Abstract
Natural killer (NK) T cells are a lymphocyte subset with a distinct surface phenotype, an invariant T cell receptor (TCR), and reactivity to CD1. Here we show that mouse NK T cells can recognize human CD1d as well as mouse CD1, and human NK T cells also recognize both CD1 homologues. The unprecedented degree of conservation of this T cell recognition system suggests that it is fundamentally important. Mouse or human CD1 molecules can present the glycolipid α-galactosylceramide (α-GalCer) to NK T cells from either species. Human T cells, preselected for invariant Vα24 TCR expression, uniformly recognize α-GalCer presented by either human CD1d or mouse CD1. In addition, culture of human peripheral blood cells with α-GalCer led to the dramatic expansion of NK T cells with an invariant (Vα24+) TCR and the release of large amounts of cytokines. Because invariant Vα14+ and Vα24+ NK T cells have been implicated both in the control of autoimmune disease and the response to tumors, our data suggest that α-GalCer could be a useful agent for modulating human immune responses by activation of the highly conserved NK T cell subset.
Keywords: CD1, natural killer T cells, antigen presentation, glycolipid, cytokines
CD1 molecules are β2-microglobulin (β2m)–associated1 transmembrane proteins that are related to MHC-encoded antigen presenting molecules. Despite their association with β2m, comparisons of primary sequences demonstrate that CD1 molecules are almost as closely related to class II molecules as they are to class I molecules (1). Therefore, CD1 molecules probably diverged from these other antigen presenting molecules early in vertebrate evolution, around the time of the class I–class II divergence. CD1 molecules are distinguished from the MHC-encoded classical class I and class II molecules by their lack of polymorphism. Of the five human CD1 genes, protein products have been identified for the CD1a, -b, -c, and -d isoforms (2). The CD1 isoforms are themselves quite divergent, although CD1a, -b, and -c are more closely related to one another in their amino acid sequences than to CD1d (1, 3). Only two CD1 genes, CD1d1 and CD1d2, have been identified in mice. They are highly related to one another and are most similar to human CD1d in sequence (4). However, there is not an extraordinarily high degree of conservation between the mouse CD1 (mCD1) and human CD1d (hCD1d) homologues. The overall percentage of sequence identity between the hCD1d and mCD1 polypeptides in the antigen binding region is 60.4% for the α1 domain and 62.4% for the α2 domain (1, 5).
One of the properties shared by mCD1 and hCD1d molecules is their ability to be recognized by NK T cells (6–11). Recently, much interest has been focused upon the NK T cell subpopulation on account of its ability to quickly produce large amounts of cytokines, suggesting a potential for these T cells to regulate immune responses. The majority of mouse NK T cells use mainly the Vβ8.2, Vβ7, or Vβ2 chain paired with an invariant Vα14Jα281 rearrangement (12). The human counterpart of the mouse NK T cells also expresses a restricted TCR repertoire, including a homologous, invariant Vα24 rearrangement paired with Vβ11, the human homologue of mouse Vβ8 (13–15). NK T cells in both species are autoreactive in vitro for CD1 molecules, in the absence of exogenous antigen. In addition to TCR repertoire and CD1 autoreactivity, NK T cells from both species resemble each other in several additional ways, including expression of intermediate TCR levels, expression of CD4 or the absence of both CD4 and CD8, expression of cell surface proteins characteristic of memory or activated T cells (16), and the presence of NK receptors, particularly NK1.1 (CD161) in mice (17, 18) and its homologue, NKRP1, in humans (11, 16).
Despite their relatively restricted TCR repertoire, a minority of NK T cells lack Vα14 expression. Moreover, Vα14 can be paired in the mouse with several different Vβs (19), and the Vβ rearrangements expressed by NK T cells have junctional diversity (12). The combination of these three factors allows for some diversity in the antigen receptors expressed by the NK T cell population, and the results from recent studies in the mouse indicate that mCD1 autoreactive T cells are in fact heterogeneous in their ability to recognize different mCD1+ cell lines and transfectants (20, 21). These data suggest that a diverse set of autologous ligands may be presented by mCD1, although other interpretations are not ruled out. Consistent with the requirement for a diverse set of ligands, it has been shown recently that mouse Vα14+ T cells can recognize the lipoglycan α-galactosylceramide (α-GalCer) presented by mCD1, whereas Vα14− but mCD1-autoreactive T cells are not responsive to this antigen (22, 23).
In this study, we have uncovered a surprising degree of conservation in the interaction of invariant NK TCRs from different species with CD1 molecules despite the extensive divergence in primary sequences of these molecules between mice and humans. We also demonstrate here that α-GalCer is presented by hCD1d to human NK T cells, providing the first evidence both for the presentation of a defined antigen by hCD1d and for a requirement for human NK T cells for a lipoglycan antigen in addition to hCD1d. As α-GalCer can induce a dramatic hCD1d dependent expansion of human NK T cells, as well as a strong release of cytokines by these cells, our data further suggest that this lipoglycan could be a useful agent for the modulation of human immune responses.
Materials and Methods
Gene Cloning and Transfection.
pSRα-neo-hCD1d (a gift from Dr. S. Balk, Beth Israel Hospital, Boston, MA), which contains a full-length hCD1d cDNA, was used as a template for PCR. After amplification, the hCD1d cDNA was sequenced and ligated into the TA cloning vector (InVitrogen, Carlsbad, CA). For expression in mammalian cells, the hCD1d cDNA was inserted into the BamHI and SalI sites of the pHβAprneo vector, which contains the human β-actin promoter. 20 μg of plasmid was linearized and electroporated (Gene Transfector 300, BTX Corp., San Diego, CA) into A20 B lymphoma, C1R, and Hela cells. Stable transfectants were chosen at 3–4 wk and stained with biotinylated anti-hCD1d mAb 42.2 (see below).
T Cell Hybridomas.
The derivation and characterization of the mCD1 autoreactive T cell hybridomas has been described previously (21, 23, 24). For the stimulation assays, 5 × 104 T hybridoma cells per well were cultured in the presence of 105 mCD1+, hCD1d+, or control stimulator cells. After 16 h, IL-2 release was evaluated in a sandwich ELISA using rat anti–mouse IL-2 mAbs (PharMingen, San Diego, CA).
Cloning of Invariant Vα24+ T Cells.
PBMCs of healthy donors were stained with purified anti-Vα24 (IgG1) and anti-Vβ11 (IgG2a) mAbs, followed by human adsorbed FITC-conjugated goat anti–mouse IgG1 and PE-conjugated goat anti–mouse IgG2a antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). Double positive cells were sorted and either immediately cloned by limiting dilution or activated as a primary bulk culture and then cloned by limiting dilution (14). Clones that coexpressed Vα24 and Vβ11 by cytofluorimetric analysis were further expanded and used for this study. Molecular typing of the TCR expressed by Vα24/Vβ11+ T cell clones was performed as previously described (25).
Activation of Vα24/Vβ11+ T Cell Clones by CD1 Transfectants.
Activations were performed in 96-well plates in 200 μl total volume containing 1 ng/ml of PMA. T cell clones were added to wells at 5 × 104 per well, along with 105 CD1 transfectants. Hela and Hela-hCD1d transfectants were fixed for 30 s in glutaraldehyde 0.05% in PBS, immediately diluted, and washed three times in complete medium. Antigen-pulsed APCs were generated by culture of hCD1d transfectants for 2 h with 100 ng/ ml of α-GalCer followed by three washes, and irradiated at 8,500 rads. To block recognition of hCD1d, the 51.1 anti-hCD1d–specific mAb, provided by Dr. S. Porcelli (Brigham and Women's Hospital, Boston, MA), or control irrelevant mouse IgG2b was added to cultures at a final concentration of 20 μg/ml.
Generation of α-GalCer–reactive Cell Lines.
Total human PBMCs were cultivated in 24-well plates in the presence of 50 U/ml of IL-2 and 100 ng/ml of α-GalCer. Expansion of the Vα24+ cells was determined upon staining with a combination of anti-CD3, anti-CD4, anti-CD8, anti-Vα24, and anti-Vβ11 mAbs.
Flow Cytometric Analysis.
Biotinylated anti-hCD1d mAb 42.2 was provided by Dr. S. Porcelli. Secondary reagents for mCD1 detection were streptavidin-PE–conjugated (Caltag, South San Francisco, CA). For staining, cells were suspended in buffer comprised of PBS, pH 7.3, containing 2% BSA (wt/vol) and 0.02% NaN3 (wt/vol) and incubated at 4°C for 20–30 min with the primary Ab, washed twice, and then further incubated with secondary reagents for another 20–30 min at 4°C. After two washes, the cells were fixed and analyzed on a FACScan® 440 flow cytometer (Becton Dickinson, San Jose, CA).
Cytokine Determination by ELISA.
Supernatants were quantified for IL-4 or IFN-γ by ELISA using commercial pairs of mAbs: BAF285 and MAB285 for IFN-γ or BAF 204 and MAB604 for IL-4 (R&D Systems, Minneapolis, MN).
Results
A Subset of mCD1–autoreactive Hybridomas Responds to hCD1d.
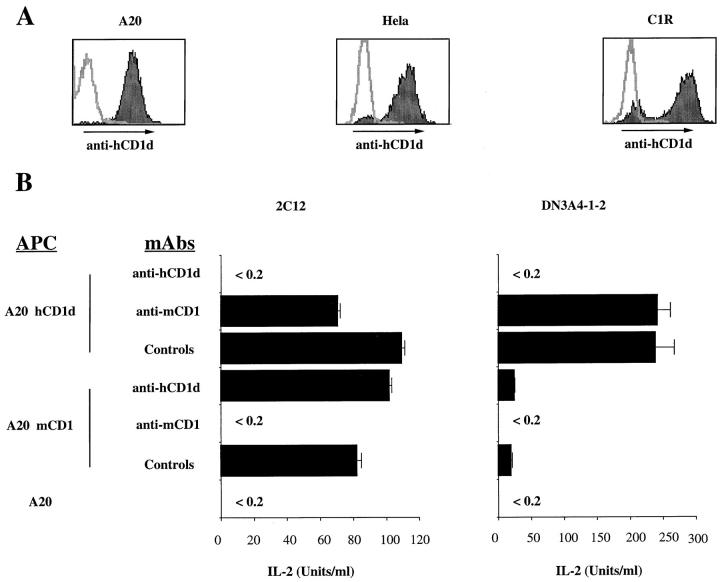
The parallels between mouse and human NK T cells led us to test for a possible cross-reactivity of mouse T cell hybridomas with hCD1d. Transfectants in several different cell types were generated and selected for approximately similar levels of surface hCD1d expression (Fig. 1 A). Each of these three transfected cell lines was used as APCs for T cell hybridomas that are mCD1 autoreactive. As shown in Table 1, two of the seven mCD1 autoreactive hybridomas also react with the three different hCD1d transfectants, whereas the other five do not. The hCD1d reactivity of these two NK T cell hybridomas has been confirmed using anti-hCD1d blocking mAb, which inhibited the reactivity to hCD1d A20 transfectants but not to mCD1 transfectants of the same cell line (Fig. 1 B). Similar antibody blocking data have been obtained using hCD1d-transfected Hela and C1R cells (data not shown).
Figure 1.
hCD1d stimulates mouse NK T cell hybridomas. (A) Flow cytometry profiles of hCD1d-transfected A20, Hela, and C1R cell lines. Stainings were carried out with biotinylated 42.2 anti-hCD1d mAb, followed by PE-conjugated streptavidin. Before each staining, cells were incubated with 1% human serum. Each panel is an overlay of the isotype control (open histogram) and the anti-hCD1d mAb (filled histogram). (B) Interspecies cross-reactivity of mouse Vα14/Vβ8 T cell hybridomas. The 2C12 and DN3A4-1.2 mCD1-autoreactive hybridomas were cultured at 5 × 104 cells/well with 105 APCs. Antibody-mediated inhibition was determined using an anti-hCD1d mAb 51.1, anti-mCD1 mAb 1B1, or isotype-matched control mAbs, all at 10 μg/ml. After 16 h of culture, IL-2 release was determined by ELISA. The experiments shown here are representative of nine different experiments.
Table 1.
A Subset of mCD1-restricted T Cells Responds to hCD1D Molecule
| Hybridoma | TCR | A20 mock | A20 mCD1 | A20 hCD1d | Hela | Hela hCD1d | C1R | C1R hCD1d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | α3.2/β9 | 0.1* | 22.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| 1A12 | α5/β14 | 0.2 | 40.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| 68 | α4/β11 | 0.3 | 6 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 | ||||||||
| DN3A4-1-4 | α14/β10 | 0.4 | 6.4 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | ||||||||
| 2C12 | α14/β8 | 0.1 | 74.6 | 97.6 | 0.1 | 4 | 0.1 | 27 | ||||||||
| DN3A4-1-2 | α14/β8 | 0.1 | 20 | 239 | 0.1 | 7.6 | 4.2 | 99 | ||||||||
| 3C3 | α14/β8 | 0.1 | 4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
IL-2 release is expressed in units per milliliter and determined according to the IL-2 standard from PharMingen.
It is noteworthy that the two hCD1d reactive mouse T cell hybridomas express the canonical Vα14/Vβ8 NK TCR, whereas the nonreactive ones either express Vα14/ Vβ8 or Vα14/Vβ10 or, in three instances, lack Vα14 expression. The lack of hCD1d reactivity of the Vα14/Vβ8+ 3C3 hybridoma is not due to a lower level of TCR expression, implicating either Vβ junctional sequences or some other factor that distinguishes this hybridoma. Surprisingly, one of the two cross-reactive T cell hybridomas, DN3A4-1-2, responds much more strongly to hCD1d than to mCD1 transfectants of A20 cells (Table 1 and Fig. 1 B). Although the amount of IL-2 this cell releases in response to mCD1 transfectants is well above the background, the ∼12-fold greater increase in IL-2 release obtained with hCD1d was typical of the results from nine different experiments. By contrast, hybridoma 2C12 reacts equally to the two CD1 homologues (Table 1 and Fig. 1 B).
A Subset of Human NK T Cell Clones Are Reactive to mCD1 Molecules.
We then asked whether the interspecies cross-reactivity of NK T cells for CD1 molecules also would be observed using human Vα24/Vβ11+ clones as responder cells. The presence or absence of the invariant Vα24-JαQ rearrangement in the T cell clones was confirmed by both heteroduplex and oligotyping analysis, and the diversity in the Vβ11 junctions was determined by sequencing (data not shown). A total of six clones from three different donors were analyzed, four of which expressed the invariant Vα24 TCR paired with Vβ11 and two of which expressed a noninvariant Vα24 also paired with Vβ11. The ability of these clones to release IFN-γ (Table 2) and IL-4 (data not shown) in response to CD1 transfectants was tested. Data from five experiments are combined and averaged. Consistent with previous data (11), we found that recognition of hCD1d by human NK T cells required PMA, and in some cases also required mild aldehyde fixation of the target cells. Therefore, all the experiments shown in Table 2 had PMA in the cultures, and the Hela cell APCs also were fixed with glutaraldehyde.
Table 2.
Invariant Vα24+ T Cell Clones Respond to mCD1-transfected Cells (IFN-γ)
| Clones | TCR | A20 | A20 mCD1 | A20 hCD1d | Hela | Hela hCD1d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD9 | I* | 230‡ | 1,050 | 620 | 50 | 1,300 | ||||||
| PD16 | I | 430 | 1,500 | 1,280 | 0 | 1,590 | ||||||
| LG2D3 | I | 0 | 0 | 0 | 0 | 1,080 | ||||||
| 15.21.2 | I | 0 | 690 | 510 | 0 | 220 | ||||||
| 15.21.3 | NI | 370 | 280 | 94 | 2,500 | 770 | ||||||
| PD18 | NI | 600 | 460 | 700 | 330 | 370 |
I, invariant Vα24/Vβ11 TCR; NI, noninvariant Vα24/Vβ11 TCR.
IFN-γ release is expressed in picogram per milliliter and determined according to the IFN-γ standard from R & D.
In agreement with the previous results, all of the clones with an invariant Vα24 TCR could respond either to one or both hCD1d transfectants (Table 2). As we and others have described for mouse NK T cells (20, 21), the reactivity of different human NK T cell clones is somewhat heterogeneous, and is more dependent on the type of hCD1d expressing APCs than on the level of hCD1d surface expression. Indeed, clone LG2D3 responds to hCD1d-transfected Hela cells but not to A20-transfected cells, whereas the other three clones respond similarly to both types of cells (Table 2). Neither of the two clones that lack the invariant Vα24 responded significantly to the hCD1d transfectants, although both showed some background reactivity against all the APCs when PMA was added. When reactive, each of the clones could synthesize significant quantities of both IFN-γ and IL-4 in response to hCD1d stimulation.Three out of the four clones with an invariant Vα24 also were stimulated significantly by transfectants expressing heterologous mCD1 in the presence of PMA (Table 2), and this reactivity could be inhibited with an anti-mCD1 mAb (data not shown).
In summary, we conclude that not only have NK T cells been conserved throughout evolution, but the ability of the invariant TCR expressed by NK T cells to recognize the CD1d-like molecules mCD1 and hCD1d also has been conserved strictly. Furthermore, if self-ligands are required for this CD1 recognition, these ligands are likely to be similar in mice and humans.
Mouse Vα14/Vβ8+ Hybridomas Respond to α-GalCer– pulsed hCD1d Molecules.
We tested the ability of mouse NK T cell hybridomas to respond to the lipoglycan α-GalCer presented by the heterologous hCD1d molecule. It has been shown recently that mouse NK T cells with an invariant Vα14 TCR can respond in an mCD1-mediated fashion to α-GalCer (22, 23). We therefore used this compound to determine if hCD1d and mCD1 can present similar compounds to NK T cells.
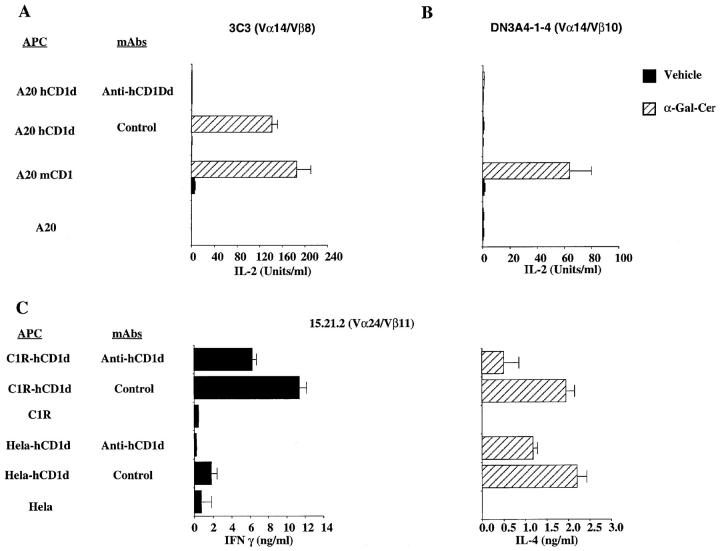
Using α-GalCer–pulsed CD1d transfectants as APCs, we analyzed the reactivity of the panel of seven mouse T cell hybridomas described in Table 1. Only three hybridomas responded to the pulsed APCs, regardless of whether the hCD1d-expressing cell line used was a transfected C1R, A20, or Hela cell. The responses of Vα14/Vβ8+ hybridomas 2C12 and DN3A4-1-2 to hCD1d plus α-GalCer were significantly increased above the level of hCD1d reactivity in the presence of the vehicle alone. Depending upon the APC used, the ratio of the IL-2 production in the presence of α-GalCer compared with the vehicle increased from 1.7- to 3-fold for hybridoma 2C12, and from 1.5- to 9.6-fold for hybridoma DN3A4-1-2. Interestingly, although the third Vα14/Vβ8+ hybridoma, 3C3, was unresponsive to hCD1d transfectants in the absence of antigen, it was strongly stimulated by α-GalCer–pulsed hCD1d cells (Fig. 2 A). Complete inhibition of the IL-2 release induced by α-GalCer pulsed APCs was obtained using an anti-hCD1d mAb, confirming the hCD1-mediated presentation of α-GalCer (Fig. 2 A). Therefore, all three Vα14/Vβ8+ T cell hybridomas tested respond to hCD1d plus α-GalCer. By contrast, the Vα14/Vβ10+ hybridoma DN3A4-1-4 responded to α-GalCer–pulsed mCD1-expressing APCs but not to α-GalCer–pulsed hCD1d-expressing cells (Fig. 2 B). In summary, these data demonstrate that hCD1d can present the same lipoglycan antigen as its mouse counterpart. Our previous studies demonstrated that mCD1-mediated recognition of α-GalCer by mouse NK T cells was effective when Vα14 was expressed with any one of these different TCR-β chains (23). The data presented here, in contrast, suggest that when presented by the heterologous hCD1d molecule α-GalCer responsiveness by mouse NK T cells may be more highly dependent upon the TCR-β chain.
Figure 2.
hCD1d presents α-GalCer to mouse and human NK T cells. (A and B) Response of a Vα14/Vβ8 hybridoma. hCD1d-transfected cells (A20 hCD1d), mCD1.1-transfected cells (A20 mCD1), or untransfected A20 cells were pulsed for 2 h either with α-GalCer or the vehicle control. These APCs were washed twice and plated at 105 cells/well. Antibodies at 10 μg/ml were added immediately before the addition of either the 3C3 (Vα14/Vβ8) or the DN3A4-1-4 (Vα14/Vβ10) T cell hybridomas at 5 × 104 cells/well. After 16 h of culture, IL-2 release was determined by ELISA. The experiments shown here are representative of four different experiments. (C) Response of a human Vα24/Vβ11 clone. hCD1d CIR transfectants (C1R hCD1d), hCD1d-transfected Hela cells (Hela hCD1d), or the untransfected parental control cells were antigen pulsed as described above. Cells were then incubated for 48 h with T cells from clone 15.21.2 at 5 × 104 cells/well. Isotype-matched control or anti-hCD1d mAb 51.1 at 20 μg/ml were added immediately before the addition of the T cells. IFN-γ and IL-4 release were determined by ELISA. The experiments shown here have been performed without the addition of PMA, and they are representative of three different experiments.
Human Invariant Vα24/Vβ11+ Clones Respond to α-GalCer–pulsed hCD1d Molecules.
We next tested if human NK T cell clones are responsive to α-GalCer presented by hCD1d. Cultures containing lipoglycan antigen-pulsed APC were carried out in the absence of either PMA or glutaraldehyde fixation. Three NK T cell clones with an invariant Vα24 TCR (15.21.2, LG2D3, and PD9) and one of the Vα24/Vβ11 clones without the invariant junction (PD18) were tested. In the absence of lipoglycan antigen there was no stimulation by hCD1d because of the absence of PMA. The results from one representative experiment of three carried out with clone 15.21.2 are shown in Fig. 2 C. α-GalCer is a potent inducer of both IFN-γ and IL-4 release by this T cell clone. The cytokine release was inhibited by an anti-hCD1d mAb (Fig. 2 C) as well as an anti-Vα24 mAb (data not shown), confirming the TCR-mediated recognition of α-GalCer–pulsed hCD1d+ APCs. Similar data were obtained with clones LG2D3 and PD9. The reactivity of the 15.21.2 and PD9 human T cell clones for heterologous mCD1 also could be detected by the addition of α-GalCer. Clone LG2D3, which did not respond to mCD1+ APCs in the presence of PMA, likewise did not respond to α-GalCer presented by mCD1 (data not shown).
α-GalCer–induced Expansion of Human Primary NK T Cells.
The results described above demonstrate that long term T cell clones, selected only on the basis of invariant Vα24 and Vβ11 expression, are specifically activated by α-GalCer presented by hCD1d. To analyze the response of T cells that have not been subject to long-term culture, we asked whether α-GalCer would induce the in vitro proliferation of human NK T cells. In these experiments, either the lipoglycan antigen or the vehicle control was added directly to cultures of unfractionated, fresh PBMCs. The percentage of Vα24-positive T cells was determined by flow cytometry at days 0, 7, 9, 11, and 12. By day 12, we systematically obtained a significant expansion in both the percentage and number of T cells expressing the Vα24/ Vβ11 TCR. A compilation of all the data from a series of experiments done with PBMCs from eight different donors is shown in Table 3. Although on day 0 the Vα24/Vβ11+ T cells were <1% of the total cell number, by day 7–12, the percentage of Vα24+ cells had expanded from 18.5- to 82-fold (Table 3). This increase in the percentage of Vα24+ cells is due to an expansion in cell number, because the total number of cells in the cultures generally was not decreased compared with day 0. This expansion required the presence of IL-2 and α-GalCer, as the number of Vα24/Vβ11+ T cells did not augment significantly when either one of these reagents was omitted (data not shown). PCR-heteroduplex analysis and oligotyping performed on aliquots of PBMCs, drawn at different time points from the different culture conditions, confirmed the expansion of Vα24/Vβ11+ T cells expressing the invariant Vα24-JαQ rearrangement (data not shown). Several of these lines were maintained in culture beyond day 12, and restimulated once with α-GalCer–pulsed autologous APCs, and further with α-GalCer–pulsed hCD1d-transfected C1R cells. These two further recalls allowed us to enrich the percentage of Vα24-positive T cells to 64% for line XC and 53% for line PB (Fig. 3). As shown in Fig. 3, lipoglycan-specific invariant Vα24/Vβ11+ T cells are either CD4+ or CD4−, consistent with previous reports on the surface phenotype of this subset (12, 14, 16). A 72% inhibition of the expansion of Vα24+ T cells was obtained for line PB when parallel cultures were set up from day 0 in the presence of the 51.1 anti-hCD1d mAb. This inhibition is consistent with the view that the α-GalCer–induced expansion of fresh T cells is hCD1d dependent. Similarly, a 62 and 60% reduction of the Vα24+ T cell expansion was obtained for lines MP and PD, respectively, when an anti-Vα24 mAb was added to the culture from day 0. These data suggest that the α-GalCer–induced T cell expansion was dependent upon engagement of the TCR. Interestingly, when cultured in the presence of α-GalCer–pulsed transfected APCs, these 2 lines released IFN-γ but not IL-4 in an hCD1d- and Vα24-dependent manner (data not shown).
Table 3.
α-GalCer Induces Expansion of Vα24/Vβ11+ T Cells
| Cell line | Day 0 | Antigen-stimulated* | ||
|---|---|---|---|---|
| PD | 0.2‡ | 5 (25)§ | ||
| MP | 0.08 | 2.7 (33) | ||
| CRG | 0.06 | 3.4 (57) | ||
| BG | 0.35 | 20 (57) | ||
| CU | 0.13 | 8.6 (66) | ||
| XC | 0.39 | 32.1 (82) | ||
| YG | 0.45 | 8.17 (18.5) | ||
| PB | 0.28 | 12.8 (45.7) | ||
Stainings were done at day 7, 9, 11, or 12 depending on the donor.
Percentage of Vα24+ T cells within CD3+ T cells.
Fold of expansion over percentage of input.
Figure 3.
NK T cells from human PBMCs expand in response to α-GalCer. Crude PBMCs were cultivated in the presence of 50 U/ml of IL-2 and 100 ng/ml of α-GalCer. At day 10 harvested cells from XC cell line (top) or PB cell line (bottom) were cultured for 5 d in the presence of IL-2 only. Proliferating T cells were then cultured for another 5 d with α-GalCer pulsed C1R-hCD1d transfectants in the presence of 50 U of IL-2. Expansion of the Vα24+ cells was determined upon staining with anti-CD4 and anti-Vα24 mAbs at days 0, 9, and 21.
Discussion
The hCD1d molecule is of particular interest on account of its recognition by human NK T cells and on account of having a broader expression pattern than do other human CD1 molecules (26). In this report, we provide the first evidence for the ability of hCD1d to present antigen. The antigen identified here is α-GalCer, a structurally well-defined glycolipid. In the presence of α-GalCer, neither the addition of PMA nor fixation of the APCs is required to stimulate NK T cell reactivity to hCD1d. Furthermore, preliminary data indicate that α-GalCer can bind in vitro to purified, soluble hCD1d and mCD1 molecules (Maher, J., O. Naidenko, W. Ernst, R. Modlin, and M. Kronenberg, manuscript in preparation). Together with the antigen presentation and antibody inhibition studies presented here, these data indicate that the effect of α-GalCer is not likely to be an indirect one, but rather that α-GalCer forms a complex with hCD1d that is recognized by the TCR on human NK T cells. It should be noted that α-GalCer was purified from an extract from a marine sponge, based upon the positive results obtained with this extract during a screen for compounds that prevent tumor metastases (27). Ceramides with an α-linked sugar are not abundant in most microbes, and they are much less abundant than those with a β-linked sugar in mammalian cells. However, β-galactosylceramide is not an antigen for CD1d presentation (22, 23). It therefore remains possible that α-GalCer is a mimic for a natural ligand that normally stimulates NK T cells, although there are no data to exclude the possibility of a low level of α-GalCer expression in mammalian cells. In mice, a glycosylphosphatidylinositol has been the only natural ligand bound to mCD1 identified so far (28), although compounds of this type apparently do not stimulate NK T cells in a CD1-dependent fashion (23).
The lack of polymorphism of nonclassical antigen-presenting molecules has led to the proposal that they may carry out some conserved and essential antigen presenting function. Although this is a plausible hypothesis, with the possible exception of mouse Qa-1b and human HLA-E (29), there is surprisingly little evidence for interspecies conservation of nonclassical class I molecules at either the sequence level or the functional level. In contrast, the CD1 family is demonstrated here to show an extremely high degree of conservation with regard to its interaction with the invariant TCRs that are expressed by NK T cells. This conservation is observed either in the absence of exogenous antigen or together with a lipoglycan antigen. From a functional viewpoint, the mouse and human invariant TCRs and CD1 molecules are nearly equivalent, indicating a strong selection upon this TCR interaction with CD1. These data are consistent with previous reports demonstrating that human and mouse NK T cells are similar in phenotype and function (11, 12, 16). This degree of conservation and cross-reactivity at the antigen recognition level is particularly striking in light of the divergence in primary sequence of both the CD1 molecules and the TCRs expressed by NK T cells (5). We conclude that despite these significant changes in primary structure, the amino acids important for either lipoglycan binding to CD1 or the interaction between the TCR and CD1 must not have diverged significantly.
The results from immune assays of mouse and human T cells permit the identification of candidate regions of the TCR that are necessary for the recognition of CD1 plus lipoglycan. Data from a previous study of TCR transgenic mice expressing Vβ8 and Vα14 and of mice deficient for Jα281 as a result of targeted mutation implicated a role for the invariant Vα14 TCR in α-GalCer recognition (22). Although several analyses of mCD1-reactive T cell hybridomas and cell lines established that Vα14 is not absolutely required for mCD1 autorecognition (20, 24, 30), only mCD1-autoreactive hybridomas with a Vα14 TCR could respond to α-GalCer plus mCD1 (23). The conservation of the Vα-Jα junction in the invariant TCR (14, 15, 18, 31) implicates this region of the TCR in contacting the CD1 molecule or a combination of CD1 and lipoglycan bound to it. Consistent with this hypothesis, we found that human T cells that express a Vα24 without the invariant V-J junctional sequence were unreactive to hCD1d transfectants in either the absence or presence of α-GalCer. Furthermore, there may be a more stringent selection upon the NK T cell β chain in humans than in mice, as the invariant Vα24 is mainly paired with Vβ11 (16, 32, 33), whereas in mice there are several predominant Vβs (12, 19, 20).
It is unprecedented to find complete cross-reactivity between mice and humans of the interaction of a TCR with a peptide–MHC complex. However, such a high degree of conservation is consistent with a superantigen type of recognition mechanism (34). In addition, the high frequency of α-GalCer–reactive cells in humans is consistent with a superantigen-like mode of action. However, there are two reasons why the data presented here do not favor the possibility that α-GalCer is recognized as a superantigen. First, the reactive T cells have a restricted V-J junctional diversity of their α chains, as well as Vα gene use. Second, the reactive T cells are selected for both Vα and for Vβ expression, particularly in humans. These findings favor a more conventional mechanism for the interaction of the α-GalCer plus CD1 complex with the TCR expressed by NK T cells.
At the functional level, NK T cells are distinguished by their ability to produce large amounts of the cytokines characteristic of memory T cells within a few hours of stimulation. It was proposed originally that the early expression of IL-4 by NK T cells could be required for Th2-type immune responses (35–36). Although NK T cells normally may contribute to the induction of Th2 responses, it is now clear that in many cases they are not required (37, 38). It also is clear from our studies and those of other investigators that NK T cells can secrete large amounts of IFN-γ in addition to IL-4 under some circumstances (39, 40). Based upon their relatively high frequency in some sites and their ability to rapidly secrete large amounts of cytokines, functions for NK T cells in the regulation of autoimmune diseases (33, 41, 42), the response to infectious agents (43), and the surveillance for tumors (44) have been proposed. With regard to autoimmune disease progression, the strongest evidence currently favors a connection between NK T cells and diabetes, in which the results from mice and humans show a striking degree of similarity (33, 42, 45). The dramatic expansion and activation of human NK T cells upon stimulation in vitro with α-GalCer is therefore of a considerable interest. Because we generally observed both IL-4 and IFN-γ in the culture supernatants of the T cell clones, but only IFN-γ from the lines, it should be possible to identify conditions in which a more polarized cytokine response develops. Therefore, one might envision the use of this molecule, or perhaps analogues (22) that might give a more polarized Th1 or Th2 response, as an attractive strategy to modulate human immune response in vivo.
Acknowledgments
The authors wish to thank Drs. S. Cardell (Lund University, Lund, Sweden), M. Bix (University of California at San Francisco, San Francisco, CA), and K. Hayakawa (Institute for Cancer Research, Philadelphia, PA) for kindly providing mCD1-restricted hybridomas. We also thank Dr. S. Porcelli for providing the anti-hCD1d mAbs. We thank Drs. S. Tangri, T. Prigozy, L. Gapin, and H. Cheroutre for critical review of the manuscript.
This work was supported by National Institutes of Health research grants CA-52511 and AI-40617 (to M. Kronenberg) and by Progetto Nazionale Tubercolosi, Istituto Superiore di Sanità (to P. Dellabona). This is manuscript no. 244 of the LIAI.
Abbreviations used in this paper
- α-GalCer
α-galactosylceramide
- β2m
β2-microglobulin
- hCD1d
human CD1d
- mCD1
mouse CD1
References
- 1.Hughes AL. Evolutionary origin and diversification of the mammalian CD1 antigen genes. Mol Biol Evol. 1991;8:185–201. doi: 10.1093/oxfordjournals.molbev.a040640. [DOI] [PubMed] [Google Scholar]
- 2.Bilsland CA, Milstein C. The identification of the β2-microglobulin binding antigen encoded by the human CD1D gene. Eur J Immunol. 1991;21:71–78. doi: 10.1002/eji.1830210112. [DOI] [PubMed] [Google Scholar]
- 3.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19:285–292. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury A, Belt KT, Neri TM, Milstein C, Calabi F. Mouse CD1 is distinct from and co-exists with TL in the same thymus. EMBO (Eur Mol Biol Organ) J. 1988;7:3081–3086. doi: 10.1002/j.1460-2075.1988.tb03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porcelli SA. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac A. Positive selection of mouse NK1+T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 9.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 10.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4- secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 11.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8−T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8−T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellabona P, Casorati G, Friedli B, Angman L, Sallusto F, Tunnacliffe A, Roosneek E, Lanzavecchia A. In vivo persistence of expanded clones specific for bacterial antigens within the human T cell receptor α/β CD4−8−subset. J Exp Med. 1993;177:1763–1771. doi: 10.1084/jem.177.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-Jα Q/Vβ 11 T cell receptor is expressed in all individuals by clonally expanded CD4−8−T cells. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8−α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davodeau F, Peyrat MA, Necker A, Dominici R, Blanchard F, Leget C, Gaschet J, Costa P, Jacques Y, Godard A, et al. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J Immunol. 1997;158:5603–5611. [PubMed] [Google Scholar]
- 17.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+T cell populations. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 19.Masuda K, Makino Y, Cui J, Ito T, Tokuhisa T, Takahama Y, Koseki H, Tsuchida K, Koike T, Moriya H, Amano M, Taniguchi M. Phenotypes and invariant αβ TCR expression of peripheral Vα14+NK T cells. J Immunol. 1997;158:2076–2082. [PubMed] [Google Scholar]
- 20.Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- 21.Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. Mouse CD1 autoreactive T cells have diverse patterns of reactivity to CD1+targets. J Immunol. 1998;160:3681–3688. [PubMed] [Google Scholar]
- 22.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 23.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 24.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+T cells in major histocompatibility complex class II–deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wack A, Montagna D, Dellabona P, Casorati G. An improved PCR-heteroduplex method permits high-sensitivity detection of clonal expansions in complex T cell populations. J Immunol Methods. 1996;196:181–192. doi: 10.1016/0022-1759(96)00114-7. [DOI] [PubMed] [Google Scholar]
- 26.Blumberg RS, Terhost C, Bleicher P, McDermott FV, Allan CH, Landau SB, Trier JS, Balk SP. Expression of a nonpolymorphic MHC class-I-like molecule, CD1d, by human intestinal epithelial cells. J Immunol. 1991;147:2518–2524. [PubMed] [Google Scholar]
- 27.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 28.Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 29.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 30.Zeng D, Dick M, Cheng L, Amano M, Dejbakhsh-Jones S, Huie P, Sibley R, Strober S. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J Exp Med. 1998;187:525–536. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Positive selection of invariant Vα14+T cells by non-major histocompatibility complex-encoded class I-like molecules expressed on bone marrow-derived cells. Proc Natl Acad Sci USA. 1995;92:1200–1204. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porcelli S, Gerdes D, Fertig AM, Balk SP. Human T cells expressing an invariant Vα24-JαQ TCRα are CD4−and heterogeneous with respect to TCRβ expression. Hum Immunol. 1996;48:63–67. doi: 10.1016/0198-8859(96)00090-0. [DOI] [PubMed] [Google Scholar]
- 33.Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 34.Acha-Orbea H, MacDonald HR. Superantigens of mouse mammary tumor virus. Annu Rev Immunol. 1995;13:459–486. doi: 10.1146/annurev.iy.13.040195.002331. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 36.Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1.1 T cells. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown DR, Fowell DJ, Corry DB, Wynn TA, Moskowitz NH, Cheever AW, Locksley RM, Reiner SL. β2-microglobulin-dependent NK1.1+T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Rogers KH, David BL. β2-microglobulin-dependent T cells are dispensable for allergen- induced T helper 2 responses. J Exp Med. 1996;184:1507–1512. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Paul WE. Cultured NK1.1+ CD4+T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 41.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gombert JM, Herbelin A, Tancrede-Bohin E, Dy M, Carnaud C, Bach JF. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur J Immunol. 1996;26:2989–2998. doi: 10.1002/eji.1830261226. [DOI] [PubMed] [Google Scholar]
- 43.Teixeira HC, Kaufmann SH. Role of NK1.1+ cells in experimental listeriosis. NK1+ cells are early IFN-γ producers but impair resistance to Listeria monocytogenesinfection. J Immunol. 1994;152:1873–1882. [PubMed] [Google Scholar]
- 44.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 45.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter DI. α/β− T cell receptor (TCR)+ CD4−CD8−(NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]