Abstract
The tyrosine kinase p56lck regulates the differentiation and proliferative expansion of pre-T cells. However, nothing is known about other signaling molecules that operate with p56lck to mediate the pleiotropic changes that occur at this stage of thymocyte development. We used a genetic strategy to examine the requirement for the GTPase Rho in p56lck-mediated signals in the thymus. By generating mice double transgenic for a constitutively activated form of p56lck (p56lckF505) and the Rho inhibitor C3 transferase we were able to compare thymocyte development in mice expressing active p56lck on a wild-type or Rho− background. Thymocytes expressing active p56lck show enhanced proliferation of pre-T cells resulting in increased numbers of late pre-T cells, however, this dramatic effect on pre-T cell proliferation is lost when the p56lck transgene is expressed in thymocytes lacking endogenous Rho GTPase function. Expression of active p56lck also generates double positive (DP) thymocytes with low levels of CD2 antigen expression. Again, p56lck cannot prevent expression of CD2 when expressed on a Rho− background. CD4+CD8+ DP cells expressing active p56lck have been shown to lack functional α/β–T cell receptor (TCR) complexes due to p56lck-mediated inhibition of TCR gene Vβ-Dβ rearrangement. This inhibition of TCR expression by active p56lck is unimpaired in the absence of Rho function. The signaling pathways that are mediated by p56lck and control thymocyte proliferation, α/β-TCR and CD2 antigen expression can thus be distinguished by their dependency on Rho function.
Keywords: pre-T cell, p56lck, Rho, signaling, development
Lymphocyte development in the thymus is dependent on signaling pathways generated by antigen receptors and cytokine receptors (1, 2). Different developmental stages within the thymus can be defined by expression of CD4 and CD8 molecules which act as receptors for MHC class II and class I molecules, respectively. The earliest T cell progenitors are found within the CD4−CD8− double negative (DN)1 population that comprises 2–3% of the total thymocyte population. These progenitor cells can be further subdivided into four developmental stages on the basis of expression of CD44 and CD25 molecules: the earliest progenitors are CD44+CD25−, followed sequentially by CD44+CD25+, CD44−CD25+, and CD44−CD25− stages. At the CD44−CD25+ stage, cells that successfully rearrange their TCR-β locus proliferate rapidly, downregulate CD25 and differentiate into CD4+CD8+ double positive (DP) cells. The process of allelic exclusion arrests further rearrangement at the TCR-β locus and ensures the generation of DP thymocytes with a single productive TCR-β chain. DP cells then undergo TCR-α chain rearrangements and upon expression of a functional α/β-TCR complex are subjected to the processes of positive and negative selection that ensures the generation of CD4+CD8− or CD4−CD8+ single positive (SP) thymocytes that exit to the periphery. The transition of cells from the CD44−CD25+ to the CD44−CD25− compartment is a critical checkpoint for thymocyte differentiation and is controlled by signals generated by the pre-TCR complex. Mice lacking expression of any of the components of this complex such as the CD3ε subunit (3), the TCR-β chain (4), or the pTα subunit (5) show a developmental block at the CD44−CD25+ stage.
The signal transduction pathways used by the pre-TCR to control T cell differentiation involve the activation of protein tyrosine kinases. Mouse strains with null mutations in the tyrosine kinases p56lck (6), ZAP70, and Syk (7), or their regulatory molecules such as CD45 (8) show defects at the pre-T cell stage of development. Several other studies in particular highlight the importance of the tyrosine kinase p56lck in the transmission of pre-TCR–mediated signals. Mice lacking p56lck or overexpressing a dominant inhibitory p56lck mutant (6, 9) show a defect in early thymopoiesis similar to that seen in pTα or TCR-β chain–deficient mice. Additionally, transgenic expression of a constitutively active form of p56lck can induce the transition of thymocyte progenitors from the CD44−CD25+ to the CD44−CD25− compartment and induce the generation of DP thymocytes in mice lacking expression of the pre-TCR complex, e.g., in RAG−/− or pTα−/− mice (10, 11). This indicates that activated p56lck is sufficient to initiate all the critical events that occur as CD44−CD25+ early pre-T cells develop into CD44−CD25− late pre-T cells: the onset of rapid cell cycle progression, allelic exclusion at the TCR-β chain locus and ultimately expression of CD4 and CD8.
We recently generated mice that lack function of the GTPase Rho in thymocytes due to tissue-specific transgenic expression of a Rho inhibitor, C3 transferase, which selectively ADP-ribosylates Rho and thereby abolishes its biological activity (12). The phenotype of these mice indicated that Rho might also be important for pre-TCR function (13). Mice lacking Rho function in the thymus were able to generate DP and SP thymocytes, but at severely reduced levels. Loss of Rho function resulted in decreased cell proliferation in CD44−CD25− late pre-T cells due to a partial block in G1/S cell cycle progression. This stage of thymocyte development is regulated by p56lck which raises questions about the positioning of Rho in the context of the p56lck signals that control the proliferation of early thymocyte progenitors. In fact, very little is known about the signaling pathways downstream of p56lck that control pre-T cell differentiation. Given the involvement of both p56lck and the GTPase Rho in the regulation of proliferation in late pre-T cells we considered it possible that Rho might function in the signaling pathways used by p56lck to regulate the checkpoints for proliferation and differentiation at the pre-T cells stage of thymocyte development. To explore these questions, we generated mice double transgenic for active p56lck (p56lckF505; reference 14) and C3 transferase (12) and examined the ability of active p56lck to drive thymocyte proliferation and differentiation in the absence of Rho function. Mice expressing active p56lck show accelerated pre-T cell proliferation and generate CD4+CD8+ DP thymocytes that fail to express α/β-TCR complexes via a process that mimics allelic exclusion. The present study reveals that the proliferative signals generated by p56lck are suppressed in mice lacking thymic Rho function. In contrast the ability of active p56lck to suppress expression of α/β-TCR complexes is unimpaired. p56lck thus uses either qualitatively or quantitatively different signals to control thymocyte development at the pre-T cell stage, p56lck-mediated signaling pathways for proliferation are dependent on the Rho GTPase whereas signals for regulation of antigen receptor expression are not.
Materials and Methods
Transgenic Mice.
Transgenic mice expressing the C3 transferase transgene under the control of the proximal p56lck promoter were generated as previously described (12). Mice transgenic for an activated form of the tyrosine kinase p56lck (p56lckF505; strain pLGF-2964, 10 copies of p56lckF505 transgene) were provided by Dr. Roger M. Perlmutter (Merck Research Labs.; 14). Expression of this transgene is also driven by the proximal p56lck promoter, restricting expression of the transgene to thymocytes. Mice were kept under SPF conditions and bred to C57BL/6/J mice to maintain the transgenic lines. Mice double transgenic for C3 transferase and p56lckF505 (pLGF/C3) were generated by cross-breeding of the parental strains.
PCR.
Offspring were genotyped for the presence of both transgenes by PCR analysis of genomic mouse DNA using transgene specific primer pairs: b-actin (actin 1: 5′-GTGGCCATCTC-CTGCTCGAAGTC-3′ and actin 2: 5′-GTTTGAGACCTTCAACACCCC-3′); C3 transferase (G9112: 5′-GCCACCATGGAGCAGAAGCTGATCTCCG-3′ and C3NT: 5′-CTGATTTGCTTAGTCCATAC-3′), and p56lckF505 (pLGF-Fwd: 5′-ATGACTTCTTCACAGCCACAGAGGG-3′ and pLGF-Rev: 5′-TTTTATTAGGACAAGGCTGGTGGGC-3′).
PCR reactions were performed in 20 mM ammonium sulphate, 75 mM Tris-HCl, pH 9.0, 0.01% Tween 20, 1.2 mM MgCl2, 200 μM dNTP, 0.5 U Taq DNA polymerase, and 1 μM of each primer. Genomic DNA was purified from mouse ear clips and used as template for 30 cycles of polymerase chain reaction (57°C annealing, 72°C elongation, 95°C denaturation). PCR products were separated in 2% agarose gels and stained with ethidium bromide.
Thymocyte Preparation.
Young adult mice of ∼4–6 wk of age were typically analyzed. Thymocytes were obtained by carefully mincing the thymus with forceps and filtering through a fine mesh filter to obtain a single cell suspension. Total cell numbers were determined by counting of trypan blue stained thymocytes within a representative volume of cell suspension using a Neubauer hemocytometer.
Flow Cytometric Analysis.
Freshly isolated thymocytes were stained with saturating concentrations of antibody in 100 μl cold PBS supplemented with 1% BSA on ice for 30 min in a 96-well V-bottom shaped microtiter plate. Cells were washed twice in between incubations with this buffer. In a first step cells were incubated with anti-FcgRII mAb in order to block any unspecific staining. All mAb used were conjugated to either FITC, PE, or biotin. Biotinylated mAb were revealed using streptavidin-Tricolor (Caltag Laboratories, South San Francisco, CA). The following mAb were used (all PharMingen, San Diego, CA): CD8 (53-5.8), CD4 (RM4-5), CD25 (IL2R α chain, 3C7), CD3ε (145-2C11), α/β-TCR (β chain, H57-597), B220 (CD45R, RA3-6B2), CD44 (Pgp-1, IM7), CD2 (LFA-2, RM2-5), Mac-1 (α chain, M1/70), Gr-1 9 (Ly-6G, RB6-8C5), and NK (2B4, 129/J). Cells stained negative for CD4, CD8, CD3, B220, Gr-1, Mac-1, and the NK-cell marker 2B4 were labeled Lin−. Stained thymocytes were analyzed on a FACS® Calibur (Becton Dickinson & Co., Sparks, MD) using CellQuest software (Becton Dickinson & Co.). Viable cells were gated on the basis of forward and side light scatter.
Cell Cycle Analysis.
Cellular DNA content was assayed by standard techniques using staining with propidium iodide. In brief, thymocytes were stained with biotinylated mAb against CD4, CD8, CD3, B220, NK, Mac-1, Gr-1, and CD44 and surface staining revealed with FITC conjugated Avidin (Sigma Chemical Co., St. Louis, MO). Cells were fixed in 70% ethanol for 30 min on ice, washed free of ethanol, RNase (1 mg/ml) treated for 15 min at room temperature, and then resuspended in propidium iodide (50 ug/ml in PBS) in order to stain DNA. Maintaining a low flow rate events were collected and propidium iodide fluorescence was measured >600 nm on a FACS®Calibur (Becton Dickinson & Co.). DNA content of FITC-negative (CD44−Lin−) thymocytes was analyzed using a doublet discrimination module.
Results
Loss of Endogenous Rho Function Inhibits p56lck-mediated Proliferative Responses and Developmental Maturation in Pre-T Cells.
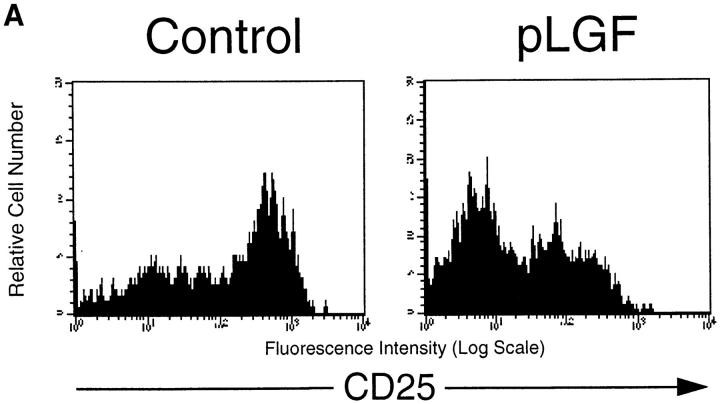
The developmental maturation of pre-T cells is associated with downregulation of the CD25 surface marker and the acquisition of CD4 and CD8 molecules (15). At this stage, only thymocytes that productively rearranged their TCR-β chain and express a complete pre-TCR complex are selected for further development and transition into the DP compartment, a process known as β-selection (16, 17). Current models propose that the pre-TCR complex triggers this maturation program via intracellular signaling pathways that involve activation of the tyrosine kinase p56lck (18). β-selection is bypassed in thymocytes that express a constitutive activated form of p56lck (19), resulting in differentiation and proliferation of all CD44−CD25+ pre-T cells, regardless of a productive or unproductive β chain rearrangement. Accordingly, pLGF mice expressing a constitutively active p56lck (p56lckF505) transgene in the thymus show enhanced generation of CD44−CD25− pre-T cells compared with normal control mice (10). Comparison of CD25 expression on CD44− triple negative (TN) thymocytes (CD44−CD4−CD8−CD3−) from pLGF mice with normal control mice shows how expression of activated p56lck results in an increased frequency of CD44−CD25− late pre-T cells and a concomitant low frequency of CD44−CD25+ early pre-T cells (Fig. 1 a).
Figure 1.
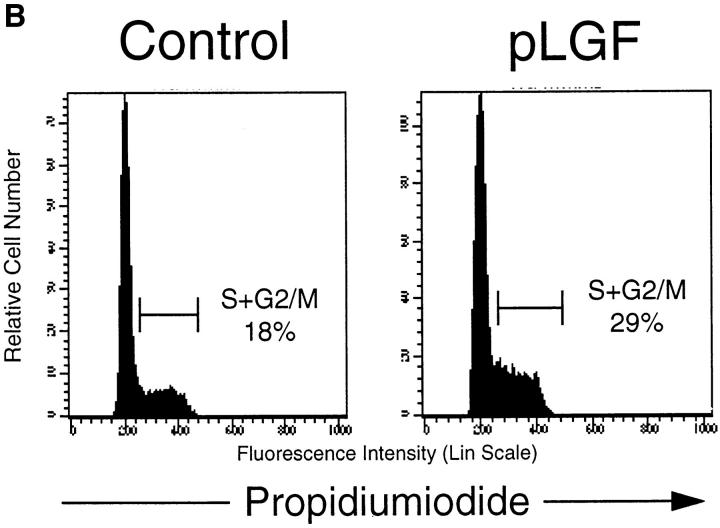
Transgenic expression of activated p56lck enhances differentiation and proliferation of pre-T cells. (A) CD25 expression profiles of CD44neg TN thymocytes in normal control mice and pLGF transgenic mice expressing active p56lck. Single cell suspensions of total thymocytes from normal control and pLGF transgenic mice were prepared and stained with mAb against a panel of mature lineage markers including CD4, CD8, CD3, B220, NK, Gr-1, Mac-1 (all biotinylated), CD44 (biotinylated), and CD25 (FITC). Cells negative for all lineage markers and CD44 were gated and examined for their CD25 expression profile. (B) Transgenic expression of active p56lck leads to increased levels of CD44neg TN thymocytes in S+G2/M phase of the cell cycle. Total thymocytes from normal and pLGF transgenic mice were prepared and stained with mAb against CD44 (biotinylated) and a panel of mature lineage markers including CD4, CD8, CD3, B220, NK, Gr-1, and Mac-1 (all biotinylated), fixed in 70% ethanol, and stained with propidium iodide (PI). Cells lacking expression of CD44 and all lineage markers were gated and then examined for their DNA profile.
Analysis of the cellular DNA content of pre-T cells from pLGF mice compared with normal control mice is shown in Fig. 1 b. We observed a marked increase of cells in the proliferative S and G2 phases of the cell cycle in CD44− TN thymocytes from pLGF mice when compared with cells of the same phenotype from normal control mice. Thymocyte-specific transgenic expression of activated p56lck thus accelerates proliferative responses in pre-T cells. The dramatic enhancement of the production of late pre-T cells by active p56lck is illustrated by a comparison of total cell numbers for this population in pLGF mice compared with control mice. A normal thymus contains an average number of CD25−CD44− late pre-T cells of 1.2 × 106, whereas in pLGF mice their numbers are typically increased to ∼6.0 × 106. There is thus a fivefold increase in total numbers of CD44−CD25− late pre-T cells in thymi from pLGF mice compared with thymi from normal control mice.
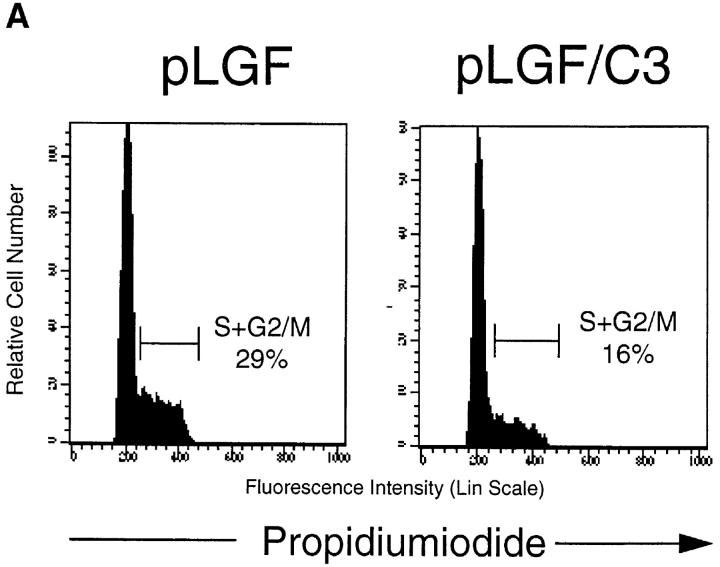
Proliferative responses in late pre-T cells are reduced in thymi lacking endogenous Rho function as a consequence of transgenic expression of C3 transferase (C3). These cells show a severe decrease in the production of late pre-T cells (12). To explore whether p56lck-mediated responses in early T cell development required endogenous Rho function we crossed pLGF mice (14) to transgenic mice with inactivated thymic Rho function (C3). We then used CD44 and CD25 markers to analyze early thymocyte progenitors in TN thymocytes of pLGF/C3 double transgenic mice. Total cell numbers and the cell cycle status of the CD44−25− pre-T cell compartment were monitored. The data in Fig. 2 a show that the increases in the percentage of cycling pre-T cells seen in pLGF mice are lost in pLGF/C3 double transgenic mice, i.e., loss of Rho function antagonizes the action of p56lck in driving cell cycle progression in pre-T cells. There are still some cycling cells in pre-T cells from pLGF/C3 double transgenic mice but the loss of Rho function appears to prevent the ability of activated p56lck to induce a hyperproliferative response in CD44−CD25− late pre-T cells.
Figure 2.
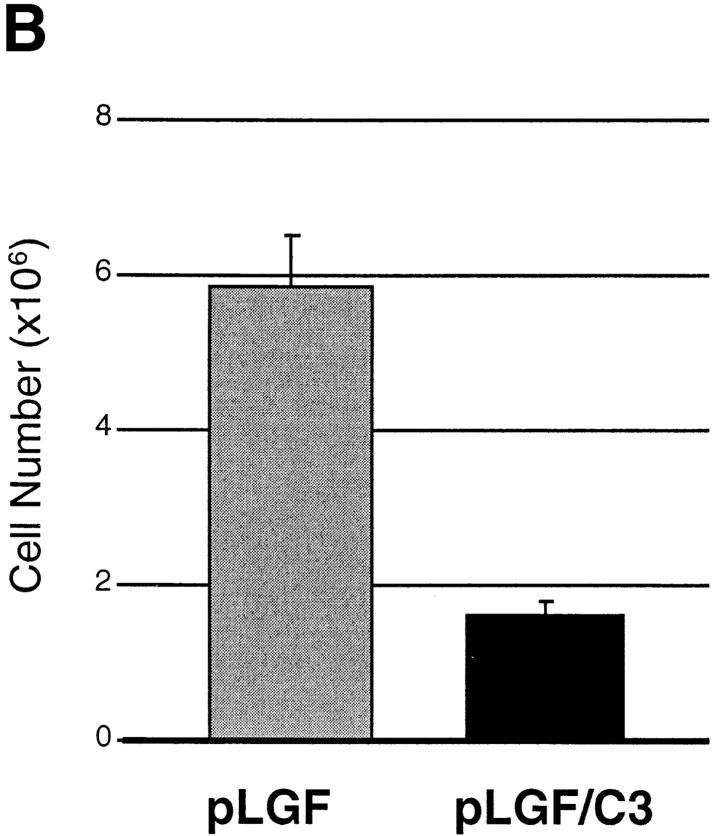
Inactivation of Rho function inhibits p56lck-initiated pre-T cell proliferation. (A) Inactivation of Rho function during early thymopoiesis prevents hyperproliferative responses in pre-T cells from pLGF transgenic mice. Flow cytometric analysis of cellular DNA content of CD44neg TN thymocytes from pLGF transgenic, and pLGF/C3 double transgenic mice as described above. (B) Loss of Rho function prevents the accumulation of CD25−CD44− pre-T cells in thymocytes expressing active p56lck. Total thymocytes from normal, pLGF transgenic and pLGF/C3 double transgenic mice were prepared and stained with mAb against a panel of mature lineage markers including CD4, CD8, CD3, B220, NK, Gr-1, Mac-1 (all biotinylated), CD25 (FITC), and CD44 (PE). Thymocytes negative for all lineage markers were gated and examined for their CD25/CD44 profiles. Absolute cell numbers in individual CD25/CD44 thymocyte subsets were calculated on the basis of average percentages obtained in these analyses and average numbers of TN thymocytes in pLGF and pLGF/C3 mice. Columns represent means of six mice analyzed, bars indicate standard deviations.
The most striking consequence of the decreased proliferative capacity of pre-T cells in pLGF/C3 double transgenic mice is presented in Fig. 2 b, which shows absolute numbers of CD44−CD25− late pre-T cells in pLGF and pLGF/C3 mice. The data reveal that in the absence of endogenous Rho function p56lck is unable to enhance the production of CD44−CD25− late pre-T cells. Thus, pLGF/C3 double transgenic mice do not show the skewed distribution of CD44−CD25− and CD44−CD25+ subpopulations seen in pLGF mice. These results indicate that Rho function is required for active p56lck to accelerate the transition of early thymocytes from the CD44−CD25+ to the CD44−CD25− compartment.
p56lck-mediated Inhibition of α/β-TCR Expression and Generation of CD4+ or CD8+ SP Thymocytes Is Not Rho Dependent.
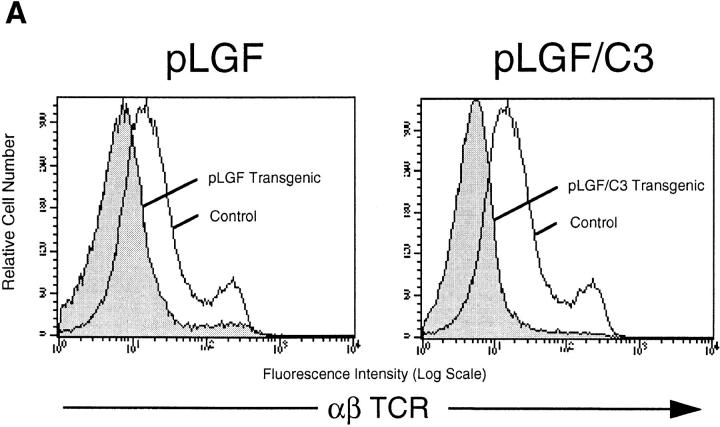
The consequence of a productive rearrangement of the TCR-β locus in thymocytes is expression of the pre-TCR complex and the initiation of the process of allelic exclusion that ensures that mature T cells express a TCR complex with only one β chain (20). p56lck function is required for this pre-TCR initiated response. Moreover, transgenic expression of activated p56lck inhibits V-DJ β-rearrangements and expression of the TCR-β chain in thymocytes. Consequently, DP thymocytes that develop in pLGF mice show defective expression of the TCR–CD3 complex (19). The data in the left panel of Fig. 3 a show α/β-TCR staining profiles of thymocytes from pLGF mice in comparison with thymocytes from control littermate mice. Normal thymocytes contain a large subpopulation of cells expressing intermediate levels of the TCR–CD3 complex and a smaller subset of cells with upregulated, higher levels of TCR expression. In contrast, thymocytes derived from pLGF mice show an abnormal α/β-TCR staining pattern with few intermediate or high α/β-TCR positive cells.
Figure 3.
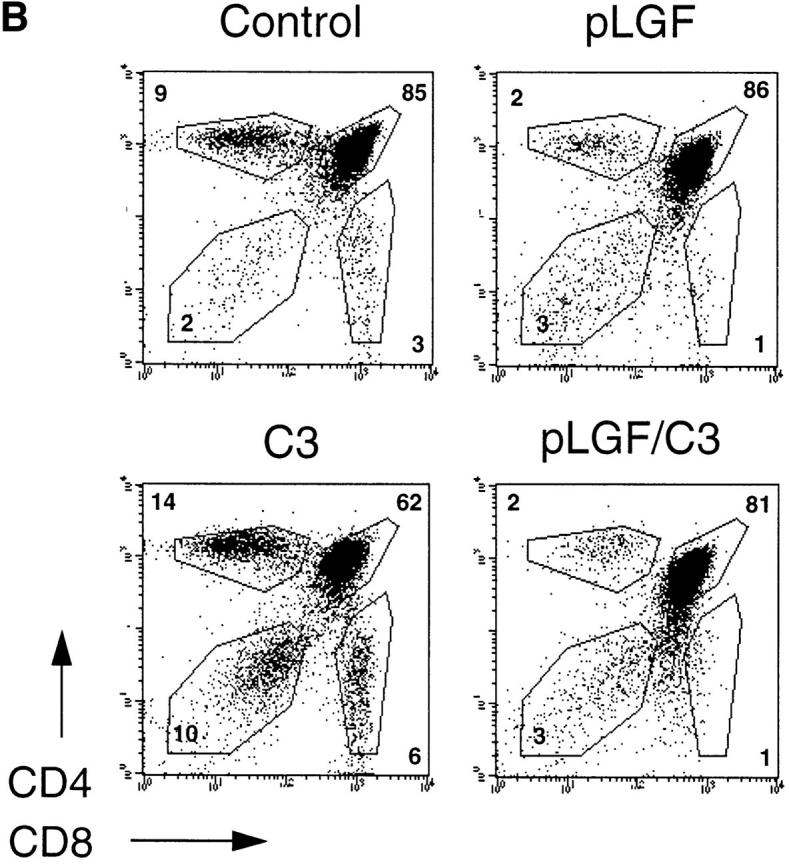
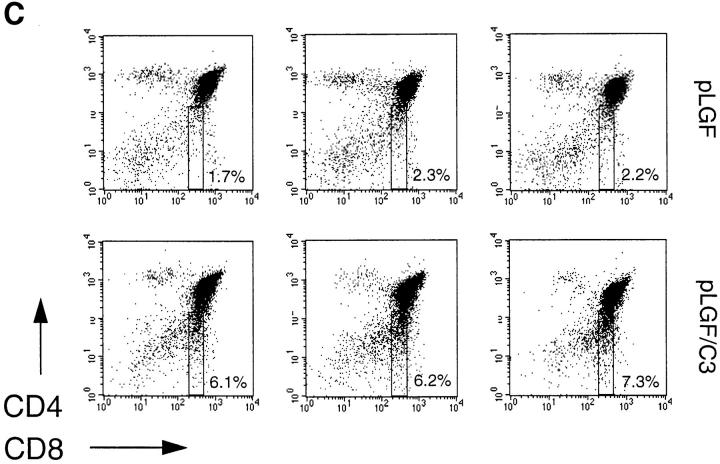
Failed expression of α/β-TCR complexes and development of mature SP cells in thymocytes expressing active p56lck is independent of endogenous Rho function. (A) α/β-TCR expression in normal and Rho− thymocytes expressing active p56lck. Total thymocytes from normal control mice, pLGF transgenic and pLGF/C3 double transgenic mice were stained for α/β-TCR expression (anti–TCR-β chain-FITC) and TCR expression profiles of transgenic mice (shaded) were compared with normal profiles (outline) by overlay. (B) Expression of CD4 and CD8 markers in normal and Rho− thymocytes expressing active p56lck. Representative dual parameter histograms for CD4 and CD8 expression of total thymocytes isolated from adult normal, C3 and pLGF transgenic and pLGF/C3 double transgenic mice. Percentages of individual CD4/CD8 subpopulations are indicated. Data shown are representative of four such experiments. (C) Frequencies of CD4−CD8low cells in normal and Rho− thymocytes expressing active p56lck. Three representative dual parameter histograms for CD4 and CD8 expression of total thymocytes isolated from adult pLGF transgenic and pLGF/C3 double transgenic mice are shown. Percentages of CD4−CD8low subpopulations are indicated.
If Rho function was required for p56lck-mediated suppression of TCR-β chain rearrangements then expression of activated p56lck in Rho− thymocytes would not prevent expression of the α/β-TCR complex. Thymocytes from pLGF/C3 double transgenic mice should then express a mature α/β-TCR complex, undergo positive selection, and generate mature SP thymocytes in the normal ratio. Conversely, if Rho function was not required for p56lck-mediated regulation of α/β-TCR expression then pLGF/ C3 thymocytes should show a pattern of TCR expression and CD4/CD8 differentiation like that seen in pLGF thymocytes that express active p56lck alone. Therefore, we analyzed expression of the TCR–CD3 complex and the development of CD4 and CD8 mature SP cells in thymocytes from pLGF/C3 double transgenic mice. As shown in the right panel of Fig. 3 a, pLGF/C3 thymocytes that express active p56lck on a Rho− background fail to normally express a mature TCR–CD3 antigen receptor complex.
The absence of a mature TCR–CD3 complex interrupts the normal processes of positive and negative selection and inhibits CD4 and CD8 SP development in the thymus of pLGF mice. As shown in the upper right panel of Fig. 3 b, thymocytes expressing active p56lck comprise CD4−CD8− DN and CD4+CD8+DP cells but abnormally low levels of CD4+ or CD8+ SP cells. We have shown previously that positive and negative selection processes of DP thymocytes occur undisturbed in the absence of Rho function (12). Adult thymi which lack endogenous Rho function show severe hypocellularity of only ∼5–10% of normal cell numbers but do contain DP and mature SP thymocytes with normal expression levels of the TCR–CD3 complex (Fig. 3 b, lower left). The lower right panel in Fig. 3 b shows the result of a representative analysis of CD4/CD8 subpopulations in pLGF/C3 thymocytes. The frequencies of DP and mature SP cells of thymocytes from pLGF/C3 double transgenic mice are identical to that of thymocytes from pLGF mice. The data in Fig. 3 thus show that introduction of an activated p56lck transgene into thymocytes lacking endogenous Rho function prevents the expression of the α/β-TCR complex and consequently, because positive selection can not occur, the generation of mature CD4 and CD8 SP thymocytes. In this context, the outcome of expression of activated p56lck in terms of inhibition of α/β-TCR complexes and suppression of the differentiation of CD4 and CD8 SP cells is the same in Rho− and normal thymocytes.
Immature CD8 single positive cells (ISP), distinguished by low levels of CD8 antigen expression but no expression of the TCR–CD3 complex, represent an intermediate stage in the transition from the CD4−CD8− DN to the CD4+CD8+ DP compartment (21, 22). These cells are not easy to discern in normal thymocytes but can accumulate in mice where there is a partial block or a delay in the DN to DP transition. For example, an accumulation of CD8lowCD3− ISPs was seen in mice lacking expression of the transcription factors Tcf-1 and Lef-1 (23). A consistent and reproducible observation was that thymocytes from pLGF/C3 double transgenic mice contained higher frequencies of CD8 low ISPs when compared with pLGF single transgenic mice (Fig. 3 c).
Activated p56lck Regulates Expression of CD2 by a Rho-dependent Pathway.
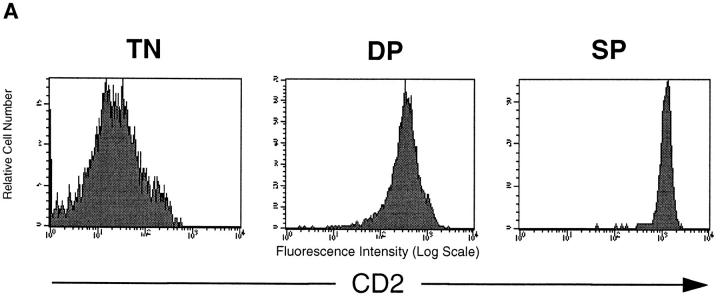
In normal thymocyte development pre-TCR–mediated downregulation of CD25 expression is accompanied by upregulation of the expression of the CD2 antigen (24, 25). The data in Fig. 4 a show CD2 expression of thymocytes isolated from a normal young C57/BL6 mouse. CD2 expression is found on CD4+CD8+ DP and SP thymocyte populations. There is a subtle but clear regulation of CD2 expression levels during normal thymocyte development in that SP thymocytes express about threefold higher levels of the CD2 antigen than DP thymocytes.
Figure 4.
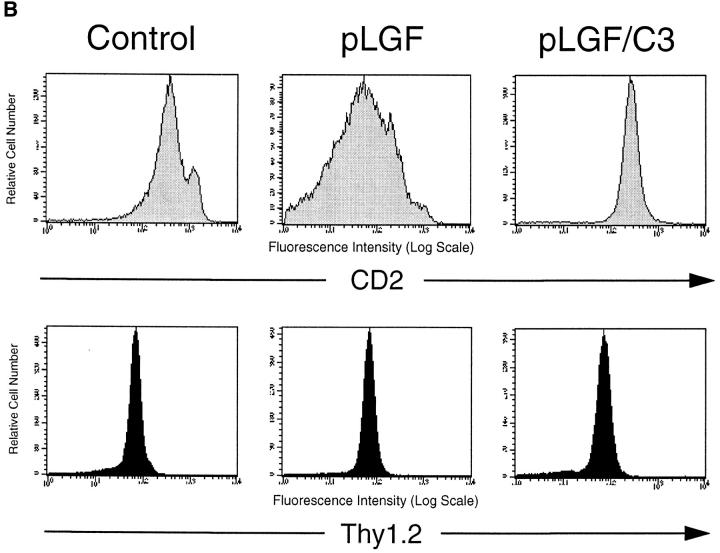
Thymocytes that express active p56lck show downregulation of CD2 antigen expression via a Rho dependent mechanism. (A) CD2 antigen expression in TN, DP, and SP subsets of normal mouse thymocytes. Normal thymocytes from C57/ BL6 mice were stained for expression of CD2 (PE), CD4 (FITC), and CD8 (biotinylated) or CD4, CD8, CD3 (all FITC), and CD2 (PE) and analyzed by flow cytometry. CD4−CD8−CD3− TN, CD4+CD8+ DP and CD4+ SP cells were gated and examined for CD2 expression. Mean fluorescence intensity for CD2 expression was 350 for DP thymocytes and 1,150 for SP thymocytes. (B) Expression of active p56lck results in downregulation of CD2 expression in normal thymocytes but not in thymocytes lacking endogenous Rho function. Total thymocytes from normal control mice, pLGF transgenic and pLGF/C3 double transgenic mice were stained for expression of CD2 (PE) and Thy1.2 (FITC) and analyzed by flow cytometry. Mean fluorescence intensity for CD2 expression in DP thymocytes from the individual mice were: 291 for controls, 108 for pLGF, and 275 for pLGF/C3.
Fig. 4 b shows a comparison of CD2 antigen expression on thymocytes from normal mice, pLGF transgenic and pLGF/C3 double transgenic mice. The representative profiles depicted here show that expression of active p56lck results in an about fourfold reduction in CD2 antigen expression levels compared with normal control thymocytes. Expression profiles of other T cell markers such as Thy-1.2 are normal in pLGF thymocytes (Fig. 4 b, lower panel). Similarly, levels of CD4 and CD8 on DP thymocytes from pLGF thymocytes are comparable to levels seen in normal control mice (Fig. 3 b). Expression of active p56lck thus selectively downregulates expression of CD2 antigen. In contrast, loss of Rho function during thymocyte development does not alter CD2 expression levels in adult thymocytes (13). To explore whether the downregulation of CD2 expression in pLGF thymocytes was a Rho dependent phenomenon we analyzed CD2 antigen levels on thymocytes from LGF/C3 double transgenic mice (Fig. 4 b, upper right panel). These experiments reveal normal levels of CD2 antigens on pLGF/C3 thymocytes. Thus, transgenic expression of active p56lck in the thymus interferes with the generation of normal levels of CD2. However, induced reduction of CD2 antigens occurs only in normal thymocytes but not in thymocytes lacking endogenous Rho function, p56lck downregulation of CD2 is thus a Rho-dependent response.
Discussion
The differentiation and proliferative expansion of pre-T cells is mediated via signaling pathways which involve activation of the tyrosine kinase p56lck (10, 11). However, nothing is known about other signaling molecules that operate with p56lck to mediate the complex molecular changes that occur at this stage of thymocyte development. To examine the role of the GTPase Rho in p56lck-mediated signals in the thymus, we generated mice double transgenic for active p56lck and the Rho inhibitor C3 transferase. This enabled us to compare thymocyte development in mice expressing active p56lck on a wild-type or Rho− background.
Mice with thymocyte-specific expression of a transgene encoding active p56lck show strong potentiation of proliferative responses in pre-T cells that results in a fivefold increase in numbers of CD44−CD25− late pre-T cells. This p56lck-mediated response is abrogated when the active p56lck transgene is expressed in thymocytes which lack endogenous Rho function. Rho function is thus required for p56lck to drive the differentiation of pre-T cells from the CD44−CD25+ into the CD44−CD25− compartment and to accelerate proliferative responses in this population. It has been shown that expression of active p56lck inhibits TCR-β chain rearrangements; DP thymocytes that develop in pLGF thymocytes fail to normally express α/β-TCR complexes (19). We now show that active p56lck can prevent expression of α/β-TCR complexes on DP thymocytes in the absence of endogenous Rho function. Interestingly, active p56lck also generates DP thymocytes that express downregulated levels of CD2 antigens when compared with normal thymocytes. However, our data show that p56lck does not prevent expression of CD2 when expressed on a Rho− background. p56lck thus regulates thymocyte development at the pre-T cell stage by both Rho-dependent and Rho-independent responses.
It has been suggested that one critical role for p56lck at the pre-T cell stage of thymocyte development is to mediate allelic exclusion at the TCR-β chain locus (19, 26, 27). Expression of a constitutive active form of p56lck can initiate thymocyte differentiation in RAG−/− or pTα−/− mice (10, 11) and switch off rearrangements at the TCR-β locus. Further evidence for the role of p56lck in preventing rearrangement at the TCR-β locus stems from observations that DP thymocytes that develop in the presence of active p56lck fail to express TCR-β subunits. They do not show rearrangements at the TCR-β locus, do not express a functional α/β-TCR complex and fail to develop into mature CD4+ or CD8+ SP thymocytes (19, 26).
The current model to explain this phenomenon is that p56lck acts as a sensor for the expression of a functional pre-TCR complex. Successful expression of a functional pre-TCR complex would normally activate p56lck and initiate the feedback mechanism that prevents further rearrangements of TCR-β chains, i.e., p56lck mediates allelic exclusion at the pre-T cell stage. The failure of TCR-β chain rearrangements in cells expressing active p56lck is thus thought to reflect the fact that such cells initiate the allelic exclusion mechanism before they have produced a competent TCR-β subunit. Once such cells differentiate into DP thymocytes there is no TCR-β chain to partner newly synthesized TCR-α subunits and consequently expression of a mature TCR–CD3 antigen receptor complex does not occur. This results in failed positive and negative selection and impaired development of mature SP thymocytes. The present data show that loss of Rho function has no influence on the ability of active p56lck to prevent expression of α/β-TCR complexes. Since the failure to express α/β-TCR complexes in thymocytes expressing active p56lck is a model for allelic exclusion, the simplest interpretation of the present data is that Rho function is not required for allelic exclusion at the TCR-β locus.
In this study, we demonstrate that expression of active p56lck in thymocytes not only prevents expression of mature α/β-TCR complexes but also regulates expression of the accessory molecule CD2. The downregulation of CD25 expression on pre-T cells is normally accompanied by upregulation of the expression of the CD2 antigen (24, 25). Active p56lck can push the differentiation processes that downregulates expression of CD25, but the resultant cells do not express normal levels of CD2. The negative regulatory effect of active p56lck on CD2 expression can be reversed by a concomitant loss of Rho function. This was a particularly striking result because loss of Rho function did not restore expression of the α/β-TCR complex on pLGF thymocytes. The ability of active p56lck to prevent CD2 antigen expression on DP thymocytes is thus dependent on Rho function whereas the ability of p56lck to inhibit expression of the α/β-TCR complex is not.
In pLGF/C3 double transgenic mice we observed a consistent increase in frequencies of CD4−CD8low cells when compared with pLGF single transgenic mice. These ISP represent an intermediate stage in the transition from the DN to the DP compartment (21, 22). This result suggests that the transition of late pre-T cells into DP cells is partially suppressed in thymocytes lacking endogenous Rho function. However, loss of Rho function does not completely suppress pre-T cell differentiation. Moreover, although it strikingly decreases proliferative responses, it does not completely abrogate proliferation in late pre-T cells. The present results thus demonstrate that Rho is a regulator of pre-T cell development but they equally illustrate that compensatory, Rho-independent proliferative and differentiation signals must operate in early thymic progenitors. There is increasing awareness that pre-T cell differentiation is controlled by overlapping and compensatory signaling pathways. For example, the tyrosine kinases ZAP70 and Syk have overlapping and compensatory roles in pre-T cells (7) as do the transcription factors Tcf and Lef (23). The present data show that Rho has an important function in p56lck-mediated signals for thymocyte development. The fact that pre-T cell differentiation is not absolutely dependent on Rho function is consistent with the fact that the thymic developmental block in mice deficient for p56lck is not absolute. In p56lck-deficient mice, the related kinase p59fyn can partially compensate for p56lck-mediated signals. Thymocytes that are deficient for both p56lck and p59fyn show a complete block in thymocyte development at the pre-T cell stage analogous to the developmental block seen in RAG−/− thymocytes (28). Therefore, one speculation is that loss of Rho function causes defects in p56lck-mediated function in pre-T cells but does not impact compensatory p59fyn signaling pathways. In this respect, the strategy used in this study to inactivate Rho function is extremely selective. C3 transferase has no inhibitory effects on the function of other Rho family GTPases such as Rac-1, Rac-2, or Cdc42 (29). It will be intriguing to know if loss of Rho function in the thymus can be compensated by the actions of these other Rho family GTPases.
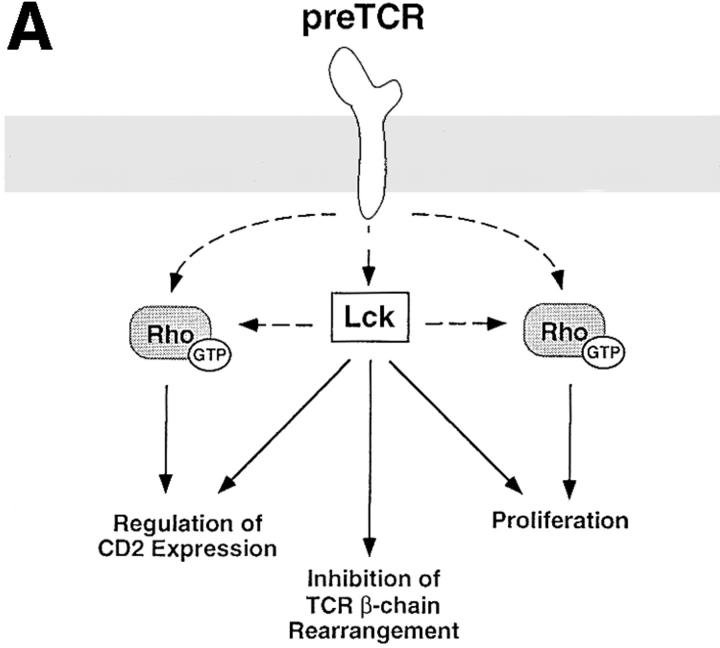
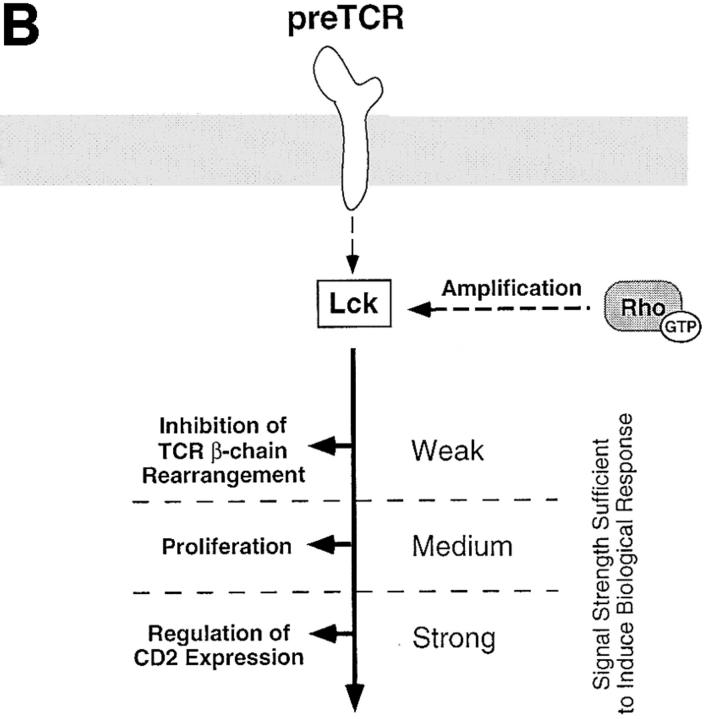
In summary, the tyrosine kinase p56lck regulates pre-T cell proliferation and differentiation and controls expression of α/β-TCR complexes and CD2 antigens. This study shows that these p56lck-mediated responses can be distinguished by their requirement for Rho function. This requirement of Rho function for p56lck-mediated regulation of pre-T cell development is consistent with a model where Rho is part of signaling pathways that emerge from p56lck and regulate cell cycle progression and CD2 expression at this developmental stage. The signaling pathways emerging from p56lck that prevent expression of α/β-TCR complexes by suppressing TCR gene rearrangement at the β-locus are apparently independent of the function of Rho. One interpretation of these results is that qualitatively different signaling pathways, some involving Rho and some not, are being used to control these different p56lck responses (Fig. 5 a). However, an alternative possibility is that Rho is regulating p56lck-signaling responses quantitatively. In this model, the same p56lck-induced signaling pathways controls thymocyte proliferation, α/β-TCR and CD2 expression but these responses require quantitatively different signaling thresholds (Fig. 5 b). Rho could thus regulate the strength of p56lck-induced signals. This model predicts that regulation of TCR-β chain rearrangements has a low signaling threshold compared with the thresholds required for proliferation and control of CD2 antigen expression. Future studies will determine whether Rho is positioned directly downstream of p56lck at the pre-T cell stage or whether Rho is part of a parallel signaling pathway required for pre-T cell development.
Figure 5.
Possible models for the positioning of Rho in p56lck-mediated signaling pathways regulating various aspects of pre-T cell development. (A) The pre-TCR initiates signaling pathways mediated by p56lck that regulate thymocyte development and control the process of allelic exclusion of the TCR-β locus in pre-T cells. A possible model consistent with our data locates Rho either directly downstream of p56lck or in parallel signaling pathways controlling proliferation and regulation of CD2 expression in pre-T cells. Other signals emerging from p56lck-regulating inhibition of TCR-β chain rearrangement are Rho independent. Dashed arrows symbolize as yet formally unproven links between the pre-TCR, p56lck and Rho. (B) An alternative model compatible with our results depicts Rho as a signal amplifier which increases the signal intensity generated by p56lck. Individual biological responses regulated by the pre-TCR would be dependent on different signal intensities and would only be initiated if the signaling strength generated by p56lck and Rho would reach their individual thresholds. Thus, signal intensity in the absence of Rho function would still be sufficient to mediate inhibition of TCR-β chain rearrangement but not to downregulate CD2 expression or drive pre-T cell proliferation.
Acknowledgments
We are indebted to Ian Rosewell from the Imperial Cancer Research Fund Transgenic Unit for microinjection of the C3 transferase transgenic construct. The p56lckF505-expressing pLGF-2964 mice were kindly provided by Dr. Roger M. Perlmutter. We also wish to thank Tracy Crafton, Gary Martin and Barbara Turner at the transgenic mouse breeding unit for excellent assistance in animal care and Derek Davies for helpful advice on flow cytometry. Drs. L. Bruno and M.J. Owen are acknowledged for helpful discussion and critical reading of the manuscript.
This work was supported by the Imperial Cancer Research Fund and the European Human Capital and Mobility Program (CHRX-CT94-0537). S.W. Henning was a fellow of the European Training and Mobility of Researchers Program (ERBFMBICT 960518).
Abbreviations used in this paper
- DN
double negative
- DP
double positive
- ISP
immature CD8 single positive cells
- SP
single positive
- TN
triple negative
References
- 1.Di Santo JP, Rodewald H-R. In vivo roles of receptor tyrosine kinases and cytokine receptors in early thymocyte development. Curr Opin Immunol. 1998;10:196–207. doi: 10.1016/s0952-7915(98)80249-5. [DOI] [PubMed] [Google Scholar]
- 2.Fehling HJ, von Boehmer H. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr Opin Immunol. 1997;9:263–275. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- 3.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-ε gene. EMBO (Embo Mol Biol Organ) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 5.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 6.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann K-U, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AM, Negishi I, Anderson SJ, Chan AC, Bolen J, Loh DY, Pawson T. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc Natl Acad Sci USA. 1997;94:9797–9801. doi: 10.1073/pnas.94.18.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byth KF, Conroy LA, Howlett S, Smith AJH, May J, Alexander DR, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+thymocytes, and in B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin SD, Anderson SJ, Forbush KA, Perlmutter RM. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO (Eur Mol Biol Organ) J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehling H-J, Iritani BM, Krotkova A, Forbush KA, Laplace C, Perlmutter RM, von Boehmer H. Restoration of thymopoiesis in pTα−/−mice by anti-CD3ε antibody treatment or with transgenes encoding activated lck or tailless pTα. Immunity. 1997;6:703–714. doi: 10.1016/s1074-7613(00)80446-x. [DOI] [PubMed] [Google Scholar]
- 11.Mombaerts P, Anderson SJ, Perlmutter RM, Mak TW, Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 12.Henning SW, Gallandrini R, Hall A, Cantrell DA. The GTPase Rho has a critical regulatory role in thymus development. EMBO (Eur Mol Biol Organ) J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galandrini R, Henning SW, Cantrell DA. Different functions of the GTPase Rho in pro-thymocytes and late pre-T cells. Immunity. 1997;7:163–174. doi: 10.1016/s1074-7613(00)80519-1. [DOI] [PubMed] [Google Scholar]
- 14.Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. Delayed thymocyte development induced by augmented expression of p56lck . J Exp Med. 1991;173:1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8−triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 16.Dudley EC, Petri HT, Shah LM, Owen MJ, Hayday AC. T cell receptor β chain rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 17.Mallick CA, Dudley EC, Viney JL, Owen MJ, Hayday AC. Rearrangement and diversity of T cell receptor β chain genes in thymocytes: a critical role for the β chain in development. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 18.Cheng AM, Chan AC. Protein tyrosine kinases in thymocyte development. Curr Opin Immunol. 1998;9:528–533. doi: 10.1016/s0952-7915(97)80106-9. [DOI] [PubMed] [Google Scholar]
- 19.Anderson SJ, Abraham KM, Nakayama T, Singer A, Perlmutter RM. Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the non-receptor protein-tyrosine kinase p56lck. EMBO (Eur Mol Biol Organ) J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M. In transgenic mice the introduced functional T cell receptor β gene prevents expression of endogenous β genes. Cell. 1988;52:831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- 21.Shortman K, Wilson A, Egerton M, Pearse M, Scollay R. Immature CD4−CD8+murine thymocytes. Cell Immunol. 1988;113:462–479. doi: 10.1016/0008-8749(88)90042-1. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald R, Budd RC, Howe RC. A CD3− subset of CD4−8+ thymocytes: a rapidly cycling intermediate in the generation of CD4+8+cells. Eur J Immunol. 1988;18:519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 23.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRα gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 24.Lalli E, Sassone-Corsi P, Ceredig R. Block of T lymphocyte differentiation by activation of the cAMP-dependent signal transduction pathway. EMBO (Eur Mol Biol Organ) J. 1996;15:528–537. [PMC free article] [PubMed] [Google Scholar]
- 25.Rodewald H-R, Awad K, Moingeon P, D'Adamio L, Rabinowitz D, Shinkai Y, Alt FW, Reinherz EL. FcγRII/II and CD2 expression mark distinct subpopulations of immature CD4−CD8−murine thymocytes: in vivo developmental kinetics and T cell receptor β chain rearrangement status. J Exp Med. 1993;177:1072–1092. doi: 10.1084/jem.177.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson SJ, Levin SD, Perlmutter RM. Protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor β-chain genes. Nature. 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- 27.Wallace VA, Kawai K, Levelt CN, Kishihara K, Molina T, Timms E, Pircher H, Penninger J, Ohashi PS, Eichmann K, Mak TW. T lymphocyte development in p56lck-deficient mice: allelic exclusion of the TCRβ locus is incomplete but thymocyte development is not restored by TCRβ or TCRαβ transgenes. Eur J Immunol. 1995;25:1312–1318. doi: 10.1002/eji.1830250527. [DOI] [PubMed] [Google Scholar]
- 28.van Oers NSC, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both lck and fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 29.Aktories K. Clostridial ADP ribosylating toxins: effect on ATP and GTP binding proteins. Mol Cell Biol. 1994;138:167–176. doi: 10.1007/BF00928459. [DOI] [PubMed] [Google Scholar]