Abstract
Epidermal Langerhans cells (LC) are immature dendritic cells (DC) located in close proximity to the site of inoculation of infectious Leishmania major metacyclic promastigotes by sand flies. Using LC-like DC expanded from C57BL/6 fetal skin, we characterized interactions involving several developmental stages of Leishmania and DC. We confirmed that L. major amastigotes, but not promastigotes, efficiently entered LC-like DC. Parasite internalization was associated with activation manifested by upregulation of major histocompatibility complex (MHC) class I and II surface antigens, increased expression of costimulatory molecules (CD40, CD54, CD80, and CD86), and interleukin (IL)-12 p40 release within 18 h. L. major–induced IL-12 p70 release by DC required interferon γ and prolonged (72 h) incubation. In contrast, infection of inflammatory macrophages (Mφ) with amastigotes or promastigotes did not lead to significant changes in surface antigen expression or cytokine production. These results suggest that skin Mφ and DC are infected sequentially in cutaneous leishmaniasis and that they play distinct roles in the inflammatory and immune response initiated by L. major. Mφ capture organisms near the site of inoculation early in the course of infection after establishment of cellular immunity, and kill amastigotes but probably do not actively participate in T cell priming. In contrast, skin DC are induced to express increased amounts of MHC antigens and costimulatory molecules and to release cytokines (including IL-12 p70) by exposure to L. major amastigotes that ultimately accumulate in lesional tissue, and thus very likely initiate protective T helper cell type 1 immunity.
Keywords: Langerhans cell, dendritic cell, Leishmania major, T helper cell type 1/T helper cell type 2 immune response, interleukin 12
evelopment of T cell–mediated immunity against foreign Ag necessitates prior Ag-nonspecific triggering of APCs. With the recognition that dendritic cells (DC) are uniquely able to initiate responses in naive T cells and that DC also participate in Th cell education (for a review, see references 1 and 2), considerable effort has been directed towards identifying DC agonists and elucidating mechanisms that mediate DC activation. We have recently identified culture conditions that allow the expansion of epidermal Langerhans cell (LC)-like immature DC from murine fetal skin (fetal skin–derived DC [FSDDC]; reference 3) and have begun to characterize the response of FSDDC to a variety of agonists (4, 5).
To study LC/DC–pathogen interactions and mechanisms responsible for pathogen-dependent DC activation, we initiated experiments with FSDDC and Leishmania major. This experimental system was chosen because L. major infection in mice is a well-established model for human cutaneous leishmaniasis (for a review, see reference 6), and previous studies implicated LC as important participants in the initiation phase of immune responses to Leishmania in vivo (7–9). Prior in vitro studies of LC–L. major interactions have been hampered by technical difficulties associated with isolating keratinocyte-free LC and the spontaneous activation that results from removing LC from their epidermal microenvironment.
In this study, we have taken advantage of the relatively stable immature phenotype of FSDDC (4, 5) to assess the DC-activating potential of the two developmental stages of L. major that might interact with skin DC in the setting of cutaneous leishmaniasis. We also evaluated cytokines produced by FSDDC and inflammatory macrophages (Mφ) in response to L. major because cytokine-dependent Th education ultimately determines the outcome of infection (for a review, see references 6, and 10–12). We observed that FSDDC preferentially ingested and were activated by L. major amastigotes, and that FSDDC activation resulted in IL-12 release. Although Mφ readily ingested amastigotes (as well as promastigotes), they were not activated by infection. These data suggest that Mφ and DC are sequentially parasitized in cutaneous leishmaniasis, and that skin DC, rather than Mφ, are responsible for Th priming and the initiation of Th education in this disease.
Materials and Methods
Propagation of FSDDC.
Immature FSDDC were generated as described previously (3). In brief, fetal skin cells from day 16 C57BL/6 mice were cultured in GM-CSF– and M-CSF–supplemented media and, after ∼2 wk, DC aggregates were isolated by 1 g sedimentation. DC aggregates were dissociated in trypsin/ EDTA (0.25%/0.1 mM; reference 3) as necessary to allow accurate determination of cell numbers.
Isolation of Inflammatory Mφ.
Inflammatory C57BL/6 tissue Mφ were elicited by subcutaneous injection of polyacrylamide beads (13). Cells infiltrating the resulting nonimmune granulomas were harvested after 3–4 d, cells were separated from beads by filtering through 70 μm nylon mesh, and Mφ were enriched by plastic adherence.
Propagation and Isolation of L. major.
L. major clone V1 (MHOM/ IL/80/Friedlin) was cultured and different developmental stages were prepared as described (13). Infectious metacyclic promastigotes were isolated from stationary cultures by negative selection using peanut agglutinin. Amastigotes were prepared from homogenates of tissue derived from BALB/c footpad lesions via differential centrifugation and were used immediately or suspended in DMEM/10% fetal bovine serum (FBS)/7.5% DMSO and stored in liquid nitrogen. Isolated parasites were opsonized with 5% normal mouse serum before infection. Levels of LPS in parasite stock preparations were below the limit of detection (<0.1 endotoxin units/ml [LAL-test; BioWhittaker, Inc., Walkersville, MD]). Parasites were diluted 1:100 before use.
Coculture Experiments with L. major and FSDDC or Mφ.
FSDDC aggregates or Mφ were subcultured in their basal media (5% FBS containing GM-CSF– and M-CSF–supplemented RPMI 1640 [3] and complete DMEM/10% FBS [13], respectively) in 24-well plates at 2 × 105 cells in 1 ml/well (or 106 cells/well for determination of IL-12 p70 levels). L. major parasites (two to three organisms/cell) or Escherichia coli LPS (100 ng/ml; provided by Dr. Stephanie Vogel, Uniformed Services University of the Health Sciences, Bethesda, MD), IFN-γ (1,000 U/ml; Genzyme Corp., Cambridge, MA), and anti-CD40 (HM40-3, 10 μg/ml; PharMingen, San Diego, CA) were added as indicated.
Microscopy.
Morphologic changes in FSDDC aggregates in the coculture experiments were documented after 18 h using a video-linked inverted phase microscope (Eclipse TE 300; Nikon, Inc., Melville, NY). Parasite internalization was quantitated in DiffQuick-stained cytospin preparations using light microscopy. FSDDC aggregates were completely dissociated in calcium- and magnesium-free HBSS containing 1 mM EDTA (30 min at 37°C) before cytocentrifugation.
Antibodies and Flow Cytometry.
Anti-CD16/CD32 (2.4G2) was provided by Julie Titus (National Cancer Institute, Bethesda). Anti–H-2Db (28-14-8), anti–I-Ab (2G9), anti-CD40 (3/23), anti-CD54 (3E2), anti-CD80 (1G10), and anti-CD86 (GL1) were purchased from PharMingen as biotin- or PE-modified mAbs. PE-streptavidin was obtained from Tago Inc. (Burlingame, CA). Cells were stained for surface Ag expression as described previously (3). Stained and paraformaldehyde (1% in PBS)-fixed cells were analyzed using a FACScan® flow cytometer equipped with CellQuest software (Becton Dickinson, Mountain View, CA).
Quantitation of Cytokine Release.
Cytokine release into 18- and 72-h FSDDC and Mφ supernatants was measured using ELISA kits specific for IL-1α, IL-12 (p70), and TNF-α (Genzyme Corp.), or IL-1β, IL-4, IL-6, IL-10, IL-12 (p40), and IFN-γ (Biosource International, Camarillo, CA). Supernatants were concentrated approximately fivefold using microconcentrators (30K Microcon; Amicon, Inc., Beverly, MA) before determination of IL-12 p70. Statistical analysis was performed using the Wilcoxon signed rank test.
Results
FSDDC Internalized L. major Amastigotes, but not Metacyclic Promastigotes.
FSDDC were incubated with the different forms of serum-opsonized L. major, and cell–parasite interactions were studied by light microscopic examination of cytospin preparations. 18 h after amastigote addition, 36 ± 8% (n = 10) of the FSDDC were infected, and each infected cell contained up to six parasites (Fig. 1 A). In contrast, coincubation of FSDDC with promastigotes led to parasite attachment (Fig. 1 B), but only very low infection rates (7 ± 3%, n = 4).
Figure 1.
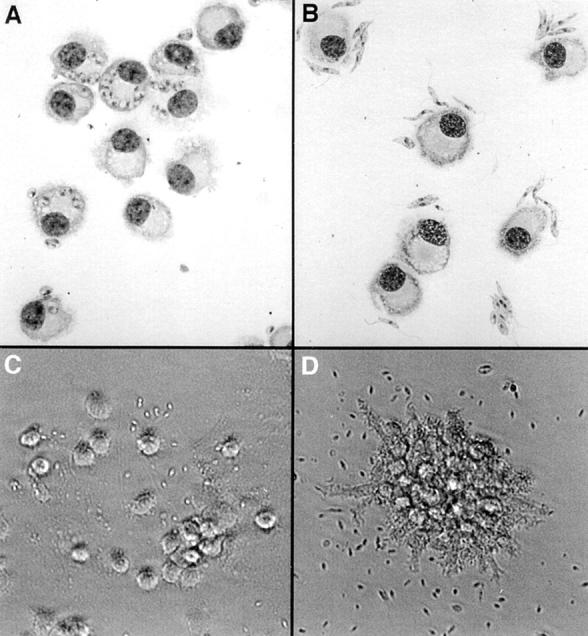
L. major amastigotes preferentially infect FSDDC and induce dissociation of FSDDC aggregates. C57BL/6 FSDDC were incubated with L. major amastigotes (A and C) or metacyclic promastigotes (B and D) for 18 h. (A and B) After complete dissociation of aggregates in EDTA, cytospins were prepared and stained with DiffQuick, and DC– parasite interactions were evaluated. (C and D) The morphology of FSDDC aggregates was assessed by phase–contrast photomicroscopy. Representative data from one of four experiments are shown.
L. major Amastigotes Activate FSDDC.
E-cadherin–mediated adhesion within aggregates of immature FSDDC can be viewed as a correlate of E-cadherin–mediated adhesion of LC to keratinocytes in epidermis (3, 14). Previous studies demonstrated that treatment of FSDDC with LPS or proinflammatory cytokines led to loss of adhesion within FSDDC aggregates and DC maturation (increased expression of MHC Ag and costimulatory molecules and enhanced APC activity [4]). Coincubation of FSDDC with L. major amastigotes for 18 h also induced dissociation of FSDDC aggregates into single, highly dendritic cells (Fig. 1 C). Although promastigotes adhered to FSDDC (Fig. 1 B), attachment was not accompanied by morphologic evidence of activation (Fig. 1 D).
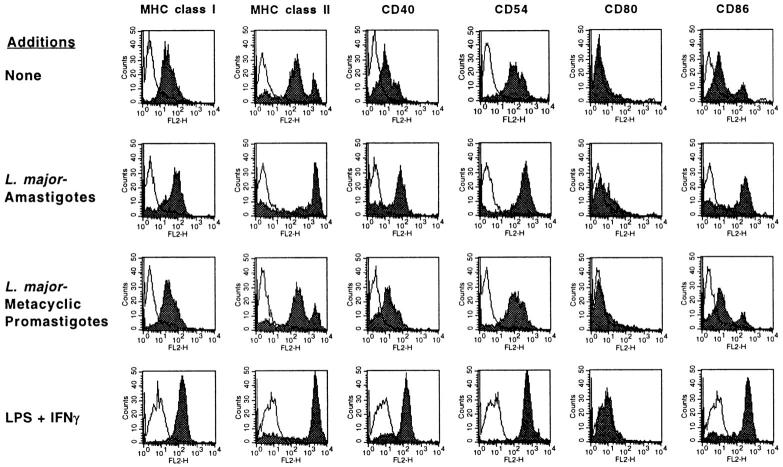
Analysis of surface Ag expression using flow cytometry confirmed that L. major amastigotes selectively induced FSDDC activation and maturation. Amastigote infection of FSDDC led to upregulation of MHC class I, class II, CD40, CD54 (intracellular adhesion molecule 1 [ICAM-1]), CD80 (B7.1), and CD86 (B7.2) analogous to that observed after addition of the known DC activators LPS and IFN-γ (Fig. 2). In contrast, coincubation of FSDDC with L. major promastigotes did not affect levels of the activation markers studied.
Figure 2.
L. major amastigotes induce upregulation of FSDDC MHC class I, class II, and costimulatory molecule expression. L. major amastigotes, metacyclic promastigotes, or LPS (100 ng/ml) and IFN-γ (1,000 U/ml) were added to FSDDC, and effects on surface Ag expression were assessed using flow cytometry 18 h later. Residual aggregates were dissociated with EDTA before analysis. Shaded areas, mAbs of interest; solid lines, isotype controls (n = 4).
Failure of L. major to Activate Inflammatory Tissue Mφ.
Both infectious metacyclic promastigotes and tissue amastigotes of L. major are efficiently internalized by Mφ (13). To determine if skin DC and Mφ were differentially activated by exposure to Leishmania, the expression of relevant surface Ag by infected Mφ was quantitated. In the absence of agonists, infiltrating tissue Mφ expressed low levels of MHC class II, CD40, CD54, and CD86 (Fig. 3), as well as MHC class I and CD80 (data not shown), consistent with their derivation from nonimmune granulomas. Although incubation of Mφ with L. major amastigotes or metacyclic promastigotes resulted in frequent infection (infection rates of 62 ± 12% [n = 4] and 53 ± 8% [n = 2], respectively) within 18 h, neither life-cycle stage led to upregulation of activation markers (Fig. 3).
Figure 3.
L. major does not activate inflammatory Mφ. Polyacrylamide bead–elicited tissue macrophages were infected with L. major amastigotes or metacyclic promastigotes, or stimulated with LPS (100 ng/ml) and IFN-γ (1,000 U/ml) for 18 h. MHC class II, CD40, CD54, and CD86 levels were analyzed after 18 h using flow cytometry. Shaded areas, mAbs of interest; solid lines, isotype control (n = 4).
Amastigote-infected FSDDC Release IL-12.
The outcome of encounters between Ag-bearing APCs and naive T cells depends, in part, on the nature of the cytokines released locally by APCs. This is particularly relevant in leishmaniasis because development of Th1-predominant protective immunity is clearly dependent on production of IL-12 (for a review, see references 6 and 11). Thus, we identified and quantitated cytokines released by Leishmania-infected FSDDC and, for comparison, cytokines released by infected Mφ. Relative to untreated FSDDC, amastigote-infected FSDDC released ∼12-fold more IL-12 p40 (P < 0.05, n = 9) and increased amounts of TNF-α (P < 0.05, n = 9) into supernatants (see Table 1), whereas incubation of FSDDC with promastigotes did not stimulate cytokine production. Small amounts of IL-1α, IL-1β, and IL-6 were also released by FSDDC (Table 1, and data not shown), but production of these cytokines was not augmented by infection with amastigotes. IL-4, IL-10, IL-12 p70, and IFN-γ were not detected in 18-h FSDDC supernatants (data not shown). Interestingly, prior infection of FSDDC with amastigotes did not inhibit IL-12 release induced by relatively high concentrations of LPS and IFN-γ (Table 1). Consistent with previous reports (15), bioactive IL-12 (p70) was detected only in 72-h supernatants of FSDDC (Table 2). Amastigote-induced FSDDC IL-12 p70 release also required addition of IFN-γ. Note that similar amounts of IL-12 p70 were released by amastigotes plus IFN-γ and maximally stimulated LPS plus IFN-γ plus anti-CD40– treated FSDDC.
Table 1.
Cytokine Release by L. major–infected FSDDC and Inflammatory Mφ
| Additions |
Cytokines analyzed | |||||||
|---|---|---|---|---|---|---|---|---|
| IL-12 (p40) | IL-12 (p70) | TNF-α | IL-6 | |||||
| FSDDC | ||||||||
| None | 34 ± 13 | <5 | <35 | 123 ± 67 | ||||
| Metacyclic promastigotes | 28 ± 17 | <5 | <35 | 163 ± 119 | ||||
| Amastigotes | 346 ± 134‡ | <5 | 112 ± 43‡ | 178 ± 107 | ||||
| LPS + IFN-γ | 1,226 ± 507‡ | <5 | 342 ± 130‡ | 1,599 ± 737‡ | ||||
| Amastigotes + LPS + IFN-γ≳ | 2,323 ± 1,584‡ | <5 | 503 ± 56‡ | 969 ± 258‡ | ||||
| Mφ | ||||||||
| None | 43 ± 11 | <5 | <35 | 1,045 ± 453 | ||||
| Metacyclic promastigotes | 27 ± 13 | <5 | <35 | 868 ± 868 | ||||
| Amastigotes | 44 ± 29 | <5 | 251 ± 251 | 701 ± 481 | ||||
| LPS + IFN-γ | 3,610 ± 1,648 | 686 ± 450 | 5,447 ± 425 | >20,000 | ||||
| Amastigotes + LPS + IFN-γ | 1,028 ± 595 | 45 ± 45 | 2,949 ± 1,561 | 11,585 ± 4,902 | ||||
FSDDC aggregates (n = 9) or Mφ (n = 4) were incubated with L. major promastigotes or amastigotes (two to three organisms/cell) or with LPS (100 ng/ml) plus IFN-γ (1,000 U/ml) as indicated. Cytokine levels in 18-h supernatants were determined by ELISA. Values represent pg/ml of cytokine produced by 2 × 105 cells/ml in 18 h (mean ± SEM).
LPS and IFN-γ were added 3 h after amastigotes.
P < 0.05 compared with untreated control.
Table 2.
Release of Bioactive IL-12 (p70) by L. major–infected FSDDC
| Additions | IL-12 (p70) | |
|---|---|---|
| None | <5 | |
| IFN-γ | <5 | |
| Amastigotes | <5 | |
| Amastigotes + IFN-γ | 56 ± 9* | |
| LPS + IFN-γ + anti-CD40 | 86 ± 7* |
FSDDC aggregates (n = 3) were incubated with L. major amastigotes (two to three organisms/cell) or with LPS (100 ng/ml), IFN-γ (1,000 U/ml), and anti-CD40 (10 μg/ml) as indicated. Cytokine levels in fivefold concentrated 72-h supernatants (106 FSDDC/ml) were determined by ELISA (pg/ml; mean ± SEM).
P < 0.05 compared with untreated control.
These results contrast with those obtained with Mφ. Infection of nonimmune tissue Mφ with L. major did not induce significantly increased production of IL-12 or other cytokines (Table 1). In addition, LPS plus IFN-γ–induced IL-12 release by Mφ was inhibited by infection with amastigotes before stimulation. These findings confirm previous observations made with Mφ infected with metacyclic promastigotes or amastigotes (6, 13, 16, 17), and highlight the differential effects of Leishmania on DC and Mφ.
Discussion
Cutaneous leishmaniasis is initiated by inoculation of small numbers of L. major metacyclic promastigotes into the dermis (for a review, see references 6 and 18). Although Mφ ingest promastigotes, they are not activated and are rendered selectively unable to produce the Th1-promoting cytokine IL-12 in response to inflammatory mediators (references 6, 13, and 16, and this study). In addition, the evidence that Leishmania-infected Mφ migrate to regional lymph nodes and trigger responses in naive T cells is not compelling (8). Thus, although dermal Mφ represent the initial site of parasite proliferation after epicutaneous inoculation and IFN-γ–activated Mφ are ultimately responsible for the destruction of organisms (6, 18), Mφ may not play a role in the induction of anti-Leishmania immunity.
The results of this and previous studies suggest that DC rather than Mφ are responsible for T cell priming in leishmaniasis. Because the L. major life form that is inoculated into skin does not parasitize immature DC (references 19 and 20, and this study), it seems likely that LC/DC infection occurs subsequent to amastigote release by Mφ. Exposure of FSDDC to amastigotes led to upregulation of MHC and costimulatory molecules and loss of E-cadherin–mediated adhesion within aggregates. The former observation is consistent with earlier results which indicated that amastigotes induced a transient increase in MHC class II biosynthesis in LC (21). The latter finding demonstrates that L. major amastigote– induced activation of FSDDC is associated with downregulation of E-cadherin expression and/or function as might be expected before mobilization of LC from epidermis to lymph nodes (4, 22). Although coincubation with amastigotes did not lead to infection of the entire FSDDC population, MHC and costimulatory molecules were upregulated on almost all cells. This may reflect a bystander effect (e.g., activation of uninfected DC by TNF-α release by infected cells [3]) or effects of organism fragments/subcellular fractions of parasites that are not visualized by light microscopy.
IL-12 plays an important role in the immunophysiology of experimental leishmaniasis and is required for the development of protective Th1-predominant immunity (for a review, see reference 6). In addition to directly facilitating Th1 education, IL-12 activates NK cells to become effectors and produce IFN-γ (23), which may also promote Th1 development and/or augment Mφ leishmaniacidal activity. Because Leishmania-infected tissue Mφ do not release IL-12 spontaneously or in response to potent stimuli (references 6, 13, 16, and 17, and this study), it is unlikely that Mφ are the primary source of IL-12 in lesional tissue.
Recent studies indicate that systemic administration of Toxoplasma gondii extracts (24) or Leishmania donovani amastigotes (25) results in rapid IL-12 p40 accumulation in DC in lymphoid tissue, but not in Mφ. These observations localize IL-12 production to the APCs and the microenvironment that are thought to be critical for T cell priming. Our results indicate that parasitized DC are a likely source of IL-12 in leishmaniasis, and demonstrate that amastigotes directly stimulate IL-12 production by skin DC. In addition to inducing IL-12 release, our data also indicate that L. major amastigotes induce DC maturation. We hypothesize that local activation of skin DC by Leishmania, mobilization of skin DC, and localization of Ag-bearing, IL-12–producing mature DC in regional lymph nodes is required for development of protective immunity. The delayed appearance of IL-12 p40 transcripts in lymph nodes draining murine skin inoculated with L. major (6) has been attributed to dissemination of amastigotes from skin to lymph nodes followed by IL-12 synthesis within Mφ. An alternative interpretation is that parasites are conveyed to lymph nodes by skin-derived DC, and that IL-12 is produced within infected DC. The scenario we propose is also consistent with previous data indicating that infected DC can be recovered from lymph nodes that drain murine skin inoculated with L. major, and that L. major–infected LC can initiate primary anti-Leishmania responses in T cells after injection into naive mice whereas infected Mφ cannot (8, 9, 26).
We envision that skin DC play a particularly important role as transporters of parasites/Ag to lymph nodes in cutaneous leishmaniasis initiated by small numbers of parasites (e.g., numbers of parasites comparable to those introduced by sand flies), where extracutaneous dissemination occurs only after significant proliferation of Leishmania in the dermis. The observation that development of protective immunity in naturally acquired infections in people (27) and in C57BL/6 mice inoculated with small numbers of metacyclic promastigotes (D.L. Sacks, unpublished observations) is delayed relative to that which occurs after administration of standard inocula suggests the existence of a threshold requirement that must be satisfied before priming of naive T cells can occur. One possibility is that recruitment of epidermal LC or dermal DC into sites of inoculation requires elaboration of proinflammatory cytokines (e.g., IL-1 or TNF-α) or chemokines (16, 28), which are released only after a significant parasite load accumulates. Alternatively (or in addition), priming may require that relatively large numbers of extracellular amastigotes are available to activate resident skin DC. The latter possibility would suggest that parasites that are effectively sequestered within Mφ in the early stages of infection would not initiate priming and also might not lead to a dramatic inflammatory response. Additional experiments will be required to distinguish between these, and other, potential explanations.
Delineation of the role that skin DC play in T cell priming in cutaneous leishmaniasis is important for several reasons. First, leishmaniasis is a significant world health problem for which no effective vaccine exists. Development of a useful vaccine will require identification of adjuvants that elicit protective responses as well as relevant Ag. Because DC are likely to be involved in Th1 education, DC agonists may be potent adjuvants. Second, it is interesting that DC and Mφ are differentially activated by L. major. This suggests that Leishmania may initiate immune responses via mechanisms that are distinct from those activated by other parasites (e.g., the potent Mφ activators T. gondii (29) and Listeria monocytogenes [30]). Elucidation of these mechanisms will further our understanding of the roles DC and Mφ play in the pathophysiology of various parasitic diseases and may also improve our ability to prevent or treat these common infections.
Acknowledgments
The authors thank Bai Nguyen and Mark Wilson for expert technical assistance, Harry Schaefer for preparing the figures, and Drs. Patricia Walker and Jonathan Vogel for helpful discussions.
Footnotes
E. von Stebut was supported by the Deutsche Forschungsgemeinschaft (Ste 833/2-1), and Y. Belkaid and T. Jakob were funded through the Fogarty International Center, National Institutes of Health.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Jakob T, Saitoh A, Udey MC. E-cadherin- mediated adhesion involving Langerhans cell-like dendritic cells expanded from murine fetal skin. J Immunol. 1997;159:2693–2701. [PubMed] [Google Scholar]
- 4.Jakob T, Udey MC. Regulation of E-cadherin-mediated adhesion in Langerhans cell-like dendritic cells by inflammatory mediators that mobilize Langerhans cells in vivo. J Immunol. 1998;160:4067–4073. [PubMed] [Google Scholar]
- 5.Jakob, T., P. Walker, A. Krieg, M. Udey, and J. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides. A role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. In press. [PubMed]
- 6.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. . Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 7.Will A, Blank C, Rollinghoff M, Moll H. Murine epidermal Langerhans cells are potent stimulators of an antigen-specific T cell response to Leishmania major, the cause of cutaneous leishmaniasis. Eur J Immunol. 1992;22:1341–1347. doi: 10.1002/eji.1830220603. [DOI] [PubMed] [Google Scholar]
- 8.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania majorfrom the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 9.Moll, H. 1995. The Immune Functions of Epidermal Langerhans Cells. R.G. Landes Company, Austin, TX. 190 pp.
- 10.Launois P, Louis J, Milon G. The fate and persistence of Leishmania majorin mice of different genetic backgrounds: an example of exploitation of the immune system by intracellular parasites. Parasitology. 1997;115:S25–S32. doi: 10.1017/s0031182097001777. [DOI] [PubMed] [Google Scholar]
- 11.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 12.Gorham JD, Gueler ML, Murphy KM. Genetic control of interleukin 12 responsiveness: implications for disease pathogenesis. J Mol Med. 1997;75:502–511. doi: 10.1007/s001090050135. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y, Butcher B, Sacks DL. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur J Immunol. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 15.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrera L, Gazzinelli RT, Badolato R, Henry S, Muller W, Kuhn R, Sacks DL. Leishmaniapromastigotes selectively inhibit interleukin 12 induction in bone marrow–derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartori A, Oliveira M, Scott P, Trinchieri G. Metacyclogenesis modulates the ability of Leishmaniapromastigotes to induce IL-12 production in human mononuclear cells. J Immunol. 1997;159:2849–2857. [PubMed] [Google Scholar]
- 18.Bogdan C, Rollinghoff M. The immune response to Leishmania: mechanisms of parasite control and evasion. Int J Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 19.Locksley RM, Heinzel FP, Fankhauser JE, Nelson CS, Sadick MD. Cutaneous host defense in leishmaniasis: interaction of isolated dermal macrophages and epidermal Langerhans cells with the insect-stage promastigote. Infect Immun. 1988;56:336–342. doi: 10.1128/iai.56.2.336-342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blank C, Fuchs H, Rappersberger K, Rollinghoff M, Moll H. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. . J Infect Dis. 1993;167:418–425. doi: 10.1093/infdis/167.2.418. [DOI] [PubMed] [Google Scholar]
- 21.Flohe S, Lang T, Moll H. Synthesis, stability, and subcellular distribution of major histocompatibility complex class II molecules in Langerhans cells infected with Leishmania major. . Infect Immun. 1997;65:3444–3450. doi: 10.1128/iai.65.8.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarzenberger K, Udey MC. Contact allergens and epidermal proinflammatory cytokines modulate Langerhans cell E-cadherin expression in situ. . J Investig Dermatol. 1996;106:553–558. doi: 10.1111/1523-1747.ep12344019. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 24.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorak PMA, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovaniinfection. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 26.Moll H, Flohe S, Rollinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol. 1995;25:693–699. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 27.Melby PC. Experimental leishmaniasis in humans: review. Rev Infect Dis. 1991;13:1009–1017. doi: 10.1093/clinids/13.5.1009. [DOI] [PubMed] [Google Scholar]
- 28.Racoosin E, Beverley S. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol. 1997;85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- 29.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]