Abstract
Interleukin (IL)-18 induces interferon (IFN)-γ synthesis and synergizes with IL-12 in T helper type 1 (Th1) but not Th2 cell development. We report here that IL-18 receptor (IL-18R) is selectively expressed on murine Th1 but not Th2 cells. IL-18R mRNA was expressed constitutively and consistently in long-term cultured clones, as well as on newly polarized Th1 but not Th2 cells. IL-18 sustained the expression of IL-12Rβ2 mRNA, indicating that IL-18R transmits signals that maintain Th1 development through the IL-12R complex. In turn, IL-12 upregulated IL-18R mRNA. Antibody against an IL-18R–derived peptide bound Th1 but not Th2 clones. It also labeled polarized Th1 but not Th2 cells derived from naive ovalbumin–T cell antigen receptor-αβ transgenic mice (D011.10). Anti–IL-18R antibody inhibited IL-18– induced IFN-γ production by Th1 clones in vitro. In vivo, anti–IL-18R antibody reduced local inflammation and lipopolysaccharide-induced mortality in mice. This was accompanied by shifting the balance from Th1 to Th2 responses, manifest as decreased IFN-γ and proinflammatory cytokine production and increased IL-4 and IL-5 synthesis. Therefore, these data provide a direct mechanism for the selective effect of IL-18 on Th1 but not Th2 cells. They also show that the synergistic effect of IL-12 and IL-18 on Th1 development may be due to the reciprocal upregulation of their receptors. Furthermore, IL-18R is a cell surface marker distinguishing Th1 from Th2 cells and may be a therapeutic target.
Keywords: T helper type 1 cells, T helper type 2 cells, interleukin 18 receptor, inflammation, septic shock
Functional heterogeneity of CD4+ T cells was recognized in the 1970's (1). Subsequently, Th1- and Th2-type T cell responses have been classified by virtue of IFN-γ/IL-2 and IL-4/IL-5 production, respectively (2). It is now generally accepted that the balance between these two T cell subsets determines the phenotype and progression of numerous experimental and clinical diseases (3–5). Therefore, elucidating their preferential induction and regulation is of considerable importance both in facilitating potential clinical immunoregulatory applications and understanding fundamental immunology. Thus, there has been an intensive search for genes and/or cell surface molecules selectively expressed on Th1 or Th2 cells which are implicated in selectively directing and regulating their biological functions.
Using differential display PCR, we have recently identified a gene encoding a cell surface molecule, originally designated ST2L/T1/DER4/Fit, expressed constitutively and stably on murine Th2 but not Th1 cells, even after stimulation with a range of immunological stimuli (6). Anti-ST2L antibody can decrease Th2 functions in vitro and in vivo. ST2L is a member of the IL-1R family with 28% amino acid sequence homology with the type I IL-1R (7). Although IL-1α acts as a cofactor in IL-12–induced Th1 development in BALB/c mice, IL-1α responsiveness is lost by committed Th1 cells and clones (8). Thus, members of the IL-1R family may be differentially expressed and thereby regulate the sequential functions of Th1 and Th2 cells. Therefore, we investigated other members of the IL-1R family for their expression and function in Th1 and Th2 cells. We have found that a gene known as IL-1R–related protein (IL-1Rrp)1 (9) with previously unknown function was selectively expressed in Th1 but not Th2 cells. During the course of this work, it was reported that human IL-18R shared complete amino acid sequence homology with IL-1Rrp and has 30% homology with ST2L (10). IL-18 is a cytokine produced by monocytic cells capable of promoting proliferation and IFN-γ production by Th1, CD8+, and NK cells in mice and in humans (11). It shares some of the biological activities of IL-12 but without significant structural homology, and serves as a costimulatory factor in the activation of Th1 but not Th2 cells (12). It appears not to drive Th1 development but synergizes with IL-12 for IFN-γ production (8). Consistent with this is the recent report that mice deficient in IL-18 gene are defective in Th1 and NK cell activities (13).
In this study, we show that IL-18R is preferentially expressed on the surface of Th1 compared with Th2 cells. IL-18 and IL-12 reciprocally upregulated each other's receptors. Furthermore, anti–IL-18R antibody decreased the functions of Th1 cells in vitro and in vivo. Therefore, these findings provide a mechanism for the synergistic effect of IL-12 and IL-18 in the development of Th1 but not Th2 cells. They also demonstrate that IL-18R is not only a selective cell surface marker for Th1 cells, distinguishing them from Th2 cells, but may also be an immunotherapeutic target.
Materials and Methods
Mice.
BALB/c and CBA mice were obtained from Harlan UK Ltd. (Bicester, Oxon, UK). OVA-TCR-αβ transgenic mice (D011.10) of the BALB/c background were provided by Dr. Ken Murphy (Washington University, St. Louis, MO).
Cell Lines and Cell Culture.
Murine T cell clones were as described previously (6). Dorris (Th1, specific for hen egg lysozyme, H-2k) and D10 (Th2, specific for conalbumin, H-2k) were obtained from American Type Culture Collection (Rockville, MD). X4 (Th1) and X12 (Th2) were both H-2d–restricted and specific against group A streptococcal M protein (14). The cells were maintained by periodic antigen stimulation with appropriate irradiated spleen cells followed by expansion in medium containing IL-2 for up to 4 wk. For short-term polarized T cell lines, CD4+ cells were purified from the spleen of OVA-TCR-αβ transgenic mice (D011.10; reference 15) by negative selection as described previously (16). They were cultured with OVA peptide (0.3 μM, OVA323–339) and irradiated BALB/c spleen cells in the presence of IL-12 (40 U/ml) plus anti–IL-4 antibody (10 μg/ml, 11B11) for Th1 line, or IL-4 (250 U/ml) plus anti–IL-12 (1/400, rabbit anti– IL-12 antiserum, R73.1) and anti–IFN-γ (5 μg/ml, R46A2) antibodies for Th2 line, for 7 d (16). IL-2 (10 U/ml) was added to the culture from day 3. Clones and T cell lines were also cultured in medium alone, in the presence of recombinant IL-2 (10 ng/ ml), IL-12 (10 ng/ml; both obtained from Genzyme Diagnostics, Kent, UK), IL-18 (30 ng/ml; PeproTech EC Ltd., London, UK), or a combination of IL-12 and IL-18.
Reverse Transcriptase PCR Southern Blot.
Primers for amplifying IL-18R were as follows. IL-18R: sense, TTAGGACCAAAGTGTGAGAAGG, and antisense, TCTCGTCTCTTTCCGCTATGCG (product of 459 bp); IL-12Rβ2: sense, AAAGCCAACTGGAAAGCATTCG, and antisense, AGTTTTGAGTCAGGGTCTCTGC (product of 466 bp); ST2L: sense, ACTTTGTTCACCACACTCTGC, and antisense, AACAGATGCCGTCTTGGAGGC (product of 450 bp); and hypoxanthine phosphoribosyltransferase (HPRT): sense, GTTGGATACAGGCCAGACTTTGTTG, and antisense, GAGGGTAGGCTGGCCTATAGGCT (product of 352 bp). PCR products were separated in 1% agarose gels and transferred to nylon membranes which were hybridized with cDNA probes labeled with [α-32P]dATP using the random primer method (Promega, Southampton, UK). The cDNA probes were generated by reverse transcription (RT)-PCR using the primers mentioned above. The PCR fragments were cloned into PCR-A/T vector (Invitrogen Corp., Carlsbad, CA), and sequences were confirmed by standard methods.
Northern Blot Analysis.
Total RNA was isolated from cells by RNA-ZolB and was separated (20 μg/lane) in 1% formaldehyde/ agarose gels, blotted to nylon membrane, and hybridized with the probes used for Southern blot above. The relative amount of the messages was compared with HPRT using an imager analyzer (Media Cybernetics, Inc., Silver Spring, MD). The membranes were stripped according to the manufacturer's recommendation and reprobed as indicated.
Anti–IL-18R Antibody.
A rabbit was immunized with the peptide corresponding to residues 247–266 of the murine IL-18R amino acid sequence (IL-1Rrp in reference 9). The peptide spanning the hydrophilic region within the extracellular domain of the molecule was selected for its uniqueness. The rabbit was immunized subcutaneously with 100 μg of the peptide conjugated to KLH emulsified in CFA, and boosted sequentially with the peptide–KLH conjugate (100 μg) in IFA and in saline. Specificity was tested by ELISA with peptide-bound 96-well plates. The biological activity of the antibody was tested by its ability to neutralize IL-18–induced IFN-γ production by cloned Th1 cells. In brief, Th1 cells (Dorris or X4) were cultured in 96-well plates in the presence of irradiated spleen cells and antigen, or with immobilized anti–murine CD3 antibody (Sigma Chemical Co., Poole, UK). To this was added recombinant murine IL-18 (30 ng/ml) and a serial dilution of the rabbit anti–IL-18R antibody. Culture supernatant was harvested at 48 h, and concentrations of IFN-γ were determined by ELISA, using paired antibodies (PharMingen, San Diego, CA). Total IgG was purified from the immune serum and preimmunized serum by ammonium sulphate precipitation.
Flow Cytometric Analysis.
Cells were incubated with anti– IL-18R or normal rabbit serum followed by biotinylated goat anti–rabbit IgG (DAKO Corp., Carpinteria, CA) and then developed with PercP-streptavidin (Becton Dickinson, Mountain View, CA). All reactions and washings were carried out at 4°C in FACS wash buffer (PBS containing FCS [5%] and NaN3 [0.1%]), and all antibodies were centrifuged (11,000 g for 5 min) immediately before use. For intracellular staining, cells were suspended at 105–106 cells/ml and stimulated with PMA (50 ng/ml; Sigma Chemical Co.) plus ionomycin (500 ng/ml; Sigma Chemical Co.) for 4 h. Brefeldin A (10 ng/ml; Sigma Chemical Co.) was added during the last 2 h. Cells were then fixed with paraformaldehyde (2%), and, after washing, permeabilized at room temperature with 0.5% saponin (Sigma Chemical Co.) in PBS and FCS (5%), then stained with FITC-conjugated anti–mouse IFN-γ (IgG2a; PharMingen) and PE-conjugated anti–mouse IL-4 (IgG2a; PharMingen), or FITC- and PE-conjugated isotype control antibodies (Becton Dickinson). After two washes with FACS wash buffer containing 0.1% saponin, samples were analyzed on a FACScan® flow cytometer (Becton Dickinson).
Local Inflammation.
Groups of BALB/c mice were injected daily with 0.5 mg i.p. of anti–IL-18R antibody or normal rabbit IgG. They were injected in the right hind footpad with 300 μg of lambda carrageenin (Sigma Chemical Co.) in 50 μl PBS 24 h after the first injection of antibody. Footpad swelling was measured over the next 3 d with a dial calliper (Kroeplin GmbH, Munich, Germany), and data are expressed as mean footpad thickness increase (right footpad − left footpad) ± SEM. Some mice were killed 24 h after carrageenin injection, and footpad and draining lymph nodes (DLN) were removed. Footpad was fixed in formaldehyde, and histology was examined for cellular infiltration after staining with hematoxylin and eosin. DLN cells were cultured with immobilized anti-CD3 antibody (Sigma Chemical Co.) for 48 h. Supernatants were collected for cytokine assay by ELISA using paired antibodies (PharMingen). T cell proliferation was analyzed by [3H]thymidine uptake during the last 6 h of culture. In some experiments, mice were killed 24 h after carrageenin injection, and peripheral blood was collected in heparin.
LPS-induced Shock.
Groups of BALB/c mice were injected daily with 0.5 mg i.p. of anti–IL-18R antibody or normal rabbit IgG. They were then challenged intraperitoneally, 24 h after the first antibody injection, with 18 mg/kg body wt of LPS (Salmonella enteritidis; Sigma Chemical Co.) in 0.2 ml PBS. Animals were observed every 6 h for general health and mortality. Experiments were terminated on day 4 after LPS injection as required by the guidelines for animal experimentation, Home Office, UK. Mice were tail-bled 2 and 24 h after LPS injection. The sera were pooled, and concentrations of IFN-γ, TNF-α, IL-4, IL-5, IL-6, and IL-12 were determined by ELISA using paired antibodies (PharMingen).
Statistical Analysis.
Statistical analysis was performed using Minitab software for Macintosh. Comparison between groups was by Mann-Whitney test or Student's t test except for lethality data, which were analyzed by the two-tailed log–rank test.
Results
IL-18R Message Is Consistently Expressed in Th1 but not Th2 Cells.
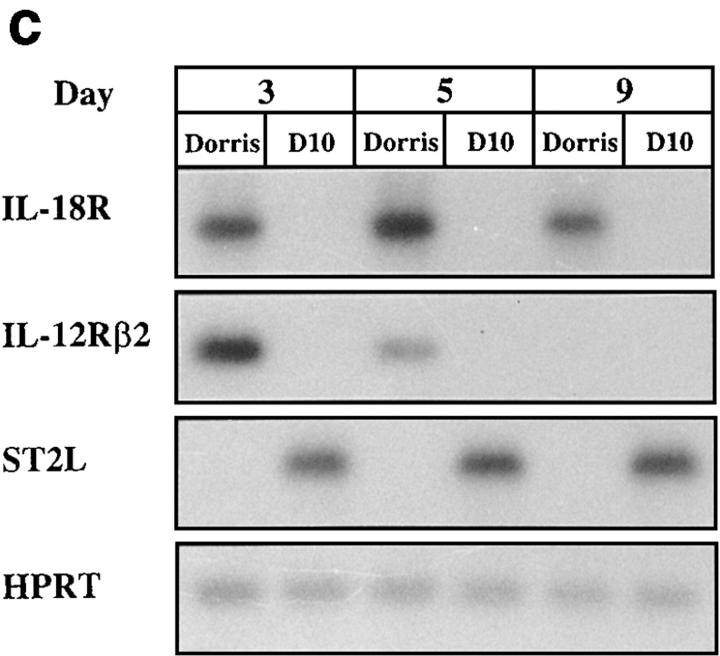
A panel of cloned Th1 and Th2 cells were stimulated with antigens and irradiated APCs. 5 d later, RNA was extracted, and the expression of IL-18, IL-12Rβ2, and ST2L was detected by the highly sensitive RT-PCR Southern blot analysis. Data presented in Fig. 1 show that, as expected (6), Th2 but not Th1 cells expressed ST2L. In contrast, Th1 but not Th2 cells expressed IL-12Rβ2 and IL-18R. IL-18R expression in Th1 cells was persistent because its message was clearly expressed for at least 28 d when the cells were maintained in culture medium containing IL-2. It should also be noted that T cell clones derived from BALB/c (X4, X12) or CBA (Dorris, D10) mice show a similar pattern of expression of IL-18R, ST2L, and IL-12Rβ2 message (Fig. 1 a).
Figure 1.
Selective expression of IL-18R message in Th1 compared with Th2 cells. (a) Cloned Th1 (Dorris, X4) and Th2 cells (D10, X12) were activated with irradiated spleen cells (APCs) and antigen. RNA were extracted 5 d later and analyzed by RT-PCR Southern blot with probes of IL-18R, IL-12Rβ2, ST2L, or HPRT. (b) CD4+ T cells from D011.10 mice were polarized with APCs and antigen in the presence of IL-12 and anti–IL-4 antibody (Th1 line), or IL-4, anti–IL-12, and anti-IFN-γ (Th2 line). The cells were harvested 3, 5, and 7 d after start of the culture or 5 d (*5) after the second round of culture. RNA was extracted and analyzed by RT-PCR Southern blot as in a above. (c) Kinetic study of the expression of messages by representative Th1 (Dorris) and Th2 (D10) clones. Cells were stimulated with APCs and antigen, and RNA was extracted for RT-PCR Southern blot analysis on days 3, 5, and 9. For cells extracted on day 9, IL-2 was added in the culture on day 3 to maintain the viability of the cells beyond day 7. Data are representative of four experiments.
We then investigated whether selective expression of IL-18R in Th1 cells also applied to newly polarized T cell lines, and whether this was due to a selective loss of the receptor in Th2 cells during development. CD4+ T cells were negatively selected from the splenic cell population of OVA-TCR-αβ transgenic mice (D011.10). They were cultured in vitro in the presence of IL-12 plus anti–IL-4 (Th1 line) or IL-4 plus anti–IL-12 and anti-IFN-γ (Th2 line). Cells were harvested on days 3, 5, and 7. Some cells were driven for a second round and harvested 5 d later. The differential expression pattern of IL-18R, IL-12Rβ2, and ST2L was evident even as early as day 3. The Th1 line progressively lost ST2L message and expressed strong IL-18R and IL-12Rβ2 messages. In contrast, the Th2 line expressed strong ST2L message but little or no IL-18R or IL-12Rβ2 mRNA (Fig. 1 b). This pattern became more polarized with prolonged culture. By day 5 of the second round of driving, IL-18R and IL-12Rβ2 message was clearly present in Th1 lines but not detectable in Th2 lines, whereas ST2L was present in Th2 but not in Th1 lines.
In parallel experiments, we investigated the relative stability of the expression of IL-18R, IL-12Rβ2, and ST2L in cloned T cell lines. The clones were stimulated in vitro with their respective antigen (peptide) and irradiated APCs, and RNA was extracted at regular intervals. Although the expressions of ST2L and IL-18R were stable beyond day 9, that of IL-12Rβ2 began to decline on day 5 and was not detectable by day 9 (Fig. 1 c).
Interactions of IL-18 and IL-12 in Th1 Cells.
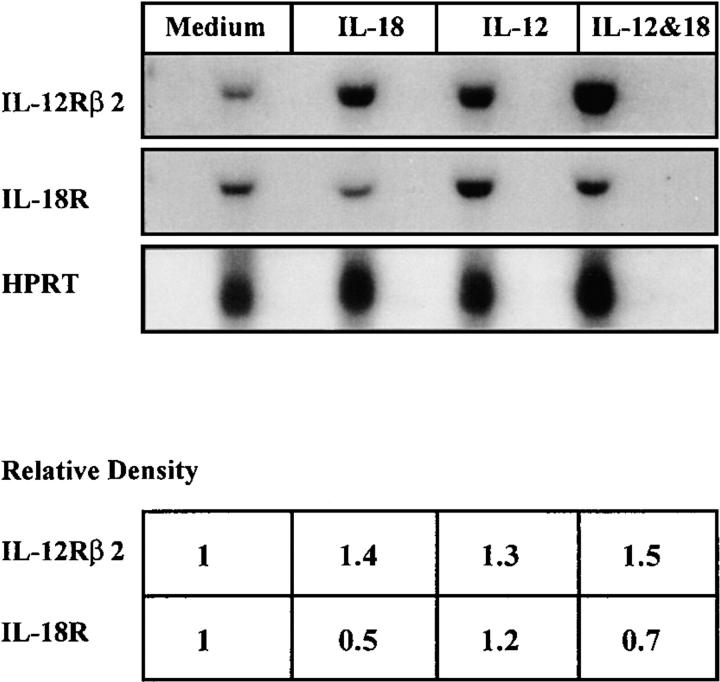
Since IL-18 synergizes with IL-12 in the development of Th1 cells (12) and the production of IFN-γ (8), we investigated the possibility that these two cytokines may influence each other's receptor expression. Th1 clones were cultured with APCs and antigen in the presence of IL-12, IL-18, or a combination of IL-12 and IL-18. Cells were harvested on days 3–7, and RNA was extracted. Northern blot analysis showed that IL-12Rβ2 was sustained and increased by the presence of IL-18, IL-12, and particularly a combination of IL-18 and IL-12, compared with cells cultured with medium alone. In contrast, IL-18R was markedly decreased by IL-18 but increased by IL-12. A combination of IL-12 and IL-18 induced a slight decrease of IL-18R (Fig. 2). The effect of IL-12 and IL-18 on IL-12Rβ2 was reflected in IFN-γ production by the treated Th1 cells. IL-18, IL-12, and especially a combination of the two cytokines significantly increased IFN-γ synthesis compared with cells cultured with medium alone (data not shown). Therefore, binding of IL-18 to IL-18R appears to transmit signals for a sustained expression of IL-12R complex and the production of IFN-γ. Conversely, binding of IL-12 to IL-12R increased the expression of IL-18R even in the presence of IL-18. This may account for the synergistic effect of IL-18 and IL-12 in Th1 expansion.
Figure 2.
Effect of IL-18 and IL-12 on the expression of IL-18R, IL-12Rβ2, and Th1 functions. A representative Th1 clone (Dorris) was stimulated with irradiated APCs and antigen in the presence of IL-18, IL-12, a combination of IL-18 and IL-12, or medium alone. RNA was extracted on day 5 and analyzed by Northern blot. The relative level of the message was compared with reference to HPRT. Message expressed by cells cultured with medium alone was normalized as 1.0. Similar results were obtained with cells cultured for 3 or 7 d.
IL-18R Is Expressed on the Surface of Th1 but not Th2 Cells.
A rabbit polyclonal antibody was raised against a peptide corresponding to the hydrophilic region of the extracellular domain of IL-18R. The antibody stained Th1 but not Th2 clones by flow cytometric analysis (Fig. 3 a). The expression was persistent, because Th1 cells remained IL-18R–positive after antigenic stimulation and prolonged culture in medium containing IL-2 alone (data not shown). To determine whether this staining pattern of IL-18R also occurs in cells derived from naive T cells, we polarized the splenic CD4+ T cells from OVA-TCR-αβ transgenic mice (D011.10) with IL-12 and anti–IL-4 (Th1 line) or IL-4 (Th2 line) in vitro in a 6-d culture and stimulated the cells with PMA and ionomycin for 4 h (16). They were then stained for cell surface IL-18R and intracellular IL-4 and IFN-γ. Th1 lines stained positive for IL-18R and IFN-γ, but negative for IL-4. In contrast, the Th2 line was negative for IL-18R and IFN-γ, but positive for IL-4 (Fig. 3 b). Titration studies showed that cell surface expression of IL-18R by Th1 cells was positively related to the concentration of IL-12 used in the culture; 40 U/ml of IL-12 induced stronger IL-18R expression than 20 or 10 U/ml. This is consistent with the results reported in Fig. 2 above.
Figure 3.
Flow cytometric analysis of cell surface expression of IL-18R. (a) Th1 (Dorris) and Th2 (D10) were stained with rabbit anti–IL-18R or preimmune serum (1/25 dilution) followed by biotinylated goat anti–rabbit IgG and were developed with PerCP-streptavidin. Similar results were obtained with X4 (Th1) and X12 (Th2) (not shown). (b) CD4+ T cells from OVA-TCR-αβ transgenic mice (D011.10) were driven to Th1 or Th2 lines for 6 d with APCs and antigen in the presence of IL-12 and anti–IL-4 antibody (Th1 line) or IL-4 (Th2 line). They were stained for cell surface IL-18R (with PerCP), and intracellular IFN-γ (with FITC) and IL-4 (with PE). All cells in b were activated with PMA/ionomycin for 4 h and Brefeldin A added in the last 2 h. Unfilled histograms, Staining with control preimmune serum. Similar results were obtained with cells driven for up to five rounds of culture.
To determine whether the anti–IL-18R antibody can affect Th1 functions, Th1 cells were cultured with APCs and antigen in vitro in the presence of IL-18 (15 ng/ml) plus IL-12 (10 ng/ml), and anti–IL-18R antibody (700 ng/ml) or normal IgG. The antibody significantly inhibited the production of IFN-γ by Th1 cells (normal IgG versus anti– IL-18R [mean ± SD]: 1,140 ± 130 vs. 640 ± 8 ng/ml, representative of four experiments]. However, the antibody had little or no effect on Th1 cell proliferation (data not shown).
Anti–IL-18R Antibody Reduces Local Inflammation.
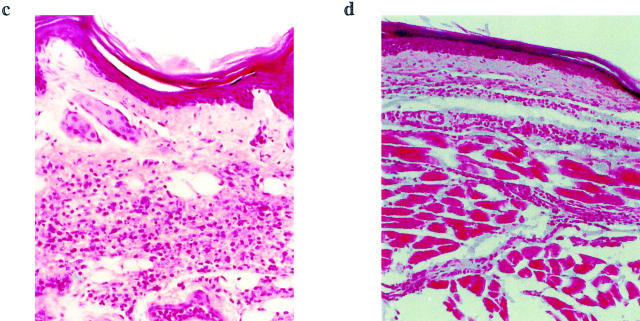
We next investigated the effect of anti–IL-18R antibody in vivo using an inflammatory model. BALB/c mice were injected in the footpad with carrageenin and treated intraperitoneally with anti–IL-18R antibody or normal rabbit IgG. Mice treated with anti–IL-18R antibody developed significantly less footpad swelling within the first 48 h compared with normal IgG-treated controls (Fig. 4 a). Histological examination revealed marked mononuclear and polymorphonuclear cellular infiltration in the control footpad. This was significantly reduced in the footpad of the anti–IL-18R antibody–treated mice (Fig. 4, b–d). DLN T cells from the antibody-treated mice also produced considerably less IFN-γ and IL-6 but more IL-4 and IL-5 than those from the control mice when stimulated with anti-CD3 antibody in vitro (Fig. 4 e). Together, these findings are consistent with the notion that IL-18 is associated with local inflammation and that anti–IL-18R antibody can downregulate Th1 activity within such inflammatory responses.
Figure 4.
Effect of anti–IL-18R antibody on local inflammation. BALB/c mice were injected in the footpad with carrageenin (300 μg/50 μl/mouse). They were also injected intraperitoneally with anti–IL-18R antibody or normal rabbit IgG (0.5 mg/mouse/d) daily for 4 d, starting 24 h before the footpad injection. (a) Footpad swelling was measured daily. Data are mean ± SEM; n = 5, *P < 0.01. Footpad thickness of the antibody-treated group was indistinguishable from the control at 72 h after injection. Thus, the antibody delayed the onset of local inflammation. (b) Inflammatory score of histological examination shows extensive cellular infiltrations in the footpad of the (c) normal IgG-treated group at 24 h after carrageenin injection. This was significantly reduced after (d) anti–IL-18R injection. Sections of the carrageenin-injected footpads were stained with hematoxylin and eosin (original magnification: ×10). The number of infiltrating cells was counted and expressed as inflammatory score (0, no inflammation; 1, patchy mild inflammation; 2, patchy extensive inflammation; 3, continuous inflammation; 4, continuous inflammation with loss of architecture; n = 5, *P < 0.01). (e) DLN were pooled (five mice per group) 24 h after carrageenin injection and stimulated in vitro with immobilized anti-CD3 antibody. Supernatant was collected 48 h later, and cytokine concentration was determined by ELISA. Data are mean ± SD; n = 3, *P < 0.05, **P < 0.01. There was no significant difference in the T cell proliferative response between the two groups of mice (data not shown). Results are representative of three experiments.
Anti–IL-18R Antibody Reduces LPS-induced Shock.
We next determined whether anti–IL-18R antibody could influence LPS-induced septic shock. BALB/c mice were injected intraperitoneally with LPS and treated with anti– IL-18R antibody or normal rabbit IgG. While the control IgG-treated mice developed substantial mortality, the disease was markedly reduced in the group treated with the anti–IL-18R antibody (Fig. 5 a). The antibody-treated mice also recovered from body weight loss more quickly (Fig. 5 b). The beneficial effect of the anti–IL-18R antibody was accompanied by reduced levels of serum IFN-γ, TNF-α, and IL-6 after LPS injection (Fig. 5 c). Thus, anti– IL-18R antibody could significantly ameliorate LPS-induced shock and in so doing could reduce the production of proinflammatory cytokines.
Figure 5.
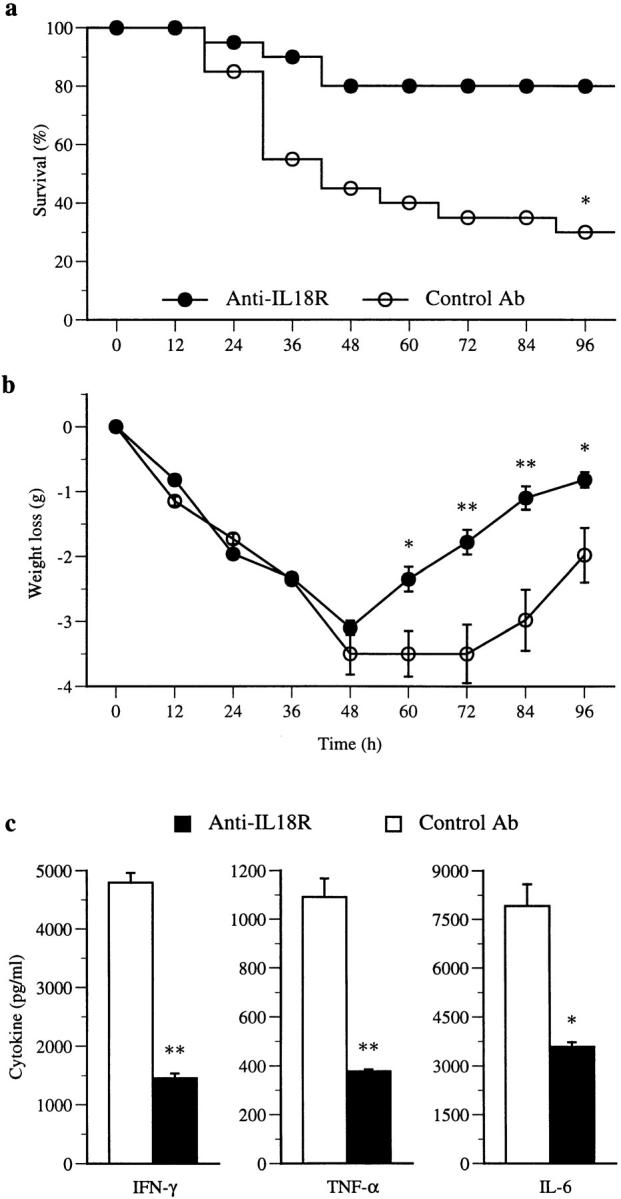
Effect of anti–IL-18R antibody on LPS-induced shock. BALB/c mice were injected intraperitoneally with 18 mg/kg body wt of LPS. They were injected daily with 0.5 mg i.p. of anti–IL-18R antibody or normal rabbit IgG, starting 24 h before LPS injection. (a) Anti–IL-18R antibody treatment significantly reduced mortality compared with control (n = 20, *P < 0.001). Results are pooled from two experiments. (b) Both groups of mice suffered similar initial weight loss. However, the antibody-treated group recovered significantly more rapidly compared with control (mean body weight of surviving mice ± SEM; *P < 0.05, **P < 0.01). (c) Sera were collected and pooled 2 and 24 h after LPS injection. Cytokine concentrations in the serum were determined by ELISA. Data are mean ± SD of triplicate assays of the 2-h sera; *P < 0.05, **P < 0.01. Similar results were obtained for the 24-h sera (data not shown).
Discussion
Data presented here demonstrate that IL-18R is selectively and persistently expressed on Th1 but not Th2 cells. Thus, IL-18R not only serves as a cell surface marker distinguishing Th1 from Th2 cells, but also provides an explanation for the selective biological effect of IL-18. In addition, our data provide a mechanism by which IL-18 synergizes with IL-12 in the expansion of Th1 cells through reciprocal modification of receptor expression, leading directly to enhanced production of IFN-γ. The persistent expression of IL-18R on Th1 cells suggests that IL-18 may play a dominant role in Th1 expansion and function. Furthermore, antibody against IL-18R affects Th1 functions in vitro and in vivo.
It is of interest that IL-18 downregulated but did not abrogate IL-18R (Fig. 2). This is consistent with a recent report that IL-18–deficient mice expressed elevated IL-18R message (13). The mechanism for this is currently unknown, but may represent a self-limiting feedback circuit to curtail overexpansion of Th1 cells, which have been implicated in a range of immunopathologies (17, 18). This possibility is currently being addressed. It should also be noted that Th1 cells from both BALB/c and CBA origin expressed substantial levels of IL-18R and IL-12Rβ2 (Fig. 1) and that IL-18 could upregulate IL-12Rβ2 (Fig. 2). This suggests that the observed sustained expression of IL-12R in CBA mice, which may account for the resistance of this mouse strain to intracellular infection (19), may be associated with innate IL-18 expression.
IL-18 is produced by monocytic cells after pathogenic infections (11). Since IL-18R is selectively present on a distinct subset of T cells, IL-18 likely plays an important role beyond providing a link between innate and adaptive immune response. The ability of IL-18 to support Th1 expansion is determined by the selective expression of IL-18R on Th1 cells. Therefore, it would be of considerable interest to determine the mechanism whereby IL-18R is preferentially expressed on Th1 but not Th2 cells during their dichotomous development from a common precursor. However, since NK cells (11, 20) and neutrophils (our unpublished data) also express IL-18R, the rapid downregulation of proinflammatory cytokines during LPS-induced shock by anti–IL-18R antibody (2 h after LPS injection) may reflect a combination of effects of the antibody on Th1 cells as well as NK cells and neutrophils. However, the antibody treatment did not significantly affect the percentage of these cells in vivo (data not shown).
It is interesting that both ST2L and IL-18R, which are preferentially and persistently expressed on Th2 and Th1 cells, respectively, belong to the type I IL-1R family. Although it is now well established that cytokines play a major role in the polarization of Th1 and Th2 cells, other factors such as MHC, antigen dose, and antigenic affinity also appear to be important (for a review, see reference 21). The precise mechanisms by which these factors interact to preferentially induce Th1 and Th2 cells remain obscure. Differential activation of the genes of the type I IL-1R family may thus provide an answer to a central question in immunology. There is considerable amino acid sequence homology between the cytoplasmic domains of human IL-1R family and the Drosophila Toll protein (22), which controls the induction of potent antimicrobial factors in the adult fly (23). Thus, the IL-1R family may govern an evolutionary ancient immune response in both insects and vertebrates. Interestingly, all members of the IL-1R family signal through the IL-1R–associated kinase–nuclear factor-κB (IRAK-NF-κB) pathway (8, 24) yet lead to differential expression and regulation of distinct T cell subsets. Unraveling these detailed signaling pathways and their transcriptional control represents an important challenge. It will also be important to determine whether the homologues of these molecules are also differentially expressed on human Th1 and Th2 cells as well as the CD8+ subsets.
Knowledge of Th1 and Th2 biology was advanced considerably by the demonstration that Th1 and Th2 cells can be polarized by culturing CD4+ T cells from transgenic mice recognizing a single peptide (25, 26), marked by differential intracellular staining of cytokines produced by the subsets (16). However, identification of stable cell surface markers, analogous to the CD4/CD8 molecules, capable of recognizing live cells and contributing towards the functions of Th1 and Th2 cells, would be of considerable importance in advancing this field. CD30 was reported to be expressed transiently and preferentially on cloned human Th2 compared with Th1 cells (27). Message for IL-12Rβ2 was induced on Th1 cells by IL-12 and IFN-γ (28, 29). However, the induction was again transitory. Th1 but not Th2 cells are able to bind P-selectin and E-selectin (30), but the ligand for the binding has not yet been clearly identified. Parallel to this finding are the recent observations that Th1 and Th2 cells differentially express chemokine receptors (31–34). CCR3 and CCR4 are preferentially expressed on Th2 cells, whereas CCR5 and CXCR3 are selectively expressed on Th1 cells. The expression of these receptors may have important implications for the migratory and activation patterns and susceptibility to HIV infection of Th1 and Th2 cells. However, these receptors are rapidly lost upon T cell activation by IL-2, anti-CD3, and anti-CD28 (31, 32). Data presented here demonstrate that IL-18R is a selective and consistent cell surface marker for Th1 cells, distinguishing them from Th2 cells.
The finding of persistent Th1 and Th2 cell surface markers directly demonstrates the validity of the Th1/Th2 classification. These markers will also facilitate investigation into the interaction between Th1 and Th2 cells, using pure populations of cells ex vivo. The antibodies will enable in vivo tracking of the migratory pattern and selective interactions of Th1 and Th2 cells with other cell types, including APCs and B cells, in the induction of humoral responses. Finally, antibodies, agonists, and antagonists for these cell surface molecules may have important clinical applications during infectious and autoimmune diseases in which the balance between Th1 and Th2 cells is known to play a critical role.
Acknowledgments
This work was supported by the Wellcome Trust, the Medical Research Council, and the Arthritis Research Council of the United Kingdom.
Abbreviations used in this paper
- DLN
draining lymph node(s)
- HPRT
hypoxanthine phosphoribosyltransferase
- IL-1Rrp
IL-1 receptor–related protein
- RT
reverse transcription
References
- 1.Liew FY, Parish CR. Lack of a correlation between cell-mediated immunity to the carrier and the carrier– hapten helper effect. J Exp Med. 1974;139:779–784. doi: 10.1084/jem.139.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 3.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 4.Liew FY, O'Donnell CA. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 6.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tominaga S. A putative protein of a growth-specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 8.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley SB, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 9.Parnet P, Garka KE, Bonnert TP, Dower SK, Sims JE. IL-1Rrp is a novel receptor-like molecule similar to the type I interleukin-1 receptor and its homologues T1/ ST2 and IL-1R AcP. J Biol Chem. 1996;271:3967–3970. doi: 10.1074/jbc.271.8.3967. [DOI] [PubMed] [Google Scholar]
- 10.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 11.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 12.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 13.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 14.Rossiter BA, Alfonso C, Kehoe MA, Robinson JH. Processing of viable group A streptococci leads to major histocompatibility complex class II presentation of T cell epitopes from the major protective antigen. Eur J Immunol. 1994;24:1244–1247. doi: 10.1002/eji.1830240537. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlothymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 16.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon AK, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91:8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Garra A, Steinman L, Gijbels K. CD4+T-cell subsets in autoimmunity. Curr Opin Immunol. 1997;9:872–883. doi: 10.1016/s0952-7915(97)80192-6. [DOI] [PubMed] [Google Scholar]
- 19.Guler ML, Gorham JD, Hsieh CS, Mackey AJ, Steen RG, Dietrich WF, Murphy KM. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 20.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 21.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 22.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to DrosophilaToll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent anti-fungal response in Drosophilaadults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 24.Kojima H, Takeuchi M, Ohta T, Nishida Y, Arai N, Ikeda M, Ikegami H, Kurimoto M. Interleukin-18 activates the IRAK-TRAF6 pathway in mouse EL-4 cells. Biochem Biophys Res Commun. 1998;244:183–186. doi: 10.1006/bbrc.1998.8236. [DOI] [PubMed] [Google Scholar]
- 25.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 26.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)–producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4–producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Prete G, De Carli M, Almerigogna F, Daniel CK, D'Elios MM, Zancuoghi G, Vinante F, Pizzolo G, Romagnani S. Preferential expression of CD30 by human CD4+T cells producing Th2-type cytokines. FASEB J. 1995;9:81–86. [PubMed] [Google Scholar]
- 28.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 32.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 33.Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]