Abstract
The B cell receptor (BCR) triggers a variety of biological responses that differ depending upon the properties of the antigen. A panel of M13 phage-displayed peptide ligands with varying affinity for the 3-83 antibody was generated to explore the role of antigen-BCR affinity in cell activation studies using primary 3-83 transgenic mouse B cells. Multiple parameters of activation were measured. T cell–independent B cell proliferation, antibody secretion, induction of germline immunoglobulin γ1 transcripts, and B cell production of interleukin (IL) 2 and interferon γ responses were better correlated with antigen-BCR affinity than with receptor occupancy. In contrast, other responses, such as upregulation of major histocompatibility complex class II and B7.2 (CD86), secretion of IL-6, and B cell proliferation in the context of CD40 signaling were only weakly dependent on antigen affinity. Biochemical analysis revealed that at saturating ligand concentrations the ability of phage to stimulate some early signaling responses, such as Ca++ mobilization and tyrosine phosphorylation of syk or Igα, was highly affinity dependent, whereas the ability to stimulate Lyn phosphorylation was less so. These data suggest that the BCR is capable of differential signaling. The possibility that differential BCR signaling by antigen determines whether an antibody response will be T independent or dependent is discussed.
Keywords: B lymphocytes, differential activation, phage library, immunoglobulin, transgenic mice
Unlike growth factor, cytokine, or hormone receptors, the B cell receptor (BCR)1 does not have a unique ligand with predefined stereochemical and affinity characteristics. This receptor can be stimulated by ligands of varied affinity or stereochemical structure, and the ability to distinguish among subtle differences may be an important evolved specialization. B lymphocytes are able to respond in a variety of ways to BCR engagement (1); for example, antigen stimulation of mature B cells can lead to functional inactivation (anergy), apoptosis, proliferation, and differentiation to Ig secretion, or to the formation of a memory response. Weak antigenic stimuli have even been postulated to be required for resting B cell survival because induced deletion of Ig H chain by cre/loxP-mediated recombination leads to rapid death of mature B cells (2). During the generation of immune tolerance in the preimmune repertoire, immature B cells can similarly undergo a range of responses to antigen including receptor editing, death, or anergy. It has also been suggested that very weak interactions with antigens in immature sIgM+ cells can drive differentiation to maturity (3–5). This complex differentiation potential, which is induced through a single antigen receptor, suggests an ability to integrate and interpret subtle differences in antigen structure.
Extensive in vitro studies using anti-IgM or anti-IgD antibodies as surrogate antigens have analyzed the requirements and steps of B lymphocyte activation (6–12). BCR-mediated activation events in mature resting B cells include a rapid tyrosine phosphorylation cascade apparently starting within conserved immunotyrosine-based motifs (ITAM) of the BCR signal transducer proteins Ig-α and Ig-β (13, 14). This phosphorylation can be mediated by the Src family kinase members lyn, blk, fyn, or fgr owing to the association of some or all of these kinases with the nonligated receptor (15, 16). Phosphorylation of conserved sites on Ig-α or β recruits and activates additional kinases, including syk, which subsequently activate important downstream targets (14, 17). Various tyrosine phosphorylations are believed to recruit additional molecules involved in B cell signaling such as phosphatidylinositol 3-kinase, phospholipase C-γ2, and Shc, which results in a propagation of signals, including phosphoinositide metabolism, calcium mobilization, activation of protein kinase C and MAP kinase cascades, and transcription factor activation (18–23). Sustained signaling stimulates a variety of events in the nucleus resulting in enhanced cell surface expression of MHC class II molecules and the T cell costimulator B7.2 (CD86), and in some cases entry into the cell cycle. Further signals regulate Ig secretion.
To initiate these events, BCR aggregation and a certain level of BCR occupancy are required. Studies analyzing B cell immune responses to T cell–independent antigens have concluded that thresholds of antigen valence, epitope density, or epitope organization exist that must be exceeded in order for a productive response to occur (24–27). Antigens with structures that fail to satisfy these putative thresholds can still provoke B cell immune responses, but these require T cell help. Similarly, thresholds of antigen structure and B cell receptor occupancy are believed to control the phenotype of immune tolerance (28). But the quantitative relationships among antigen structure, biological response, and intracellular signaling events have not yet been systematically studied in B cells.
The ability of antigen receptors to generate a range of responses dependent on antigen/receptor affinity (or avidity) has been intensively studied in T cells, which can respond biologically in a variety of ways to ligands of subtly differing structure. When presented in the peptide-binding groove of MHC molecules, certain peptide ligands, termed agonists, induce a robust proliferative response in mature T cells. Slight modifications of the sequences of the agonist peptides, which usually also lower affinity for the TCR, can yield partial agonists peptides that fail to induce T cell proliferation, but stimulate some biological activities, such as specific lymphokine secretion (29). Altered peptide ligands can sometimes also specifically antagonize T cell responses to agonists (30, 31). Even remarkably low affinity interactions can yield significant biological responses, such as the critical positive selection step that regulates differentiation and survival of maturing thymocytes (32, 33). Biochemical analysis indicates that these differential responses to ligands of very low affinity are associated with partial signaling through the TCR and associated molecules (18, 29, 34, 35).
To determine if B cells have a similar capacity for differential responsiveness and to understand the biochemistry of these processes, we have taken advantage of two powerful tools to evaluate BCR–ligand interactions. First, we have used a homogeneous splenic B cell population isolated from mice transgenic for genes encoding the 3-83 antibody, which was raised against the H-2Kk MHC class I molecule (36). This B cell population is virtually monoclonal because the transgenic Ig mediates feedback suppression of endogenous Ig gene rearrangements (37). Second, in order to characterize BCR stimulation by antigen instead of anti-BCR antibodies, we have screened bacteriophage-displayed peptide libraries to identify alternative ligands for the 3-83 antibody. These phages contain 12– or 15–amino acid peptide inserts in the pIII protein, which is present in four copies per phage particle and is essential for phage infectivity (38, 39). We have been able to isolate and characterize phages that vary in their relative affinity for 3-83, but that are otherwise identical in valence and overall structure, and therefore represent a useful system to compare B lymphocyte activation based on affinity differences. In this study, we use these reagents to test the hypothesis that B cell receptors can mediate differential signaling.
Materials and Methods
Mice.
The 3-83 transgenic mice (36), which express IgM and IgD forms of the 3-83 antibody, were bred and maintained in the animal care facility at the National Jewish Center Medical and Research Center. 3-83 mice were backcrossed a minimum of 10 times onto a B10.D2nSn/J background.
Phage-displayed Peptide Libraries.
Generation of the 12mer and 15mer libraries have been described previously (40). In brief, degenerate oligonucleotides encoding the peptide insert sequences and flanking restriction sites were annealed, cleaved with restriction enzymes, purified, and ligated into the M13 DNA vector digested with the same restriction enzymes: BglI for the 15mer library and XhoI/XbaI for the 12mer library. Ligation reactions were electroporated into competent DH5αF′ bacteria, which were mixed with top agar, poured onto petri plates and incubated for 12 h at 37°C to allow plaque formation. The phage particles were then recovered by diffusion into PBS that was added to the surface of the plates and incubated 4 h at 4°C with gentle rocking. After centrifugation at 4,000 rpm to remove contaminating bacterial cells, phages were precipitated with polyethylene glycol/NaCl, resuspended in PBS and used for subsequent affinity selection.
Selection of 3-83 Binding Phages from Phage-Displayed Peptide Libraries.
Library screenings were performed as previously described (40). In brief, microtiter plate wells were coated with 10 μg/ml of 3-83 antibody in 100 mM NaHCO3 for 3 h, then blocked with a solution of 5 mg/ml BSA in 0.1 M NaHCO3 for 1 h. Approximately 5 × 1011 infectious particles were incubated with the 3-83-coated wells in 50 μl PBS and 0.1% Tween 20 for 2 h. The wells were washed five times with PBS and 0.1% Tween 20, and bound phages were eluted with 50 mM glycine and HCl, pH 2.2, and neutralized with 50 μl of 200 mM sodium phosphate, pH 7.4. Recovered phages were affinity selected two more times as described above.
Large Scale Bacteriophage Purification.
Phages were purified using a modified version of a protocol described previously (41). In brief, DH5αF′ competent cells were infected with phage and grown overnight in 250 ml LB, 10 μg/ml kanamycin. After centrifugation to remove bacteria, the supernatants were adjusted to 0.5 M NaCl, 5% polyethylene glycol 6000, and the phages were allowed to precipitate overnight at 4°C. Phages were recovered by centrifugation for 45 min at 8,000 rpm, and the pellet was resuspended in 10 ml of 0.01 M Tris-HCl, pH 7.6, 1 mM EDTA, 0.1% sarkosyl, and shaken slowly at room temperature for 30 min. The solution was adjusted to 0.5 M NaCl, 5% polyethylene glycol and after 2 h at 4°C, the precipitated phages were recovered by centrifugation for 1 h at 8,000 rpm. The pellet was resuspended in 10 ml of NET buffer (0.1 M NaCl, 1 mM EDTA, and 0.01 M Tris-HCl, pH 7.6), mixed well and the phage repelleted by centrifugation at 15,000 rpm for 1 h. The phage pellet was finally resuspended in NET buffer and an OD at 260 nm reading was taken to measure the phage concentration (1 OD unit = 1 mg/ml of phage).
ELISA.
Culture supernatants were taken at the specified time for each experiment. Analysis of 3-83 production was performed using plates coated with 5 μg/ml of 54.1 rat anti-3-83 idiotypic antibody in PBS. After blocking with PBS containing 0.5% BSA, 0.4% Tween 20, supernatants at different dilutions were incubated with the coated wells overnight. After several washes, the wells were incubated with goat anti–mouse IgM coupled to horseradish peroxidase (Southern Biotechnology Associates Inc., Birmingham, AL). Bound peroxidase was developed by colorimetric substrate 2.2′-azino-di-3-ethyl-benzthiazoline-6-sulfonic acid (Sigma Chemical Co., St. Louis, MO) in McIlvain's buffer (84 mM Na2PO4, 48 mM citrate, pH 4.6) containing 0.005% H2O2. The reactions were quantitated in an automated plate reader at 410 nm with a reference wavelength of 490 nm (Dynatech, Alexandria, VA). For 3-83 concentration, standard curves were generated using a supernatant from Cos lin D1, an Sp2/0 myeloma transfected with the 3-83 IgM heavy and light chain genes.
Primary B Cell Purification.
Resting B lymphocytes were isolated as previously described (42). In brief, spleens from 3-83 transgenic mice were removed aseptically and single cell suspensions were prepared. Red blood cells were depleted by incubation for 5 min in Tris-ammonium chloride, pH 7.2, and T cell depletion was done by incubating the cells with anti-Thy antibodies (HO13.4.9 and T24/40) plus rabbit complement (GIBCO BRL, Gaithersburg, MD) and 10 μg/ml DNAse (Sigma Chemical Co.) at 37°C for 45 min. The resting B cells were finally isolated by centrifugation through Percoll at a density of 1.079 g/ml.
B Cell Culture.
Cells were cultured in 24-well plates at a final concentration of 1 × 106 cells/ml in IMDM 10% FCS, in the presence of polymyxin B (Sigma Chemical Co.) at a concentration of 50 μg/ml. The phages were diluted to a concentration of 1 mg/ml in IMDM 10% FCS, passed through a 0.45-μm filter and added to the cells at the specified final concentrations. The anti-Fd antibody (Sigma Chemical Co.), which cross-reacts with and cross-links M13 phage, was added to each well at a concentration of 5 μg/ml. After various times of incubation, supernatants were analyzed for 3-83 IgM production and the cells were stained for several cell surface markers. Proliferation was monitored using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay as described below. Anti-CD40 antibody (a gift of Dr. Ton Rolink, Basel Institute for Immunology, Basel, Switzerland) was added as supernatant of FGK45 hybridoma culture, at 100 μl per 106 spleen cells.
Flow Cytometry.
5 × 105 cells were stained on ice for 20 min with the indicated antibodies in PBS supplemented with 1% BSA, 0.02% NaN3. The following antibody reagents were used: mouse anti-IgM-FITC (Zymed Laboratories, Inc., South San Francisco, CA), mouse anti-I-Ad–FITC (PharMingen, San Diego, CA), mouse anti-B220-PE (Caltag Laboratories, Burlingame, CA), biotin-54.1, biotin-anti-B7.2 (PharMingen), or biotin-anti-IgD (PharMingen). After two washes, cells were incubated with streptavidin-tri-Color (Caltag) for 20 min on ice to visualize bound biotinylated antibodies, then washed twice and fixed in a solution of PBS/2% paraformaldehyde. Flow cytometric analysis was done using a FACScan® (Becton Dickinson & Co.) and the data analyzed using the Cell Quest software.
PCR Assay.
RNA isolation using RNazol B (Tel-Test, Friendsville, TX), cDNA first strand synthesis, and GαS PCR were carried out as previously described (37). The γ1 switching transcript PCR assay was as described in (43).
RNase Protection Assay.
The RiboQuant kit (PharMingen) was used for this assay with the mCK-1 probe set, according to the protocol recommended by the manufacturer. 7 × 106 purified B cells were stimulated for 3 or 6 h in presence of 500 μg of the various phages and 5 μg/ml anti-Fd antibody (Sigma Chemical Co.). RNA was isolated using RNazol B (Tel-Test), and used for RNase protection assay.
Cytokine Staining.
3-83 splenic cells were stimulated 4 h with Pwt or P31 phage and stained first with B220-PE antibody (Caltag) then stained intracellularly in presence of 0.3% saponin with anti-IFN-γ–FITC, anti-IL-2–FITC, Rat IgG1–FITC (PharMingen) or anti-IgM–FITC (Zymed Laboratories, Inc.). After several washes in presence of saponin 0.1%, the cells were placed on poly-d-lysine (Sigma Chemical Co.) coated cover slips, fixed with 1% paraformaldehyde in PBS, mounted and analyzed with a 100× planApo 1.4 oil objective using a Nikon microscope. Photos were taken using a Photometric camera and IPlab software (Signal Analytics Co., Vienna, VA).
Calcium Mobilization Analysis.
For measurement of intracellular free calcium, K46J (1 × 106 cells/ml) cells were loaded with Indo-1 AM (Molecular Probes, Inc., Eugene, OR) and subsequently stimulated with either a mouse anti-Ig κ antibody (Southern Biotechnology Associates Inc., Birmingham, AL) or with various concentrations of the different phage followed as specified by anti-Fd antibody (Sigma Chemical Co.). Calcium mobilization was monitored by flow cytometry for 10–15 min (Cytofluorograf 30/50; Ortho Diagnostic Systems, Westwood, MA). The percent of responding cells and the 490/390 nm mean ratio were calculated using the MultiTime software (Phoenix Flow Systems, San Diego, CA).
Phosphotyrosine, Immunoprecipitation, and Immunoblot Analysis.
3-83 primary B cells (3 × 106 cells) were stimulated for various amounts of time at 37°C with goat anti–mouse IgM antibody (Southern Biotechnology Associates Inc.) or one of the phage preparations followed by cross-linking with anti-Fd antibody (Sigma Chemical Co.). Cells were lysed in 1% NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 10 mM Tris-HCl, pH 7.4, 2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 0.4 mM EDTA, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml each of aprotinin, leupeptin, and α-1-antitrypsin) and centrifuged for 10 min at 14,000 rpm to remove detergent-insoluble material. Supernatants were then mixed with SDS reducing buffer. Whole cell lysates were fractionated on 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. Anti-phosphotyrosine immunoblotting was carried out by incubating the membrane with the Ab2 antibody (Calbiochem Novabiochem Corp., La Jolla, CA) in 5% BSA in TBS (10 mM Tris-HCl, pH 8.0, 150 mM NaCl) for 2 h at 25°C, followed by rat anti–mouse IgG coupled to horseradish peroxidase (Zymed Laboratories, Inc.) in 5% milk in TBS for 1 h at 25°C. The blot was then developed using ECL system (Amersham Pharmacia Biotech Inc., Piscataway, NJ).
Immunoprecipitations of Syk, Lyn, and Igα were performed on lysates of 9 × 106 cells using 5–10 μg of affinity-purified antibodies coupled to Sepharose beads. Immunoprecipitates were washed three times in lysis buffer, resuspended in 50 μl of reducing SDS-PAGE sample buffer, then fractionated using 10% SDS-PAGE gels. Electrophoretically fractionated proteins were transferred to PVDF membranes which were then treated as described above.
Results
Identification of Alternative Ligands for the 3-83 Antibody.
Two M13 phage-display random peptide libraries containing either 15mer or 12mer insert peptides were screened for phages that bound to the 3-83 antibody. This led to the isolation of a single phage bearing a 15mer peptide and 13 unique phages containing 12mer inserted peptides. The inserted peptide sequences of affinity selected phages are listed in Fig. 1 A. The isolated phages bound specifically to the 3-83 heavy plus light chain combination, but not to isotype-matched antibodies or other antibodies bearing similar light or heavy chains (data not shown). Thus, these phages most likely bind to the 3-83 antigen binding site.
Figure 1.
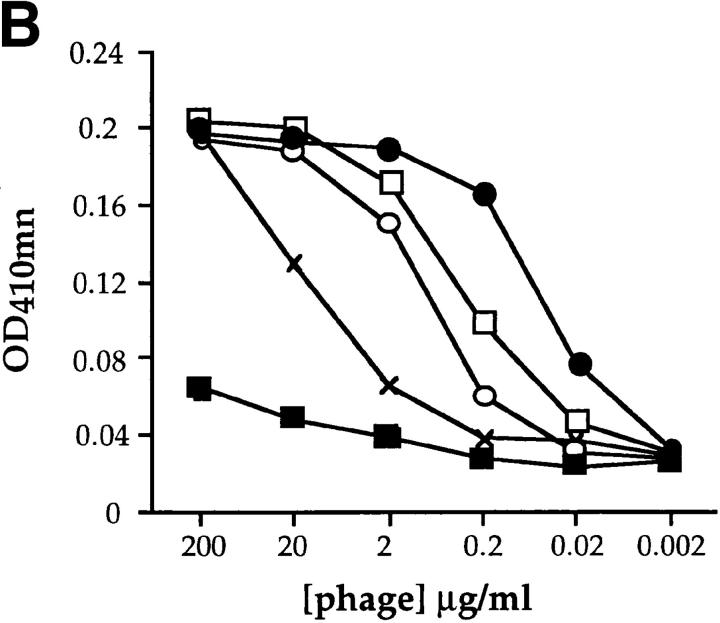
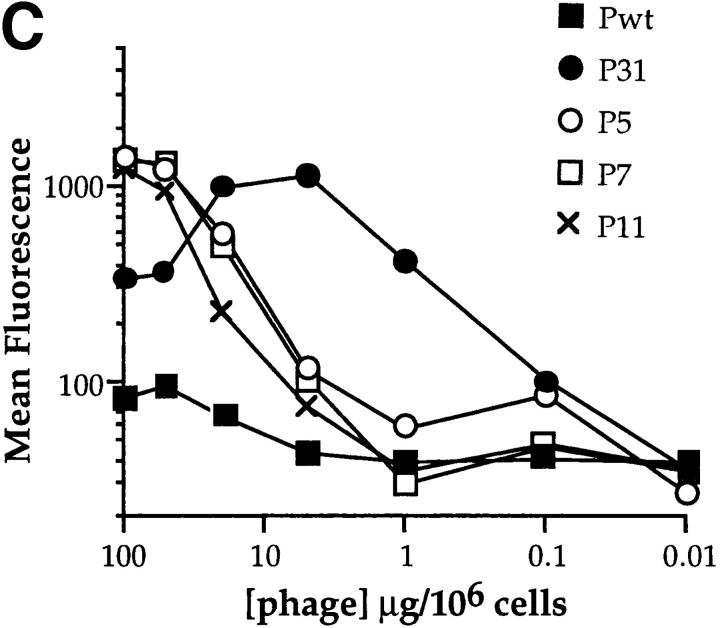
3-83 peptide ligands isolated from phage-display libraries. (A) Peptide sequences inserted into the PIII minor coat protein of M13 phages isolated by affinity purification with the 3-83 antibody are shown. The shadowed boxes highlight the common motifs found in the 12mer peptides. Designations of the phages referred to elsewhere in the text are shown at left. (B) ELISA binding analysis of the relative affinities of phages named P31, P5, P7, and P11. Serial dilutions of each purified phage were reacted with 3-83 Ig–coated plates and revealed with an anti-bacteriophage Fd-HRP. Pwt phage, which contains no inserted peptide, was used as a control to account for nonspecific background. (C) Relative binding of the various phages to 3-83 splenic B cells. 3-83 spleen cells were stained with B220-PE antibody and decreasing amount of the different peptide expressing-phages, which were revealed with anti-Fd antibody followed by goat anti–rabbit FITC antibody. Mean fluorescence intensities were measured on gated B220+ cells. Data shown are representative of several experiments.
Homology searches of GENBANK did not reveal any similarities between these peptide sequences and any MHC genes. None of the 12mer sequences matched any known database sequences. Comparison of the 12mer peptide sequences to each other allowed us to identify three motifs (Fig. 1 A, boxed sequences). The first motif, asn-trp-asn-xxx-val/leu-gln, was found in five sequences at various positions within the peptide, whereas the remaining amino acids in the peptides appeared to be totally unrelated. The second motif, pro-gly-gly-trp-gln-xxx-xxx-trp, was found at different positions in two peptides. Again, the amino acids outside the motif were distinct. The last motif, gln-asp-tyr, was likewise found in two sequences, but in this case at the same position within the peptides. Collectively, these data reveal that it is possible to identify linear epitopes that bind to an antibody known to recognize a conformational epitope, and that many linear epitopes specific for 3-83 can be found that possess almost no sequence similarities among themselves or with the initial immunogen.
Recombinant Phages Bind to the 3-83 Antibody with Differing Relative Affinities.
To define among the selected phages (Fig. 1 A) ligands with distinct affinities for the 3-83 antibody, we measured phage binding in solid phase ELISA using plate-bound 3-83 antibody (Fig. 1 B) or in binding assays to 3-83-bearing B cells (Fig. 1 C). The 15mer peptide-bearing phage P31 had the highest affinity for 3-83, whereas the 12mer-bearing phages exhibited a wide range of affinities (data not shown). Four representative phages were chosen for detailed analysis: P31, which has the highest relative affinity, P11 with the lowest relative affinity, and P5 and P7, which possess intermediate affinities for the 3-83 antibody. P5 and P7 binding to 3-83+ B cells was inhibited by a synthetic peptide containing the P31 sequence, indicating that P31, P5, and P7, bind to the same region of the 3-83 Ig (data not shown). A phage lacking a peptide insert, Pwt, was used as a negative control in all the experiments. Thus, phages of differing relative affinity to the 3-83 BCR were identified for detailed studies of B cell activation.
Recombinant Phages Can Induce Full or Partial B Cell Activation In Vitro.
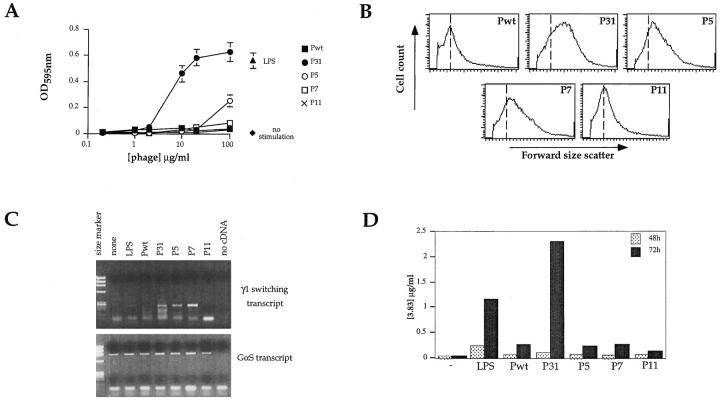
To test if the variable affinities of the phage ligands for 3-83 had any relevance to B cell activation, we analyzed the biological effects of interaction between phages and B lymphocytes in short term cultures. Splenic B cells from 3-83 Tg mice were stimulated with a broad range of concentrations (0.2–100 μg/ml) of highly purified phages. Concentrations >50 μg/ml are saturating for all the phages (Fig. 1 C). As the phages tend by nature to partially aggregate, they were incubated with cells in the presence of anti-phage antibody to promote extensive and uniform cross-linking. After 72 h of culture, cell count, proliferation, and blastogenesis were monitored for each culture condition. P31 was able to induce strong cellular proliferation (Fig. 2 A), whereas P5 induced only a very modest proliferation at the highest concentration. No proliferation was observed with P7 or P11 phages (Fig. 2 A), even at concentrations that saturated the BCR (Fig. 1 C). The absolute number of B220+ cells recovered at the end of culture correlated well with the proliferative response, as only P31-stimulated cultures demonstrated an increase in B cell recovery over input (data not shown). After 72 h of culture with P31, and to a lesser extent with P5 and P7, B lymphocytes were in a blasting state as shown by their increased forward light scatter characteristics (Fig. 2 B), whereas no blastogenesis was observed when B cells were cultured with Pwt or with receptor saturating amounts of the low affinity phage, P11.
Figure 2.
3-83 Ig affinity for peptide-expressing phages partially correlates with the degree of in vitro B cell activation. 3-83 transgenic spleen cells were cultured in the presence of increasing concentrations of the various phage or with 10 μg/ml LPS. After 72 h, (A) cell viability and proliferation was assessed using a MTT assay. Proliferation data are mean ± SD from triplicate wells. (B) Forward size scatter of cells stimulated with phage at 100 μg/ml was analyzed by flow cytometry. Data shown come from the B220+ gated population and are representative of 5 independent experiments. (C) Germline γ1 transcripts were analyzed by RT-PCR on RNA from cells stimulated 48 h with phage at 100 μg/ml. GαS PCR was used as an internal control. Data shown are representative of four different experiments. (D) Supernatants of splenic stimulated-cultures were tested by ELISA after 24, 48, and 72 h of stimulation. Serial dilutions of each supernatant were reacted on 54.1 antibody (3-83 anti-idiotype)-coated plates and revealed with either an anti-IgM or an anti-κ antibody. Data shown represent one of several experiments for the 48- and 72-h time points. Supernatant from cultures stimulated with LPS (20 μg/ml) was used as a positive control of Ig production.
An important marker of B cell differentiation is the production of germline Ig constant region heavy chain mRNA, which precedes heavy chain gene class switching (43). P31, P5, and P7 were all able to stimulate transcription of the germline γ1 locus, whereas neither P11 nor LPS were able to do so (Fig. 2 C).
The ability of 3-83-binding phages to stimulate B cell effector function, i.e., antibody secretion, was tested (Fig. 2 D). Significant 3-83 IgM secretion was detected in cultures stimulated for 72 h by P31 or LPS, whereas none of the other 3-83-binding phages was able to induce a significant level of 3-83 production over that induced by Pwt. IgM secretion was also measured as a function of increasing concentrations of phage: both P5 and P7 induced a response at a concentration of 100 μg/ml, but it never reached the level of response observed with P31, which could be induced with concentrations as low as 2 μg/ml (data not shown). BCR saturating concentrations of P11 never induced a significant response, despite its ability to bind the BCR (Fig. 2 D).
Collectively, these data indicate that the levels of B cell proliferation, T cell independent antibody secretion, and initiation of class switching correlate with the relative affinity of the BCR for its ligand, rather than with BCR occupancy. P31 was able to induce full stimulation of 3-83+ B cells, whereas P5 and P7 phages were able to induce only partial activation responses, as indicated by blastogenesis and production of sterile γ1 transcripts.
Peptide-Expressing Phages Induce Differing Patterns of Lymphokine Expression.
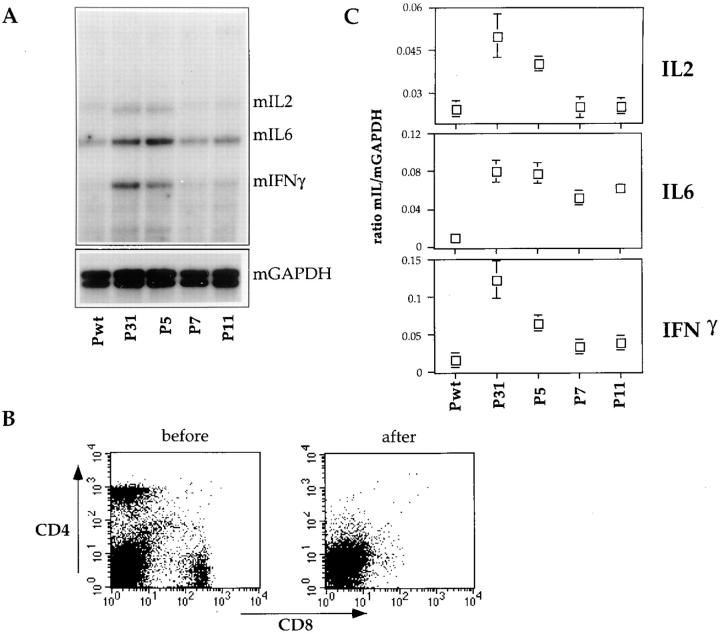
We tested the ability of the various phages to stimulate lymphokine gene expression in purified resting 3-83 B cells both because lymphokines have potent biological effects and because partial T cell agonists can induce lymphokine secretion, but not cell proliferation. After 3 and 6 h of phage stimulation, mRNA levels in the B cells was analyzed by RNase protection assays using a large set of lymphokine probes. No antigen-induced changes were detected in the levels of the following mRNAs: IL-4, IL-5, IL-9, IL-10, IL-13, and IL-15. Little induction could be seen in any of the samples at the 3-h timepoint (data not shown). But after 6 h of stimulation with P31, a clear increase in mRNAs was observed for IL-6, IL-2, and IFN-γ (Fig. 3, A and C). P5 was able to induce a similar pattern of lymphokine mRNA upregulation, however, IL-2 and IFN-γ transcription was stimulated to a slightly lower level than with P31. By contrast, P7 and P11 stimulation only increased IL-6 mRNA levels and failed to stimulate IL-2 and IFN-γ transcription (Fig. 3, A and C). The rise in IL-2 and IFN-γ mRNA in P31- or P5-induced cultures was not likely the result of T cell activation, as the cell preparations were virtually devoid of T cells as a result of the specific depletion of Thy 1+ cells (Fig. 3 B); in addition experiments using whole spleen cells resulted in lower signals than in the purified B cell cultures (not shown). However, to rule out the possibility that the observed mRNA upregulation might have been the indirect activation of contaminating non-B cells, total splenic cells were costained for B220 and IL-2 or IFN-γ after 4 h of stimulation with P31 or Pwt (Fig. 4). About 5% of the P31-stimulated cells costained for B220 and IL-2 or IFN-γ, whereas no brightly stained cells were observed upon staining with an isotype control antibody. Furthermore, no B220/IFN-γ or B220/ IL-2 costained cells were observed in Pwt stimulated cultures (data not shown). The low percentage of B cells expressing IL-2 or IFN-γ seemed to indicate a transient state of lymphokine production and correlated well with the low level of mRNA expression detected in RNase protection assay (Fig. 3). B cells are reported to be capable of producing IL-2, IL-6, and IFN-γ (44–48), but the ability of B cells to secrete IL-2 and IFN-γ is not widely accepted. Only P31 and P5 were able to upregulate IL-2 and IFN-γ, whereas all of the 3-83-binding phage induced an increase IL-6 mRNA. These data strongly suggest that the various phages induce qualitatively distinct lymphokine expression responses based on BCR-ligand affinity rather than on receptor occupancy.
Figure 3.
RNase protection assay reveals differential lymphokine expression patterns induced by the various phages. (A) RNA isolated from purified 3-83 primary B cells stimulated for 6 h with 100 μg phage was assayed for RNase protection using the multilymphokine mCK-1 probe. GAPDH mRNA served as an internal control of RNA input. (B) Cells were stained with anti CD4-PE and anti CD8-FITC before and after B cell purification to monitor the extent of residual T cell contamination. (C) Quantitation of lymphokine gene mRNA levels. Mean ± SD of five independent experiments. After densitometry analysis on an Eagle III eye apparatus (Stratagene), the ratio between the GAPDH and the lymphokine signals were calculated for each phage.
Figure 4.
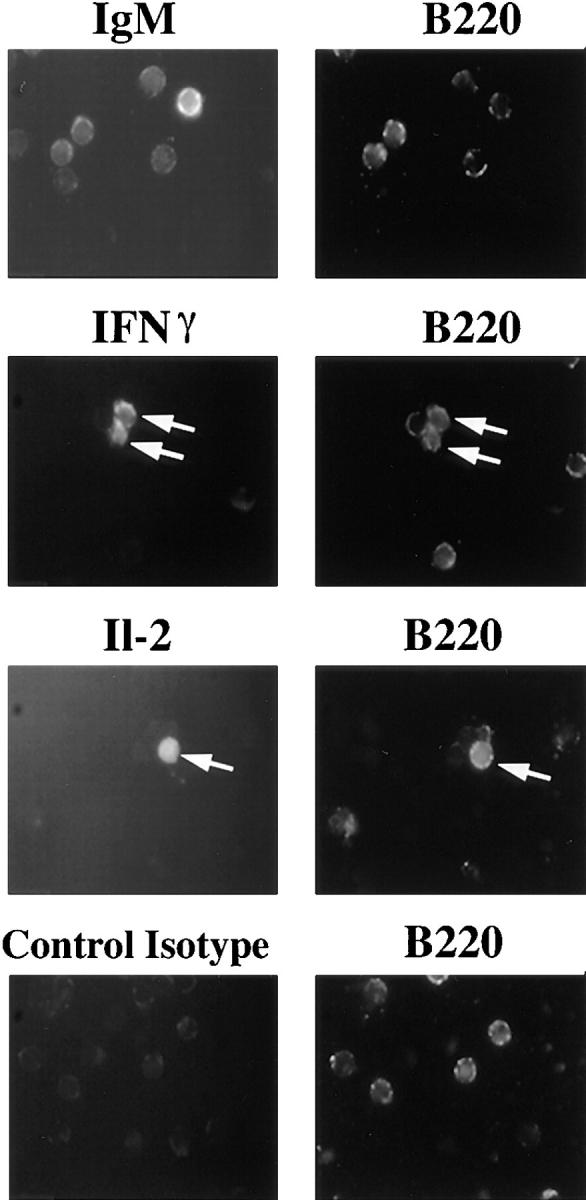
Intracellular staining of B lymphocytes for IL-2 and IFN-γ cytokines. After 4 h of stimulation with P31 at 100 μg/ ml, 3-83+ splenic cells were stained with B220-PE ab and anti-IgM–FITC, anti-IFN-γ–FITC, anti-IL-2–FITC or Rat IgG1/κ– FITC. Data shown are representative of six independent experiments.
All Recombinant 3-83 Binding Phages Were Able to Initiate Signs of T-dependent Response In Vitro.
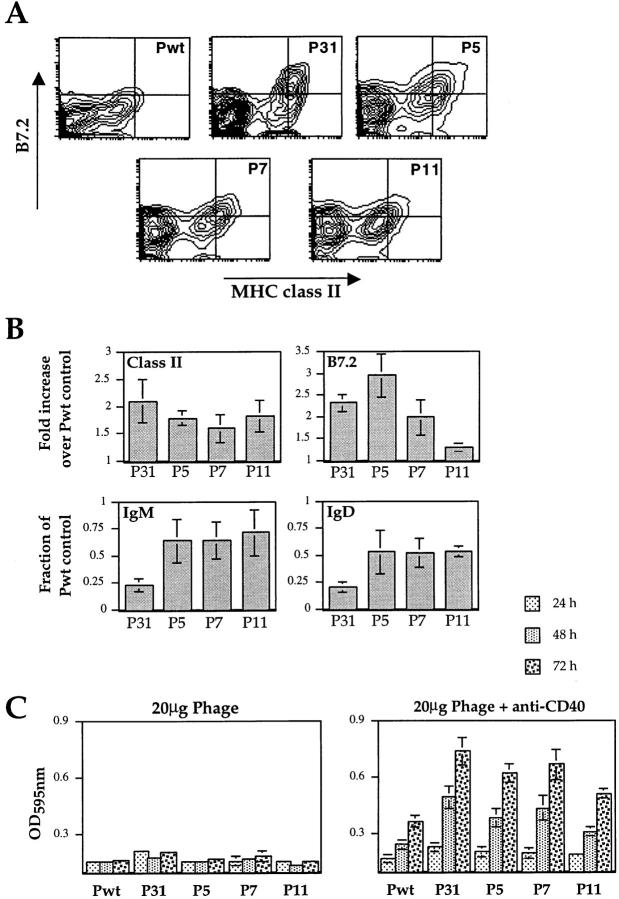
After 24 h of culture at saturating phage concentration, B cells were analyzed for expression of surface proteins known to be upregulated early during activation (Fig. 5, A and B). The relatively high basal MHC class II I-A protein expression was specifically increased 1.5–2-fold on B cells cultured with all of the 3-83 reactive phage, but not with Pwt. Expression of B7.2 (CD86), which is involved in costimulation during B-T interactions (49, 50), was increased from undetectable levels to fluorescence levels that were two- to threefold above background upon interaction with P31, P5, and P7 phage, whereas P11 induced a lower (1.3-fold), but consistent stimulation of B7.2 expression. The ability of phage stimulation to downmodulate sIgM and sIgD levels was also measured using a flow cytometry assay (Fig. 5 B). Like the induction of MHC class II and B7.2 molecules, Ig internalization of its ligand is important for B cell antigen presentation to T cells. The high affinity phage P31 markedly downregulated sIgM and sIgD cell surface expression, whereas the lower affinity phages P5, P7, or P11 only modestly downmodulated sIgM and sIgD (Fig. 5 B). Phage binding failed to directly inhibit staining with anti-IgM and anti-IgD antibodies (data not shown) indicating that specific phage binding actively induced the down modulation of these molecules.
Figure 5.
Ability of phages to induce signs of T-dependent response in vitro. (A) Surface expression of B7.2 and class II markers was assessed by immunofluorescence staining on cells stimulated with phages at 100 μg/ ml. (B) The mean surface expression of the indicated markers on B cells stimulated with the various phage expressed relative to the expression levels of cells cultured with Pwt. For all markers analyzed, the mean fluorescence intensity was measured on the B220+ gated population, after 24 h of stimulation. Data shown represent a mean average ± SD of four independent experiments. (C) 3-83 transgenic spleen cells were stimulated with the various phage at 20 μg/ml in absence of anti-Fd cross-linking ab, with or without anti-CD40 ab. Proliferation was assessed using a MTT assay at 24, 48, and 72 h of stimulation. Data are mean ± SD from triplicate wells from three independent experiments.
To determine if the 3-83-binding phages that were not directly mitogenic could induce B cell proliferation in conditions that mimic T cell help, we tested their ability to promote growth in synergy with anti-CD40 antibody (Fig. 5 C). 3-83 splenic cells were cultured with subsaturating concentration of phage (20 μg/ml), without anti-Fd cross-linking antibody, in the presence or absence of an agonistic anti-CD40. Engagement of the CD40 molecule on B lymphocytes, alone or in the presence of Pwt, induced some proliferation, as expected from prior studies (51). Importantly, in the presence of anti-CD40 all of the 3-83 binding phages induced significant augmentation in proliferation (Fig. 5 C). Flow cytometry analysis with B cell markers confirmed the specific increase and blasting state of the B cell population (data not shown).
These data demonstrate that, regardless of their affinity for the BCR, all of the 3-83-binding phages were able to increase the expression of MHC class II, CD86, and IL-6, and to stimulate B cell proliferation in presence of anti-CD40 antibody. Thus, some B cell activation responses are not strongly affected by the affinity of the BCR for its ligand. Interestingly, this subset of responses is associated with the T cell–dependent B cell response.
Phage-Induced Ca++ Mobilization Is Affinity Dependent.
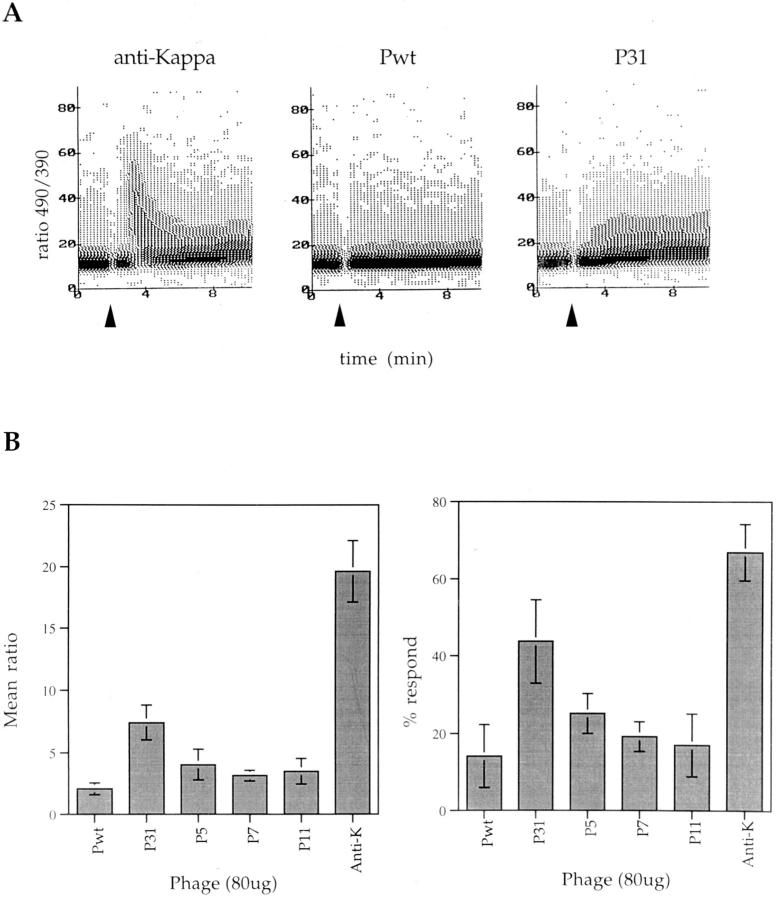
That the 3-83-reactive phages induced distinct and reproducible biological responses prompted us to test if these responses were correlated with early signaling events. The ability of the phages to mobilize calcium upon BCR signaling was tested using a transfectant of the K46 B cell lymphoma expressing the 3-83 BCR, K46J (42, 52). As expected, the high affinity phage P31 induced a significant calcium flux (Fig. 6 A), but this response differed in several ways from the response elicited by anti-Ig κ. The initial peak of Ca++ induced by the anti-κ treatment, which represents primarily a mobilization of the intracellular stores (53), was absent or very weak in the P31-induced response, and the anti-κ response was stronger than the P31 response both in terms of the percentage of responding cells and the level of calcium mobilized (Fig. 6 B). Neither increasing the dose of phage P31, nor cross-linking with an anti-phage antibody provoked an increase in the response above a certain plateau, which in the absence of cross-linking was reached with 40 μg of phage per 2 × 105 cells (data not shown). P5, P7, and P11 all induced a weak calcium mobilization at BCR saturating concentrations (Fig. 6 B). Thus, the extent of calcium mobilization induced by the phages corresponded to their relative affinities for the 3-83 BCR and was distinct from the response induced by anti-BCR antibodies.
Figure 6.
Calcium mobilization induced by the peptide-expressing phage ligands. For each assay, K46J cells loaded with Indo-1 AM were run on a Cytofluorograf 30/50 (Ortho Diagnostic Systems) to define a base line of 490/390 ratio which is a direct measurement of calcium concentration. (A) After 2 min, a stimulus (indicated by the arrow) was added, either 2 μg of anti-κ antibody as a positive control, or 80 μg of Pwt or P31 phage, and the 490/390 ratio modification was followed over a 10-min period. Data shown is representative of five independent experiments. (B) This assay was repeated for the various phage, and the mean 490/390 ratio and the percentage of responding cells were calculated using the MultiTime software (Phoenix Flow Systems). The data presented are mean values ± SD of four experiments.
Affinity-dependent Induction of Differential Patterns of Syk and Lyn Phosphorylation in Primary B Cells.
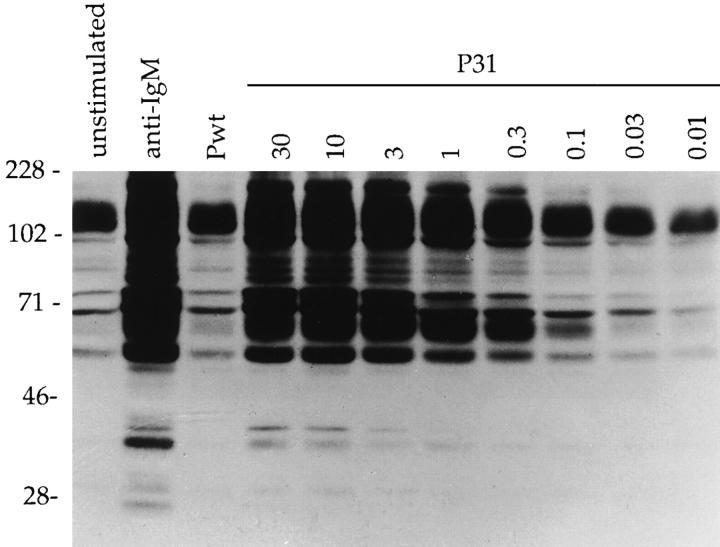
To further characterize the correlation between biological response and BCR signaling, we investigated induction of protein- tyrosine phosphorylation in purified splenic resting 3-83 B cells stimulated with the panel of phages. P31 induced the rapid phosphorylation of many proteins in a pattern similar to that observed with anti-IgM stimulation (Fig. 7), whereas the control Pwt did not induce any tyrosine phosphorylation over the background detected in unstimulated cells. P31 induced a strong pattern of protein-tyrosine phosphorylation over a wide concentration range; subsaturating concentrations as low as 0.3 μg/106 cells were still able to induce significant tyrosine phosphorylation (Fig. 7). Intermediate affinity phage P5 induced a pattern of protein-tyrosine phosphorylation that was similar to, but weaker, than that achieved with P31 (Fig. 8 A). Saturating concentrations of P7 or P11 induced a very limited and weak pattern of phosphorylation (with the possible exception of a high molecular weight species, indicated by an arrow; Fig. 8 A). No changes or increases were observed in a 30-min time course stimulation for any of the low affinity phages (data not shown).
Figure 7.
The high affinity phage P31 induces a strong pattern of tyrosine phosphorylation. Whole cell lysates were prepared from 3-83 primary B cells 5 min after stimulation with 3 μg of anti-IgM antibody, 60 μg of Pwt phage, or with the indicated amount of P31 phage. Samples were fractionated on an SDS-PAGE and after transfer to a PVDF membrane, the phosphotyrosine-containing proteins were detected with the Ab2 antibody.
Figure 8.
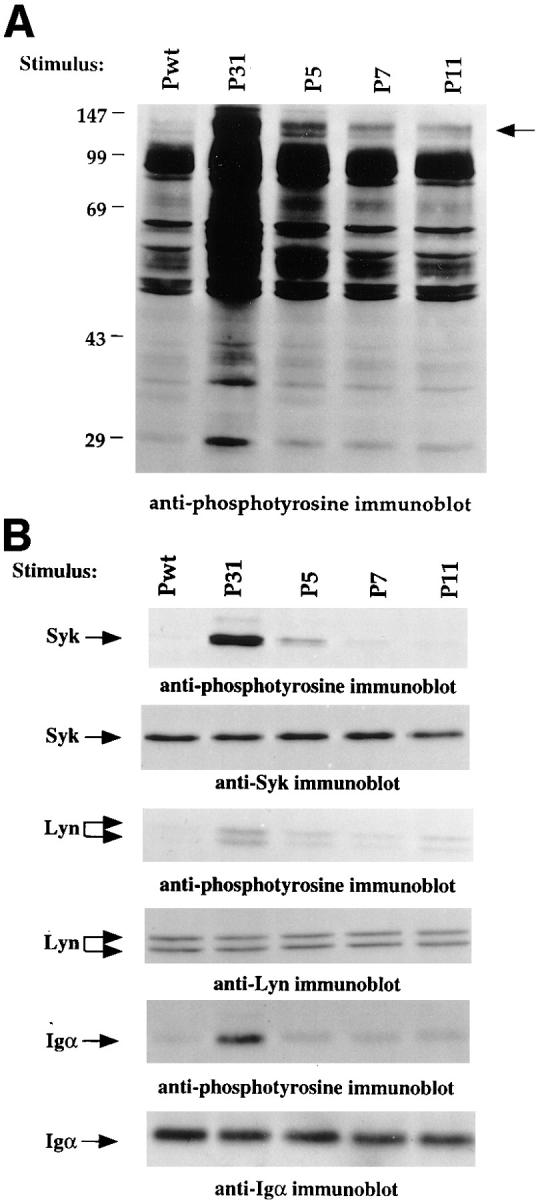
Differential tyrosine phosphorylation pattern induced by peptide-expressing phage of varying affinities. (A) Whole cell lysates were prepared from 3-83 primary B cells 5 min after stimulation with 3 μg of anti-IgM or with 60 μg phage. Samples were loaded on an SDS-PAGE, transferred to a PVDF membrane, and the phosphotyrosine-containing proteins were detected with the Ab2 antibody. (B) Immunoprecipitations of Syk, Lyn, and Igα were performed on lysates of 1.2 × 107 stimulated cells using 5–10 μg of affinity-purified Ab coupled to Sepharose beads. Electrophoretically fractionated proteins were transferred to PVDF membrane and revealed using the indicated antibodies. Data shown are representative of three independent experiments.
P31 induced the tyrosine phosphorylation of Igα, Lyn, and Syk as identified by specific immunoprecipitation (Fig. 8 B). Upon stimulation with P5, both Lyn and Syk became phosphorylated, but to a lesser extent than with high affinity phage, whereas Igα was not detectably phosphorylated. P7 and P11 induced the phosphorylation of Lyn to a level equivalent to that of P5, but failed to detectably phosphorylate Igα or Syk (Fig. 8 B). These data reveal differential induction of tyrosine phosphorylation by the various ligands. High affinity phage P31 induced a full and robust pattern of response, P5 induced phosphorylation of Lyn, weak phosphorylation of Syk, and no Igα phosphorylation, whereas P7 and P11 were able to stimulate phosphorylation of Lyn in the absence of tangible Igα and Syk phosphorylation. Thus, these early tyrosine phosphorylation events were not strictly dependent upon BCR occupancy, but were more closely correlated with ligand-BCR affinity and later biological responses.
Discussion
It is important to develop reproducible techniques to analyze antigen receptor responses to weak ligands because there is increasing evidence that these responses are both biologically significant, and biologically distinct, from responses to strong ligands. To more precisely assess the relationship between BCR-ligand affinity and its functional consequences on B cell activation, we have isolated several phage-bound peptide ligands that possess a wide range of relative affinities for a particular BCR, but are otherwise comparable in overall structure and valence, and used them to stimulate specific primary B cells. These peptide-expressing phages are relatively easy to produce and provide highly specific, reproducible reagents for the analysis of antigen receptor signaling. We find in this study that B lymphocyte activation is better correlated with the strength of the BCR-ligand interaction than with receptor occupancy. This differential induction was observed in the early signaling events of tyrosine phosphorylation and Ca++ mobilization, as well as at the level of lymphokine gene transcription, cell surface marker modulation, blastogenesis, proliferation, and antibody production (Table 1). The responses of 3-83 B cells stimulated by phage-bound ligands fell into distinct categories depending upon the stimulus. The differences observed between P31 and P11 in their ability to stimulate B cells also appear to be relevant in vivo as P11, but not P31, induced secondary light chain rearrangement in the peripheral lymphoid organs of immunized 3-83 transgenic mice (54). Collectively, these data suggest the existence of weak and partial BCR agonists, as some of the peptide ligands identified seem to stimulate only some, but not all of the responses induced by a full agonist. These results further suggest that, like the T cell receptor, the BCR possesses the capacity for differential or partial signaling.
Table 1.
Summary of the Responses Induced by the Various Phage
| Binding by ELISA |
Syk | Lyn | Calcium |
Lymphokine transcription | Downreg-ulation | Upregulation | γ1 transcript | 3.83 production | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phage | phosphorylation | IL-2 | IL-6 | IFN-γ | IgM | IgD | B7.2 | Class II | ||||||||||||||||||
| P31 agonist | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | |||||||||||||
| P5 weak agonist | ++ | + | + | + | + | ++ | + | + | + | ++ | ++ | + | ± | |||||||||||||
| P7 partial agonist | ++ | − | + | ± | − | ++ | − | + | + | ++ | ++ | + | ± | |||||||||||||
| P11 partial agonist | + | − | + | ± | − | ++ | − | + | + | + | ++ | − | − | |||||||||||||
| Pwt | − | − | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||
Although the responses we observed are correlated with affinity, this correlation is not perfect and affinity might not be the most critical parameter influencing the observed responses. The two intermediate affinity phages, P5 and P7, have a very similar relative affinity for 3-83, but are distinct in terms of their ability to induce lymphokine gene transcription and tyrosine phosphorylation, even at receptor saturating concentrations (Table 1). It is conceivable that it is not the equilibrium affinity but the on or off rate of the BCR-ligand interaction that determines the outcome of activation responses, if, for example, ligands with rapid dissociation rates could not trigger the BCR for a long enough period of time to induce a full range of effects. The duration of receptor occupancy is thought to play an important role in the differential signaling of the TCR (55– 57). Alternatively, the phage ligands may differ in their ability to induce a critical conformational change in the receptor. Because of the relative simplicity of the BCR-antigen interaction compared with TCR-antigen interactions, our experimental system may provide advantages in approaching the biochemistry of differential antigen receptor signaling.
Some of the biological effects induced by the high affinity phage P31, such as blastogenesis and antibody production, were also reproduced using 3-83 transgenic RAG- deficient spleen cells (data not shown), which are totally devoid of T lymphocytes because the RAG mutation precludes any rearrangement of the TCR-α, -β, -γ, and -δ loci (58). These data indicate that the B cell response induced by P31 is fully T cell independent. The dependence of this response on cross-linking with anti-phage antibodies also points to a T-independent type response, which is known to require a high level of BCR cross-linking (26). The observed distinctions between agonist and partial agonist ligands documented in this study apply to a T-independent response and may be different in the context of T cell dependent responses. It is probably significant that even the lowest affinity 3-83-binding phage was able to stimulate proliferation in presence of anti-CD40 and to upregulate B cell expression of B7.2 and MHC class II molecules because these molecules are critical for stimulatory interactions with T cells. The ability of BCR ligands to act as full rather than partial agonists might play an important role, in vivo, in initiating T-independent rather than T-dependent B cell responses. Furthermore, the observed production by B cells of IFN-γ and IL-2 in response to the two highest affinity phages might represent an autocrine pathway allowing for T cell–independent survival, differentiation, or growth.
We describe the isolation and characterization of several peptide ligands for the 3-83 antibody, which was originally raised against a conformational determinant on the polymorphic α1-α2 domains of particular MHC class I molecules (59). None of the peptide sequences share homology with MHC genes. Although binding motifs can be seen among some of the peptides (Fig. 1 A), examination of the sequences reveals that the 3-83 antibody can bind to a variety of structures. A surprising feature of the 3-83-binding peptides is the high representation of tryptophan (trp) residues. Among the identified peptides, eight sequences contain one trp residue, four peptides contain two, one peptide contains three, whereas only one does not contain trp. This is probably not due to a bias in the libraries since in both libraries each amino acid is randomly encoded using the N/ N/(C or G) coding scheme, where N represents an equimolar mixture of the four deoxyribonucleotides (40). Furthermore, mutational analysis of the 15mer peptide indicated that trp at position 3 was critical for 3-83 binding (unpublished data). A major conclusion to be drawn is that a wide variety of short linear peptides are capable of mimicking a conformational antigenic determinant, albeit with a wide range of affinities. The phage library approach has been shown to be effective in isolating surrogate ligands to many types of receptors and proteins (38, 60–63). Our study further extends these findings to demonstrate that many such surrogate ligands potentially exist and can induce functionally distinct signals to reactive B cells.
The fact that an enormous diversity of peptide sequences can functionally interact with a particular BCR has important theoretical and practical implications. First, it implies that antibody cross-reactivity to unrelated antigens is unavoidable. Second, it suggests that antigenic mimicry by microbes could often promote autoimmunity and that such mimicry may be independent of amino acid sequence similarity. Third, it suggests that cross-reactivity must pose an important problem for immune self-tolerance at multiple stages in B cell development. For T lymphocytes, it has been proposed that the potential problems caused by this broad cross-reactivity may be partly mitigated by the fact that the more numerous, low affinity interactions can be antagonistic. It remains to be seen to what extent B cell-ligand antagonism can occur.
Acknowledgments
We thank David Russell for technical assistance; Shirley Sobus and Bill Townsend for flow cytometry assistance; and Dr. G.L. Johnson, Dr. L. Wysocki, Susan Chan, and members of the lab for critical review of the manuscript.
This work was supported by National Institutes of Health grants (RO1 GM44809, KO4 AI01161, and PO1 AI22295). V. Kouskoff was supported by a European Molecular Biology Organization fellowship.
Abbreviations used in this paper
- BCR
B cell receptor
- ITAM
immunotyrosine-based motifs
- PVDF
polyvinylidene difluoride
- trp
tryptophan
References
- 1.Nossal GJ. Choices following antigen entry: antibody formation or immunologic tolerance? . Annu Rev Immunol. 1995;13:1–27. doi: 10.1146/annurev.iy.13.040195.000245. [DOI] [PubMed] [Google Scholar]
- 2.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 3.Cyster JG, Healy JI, Kishihara K, Mak TW, Thomas ML, Goodnow CC. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 4.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma A, Fisher P, Dildrop R, Oltz E, Rathbun G, Achacoso P, Stall A, Alt FW. Surface IgM mediated regulation of RAG gene expression in E mu-N-myc B cell lines. EMBO (Eur Mol Biol Organ) J. 1992;11:2727–2734. doi: 10.1002/j.1460-2075.1992.tb05338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cambier JC, Monroe JG. B cell activation. V. Differentiation signaling of B cell membrane depolarization, increased I-A expression, G0 to G1 transition, and thymidine uptake by anti-IgM and anti-IgD antibodies. J Immunol. 1984;133:576–581. [PubMed] [Google Scholar]
- 7.DeFranco AL, Raveche ES, Paul WE. Separate control of B lymphocyte early activation and proliferation in response to anti-IgM antibodies. J Immunol. 1985;135:87–94. [PubMed] [Google Scholar]
- 8.Goroff DK, Stall A, Mond JJ, Finkelman FD. In vitro and in vivo B lymphocyte-activating properties of monoclonal anti-δ antibodies. I. Determinants of B lymphocyte-activating properties. J Immunol. 1986;136:2382–2392. [PubMed] [Google Scholar]
- 9.Hamano T, Asofsky R. Functional studies on B cell hybridomas with B cell surface antigens. I. Effects of anti-immunoglobulin antibodies on proliferation and differentiation. J Immunol. 1983;130:2027–2032. [PubMed] [Google Scholar]
- 10.Maruyama S, Kubagawa H, Cooper MD. Activation of human B cells and inhibition of their terminal differentiation by monoclonal anti-μ antibodies. J Immunol. 1985;135:192–199. [PubMed] [Google Scholar]
- 11.Mongini PK, Blessinger CA, Dalton JP. Affinity requirements for induction of sequential phases of human B cell activation by membrane IgM-cross-linking ligands. J Immunol. 1991;146:1791–1800. [PubMed] [Google Scholar]
- 12.Rudich SM, Winchester R, Mongini PK. Human B cell activation. Evidence for diverse signals provided by various monoclonal anti-IgM antibodies. J Exp Med. 1985;162:1236–1255. doi: 10.1084/jem.162.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 14.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 15.Pleiman CM, Abrams C, Gauen LT, Bedzyk W, Jongstra J, Shaw AS, Cambier JC. Distinct p53/56lyn and p59fyn domains associate with nonphosphorylated and phosphorylated Ig-alpha. Proc Natl Acad Sci USA. 1994;91:4268–4272. doi: 10.1073/pnas.91.10.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 17.Chan AC, Shaw AS. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr Opin Immunol. 1996;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- 18.Alberola IJ, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- 19.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 20.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 21.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 22.Law CL, Craxton A, Otipoby KL, Sidorenko SP, Klaus SJ, Clark EC. Regulation of signalling through B-lymphocyte antigen receptors by cell-cell interaction molecules. Immunol Rev. 1996;153:123–154. doi: 10.1111/j.1600-065x.1996.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 23.Monroe JG. Tolerance sensitivity of immature-stage B cells: can developmentally regulated B cell antigen receptor (BCR) signal transduction play a role? . J Immunol. 1996;156:2657–2660. [PubMed] [Google Scholar]
- 24.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 25.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci USA. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 27.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 28.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 30.Sette A, Alexander J, Ruppert J, Snoke K, Franco A, Ishioka G, Grey HM. Antigen analogs/MHC complexes as specific T cell receptor antagonists. Annu Rev Immunol. 1994;12:413–431. doi: 10.1146/annurev.iy.12.040194.002213. [DOI] [PubMed] [Google Scholar]
- 31.Evavold BD, Sloan LJ, Allen PM. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 32.Fremont DH, Rees WA, Kozono H. Biophysical studies of T-cell receptors and their ligands. Curr Opin Immunol. 1996;8:93–100. doi: 10.1016/s0952-7915(96)80111-7. [DOI] [PubMed] [Google Scholar]
- 33.Jameson SC, Bevan MJ. T cell receptor antagonists and partial agonists. Immunity. 1995;2:1–11. doi: 10.1016/1074-7613(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 34.De Magistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 35.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 36.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self- reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparks AB, Quilliam LA, Thorn JM, Der CJ, Kay BK. Identification and characterization of Src SH3 ligands from phage-displayed random peptide libraries. J Biol Chem. 1994;269:23853–23856. [PubMed] [Google Scholar]
- 39.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 40.Sparks AB, Adey NB, Quilliam LA, Thorn JM, Kay BK. Screening phage-displayed random peptide libraries for SH3 ligands. Methods Enzymol. 1995;255:498–509. doi: 10.1016/s0076-6879(95)55052-6. [DOI] [PubMed] [Google Scholar]
- 41.Lin TC, Webster RE, Konigsberg W. Isolation and characterization of the C and D proteins coded by gene IX and gene VI in the filamentous bacteriophage fl and fd. J Biol Chem. 1980;255:10331–10337. [PubMed] [Google Scholar]
- 42.Justement LB, Kreiger J, Cambier JC. Production of multiple lymphokines by the A20.1 B cell lymphoma after cross-linking of membrane Ig by immobilized anti-Ig. J Immunol. 1989;143:881–889. [PubMed] [Google Scholar]
- 43.Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan IC. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tosato G, Tanner J, Jones KD, Revel M, Pike SE. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990;64:3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schena M, Gaidano G, Gottardi D, Malavasi F, Larsson LG, Nilsson K, Caligaris-Cappio F. Molecular investigation of the cytokines produced by normal and malignant B lymphocytes. Leukemia. 1992;6:120–125. [PubMed] [Google Scholar]
- 46.Pang Y, Norihisa Y, Benjamin D, Kantor RR, Young HA. Interferon-γ gene expression in human B-cell lines: induction by interleukin-2, protein kinase C activators, and possible effect of hypomethylation on gene regulation. Blood. 1992;80:724–732. [PubMed] [Google Scholar]
- 47.Kindler V, Matthes T, Jeannin P, Zubler RH. Interleukin-2 secretion by human B lymphocytes occurs as a late event and requires additional stimulation after CD40 cross-linking. Eur J Immunol. 1995;25:1239–1243. doi: 10.1002/eji.1830250516. [DOI] [PubMed] [Google Scholar]
- 48.Katano M, Morisaki T, Matsuo T, Nakamura M, Kubota E, Hisatsugu T. Interleukin-2 (IL-2) production by human B-cell line. Cell Immunol. 1994;159:262–270. doi: 10.1006/cimm.1994.1312. [DOI] [PubMed] [Google Scholar]
- 49.Lenschow DJ, Sperling AI, Cooke MP, Freeman G, Rhee L, Decker DC, Gray G, Nadler LM, Goodnow CC, Bluestone JA. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–1997. [PubMed] [Google Scholar]
- 50.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 51.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemazee, D.A. 1987. Proceedings of the EMBO Workshop on Tolerance. P. Matzinger, M. Flajlnik, D.A. Nemazee, H.-G. Rammensee, G. Stockinger, T. Rolink, and L. Nicklin, editors. 2:52–54.
- 53.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 54.Hertz, M.W., V. Kouskoff, T. Nakamura, and D. Nemazee. 1998. V(D)J recombinase induction in splenic lymphocytes is inhibited by antigen receptor signaling. Nature. In press. [DOI] [PMC free article] [PubMed]
- 55.Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/ I-Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valitutti S, Lanzavecchia A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 57.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 58.Spanopoulou E. Cellular and molecular analysis of lymphoid development using Rag-deficient mice. Int Rev Immunol. 1996;13:257–288. doi: 10.3109/08830189609061752. [DOI] [PubMed] [Google Scholar]
- 59.Ozato K, Mayer N, Sachs DH. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980;124:533–540. [PubMed] [Google Scholar]
- 60.Schellekens GA, Lasonder E, Feijlbrief M, Koedijk DG, Drijfhout JW, Scheffer AJ, Welling-Wester S, Welling GW. Identification of the core residues of the epitope of a monoclonal antibody raised against glycoprotein D of herpes simplex virus type 1 by screening of a random peptide library. Eur J Immunol. 1994;24:3188–3193. doi: 10.1002/eji.1830241241. [DOI] [PubMed] [Google Scholar]
- 61.Kay BK, Adey NB, He YS, Manfredi JP, Mataragnon AH, Fowlkes DM. An M13 phage library displaying random 38-amino-acid peptides as a source of novel sequences with affinity to selected targets. Gene (Amst) 1993;128:59–65. doi: 10.1016/0378-1119(93)90153-t. [DOI] [PubMed] [Google Scholar]
- 62.Hoess RH, Mack AJ, Walton H, Reilly TM. Identification of a structural epitope by using a peptide library displayed on filamentous bacteriophage. J Immunol. 1994;153:724–729. [PubMed] [Google Scholar]
- 63.Devlin JJ, Panganiban LC, Devlin PE. Random peptide libraries: a source of specific protein binding molecules. Science. 1990;249:404–406. doi: 10.1126/science.2143033. [DOI] [PubMed] [Google Scholar]