Abstract
The neisserial porin P.I is a GTP binding protein that forms a voltage-gated channel that translocates into mammalian cell membranes and modulates host cell signaling events. Here, we report that P.I confers invasion of the bacterial pathogen Neisseria gonorrhoeae into Chang epithelial cells and that this event is controlled by GTP, as well as other phosphorus-containing compounds. Bacterial invasion was observed only for strains carrying the P.IA subtype of porin, which is typically associated with the development of disseminated neisserial disease, and did not require opacity outer membrane proteins, previously recognized as gonococcal invasins. Allelic replacement studies showed that bacterial invasiveness cotransferred with the P.IA (por1A) gene. Mutation of the P.I-associated protein Rmp did not alter the invasive properties. Cross-linking of labeled GTP to the porin revealed more efficient GTP binding to the P.IA than P.IB porin subtype. GTP binding was inhibited by an excess of unlabeled GTP, ATP, and GDP, as well as inorganic phosphate, but not by UTP or beta-glycerophosphate, fully in line with the respective invasion-inhibitory activities observed for these compounds. The P.IA-mediated cellular invasion may explain the more invasive behavior of P.IA strains in the natural infection and may broaden the basis for the development of a P.I-based gonococcal vaccine.
Keywords: Neisseria gonorrhoeae, porin, GTP binding protein, Rmp protein, bacterial invasion
The bacterial pathogen Neisseria gonorrhoeae has evolved an array of sophisticated machineries to optimally survive within the human host. Some of its more prominent adaptive strategies are the maintenance of extensive repertoire of antigenic variation, which may serve to evade the human host defense, and the controlled on- and off-switching (phase variation) of distinct cell surface molecules, which may enable the pathogen to colonize various infection niches (for reviews see references 1–3). The phenotypic diversity is largely driven by genetic mechanisms, including allelic exchange between related loci (4, 5) and a mispairing of nucleotide repeats located within, or in close proximity to, the coding regions for relevant surface antigens. The latter mechanism leads to reversible shifts of open reading frames (ORFs)1 and thus variable expression of the antigens (6–10). Major variable surface components include pili (fimbriae), the pilus-associated adhesin PilC, the opacity protein (Opa) family of bacterial adhesins, and LPS. These factors are thought to be key players in bacterial adherence to and invasion of mammalian cells and to contribute to the cell tropism displayed by the pathogen, and may confer bacterial resistance to complement-mediated killing (11–19). Evidence is accumulating that the intrinsic neisserial surface variation may be further complicated by a bacterial phenotype-dependent recruitment of host molecules such as sialic acids, vitronectin, fibronectin, transferrin, sulfated polysaccharides, and complement factors (16, 17, 20–27). This strategy may further limit bacterial antigen exposure and determine the sensitivity of the pathogen to host defense mechanisms. The binding of several of these factors by the microorganisms has been reported to open additional pathways for cellular invasion (20–23, 26). The highly flexible neisserial phenotype is thought to be one of the major obstacles in the development of a protective host immune response.
One of the neisserial surface antigens that is stably expressed by a given strain is the principal outer membrane protein P.I (for review see reference 28). Because of its invariable presence at the bacterial cell surface, its comparatively conserved nature, and its abundance in the outer membrane, P.I has gained much attention as a potential vaccine candidate antigen (29–34). Indeed, molecular epidemiology studies suggest that P.I-specific antibodies that develop during the natural infection may provide partial protection against reinfection with gonococci of the same P.I serotype (35). On the basis of structural and immunochemical characteristics, two major subtypes of P.I have been recognized, termed P.IA and P.IB, which are encoded by the mutually exclusive alleles por1A and por1B. The P.I subtypes vary slightly in size among strains with molecular masses ranging from 32,000 to 38,000 daltons and act as membrane pores enabling the flux of ions and small macromolecules across the membrane barrier (36, 37). The porin function of P.I has been confirmed by conductance experiments in which purified P.I was incorporated into lipid bilayers and demonstrated to form a voltage-gated channel with slight anion selectivity (38, 39). Interestingly, these channels also form when viable microorganisms are added to the lipid bilayer system, suggesting transfer of the protein from the bacteria into the artificial membrane (40). Purified P.I also inserts into plasma membranes of eukaryotic cells. The addition of purified P.I to mammalian cells causes a transient change in transmembrane potential and modulates host cell signaling events (36, 41–44), but direct evidence for insertion of a functional pore upon infection of mammalian cells with intact microorganisms is not available.
The possible function of P.I in the natural infection is not well established. Strains bearing P.IA are much more commonly isolated from patients with disseminated gonococcal disease than P.IB strains (45–48), suggesting a difference in virulence related to the P.I subtype. The molecular basis for the more invasive behavior of P.IA strains is unknown, although these strains are usually more resistant to killing by normal human serum (48–51) and show up to a 10-fold increase in the rate of P.I translocation into lipid bilayers compared with P.IB strains (40). On theoretical grounds, the latter event has been speculated to facilitate bacterial invasion of mammalian cells, possibly by spiking the plasma membrane and activating a phagocytosis-like event (36, 44, 52, 53). However, true evidence for a role of P.I in neisserial invasion of human cells is lacking. In this study, we addressed this topic and identified a novel invasion mechanism for Neisseria gonorrhoeae that involves P.I and that is unique to strains bearing P.IA. This uptake mechanism does not require the family of Opa proteins previously recognized as gonococcal invasins and becomes apparent under conditions of low phosphate only. Our data provide the first direct evidence for a role of porins in bacterial invasion of eukaryotic cells and may explain the ability of the pathogen to spread from the primary focus of infection towards distant body sites, a feature that is typically associated with P.IA strains. The data lend further support for the development of a P.I-based gonococcal vaccine.
Materials and Methods
Bacterial Strains and Cell Culture.
The strains used in this study are listed in Table 1. The VP1-Opa variants have been described previously (12, 13). All microorganisms were routinely grown on GC agar plates (26) for 12 to 14 h at 37°C in a 5% CO2 atmosphere. All strains were nonpiliated. Opa variants were selected based on colonial opacity and the Opa phenotype was verified by SDS-PAGE, immunoblotting, and proteoglycan receptor binding (see below). For use in infection experiments, bacteria were grown to exponential growth phase in 50-ml polypropylene tubes containing 10 ml of Hepes medium (26) enriched with 0.5% IsoVitaleX (BBL, Cockeysville, MD) for 3 h at 37°C in a gyrotory water bath shaker (125 rpm) to remove contaminating agar polysaccharides (24). Iron starvation of strain VP1 was imposed by adding Deferoxamine (30 μM final concentration; Sigma Chemical Co., St. Louis, MO) to Hepes medium lacking IsoVitaleX. Arginine-hypoxanthine-uracil (AHU) starvation was imposed by growing the AHU-requiring strain FA19 in Hepes medium lacking IsoVitaleX for 3 h. Use of various phosphate sources for bacterial growth was determined using a chemically defined medium (54) in which phosphate was made the growth limiting constituent. Human Chang conjunctiva epithelial cells (CCL20.2; American Type Culture Collection, Rockville, MD) were maintained in 25-cm2 tissue culture flasks (Corning Glass Works, Corning, NY) in 5 ml RPMI 1640 (GIBCO BRL, Gaithersburg, MD) plus 5% fetal bovine serum (FBS) at 37°C in 10% CO2 incubator. For use in infection experiments, cells were cultured for 2 to 3 d to near confluence on circular (12-mm diameter) glass coverslips in RPMI 1640 plus 5% FCS in 24-well tissue culture plates.
Table 1.
Bacterial Strains Used in this Study
| Strain | P.I phenotype | Source | Reference | |||
|---|---|---|---|---|---|---|
| VP1 | P.IA | J.P.M. van Putten | 12, 52 | |||
| NRL 7122 | P.IA | T.M. Buchanan | 77 | |||
| NRL 8658 | P.IA | T.M. Buchanan | 77 | |||
| NRL 7929 | P.IA | T.M. Buchanan | 77 | |||
| 80 | P.IA | J.P.M. van Putten | – | |||
| FA19 | P.IA | P.F. Sparling | 49 | |||
| MS11 | P.IB | T.F. Meyer | 12 | |||
| VP2 | P.IB | J.P.M. van Putten | – | |||
| 5590 | P.IB | J.P.M. van Putten | 78 | |||
| F-62 | P.IB | T.M. Buchanan | 77 | |||
| 1291 | P.IB | M.A. Apicella | 77 | |||
| FA1090 | P.IB | J.G. Cannon | 79 | |||
| FA6616 (MS11) | P.IB(MS11) | C. Elkins | 49 | |||
| FA6611 (MS11) | P.IA(FA19) | C. Elkins | 49 | |||
| FA6564 (FA19) | P.IA(FA19) | C. Elkins | 49 | |||
| FA6571 (FA19) | P.IB(MS11) | C. Elkins | 49 |
Genetic Engineering.
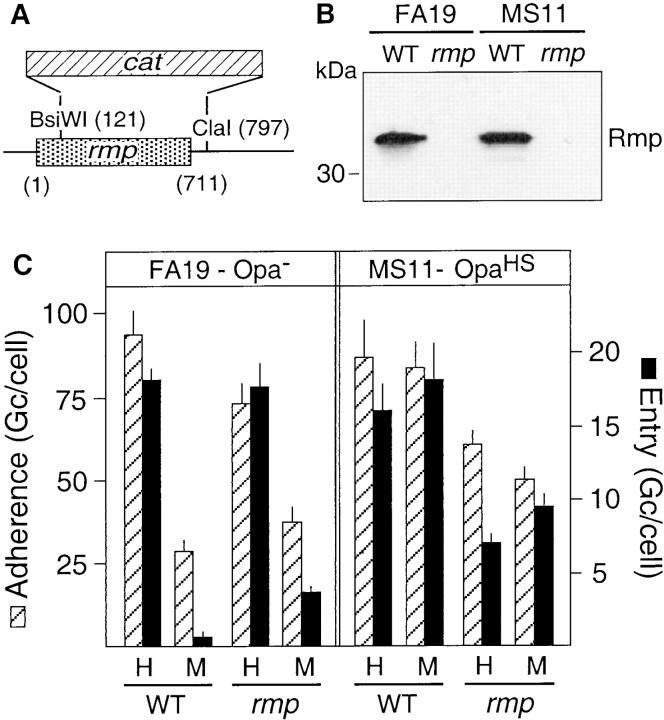
The construction of the strains FA6611, FA6616, FA6564, and FA6571 carrying recombinant P.I protein with a minitransposon mTn3-Cm-3 located downstream of the por gene has been described previously (49). For construction of Rmp null mutants, the rmp gene of strain MS11 was PCR amplified with the upstream primer JHC100 (5′-CTATCCGATTTGCCGCCATGTTTC-3′) in combination with the downstream primer JHC101 (5′-CCGCGGGGTTTCAACCGAAAAGGG-3′) using the Expand™ High Fidelity PCR System (Boehringer Mannheim Corp., Indianapolis, IN), and cloned into the pCR2.1 vector (Invitrogen, Carlsbad, CA). A mutated rmp was created by removing a 675-bp internal fragment from the subclone using the unique BsiWI site in rmp and a flanking ClaI site, thus deleting all but 121 bp on the 5′ end of the rmp ORF, and replacing it with a 1,098-bp fragment containing a chloramphenicol resistance cat cassette (see Fig. 4 A). This cassette was originally PCR amplified from plasmid pACYC184 (New England Biolabs, Beverly, MA) using the primers JHC110 (5′-CCAAGCTTCAGACGGCGAATTTCTGCCATTCATCCGC-3′) and JHC123 (5′-GCTGGTAATGTTCTTGCATGGTC-3′), which introduced terminal HindIII sites and a gonococcal DNA uptake sequence. Insertion in the rmp gene was established by filling in the sticky ends through the use of DNA polymerase I (Klenow fragment), followed by blunt-end ligation, creating plasmid pCR2.1-Δrmp::cat, in which the cat insert was in the same direction as the rmp ORF. Transformation of the mutant allele into MS11 recombinant strains carrying either P.IA or P.IB was done by transformation of linearized, AvaII-digested plasmid. Deletion of rmp was verified by PCR, restriction analysis of the PCR product, and immunoblotting using an Rmp-specific antibody (see Fig. 4 B).
Figure 4.
Role of the P.I-associated protein Rmp in the P.I-mediated invasion of epithelial cells. (A) Diagram of the deletion in rmp and insertion of the chloramphenicol resistance cassette (cat) resulting in the Rmp-negative phenotype. The numbering of nucleotides is relative to the start of the ORF. (B) Western blot of whole cell lysates of the parent (WT) and Rmp-deficient (rmp) FA19 (P.IA) and MS11 (P.IB). Rmp was detected with the mAb 4C7E. (C) Effect of mutation of rmp on the P.IA (FA19-Opa−) and proteoglycan-mediated (MS11-OpaHS) adherence and invasion of Chang cells (2 h of infection). Assays were performed in Hepes buffer (H) and tissue culture medium (M). Infection with the parental strains (WT) served as controls. Data are the mean ± SEM of three to five experiments.
Electrophoresis, Immunoblotting, and ELISA.
The P.I, Opa, and Rmp phenotypes of the various strains were verified by SDS-PAGE and/or immunoblotting of whole cell lysates as previously described (13). After protein transfer, nitrocellulose blots were incubated with the appropriate P.IA (mAb 1EA), P.IB (mAb 5.51), Rmp (mAb 4C7E), or Opa (mAb4B12/CII) protein-specific mAbs, and antibody binding was detected with goat anti–mouse IgG (Fab specific) horseradish peroxidase (HRP) conjugate (1: 2,500 dilution; Sigma Chemical Co.) and Supersignal (Pierce Chemical Co., Rockford, IL). The mAbs 5.51 and 1EA were provided by Dr. C. Elkins (University of North Carolina, Chapel Hill, NC); mAb 4B12/CII was a gift from Dr. M. Achtman (Max-Planck-Institut für molekulare Genetik, Berlin, Germany). mAb 4C7E was raised as previously described (13). The P.I phenotype of the various strains was verified by whole-cell ELISA, which involved the air-drying of a suspension of gonococci in Dulbecco's PBS (DPBS; 108 gonococci per well) onto Immulon 4 microtiter plates (Dynatech Labs., Inc., Chantilly, VA), followed by sequential incubations (1 h, 37°C) in 100 μl of DPBS/ 3% BSA, 100 μl of DPBS/0.5% BSA/0.05% Tween 20 plus P.IA- or P.IB-specific mAb, and 100 μl of DPBS/0.5% BSA/ 0.05% Tween 20 plus goat anti–mouse IgG peroxidase conjugate. Antibody binding was detected by the addition of 150 μl of O-phenylenediamine in citric acid phosphate buffer (pH 5.0). Reactions were stopped with 50 μl of 4 N H2SO4, and results were read with a Titertek Multiscan ELISA reader (Titertek Instruments, Huntsville, AL). LPS phenotypes were analyzed by Urea–SDS-PAGE and silverstaining (16).
Proteoglycan Receptor Binding.
Binding of epithelial heparan sulfate proteoglycan receptors by gonococcal Opa variants was assessed as described previously (55). In brief, Chang cells were metabolically labeled during growth in 75-cm2 tissue culture flasks in 10 ml of Basal Medium Eagle supplemented with 1% FCS, 1% nonessential amino acids, and 30 μCi 35SO4. After 48 h, cells were washed with medium and the extracellular proteoglycan receptor fragment (ectodomain) was isolated from the cells using trypsin. Ectodomain binding was measured by incubating 5 × 107 gonococci with labeled receptor fragment in 150 μl of Hepes buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM KCl, 5 mM glucose, 1 mM CaCl2, and 1 mM MgCl2, pH 7.4), for 10 min at 20°C, followed by removal of unbound receptor by centrifugation (2 min, 12,000 g, 20°C) and counting of bacteria-associated radioactivity in a Beckman liquid scintillation counter (Beckman Instruments, Fullerton, CA). Radioactivity bound in the presence of 100 μg/ml heparan sulfate was considered nonspecific (usually ∼200 cpm) and was subtracted.
Infection Assays.
Infection of Chang cells maintained on coverslips in 24-well plates in 1 ml of Hepes buffer or RPMI 1640 (tissue culture medium) plus 0.1% IsoVitaleX was initiated by adding gonococci at a bacterium to epithelial cell ratio of 100:1. After 2 or 3 h of infection, cells were rinsed three times with 1 ml DPBS and fixed in 0.1% glutaraldehyde/1% paraformaldehyde in DPBS for at least 1 h at 20°C. The number of extra- and intracellular bacteria was determined microscopically after immunogold silverstaining and/or crystal violet staining as detailed previously (55, 56). Values are given as the mean number of gonococci per epithelial cell and represent the mean ± SEM of at least three experiments. When appropriate, phosphorus containing compounds (sodium phosphate (NaH2PO4 + Na2HPO4, pH 7.4), GTP, GDP, GMP, ATP, UTP, sodium tripolyphosphate, and sodium β-glycerophosphate) from buffered stock solutions (pH 7.4) were added just before the addition of the bacteria. When AHU-starved bacteria were tested, no IsoVitaleX was present during the assay. Infections with iron-starved bacteria were performed in Hepes buffer plus 30 μM Deferoxamine.
GTP-binding Assay.
Binding of GTP to gonococci was determined by cross-linking of the ribose moiety of the nucleotide to lysine residues in the vicinity of the nucleotide binding site after the procedure of Peter et al. (57) as applied by Rudel et al. (44) with some modifications. In brief, 2 × 108 agar plate–grown gonococci were suspended in Hepes buffer, washed once, and resuspended in 50 μl Hepes buffer. Labeling was initiated by adding 2.5 μCi (10 nM, final concentration) of γ-[32P]GTP (5,000 Ci/ mmol, Amersham Pharmacia Biotech, Arlington Heights, IL). After 8 min of incubation in a 37°C waterbath, the ribose moiety was oxidized (1 min) by the addition of 1 mM of sodium periodate, resulting in the formation of a reactive dialdehyde. The Schiff base formed between the oxidized ribose and a nearby lysine residue was stabilized (1 min) with 20 mM of sodium cyanoborohydride (Sigma Chemical Co.), and the reaction was stopped by the addition of 20 mM of sodium borohydride, followed by 5 min of incubation on ice, and centrifugation (2 min, 12,000 g, 20°C). Pellets were washed three times with 150 μl Hepes buffer to remove unbound label, lysed in sample buffer, and 5 × 107 bacteria were subjected to SDS-PAGE (12.5% gels) and autoradiography. Reactive bands were analyzed by densitometry using an Alpha Imager 2000 (Alpha Innotech, San Leandro, CA) and accompanying software. When appropriate, 2 μl of unlabeled phosphorus containing compound (or an equivalent amount of buffer) was added 2 min before the addition of the γ-[32P]GTP to complete the binding of label. Experiments performed without the addition of sodium cyanoborohydride served as controls.
Results
Opa-independent Gonococcal Invasion of Human Epithelial Cells.
Infection of cultured Chang epithelial cells with isogenic variants of gonococcus strain VP1 that carry different Opa protein phenotypes resulted in efficient bacterial invasion of VP1-Opa27.5 but none of the other variants when the experiments were performed in tissue culture medium (Fig. 1, A and B). These data are consistent with the proposed role for this Opa protein as a gonococcal adhesin/invasin (12, 13) and confirm the notion that Opa protein variation contributes to the cell tropism displayed by gonococci (11, 15, 55). However, during our investigations of the molecular mechanism behind the invasion event, we noticed that when the infection experiments were performed in Hepes-buffered saline instead of tissue culture medium, gonococcus strain VP1 was efficiently endocytosed by the eukaryotic cells irrespective of the Opa phenotype. Analysis of the invasive behavior of the same set of isogenic VP1 variants described above clearly demonstrated that under these conditions Opa proteins were not required to facilitate bacterial internalization (Fig. 1 C). Similar results were obtained for gonococcus strain FA19 (Fig. 2). In contrast, when Opa variants of the widely investigated strain MS11 were tested, the characteristic MS11-Opa30 dependence of the entry process (12) was maintained and MS11-Opa− variants were unable to invade the epithelial cells even when the assay was performed in Hepes buffer (Fig. 2), suggesting that only certain gonococcal strains have evolved an alternative, Opa-independent invasion mechanism.
Figure 1.
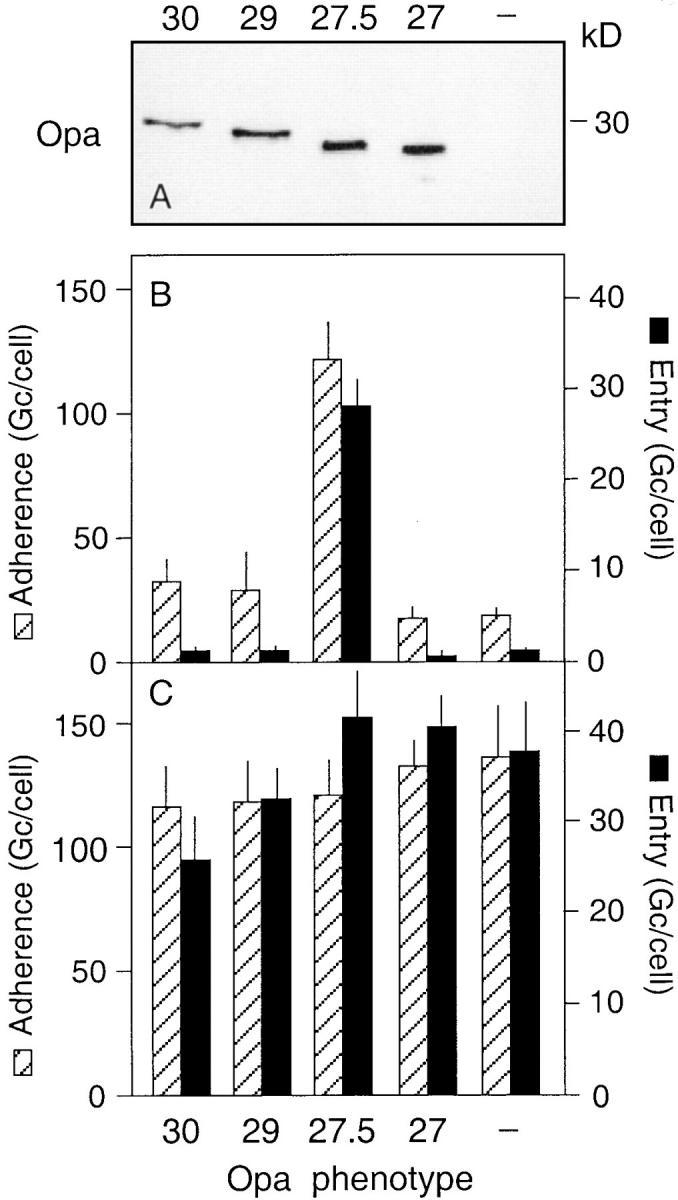
Opa-independent invasion of Chang cells by gonococcus strain VP1. (A) Immunoblot of whole cell lysates of the VP1–Opa variants (Opa30, Opa29, Opa27.5, Opa27, Opa−) used in the infection experiments. Opa proteins were detected using the mAb 4B12/CII, HRP-conjugated goat anti–mouse IgG, and Supersignal. (B and C) Bacterial adherence to and entry of the VP1–Opa variants into Chang epithelial cells maintained in tissue culture medium (B) and Hepes buffer (C) at 2 h of infection. Data are expressed as the number of gonococci (Gc) per cell and are the mean ± SEM of 6–10 experiments.
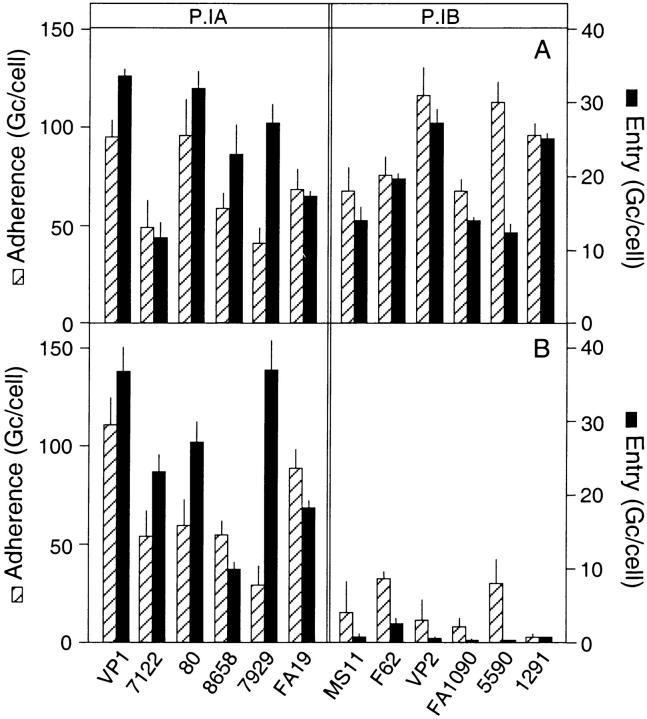
Figure 2.
Relationship between P.I phenotype and Opa-independent gonococcal invasion. Bacterial adherence to and entry into Chang cells was determined at 2 h of infection in Hepes buffer for 12 gonococcal isolates with a P.IA or P.IB phenotype. For each of the strains, variants that expressed either (A) a heparan sulfate–binding Opa (OpaHS) that confers entry through proteoglycan receptors or (B) no Opa protein were tested. The OpaHS phenotype was confirmed through binding assays using purified 35SO4-labeled proteoglycan ectodomain isolated from Chang epithelial cells as a ligand (reference 55 and data not shown). Values are the mean ± SEM of three to eight experiments.
Potential Role of the Bacterial Porin P.IA in Gonococcal Invasion of Epithelial Cells.
In search for the bacterial ligand(s) that facilitated the Opa-independent invasion, we focused on the apparent variability in the activity of this invasion mechanism among gonococcal strains. For this purpose, we selected isogenic variants of 12 gonococcal isolates that either lacked Opa protein (Opa−) or produced a member of the Opa protein family that bound epithelial cell proteoglycan-receptors (OpaHS), a feature that we previously demonstrated to be required for Opa-mediated entry into various cell types (55). Infection experiments with the isolates showed that all 12 strains were able to invade Chang epithelial cells in an OpaHS-dependent fashion (Fig. 2 A). In contrast, only 6 out of 12 variants that lacked Opa protein were able to enter the cells and again only when the assays were performed in Hepes buffer (Fig. 2 B). Detailed comparison of the characteristics of the various strains, including auxotype, electrophoretic outer membrane profile, P.I subtype, and LPS phenotype, revealed a 100% correlation between the Opa-independent invasion and the type of P.I porin produced by the strains. All six strains that carried the P.IA subtype of porin were able to enter the cells in the absence of Opa protein, whereas all strains with a P.IB type of porin required an OpaHS for bacterial invasion of Chang cells (Fig. 2). This finding is of particular interest as molecular epidemiological studies indicate an association between the P.IA protein and the intrinsic ability of a gonococcal strain to disseminate from the initial site of infection to other body sites (45–48).
Allelic Replacement of P.IA and P.IB Genes.
To ascertain that P.IA, rather than possible associated traits, conferred gonococcal invasion of epithelial cells, we determined the invasive behavior of a complete set of gonococcal recombinants in which the P.IA gene (por1A) of strain FA19 and the P.IB gene (por1B) of strain MS11 were interchanged by allelic replacement (49). Strains in which the por gene was replaced with its endogenous copy with a genetically linked resistance marker, served as controls. The P.I subtype of the recombinant strains was confirmed by SDS-PAGE and Western blotting using P.IA- and P.IB-specific antibodies (Fig. 3, A and B). Electrophoretic analysis revealed no detectable changes in LPS associated with the conversion of the P.I phenotype (data not shown). Using Opa− variants, recombinant FA19 that carried its original por1A was highly invasive, whereas the recombinant MS11-P.IB was not internalized by the epithelial cells, consistent with the behavior of their corresponding parents (Fig. 3, C and E). Of particular interest, expression of por1B derived from MS11 in the FA19-Opa− background resulted in a complete loss of bacterial invasiveness from this strain. Conversely, MS11-Opa− that had acquired the por1A of FA19 gained the ability to enter the host cells (Fig. 3, C and E). To ensure that the allelic replacement procedure had not caused a general defect in bacterial invasion, recombinants with OpaHS phenotypes were isolated and assessed for Opa-dependent invasion. Infection experiments showed that all four recombinants (MS11-P.IB, MS11-P.IA, FA19-P.IA, and FA19-P.IB) were able to enter the Chang epithelial cells in an Opa-dependent fashion (Fig. 3, D and F). Together, these data strongly suggest that P.I is a conclusive determinant of the Opa-independent entry mechanism.
Figure 3.

Invasion of gonococci carrying recombinant P.I proteins. A/B. Western blot of whole cell lysates of the P.I recombinants demonstrating the presence of P.IA and P.IB in the MS11 and FA19 backgrounds. Blot A was incubated with the P.IA-specific antibody 1EA; blot B with the P.IB-specific antibody 5.51. (C and D) Binding of 35SO4-labeled heparan sulfate proteoglycan (HSPG) receptor to selected P.I recombinants that produce the heparan sulfate–binding Opa (OpaHS) or lack Opa proteins (Opa−). Binding was measured after 10 min of incubation and data are the mean ± SEM of three to eight experiments. (E and F) Adherence to and invasion of Chang cells by the P.I recombinant strains with the different Opa phenotypes at 2 h infection in Hepes buffer. Values are mean ± SEM of 5–12 experiments.
Invasive Behavior of Rmp Knockout Mutants.
In the bacterial outer membrane, most of the P.I porins are present as trimers that are noncovalently complexed with the Rmp outer membrane protein (58, 59), and Rmp surface exposure (60) may vary with the P.I phenotype. Thus, it can be argued that P.IA-dependent invasion may actually be mediated through Rmp. To investigate this concept, we constructed a set of gonococcal recombinants in which 196 out of 214 codons encoding the mature Rmp were deleted and replaced by a chloramphenicol resistance cassette (Fig. 4 A). Mutation of Rmp was verified by genetic analysis (data not shown) and Western blotting using an Rmp-specific antibody (Fig. 4 B). Infection experiments with rmp knockouts that lacked Opa protein at their surface demonstrated efficient P.IA-mediated invasion of Chang cells in Hepes buffer, but not in tissue culture medium (Fig. 4 C). The corresponding Rmp-deficient MS11-P.IB strain was still unable to enter epithelial cells irrespective of the medium employed (Fig. 4 C), indicating that Rmp did not conceal possible invasive properties of P.IB. It should be noted that infection assays with variants with the appropriate OpaHS phenotype showed up to a 50% reduction in Opa-mediated gonococcal uptake for the Rmp mutants compared with their parents in both Hepes buffer and tissue culture medium (Fig. 4 C). This effect was probably associated with the reduced growth rate observed for these recombinant strains, as efficient bacterial growth is required to achieve maximal functional OpaHS activity (24). Together, the results strongly support the notion that the P.IA molecule drives the Opa-independent invasion event.
Regulation of P.IA-mediated Gonococcal Invasion by Phosphate.
Systematic complementation of the Hepes buffer used in the invasion assay with constituents of the tissue culture medium revealed that the addition of phosphate completely abrogated the activity of the P.IA-dependent entry mechanism. Dose–response experiments showed half-maximal inhibition of the VP1-Opa− entry at 0.8 mM sodium phosphate, whereas >95% inhibition was achieved at 5.0 mM of phosphate (Fig. 5 A). The addition of phosphate to the infection system did not affect the Opa-dependent entry process (Fig. 5 A), explaining the efficient entry of VP1-Opa27.5 that was observed when infection assays were performed in tissue culture medium, which contains relatively high concentrations of sodium phosphate (5.6 mM in RPMI 1640).
Figure 5.
Sensitivity of the P.I-mediated invasion to phosphate. (A) Adherence and entry into Chang cells of VP1 lacking Opa protein (Opa−) and a variant producing OpaHS in the absence and presence of increasing concentrations of sodium phosphate (Pi). (B) Effect of iron (Fe −) (strain VP1) and AHU starvation (strain FA19) on the P.IA-mediated bacterial adherence and entry into Chang cells in the absence and presence of 5 mM phosphate. Bacteria grown in Hepes medium served as controls (Ctrl). Values are the mean ± SEM of four to eight experiments.
The identification of a phosphate-sensitive invasion mechanism in strains bearing P.IA, but not P.IB, suggested that phosphate modulates the invasive behavior of the bacteria rather than the phagocytic activity of the epithelial cells. Theoretically, phosphate limitation may facilitate P.IA- mediated invasion through changes related to the starvation induced cessation of bacterial growth, a phosphate-specific adaptive response, or, possibly, a direct modulation of porin function. Detailed analysis of the outer membrane profiles of phosphate-starved and -nonstarved gonococci revealed no obvious differences (data not shown). Possible effects of bacterial growth rate were examined by testing bacterial invasiveness after introduction of other forms of starvation. Iron-deprivation, induced by the addition of the iron chelator Deferoxamine (30 μM) to the medium (strain VP1), or depletion of the bacteria for essential arginine, hypoxanthine, and uracil (strain FA19), which resulted in similar low bacterial growth rates as caused by phosphate starvation (data not shown), did not facilitate Opa-independent invasion unless additional phosphate limitation was imposed (Fig. 5 B). Thus, the invasion mechanism appeared to be active under low phosphate conditions only.
Infection assays in the presence of a number of different phosphorus-containing compounds demonstrated that, in addition to inorganic phosphate, the nucleotides ATP, GTP, GDP, and GMP, and tripolyphosphate effectively inhibited P.IA-mediated gonococcal invasion (Table 2). Other compounds, such as UTP, and β-glycerophosphate allowed efficient invasion of VP1-Opa− at molar concentrations at which sodium phosphate was inhibitory (Table 2). Further analysis of the effect of the nucleotides showed an apparent direct correlation between the number of phosphate groups in the guanosine moiety and the level of invasion with values for half maximal inhibition of invasion (Inv50) at 0.05 mM, 0.4 mM, and 0.8 mM for GTP, GDP, and GMP, respectively (Table 2). Additional experiments with the highly effective inhibitor tripolyphosphate (Inv50 at 0.05 mM) indicated that this compound was not efficiently metabolized by the gonococci as inferred from its inability to support bacterial growth when added as a sole phosphate source to a phosphate-deficient chemically defined medium (data not shown). Thus, metabolization of phosphate compounds appeared not to be required for inhibition of the invasion event.
Table 2.
Inhibitory Effect of Phosphorus-Containing Compounds on P.IA-mediated Invasion
| Compound | Dose | Invasion | Inv50 * | |||
|---|---|---|---|---|---|---|
| mM | Gc/cell | mM | ||||
| None | 0 | 15.8 ± 1.2 | – | |||
| Pi | 5 | 2.5 ± 0.3 | 0.95 | |||
| GTP | 0.5 | 0.2 ± 0.1 | 0.03 | |||
| GDP | 1 | 1.0 ± 0.5 | 0.09 | |||
| GMP | 1 | 2.3 ± 1.2 | 0.57 | |||
| ATP | 5 | 0 | ND | |||
| UTP | 5 | 16.6 ± 1.6 | – | |||
| TPP | 0.5 | 1.3 ± 0.5 | 0.05 | |||
| βG-P | 5 | 9.4 ± 2.0 | >5 |
Gonococcal invasion was determined at 3 h of infection of Chang cells with VP1-Opa− in the absence and presence of various putative inhibitory reagents. Values are the mean ± SEM of four to eight experiments.
Inv50, dose at which 50% reduction of invasion occurs; Gc, gonococci; Pi, sodium phosphate; TPP, tripolyphosphate; βG-P, β-glycerophosphate.
GTP Binding to P.I Is Sensitive to Phosphate.
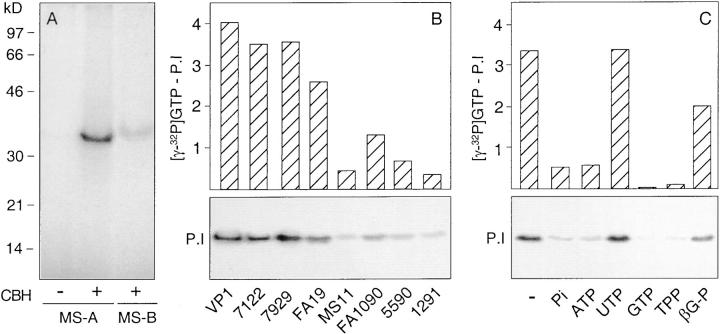
The gonococcal porin has been demonstrated to bind GTP and this binding appears to regulate the function of the ion channel (44). We took advantage of this property to test whether the inhibition of the bacterial invasion by phosphate could have occurred through a direct effect on the porin. Thus, we covalently cross-linked γ-[32P]GTP to the recombinant P.IA and P.IB proteins produced by strain MS11 in the absence and presence of phosphate, using the cyanoborohydride coupling procedure (57). Analysis of labeled bacterial cell lysates separated by SDS-PAGE and subjected to autoradiography showed that in the absence of phosphate, γ-[32P]GTP was cross-linked to both the P.IA and P.IB porins, with coupling to P.IA approximately five times more effective than to P.IB (Fig. 6 A). This difference in coupling efficiency among P.I subtypes was maintained for all strains that were tested (four P.IA and four P.IB; Fig. 6 B). In contrast, when inorganic phosphate was included in the coupling procedure, GTP binding to P.IA was strongly reduced (Fig. 6 C). A similar inhibition was observed for ATP, GTP, and tripolyphosphate (GDP and GMP were not tested) (Fig. 6 C). In contrast, UTP and β-glycerophosphate, compounds that were unable to block the invasion process, did not interfere with the cross-linking of GTP (Fig. 6 C). Thus, phosphate compounds that blocked P.IA-mediated invasion also inhibited the binding of GTP to the porin. Together, the data are consistent with a regulatory mechanism in which distinct phosphorus containing molecules modulate the P.IA-mediated invasion through an effect on the porin, possibly by mimicking the effect of GTP on porin function.
Figure 6.
Binding of GTP to P.I and its sensitivity to phosphorus-containing compounds. (A) Autoradiogram of electrophoresed whole cell lysates of MS11 carrying either recombinant P.IA (MS-A) or P.IB (MS-B) after cross-linking of γ-[32P]GTP to the bacteria. Binding in the absence of sodium cyanoborohydride (CBH) served as a control. (B) Autoradiogram showing the binding of γ-[32P]GTP to P.I of four P.IA (VP1, 7122, 7929, and FA19) and four P.IB (MS11, FA1090, 5590, and 1291) isolates (bottom). Binding was quantitated by densitometry setting binding in the absence of CBH at zero value (top). (C) Autoradiogram showing the binding of γ-[32P]GTP to MS11-P.IA (MS-A) in the absence (−) and presence of 20 mM sodium phosphate (Pi), 2 mM ATP, 2 mM UTP, 2 mM GTP, 2 mM sodium tripolyphosphate (TPP), and 20 mM sodium β-glycerophosphate (βG-P) (bottom). Binding was quantitated by densitometry setting binding in the absence of CBH at zero value (top).
Discussion
Porins of Gram-negative bacteria function as aqueous transmembrane protein channels that facilitate transport of certain solutes and macromolecules across the outer membrane barrier. In the past decade, there has been much speculation about additional biological functions of porins, particularly in bacterial pathogenesis. For the neisserial P.I porins, these speculations were largely based on observations that P.I proteins spontaneously translocate as functional voltage-gated ion channels into planar lipid bilayers and plasma membranes of eukaryotic cells (36, 40, 44), and impair neutrophil function by causing a transient change in membrane potential and interference with cell signaling events (41–43). By a mechanism seemingly unrelated to its channel forming activity (61), the neisserial P.I proteins also exhibit mitogenic potential and stimulate immunoglobulin secretion by lymphocytes, in line with their strong efficacy as adjuvants (62, 63). However, these interesting and important findings concerning P.I biology were obtained primarily with purified protein and have not been demonstrated for P.I in the context of a viable microorganism. Thus, their significance for the pathogenesis of neisserial disease and their value as a potential target for infection intervention at the cellular level remain elusive.
Here we provide evidence that the gonococcal P.I porin facilitates bacterial invasion into eukaryotic cells. This novel mechanism of invasion was specific for strains bearing the P.IA subtype of porin, independent of the Opa phenotype, and was only operational under conditions of low phosphate. The invasive phenotype was generated by expression of the por1A gene, whereas invasiveness was lost when por1A was replaced with por1B. This, in conjunction with the unaltered behavior of mutants deficient in the P.I-associated protein Rmp, confirmed P.IA as the prime determinant of the invasion event. The striking resemblance in modulation of the bacterial invasion and of the binding of the putative regulator of channel function GTP to the P.I protein by a panel of phosphate-containing compounds suggested the occurrence of a direct effect of phosphate on porin function, thus providing a potential basis for the phosphate sensitivity of this invasion mechanism. The specific invasion of epithelial cells by P.IA-bearing strains may be of particular interest as it coincides with the more invasive behavior of these strains in the setting of the clinical infection. The propensity of gonococci to disseminate from the initial focus of infection towards various body sites is typically associated with the isolation of strains with a P.IA phenotype (46–49).
The porin-mediated invasion mechanism reported here clearly differs from the well-documented uptake conferred by gonococcal Opa outer membrane proteins. Opa proteins comprise a family of up to 11 members that are variably expressed and are structurally composed of relatively conserved transmembrane protein segments interspersed with several variable surface-exposed regions that provide cell binding specificity. Opa proteins facilitate bacterial invasion of mammalian cells through recognition of distinct cell surface receptors, including heparan sulfate proteoglycans (55, 64) and various members of the CD66 (CEA) receptor family (65–68), dependent on the Opa phenotype. Our data confirm the requirement for heparan sulfate–specific Opa proteins (OpaHS) for proteoglycan-mediated entry into Chang epithelial cells, and indicate that this class of proteins is ubiquitous among gonococcal isolates. Based on immunomorphological observations, it has been postulated that P.I may participate in the Opa-mediated uptake (52). However, if present at all, this event is seemingly unrelated to the P.IA-mediated invasion mechanism, as our findings demonstrate that the Opa-dependent uptake was equally effective for P.IA- and P.IB-bearing strains, and independent of the phosphate concentration in the medium, in contrast to the P.IA-mediated event. The Opa-mediated uptake via the CD66 receptor pathway clearly does not require P.I, as evidenced by the efficient internalization of E. coli–Opa recombinants that lack gonococcal porin (66, 68).
In the bacterial outer membrane, the gonococcal porin is intimately associated with the outer membrane protein Rmp and with lipopolysaccharide (58, 59, 69). Rmp is a 31,000-dalton outer membrane protein that is conserved among the pathogenic Neisseria species and has gained much attention as a target of so-called blocking antibodies. Binding of these antibodies to the bacterial cell surface does not lead to the formation of a lytic complement attack complex and blocks the bactericidal activity of antibodies directed against other surface antigens, including P.I (50, 70). Because of the tight association with the porin protein, it was essential to discern whether the uptake event was mediated through P.I and/or Rmp, particularly as antibodies to both P.I and Rmp have been reported to afford Chang cells some protection against gonococcal challenge (71–73). However, the efficient P.IA dependent uptake of the Rmp knockout mutant unequivocally excluded Rmp as a participant in the entry process. These experiments also demonstrated that Rmp does not conceal cryptic P.IB invasion promoting activity, as MS11-P.IB deficient in Rmp was still unable to invade the epithelial cells. Whether LPS contributes to the invasive behavior of P.IA strains remains to be explored. LPS analysis of the P.I recombinant strains showed identical LPS profiles for both P.IA- and P.IB-carrying strains, but subtle P.I phenotype-related changes in LPS structure cannot be excluded.
A key observation in our work was that P.IA-mediated invasion was only apparent under conditions of low phosphate availability, even when iron or amino-acid starvation were imposed on the bacteria. Experiments in which phosphate availability was limited either before or during the invasion assay suggested that the presence of phosphate in the infection assay was the critical determinant of invasion (data not shown). Titration of the phosphate indicated that its modulatory effect occurred within the physiological range of phosphate concentrations present in human serum, with half maximal inhibition of bacterial entry at ∼0.8 mM of inorganic phosphate. The exact molecular basis for the phosphate sensitivity has yet to be resolved. All our data are consistent with a model in which phosphate directly targets P.IA. The phosphate-sensitive invasion was associated with transfer of the por1A gene, as indicated by the allelic replacement experiments, and cross-linking experiments indicated that inorganic phosphates as well as several other phosphorylated molecules prevented the binding of GTP to the porin. The possibility that the phosphate limitation induced a specific metabolic response modulating the invasion process is less likely as evidenced by the effective inhibition of bacterial invasion by tripolyphosphate at concentrations that did not support bacterial growth.
Several mechanisms can be envisioned by which phosphate modulates porin function. The simplest mechanism may be that P.IA carries a phosphate binding site that, if occupied, prevents the interaction of the P.IA porin with a specific cell surface receptor. This concept demands the presence of a surface-exposed binding domain on P.I that is conserved among P.IA strains and absent from P.IB. This is not improbable, as certain mAbs directed against surface-exposed epitopes are specific for, and broadly cross-reactive among, P.IA strains (72). In a more complex scenario, the modulatory effect of phosphate may be related to the channel-forming activity of the porin and its ability to insert into mammalian cell membranes. The neisserial P.I protein differs from most bacterial porins in that its gating function appears to be modulated by binding of nucleoside triphosphates, i.e., GTP and ATP (44). Patch clamp studies using purified P.IA have demonstrated that GTP may regulate the substate (open/closed) of the channel (44). Our cross-linking experiments indicate that GTP binding is much more efficient in P.IA versus P.IB strains and is inhibited by various phosphorus containing molecules. This inhibition correlates remarkably well with their effect on bacterial invasion. We currently favor the working hypothesis that the function of P.IA as an invasin may require the porin to be in an open state, making potential receptor binding sites accessible and/or allowing efficient insertion of functional ion channels into the host cell plasma membrane. In this model, the inhibition of invasion by phosphorylated compounds would be caused by a phosphate-induced closure of channels reminiscent of the reported closing effect of GTP on P.IA (44). Conversely, in the absence of the regulatory phosphorylated compounds maximum opening of the porins would be achieved, facilitating effective bacterial invasion. The proposed phosphate-dependent regulation in P.I channel activity is consistent with the notion that, in other bacterial species, the vast majority of porins in the outer membrane may be in the closed state when the microorganism are grown in rich media and only open under certain environmental conditions such as a shortage of nutrients (74–76). Whether the observed increased binding of GTP (Fig. 6) and the reported 10 to 20 times higher rate of translocation into lipid membranes of the P.IA porin versus its P.IB counterpart (40) are sufficient to account for the unique invasive properties associated with P.IA strains awaits future investigation.
Acknowledgments
We thank Professor P.F. Sparling, Dr. C. Elkins, and Dr. M. Achtman for providing strains and/or antibodies, and Dr. J. Swanson and Dr. M.P. Bos for critical reading of the manuscript.
Abbreviations used in this paper
- AHU
arginine-hypoxanthine-uracil
- DPBS
Dulbecco's phosphate buffered saline
- Opa
opacity outer membrane protein
- ORF
open reading frame
References
- 1.Meyer TF, Pohlner J, van Putten JPM. Biology of the pathogenic neisseriae. Curr Top Microbiol Immun. 1994;192:283–317. doi: 10.1007/978-3-642-78624-2_13. [DOI] [PubMed] [Google Scholar]
- 2.Nassif X, So M. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin Microbiol Rev. 1995;8:376–388. doi: 10.1128/cmr.8.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Putten JPM, Duensing TD. Infection of mucosal epithelial cells by Neisseria gonorrhoeae. . Rev Med Microbiol. 1997;8:51–59. [Google Scholar]
- 4.Haas R, Meyer TF. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 5.Hagblom P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. . Nature. 1985;315:156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 6.Stern A, Brown M, Nickel P, Meyer TF. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 7.Swanson J, Robbins K, Barrera O, Koomey JM. Gene conversion variations generate structurally distinct pilin polypeptides in Neisseria gonorrhoeae. . J Exp Med. 1987;165:1016–1025. doi: 10.1084/jem.165.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy GL, Connell TD, Barritt DS, Koomey M, Cannon JG. Phase variation of gonococcal protein II: regulation of gene expression by slipped strand mispairing of a repetitive DNA sequence. Cell. 1989;56:539–547. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson A-B, Nyberg G, Normark S. Phase variation of gonococcal pili by frame shift mutation in pilC, a novel gene for pilus assembly. EMBO (Eur Mol Biol Organ) J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q-L, Gotschlich EC. Variation of gonococcal lipooligosaccharide structure is due to alterations in poly-G tracts in lgtgenes encoding glycosyl transferases. J Exp Med. 1996;183:323–327. doi: 10.1084/jem.183.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virji M, Heckels JE. The effect of protein II and pili on the interaction of Neisseria gonorrhoeaewith human polymorphonuclear leucocytes. J Gen Microbiol. 1986;132:503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- 12.Makino SM, van Putten JPM, Meyer TF. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeaeinto human epithelial cells. EMBO (Eur Mol Biol Organ) J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weel JFL, Hopman CTP, van Putten JPM. In situ expression and localization of Neisseria gonorrhoeaeopacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J Exp Med. 1991;173:1395–1405. doi: 10.1084/jem.173.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudel T, van Putten JPM, Gibbs CP, Haas R, Meyer TF. Interaction of two variable proteins (PilE and PilC) required for pilus–mediated adherence of Neisseria gonorrhoeaeto human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 15.Kupsch E-M, Knepper B, Kuroki T, Heuer I, Meyer TF. Variable opacity (Opa) outer membrane proteins account for the cell tropism displayed by Neisseria gonorrhoeaefor human leucocytes and epithelial cells. EMBO (Eur Mol Biol Organ) J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Putten JPM. Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae. . EMBO (Eur Mol Biol Organ) J. 1993;12:4043–4051. doi: 10.1002/j.1460-2075.1993.tb06088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandrell RE, Apicella MA. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson A-B, Ilver D, Falk P, Pepose J, Normark S. Sequence changes in the pilus subunit lead to tropism of Neisseria gonorrhoeaeto human tissue. Mol Microbiol. 1994;13:403–416. doi: 10.1111/j.1365-2958.1994.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 19.Rudel T, Scheuerpflug I, Meyer TF. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 20.Heine RP, Elkins C, Wyrick PB, Sparling PF. Transferrin increases adherence of iron-deprived Neisseria gonorrhoeaeto human endometrial cells. Am J Obstet Gynecol. 1996;174:659–666. doi: 10.1016/s0002-9378(96)70446-5. [DOI] [PubMed] [Google Scholar]
- 21.Duensing TD, van Putten JPM. Vitronectin mediates internalization of Neisseria gonorrhoeaeby Chinese hamster ovary cells. Infect Immun. 1997;65:964–970. doi: 10.1128/iai.65.3.964-970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Duarte OG, Dehio M, Guzman CA, Chatwal GS, Dehio C, Meyer TF. Binding of vitronectin to Opa-expressing Neisseria gonorrhoeaemediates invasion of HeLa cells. Infect Immun. 1997;65:3857–3866. doi: 10.1128/iai.65.9.3857-3866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowicki S, Ram P, Pham T, Goluszko P, Morse S, Anderson GD, Nowicki B. Pelvic inflammatory disease isolates of Neisseria gonorrhoeaeare distinguished by C1q-dependent virulence for newborn rats and by the sac-4 region. Infect Immun. 1997;65:2094–2099. doi: 10.1128/iai.65.6.2094-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Putten JPM, Hayes SF, Duensing TD. Natural proteoglycan receptor analogs determine the dynamics of Opa adhesin-mediated gonococcal infection of Chang epithelial cells. Infect Immun. 1997;65:5028–5034. doi: 10.1128/iai.65.12.5028-5034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. . J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Putten JPM, Duensing TD, Cole RL. Entry of OpaA+gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin, and integrin receptors. Mol Microbiol. 1998;29:369–380. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 27.Duensing TD, van Putten JPM. Vitronectin binds to the gonococcal adhesin OpaA through a glycosaminoglycan molecular bridge. Biochem J. 1998;334:133–139. doi: 10.1042/bj3340133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judd RC. Protein I: structure, function and genetics. Clin Microbiol Rev. 1989;2:S41–S48. doi: 10.1128/cmr.2.suppl.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heckels JE, Virji M, Tinsley CR. Vaccination against gonorrhea: the potential protective effect of immunization with a synthetic peptide containing a conserved epitope of gonococcal outer membrane protein PIB. Vaccine. 1990;8:225–230. doi: 10.1016/0264-410x(90)90050-v. [DOI] [PubMed] [Google Scholar]
- 30.Gulati, S., P.A. Rice, M.S. Blake, S.K. Sarafian, S.A. Morse, M.J. Quentin-Millet, and F. Arminjon. 1991. Antibody responses in six volunteers immunized with a gonococcal protein I vaccine. In Neisseriae 1990 - Proceedings of the Seventh International Pathogenic Neisseria Conference. M. Achtman, P. Kohl, C. Marchal, G. Morelli, A. Seiler, and B. Thiesen, editors. Walter de Gruyter, Berlin, Germany. 229–234.
- 31.Wetzler LM, Blake MS, Barry K, Gotschlich EC. Gonococcal porin vaccine evaluation: comparisons of Por proteosomes, liposomes, and blebs isolated from rmpdeletion mutants. J Infect Dis. 1992;166:551–555. doi: 10.1093/infdis/166.3.551. [DOI] [PubMed] [Google Scholar]
- 32.Sparling PF, Elkins C, Wyrick PB, Cohen MS. Vaccines for bacterial sexually transmitted infections: a realistic goal? . Proc Natl Acad Sci USA. 1994;91:2456–2463. doi: 10.1073/pnas.91.7.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkins CE, Carbonetti NH, Coimbre AJ, Thomas CE, Sparling PF. Cloning and constitutive expression of structural genes encoding gonococcal porin protein in Escherichia coli and attenuated Salmonella typhimuriumvaccine strains. Gene. 1994;138:43–50. doi: 10.1016/0378-1119(94)90781-1. [DOI] [PubMed] [Google Scholar]
- 34.Blake MS, Wetzler LM. Vaccines for gonorrhea: where are we on the curve? . Trends Microbiol. 1995;3:469–474. doi: 10.1016/s0966-842x(00)89012-5. [DOI] [PubMed] [Google Scholar]
- 35.Plummer FA, Simonsen JN, Chubb H, Slaney L, Kimata J, Bosire M, Ndinya-Achola JO, Ngugi EN. Epidemiological evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989;83:1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake MS, Gotschlich EC. Gonococcal membrane proteins: speculation on their role in pathogenesis. Prog Allergy. 1983;33:298–313. [PubMed] [Google Scholar]
- 37.Gotschlich, E.C., M.E. Seiff, and M.S. Blake. 1988. Studies of gonoccoccal protein I. In Bacteria–Host Cell Interaction. M.A. Horwitz, editor. Alan R. Liss, Inc., New York. 63–73.
- 38.Douglas JT, Lee MD, Nikaido H. Protein I of Neisseria gonorrhoeaeouter membrane is a porin. FEMS Microbiol Lett. 1981;12:305–309. [Google Scholar]
- 39.Young JD, Blake M, Mauro A, Cohn ZA. Properties of the major outer membrane protein from Neisseria gonorrhoeaeincorporated into model lipid membranes. Proc Natl Acad Sci USA. 1983;80:3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch EC, Blake MS, Gotschlich EC, Mauro A. Studies of porins spontaneously transferred from whole cells and reconstituted from purified proteins of Neisseria gonorrhoeae and Neisseria meningitidis. . Biophys J. 1984;45:104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haines KA, Yeh L, Blake MS, Cristello P, Korchak H, Weissmann G. Protein I, a translocatable ion channel from Neisseria gonorrhoeae, selectively inhibits exocytosis from human neutrophils without inhibiting O2 −generation. J Biol Chem. 1988;263:945–951. [PubMed] [Google Scholar]
- 42.Haines KA, Reibman J, Tang XY, Blake M, Weissmann G. Effects of protein I of Neisseria gonorrhoeaeon neutrophil activation: generation of diacylglycerol from phosphatidylcholine via a specific phospholipase C is associated with exocytosis. J Cell Biol. 1991;114:433–442. doi: 10.1083/jcb.114.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjerknes R, Guttormsen H-K, Solberg CO, Wetzler L. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect Immun. 1995;63:160–167. doi: 10.1128/iai.63.1.160-167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudel T, Schmid A, Benz R, Kolb HA, Lang F, Meyer TF. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell. 1996;85:391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 45.Cannon JG, Buchanan TM, Sparling PF. Confirmation of association of protein I serotype of Neisseria gonorrhoeaewith ability to cause disseminated infection. Infect Immun. 1983;40:816–819. doi: 10.1128/iai.40.2.816-819.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandström EG, Knapp JS, Reller LB, Thompson SE, Hook EW, Holmes KK. Serogrouping of Neisseria gonorrhoeae: correlation of serogroup with disseminated gonococcal infection. Sex Transm Dis. 1984;11:77–80. doi: 10.1097/00007435-198404000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Brunham RC, Plummer F, Slaney L, Rand F, DeWitt W. Correlation of auxotype and protein I type with expression of disease due to Neisseria gonorrhoeae. . J Infect Dis. 1985;152:339–343. doi: 10.1093/infdis/152.2.339. [DOI] [PubMed] [Google Scholar]
- 48.Morello JA, Bohnhoff M. Serovars and serum resistance of Neisseria gonorrhoeaefrom disseminated and uncomplicated infections. J Infect Dis. 1989;160:1012–1017. doi: 10.1093/infdis/160.6.1012. [DOI] [PubMed] [Google Scholar]
- 49.Carbonetti N, Simnad V, Elkins C, Sparling PF. Construction of isogenic gonococci with variable porin structure: effects on susceptibility to human serum and antibiotics. Mol Microbiol. 1990;4:1009–1018. doi: 10.1111/j.1365-2958.1990.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 50.Rice PA, McQuillen DP, Gulati S, Jani DB, Wetzler LM, Blake MS, Gotschlich EC. Serum resistance of Neisseria gonorrhoeae. Does it thwart the inflammatory response and facilitate the transmission of infection? . Ann NY Acad Sci. 1994;730:7–14. doi: 10.1111/j.1749-6632.1994.tb44234.x. [DOI] [PubMed] [Google Scholar]
- 51.Schoolnik GK, Buchanan TM, Holmes KK. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976;58:1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weel JF, Hopman CTP, van Putten JPM. Bacterial entry and intracellular processing of Neisseria gonorrhoeaein epithelial cells: immunomorphological evidence for alterations in the major outer membrane protein P.IB. J Exp Med. 1991;174:705–715. doi: 10.1084/jem.174.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weel JF, van Putten JP. Fate of the major outer membrane protein P.IA in early and late events of gonococcal infection of epithelial cells. Res Microbiol. 1991;142:985–993. doi: 10.1016/0923-2508(91)90009-y. [DOI] [PubMed] [Google Scholar]
- 54.Wong TP, Shockley RK, Johnston KH. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. . J Clin Microbiol. 1980;11:363–369. doi: 10.1128/jcm.11.4.363-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Putten JPM, Paul SM. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeaeentry into human mucosal cells. EMBO (Eur Mol Biol Organ) J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Putten JPM, Weel JFL, Grassmé HUC. Measurements of invasion by antibody labeling and electron microscopy. Methods Enzymol. 1994;236:420–437. doi: 10.1016/0076-6879(94)36031-6. [DOI] [PubMed] [Google Scholar]
- 57.Peter ME, Hall C, Ruhlmann A, Sancho J, Terhorst C. The T-cell receptor zeta chain contains a GTP/GDP binding site. EMBO (Eur Mol Biol Organ) J. 1992;11:933–941. doi: 10.1002/j.1460-2075.1992.tb05132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newhall WJ, Sawyer WD, Haak RA. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. . Infect Immun. 1980;28:785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDade RL, Johnston KH. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. . J Bacteriol. 1980;141:1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanson J, Mayer LW, Tam MR. Antigenicity of Neisseria gonorrhoeaeouter membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982;38:668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulmer JB, Burke CJ, Shi C, Friedman A, Donnelly JJ, Liu MA. Pore formation and mitogenicity in blood cells by the class 2 protein of Neisseria meningitidis. . J Biol Chem. 1992;267:19266–19271. [PubMed] [Google Scholar]
- 62.Liu MA, Friedman A, Oliff AI, Tai J, Martinez D, Deck RR, Shieh JTC, Jenkins TD, Donnelly JJ, Hawe LA. A vaccine carrier derived from Neisseria meningitidiswith mitogenic activity for lymphocytes. Proc Natl Acad Sci USA. 1992;89:4633–4637. doi: 10.1073/pnas.89.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snapper CN, Rosas FR, Kehry MR, Mond JJ, Wetzler LM. Neisserial porins may provide critical second signals to polysaccharide-activated murine B cells for induction of immunoglobulin secretion. Infect Immun. 1997;65:3203–3208. doi: 10.1128/iai.65.8.3203-3208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen T, Belland RJ, Wilson J, Swanson J. Adherence of pilus− Opa+gonococci to epithelial cells in vitro involves heparan sulfate. J Exp Med. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Virji M, Makepeace K, Ferguson DJP, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;29:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 66.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bos MP, Grunert F, Belland RJ. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. . Infect Immun. 1997;65:2353–2361. doi: 10.1128/iai.65.6.2353-2361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. . Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 69.Hitchcock PJ. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. . Infect Immun. 1984;46:202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rice PA, Vayo HE, Tam MR, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeaeby immune serum. J Exp Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Virji M, Zak K, Heckels JE. Monoclonal antibodies to gonococcal outer membrane protein P.IB: use in investigations in the potential protective effect of antibodies against conserved and type-specific epitopes. J Gen Microbiol. 1986;132:1621–1629. doi: 10.1099/00221287-132-6-1621. [DOI] [PubMed] [Google Scholar]
- 72.Virji M, Fletcher JN, Zak K, Heckels JE. The potential protective effect of monoclonal antibodies to gonococcal outer membrane protein IA. J Gen Microbiol. 1987;133:2639–2646. doi: 10.1099/00221287-133-9-2639. [DOI] [PubMed] [Google Scholar]
- 73.Virji, M., K. Zak, K., and J.E. Heckels. 1987. Outer membrane protein III of Neisseria gonorrhoeae: variations in biological properties of antibodies directed against different epitopes. J. Gen. Microbiol. 133:3393–3401. [DOI] [PubMed]
- 74.Schindler H, Rosenbusch JP. Matrix protein from Escherichia coliouter membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci USA. 1978;75:3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buechner M, Delcour AH, Martinac B, Adler J, Kung C. Ion channel activities in the Escherichia coliouter membrane. Biochim Biophys Acta. 1990;1024:111–121. doi: 10.1016/0005-2736(90)90214-9. [DOI] [PubMed] [Google Scholar]
- 76.Delcour AH, Adler J, Kung C, Martinac B. Membrane-derived oligosaccharides (MDO's) promote closing of an E. coliporin channel. FEBS Lett. 1992;304:216–220. doi: 10.1016/0014-5793(92)80622-n. [DOI] [PubMed] [Google Scholar]
- 77.Evins GM, Knapp J. Characterization of Neisseria gonorrhoeaereference strains used in development of serologic classification systems. J Clin Microbiol. 1988;26:358–363. doi: 10.1128/jcm.26.2.358-363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tjia KF, van Putten JPM, Pels E, Zanen HC. The interaction between Neisseria gonorrhoeaeand the human cornea in organ culture. An electron microscopic study. Graefe's Arch Clin Exp Ophthalmol. 1988;226:341–345. doi: 10.1007/BF02172964. [DOI] [PubMed] [Google Scholar]
- 79.Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]