (16, 17), support the model that cell surface receptors with same or related ligand-binding specificity transmit both activation and inhibitory signals through ITAM and ITIM sequences, respectively (26). Reminiscent of this type of regulation is Fc receptors; FcγRIIIA transmits the activation signal, whereas FcγRIIB inhibits the ITAM-induced activation signal despite the same ligand specificity between these two receptors (25).
FcRγ chain is physically and functionally associated with FcγRIII–PIR-A. Since the γ chain has a very short extracellular domain (18), this association is presumably mediated by transmembrane–transmembrane interactions. Supporting this notion, the transmembrane region of another γ chain–interacting receptor, FcαR, is very similar to that of PIR-A in that positively charged Arg residue is located in the same position, although their extracellular and cytoplasmic domains are divergent (27). Given the evidence that HA-tagged PIR-A is also associated with γ chain (Fig. 2 B), our data strongly suggest that native PIR-A associates with FcRγ chain in mast cells.
Functional interaction was demonstrated by requirement of γ chain in FcγRIII–PIR-A-induced calcium mobilization. More importantly, ITAM of the γ chain was tyrosine phosphorylated upon receptor aggregation (Fig. 4 B), indicating that the PIR-A complex transmits the activation signal through an ITAM-dependent mechanism. Thus, PIR-A uses src- and syk-family PTKs to transmit the positive signal, in a similar manner to the situation with other ITAM-bearing receptors such as TCR, BCR, or FcRs, whereas PIR-B mediates the negative signal through recruitment of SHP-1 and SHP-2 to phosphorylated ITIMs in its cytoplasmic domain.
Both PIR-A and PIR-B appear to be expressed in B cells and myeloid lineage cells, as assessed by reverse transcriptase PCR (15). The RNA content of PIR-A and PIR-B may not necessarily reflect the cell surface expression of each molecule, because associated molecules such as γ chain may enhance the transport of PIR-A to cell surface, as seen in this study. Since the relative expression level of PIR-A and PIR-B on cell surface is one of the critical determinants for stimulatory or inhibitory responses, associated chains such as γ participate in PIR functions by not only conferring the stimulatory capability on PIR-A but also promoting its cell surface expression.
Acknowledgments
We would like to thank N. Yamamoto and T. Yasuda for providing us with RBL-2H3 cells and Dr. T. Takai (Tohoku University, Sendai, Japan) for communicating his unpublished results.
This work was supported by grants to T. Kurosaki from the Ministry of Education, Science, Sports, and Culture of Japan, the Sumitomo Foundation, and the Uehara Memorial Foundation.
Footnotes
Note added in proof. The results that PIR-A is able to transmit a stimulatory signal have been obtained independently (Yamashita, Y., M. Ono, and T. Takai. J. Immunol. In press).
References
- 1.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 2.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 3.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 4.Cambier JC. Inhibitory receptors abound? . Proc Natl Acad Sci USA. 1997;94:5993–5995. doi: 10.1073/pnas.94.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama WM. What goes up must come down: the emerging spectrum of inhibitory receptors. J Exp Med. 1997;186:1803–1808. doi: 10.1084/jem.186.11.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Daëron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 8.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitory receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL. Natural killer cell receptors and MHC class I interactions. Curr Opin Immunol. 1997;9:126–131. doi: 10.1016/s0952-7915(97)80169-0. [DOI] [PubMed] [Google Scholar]
- 10.Moretta A, Moretta L. HLA class I specific inhibitory receptors. Curr Opin Immunol. 1997;9:694–701. doi: 10.1016/s0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 11.Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, Vitale M, Conte R, Poggi A, Moretta A, Moretta L. The human leukocyte antigen (HLA)-C–specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645–650. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olcese L, Cambiaggi A, Semenzato G, Bottino C, Moretta A, Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 13.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 14.Hayami K, Fukuta D, Nishikawa Y, Yamashita Y, Inui M, Ohyama Y, Hikita M, Ohmori H, Takai T. Molecular cloning of a novel murine cell-surface glycoprotein homologous to killer cell inhibitory receptors. J Biol Chem. 1997;272:7320–7327. doi: 10.1074/jbc.272.11.7320. [DOI] [PubMed] [Google Scholar]
- 15.Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. doi: 10.1073/pnas.94.10.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bléry M, Kubagawa H, Chen C-C, Vély F, Cooper MD, Vivier E. The paired Ig-like receptor PIR-B is an inhibitory receptor that recruits the protein-tyrosine phosphatase SHP-1. Proc Natl Acad Sci USA. 1998;95:2446–2451. doi: 10.1073/pnas.95.5.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of tyrosine phosphatase SHP-1 and SHP-2 for PIR-B–mediated inhibitory signal. J Exp Med. 1998;187:1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ra C, Jouvin M-H, Kinet JP. Complete structure of the mouse mast cell receptor for IgE (FcεRI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J Biol Chem. 1989;264:15323–15327. [PubMed] [Google Scholar]
- 19.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor–coupled Ca2+mobilization through distinct pathways. EMBO (Eur Mol Biol Organ) J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO (Eur Mol Biol Organ) J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravetch JV, Perussia B. Alternative membrane forms of FcγRIII (CD16) on human natural killer cells and neutrophils: cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleit HB, Wright SL, Unkeless JC. Human neutrophil Fcγ receptor distribution and structure. Proc Natl Acad Sci USA. 1982;79:3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurosaki T, Gander I, Ravetch JV. A subunit common to an IgG Fc receptor and the T-cell receptor mediates assembly through different interactions. Proc Natl Acad Sci USA. 1991;88:3837–3841. doi: 10.1073/pnas.88.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 25.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 26.Colonna M. Unmasking the killer's accomplice. Nature. 1998;391:642–643. doi: 10.1038/35515. [DOI] [PubMed] [Google Scholar]
- 27.Morton HC, van den Herik-Oudijk IE, Vossebeld P, Snijders A, Verhoeven AJ, Capel PJA, van de Winkel JGJ. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR γ chain. J Biol Chem. 1995;270:29781–29787. doi: 10.1074/jbc.270.50.29781. [DOI] [PubMed] [Google Scholar]