Abstract
A conserved subset of mature circulating T cells in humans expresses an invariant Vα24-JαQ T cell receptor (TCR)-α chain rearrangement and several natural killer (NK) locus–encoded C-type lectins. These human T cells appear to be precise homologues of the subset of NK1.1+ TCR-α/β+ T cells, often referred to as NK T cells, which was initially identified in mice. Here we show that human NK T cell clones are strongly and specifically activated by the same synthetic glycolipid antigens as have been shown recently to stimulate murine NK T cells. Responses of human NK T cells to these synthetic glycolipids, consisting of certain α-anomeric sugars conjugated to an acylated phytosphingosine base, required presentation by antigen-presenting cells expressing the major histocompatibility complex class I–like CD1d protein. Presentation of synthetic glycolipid antigens to human NK T cells required internalization of the glycolipids by the antigen-presenting cell and normal endosomal targeting of CD1d. Recognition of these compounds by human NK T cells triggered proliferation, cytokine release, and cytotoxic activity. These results demonstrate a striking parallel in the specificity of NK T cells in humans and mice, thus providing further insight into the potential mechanisms of immune recognition by NK T cells and the immunological function of this unique T cell subset.
Keywords: natural killer T cell, human, CD1, antigen presentation, glycolipid
Natural killer (NK) T cells are a recently described subpopulation of TCR-α/β+ CD4+ or CD4−8− T lymphocytes that have distinctive phenotypic and functional properties (1). These T cells were first identified in mice, where they can be distinguished from conventional T cells by their expression of the NK locus–encoded C-type lectin molecule NK1. Another hallmark of murine NK T cells is their extremely restricted TCR repertoire, with the great majority expressing an invariant TCR-α chain structure (Vα14-Jα281) paired preferentially with Vβ8, 7, or 2 (2, 3). Recently, it has been demonstrated that the human immune system contains a population of T cells that show striking conservation of most of the key features of murine NK T cells (4–6). These human NK T cells express an invariant Vα24-JαQ TCR-α chain, which is highly homologous to the invariant TCR-α chain of murine NK T cells (2). In addition, human NK T cells express a restricted Vβ repertoire (7, 8) and several NK locus–encoded C-type lectins, including NKR-P1A (CD161), which is the homologue of murine NK1 (4).
At present, the function of NK T cells in the immune response remains incompletely resolved. However, much interest has been generated by the finding that murine and human NK T cells are able to produce large amounts of both IL-4 and IFN-γ rapidly upon activation, and may represent a major source of early IL-4 in certain immune responses (4, 9). Recent results also suggest major roles for murine NK T cells in the rejection of malignant tumors (10) and in regulating autoimmunity (11–13). Given these potentially important activities of NK T cells, it has become of great interest to resolve the question of what foreign or self-antigens these T cells recognize. Initial insight into this question came with the finding that both murine and human NK T cells are capable of specifically recognizing the MHC class I–like CD1d protein (4, 14). As other members of the CD1 family have recently been shown to mediate the presentation of foreign lipids and glycolipids to T cells (15–17), this suggested the possibility that the actual target ligands of NK T cells may be CD1 proteins that have bound foreign or endogenous lipids.
Recently, Kawano et al. reported the CD1-dependent recognition of a family of synthetic glycolipid antigens by murine NK T cells, thus providing the first insight into the structure of foreign antigens recognized by this T cell subset (18). The glycolipids that were active in their experiments were composed of an α-anomeric sugar linked to a ceramide type acylphytosphingosine (APS) lipid, and are structurally related but distinct from the abundant ceramide-containing glycolipids (e.g., gangliosides) found in normal mammalian tissues (19). In this study, we show that human CD1d-restricted T cells also respond to these α-glycosyl-APS, and have a fine specificity for different compounds in this family that is remarkably similar to that shown for mouse NK T cells. Recognition of these synthetic glycolipids was restricted exclusively by human CD1d, as other members of the human CD1 family (CD1a, -b, and -c) were unable to present these antigens to human NK T cells. Our results provide further evidence for the conservation of a functional NK T cell subset in humans, and support the hypothesis that the immunological role of these T cells involves recognition of foreign or endogenous glycolipids.
Materials and Methods
Human NK T Cell Clones and CD1 Transfectants.
Human NK T cell clones DN2.B9, DN2.C7, and DN2.D5 were derived, maintained, and phenotypically analyzed as previously described (4, 8). The cervical carcinoma cell line HeLa was transfected with the expression vector pSRα-Neo containing cDNA inserts encoding CD1a, CD1b, CD1c, or CD1d according to previously described methods (20). The HeLa CD1d/a transfectant expressed a chimeric form of CD1d with the extracellular domains of CD1d fused to the transmembrane plus cytoplasmic domains of CD1a. This stable transfectant was produced by the introduction into HeLa cells of a previously described expression construct encoding this chimeric protein (4).
T Cell Proliferation Assays.
5 × 104 T cells were plated in triplicate in flat-bottomed 96-well plate wells with 104 mitomycin C–treated APCs (0.1 mg/ml for 1 h at 37°C; Sigma Chemical Co., St. Louis, MO). Media for all T cell cultures was RPMI 1640 (GIBCO BRL, Gaithersburg, MD) plus 10% fetal bovine serum (HyClone Labs., Logan, UT) and other additives as previously detailed (21). The synthetic glycolipid antigens used in this study were provided by the Pharmaceutical Research Laboratory, Kirin Brewery Co., Ltd. (Gunma, Japan), and have been described in detail previously (18). Although these were previously designated as glycosyl-ceramides, here we have referred to them as glycosyl-APS derivatives because of the hydroxylations of the third and fourth carbons in their sphingosine base that are characteristic of phytosphingosines (19). Synthetic glycolipids were dissolved at 100 μg/ml in DMSO, and diluted into culture medium to the indicated final concentrations. For mAb blocking experiments, the following mAbs were added as purified IgG to a final concentration of 20 μg/ml: W6/32 (anti-HLA-A, -B, and -C; reference 22); CD1d51 (anti-CD1d; reference 4); and P3 (nonbinding IgG1 control; reference 23). For light fixation of the APC surface, CD1d-transfected HeLa cells were incubated for 1 h on ice in 2 ml of 0.9% NaCl containing 75 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide-HCl (ECDI; Pierce Chemical Co., Rockford, IL), followed by extensive washing in RPMI 1640 medium. Cultures were incubated at 37°C in a 5% CO2 incubator, pulsed with 1 μCi of [3H]thymidine (2 Ci/mmol) on day 2 and harvested 16 h later for β scintillation counting using a Tomtec harvester and a Betaplate scintillation counter (Wallac, Gaithersburg, MD).
Cytokine and Cytotoxic T Cell Assays.
5 × 104 T cells were cultured in triplicate wells with 104 mitomycin C–treated CD1d-transfected HeLa cells in the absence or presence of 100 ng/ml of α-galactosyl-APS (αGalAPS). Phytohemagglutinin (PHA-P; Difco, Detroit, MI) was added to some cultures (1:2,000 final dilution) as a positive control. Supernatants were harvested after 48 h, and cytokine levels determined by capture ELISA as described (4). T cell cytotoxic activity was measured using a standard 4-h 51Cr-release assay (21). Mock-transfected or CD1d-transfected HeLa cells were labeled with 200 μCi of 51Cr for 2 h at 37°C, followed by incubation for 12 h at 37°C in complete medium with or without 200 ng/ml of αGalAPS, and then washed and used as target cells. Assays were performed in triplicate and results were expressed as percentage of maximum specific 51Cr release.
Results and Discussion
Specific Recognition of Synthetic Glycolipid Antigens by Human NK T Cell Clones.
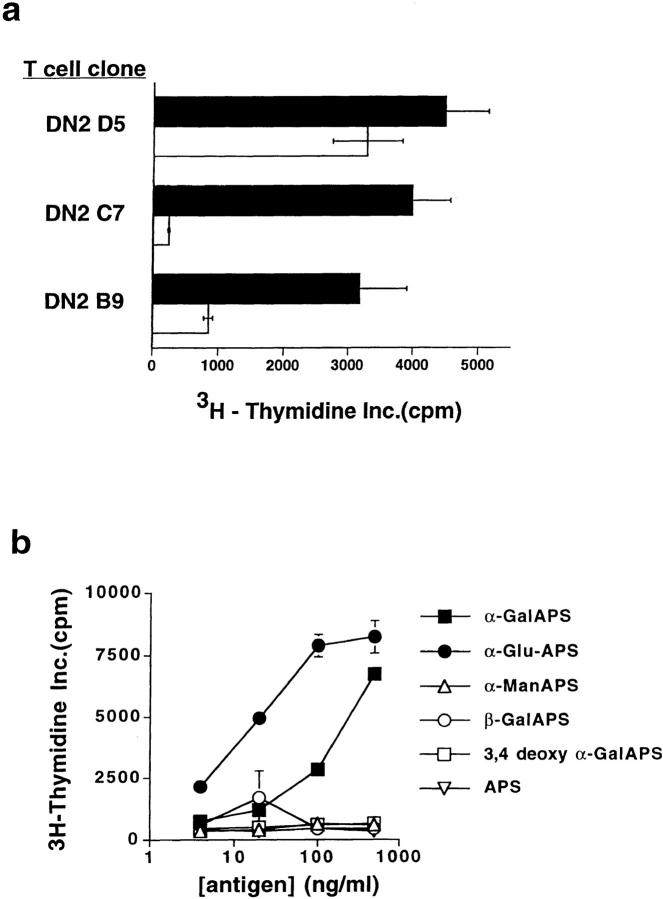
Three previously characterized human NK T clones (DN2.B9, DN2.C7, and DN2.D5; reference 4) bearing the invariant Vα24-JαQ TCR-α chain paired with different Vβ11+ TCR-β chains were tested for their ability to respond to synthetic APS-containing glycolipid antigens presented by CD1d-transfected HeLa cells. Because human NK T cells generally have intrinsic autoreactivity to CD1d-expressing cells (4), modifications of the culture conditions were required for optimal demonstration of the response to synthetic glycolipids. Preliminary experiments (data not shown) revealed that CD1d-transfected HeLa cells were particularly suited as APCs for these studies, since they did not require additional exogenous signals such as PMA to support the proliferation of human NK T cells. Using a relatively low APC/T cell ratio (1:5), we observed low to moderate autoreactivity and consistent augmentation of the proliferation of human NK T cells clones when the synthetic glycolipid αGalAPS was added (Fig. 1 a).
Figure 1.
Human NK T cell clones specifically respond to synthetic glycolipid antigens. (a) Proliferative responses of three different human NK T cell clones to CD1d-transfected HeLa cell APCs were significantly augmented by addition of the synthetic glycolipid αGalAPS. White bars show proliferation in response to CD1d+ HeLa cells plus vehicle (DMSO) alone; black bars show proliferation in response to CD1d+ HeLa cells plus 100 ng/ml αGalAPS. Results are shown as the mean and one standard deviation of triplicate values in this and all subsequent figures. (b) Proliferative responses of human NK T cell clone DN2.C7 to a panel of structurally related synthetic glycolipid antigens. APS is the nonglycosylated acyl-phytosphingosine lipid. αGalAPS, αGluAPS, and αManAPS indicate compounds in which α-anomeric galactose, glucose, and mannose, respectively are conjugated at position 1 of the hexose ring to the APS moiety. 3,4-deoxy αGalAPS is identical to αGalAPS except for the absence of the hydroxyl groups on carbons 3 and 4 of the phytosphingosine base. For complete structures of the synthetic glycolipids, see Kawano et al. (18). Thymidine incorporation in the absence of synthetic glycolipid antigen was 500 cpm in the experiment shown.
The response to αGalAPS appeared to be specific for NK T cell clones, since this compound did not stimulate proliferation of a panel of other human T cell clones that did not express the invariant Vα24-JαQ TCR-α chain (data not shown). The specificity of the response to synthetic glycolipids was also assessed by examining the responses of NK T cell clones to a panel of structurally related glycolipids. These included two previously demonstrated strong stimulators of murine NK T cells (αGalAPS and αGluAPS), as well as four related analogues that were nonstimulatory or weakly stimulatory in the murine system (APS, αManAPS, βGalAPS, and 3,4-deoxy αGalAPS) (18). NK T cell clones responded strongly to αGalAPS and αGluAPS but not to the other analogues (Fig. 1 b, and additional data not shown), demonstrating a pattern very similar to that observed for murine NK T cells. Thus, the specific recognition of synthetic glycolipids by a human NK T cell clone required an α-anomeric sugar (either galactose or glucose) and the presence of hydroxylations at positions 3 and 4 of the phytosphingosine base, exactly as described for murine NK T cells.
CD1 Restriction of Synthetic Glycolipid Recognition by Human NK T Cells.
In mice, two CD1 genes encode extremely homologous proteins, both of which are most closely related to human CD1d. In contrast, humans have a more diversified family of CD1 proteins, with genes encoding five distinct isoforms designated CD1a, -b, -c, -d, and -e (24). Previous work has shown that the human CD1d protein is a target for direct recognition by human NK T cell clones (4), whereas CD1a, -b, and -c present lipid and glycolipid antigens to other populations of T cells (15–17, 21). To determine if the human NK T cell response to α-glycosyl-APS was restricted only by the CD1d protein, or if other human CD1 isoforms could also support this response, we tested the ability of HeLa cells stably transfected with each of the human CD1 proteins to present αGalAPS. For all three human NK T cell clones tested, the response to αGalAPS was seen only when CD1d+ HeLa cells were used as APCs (Fig. 2 a and data not shown). Confirmation of this CD1d-restriction was obtained by mAb blocking experiments, which demonstrated that an mAb against the cell surface expressed conformation of CD1d protein (CD1d51) significantly inhibited the response to αGalAPS (Fig. 2 b). Thus, responses of human NK T cells to glycosyl-APS derivatives were dependent on APC expression of CD1, and CD1d was the only human CD1 isoform that presented these glycolipids to NK T cells.
Figure 2.
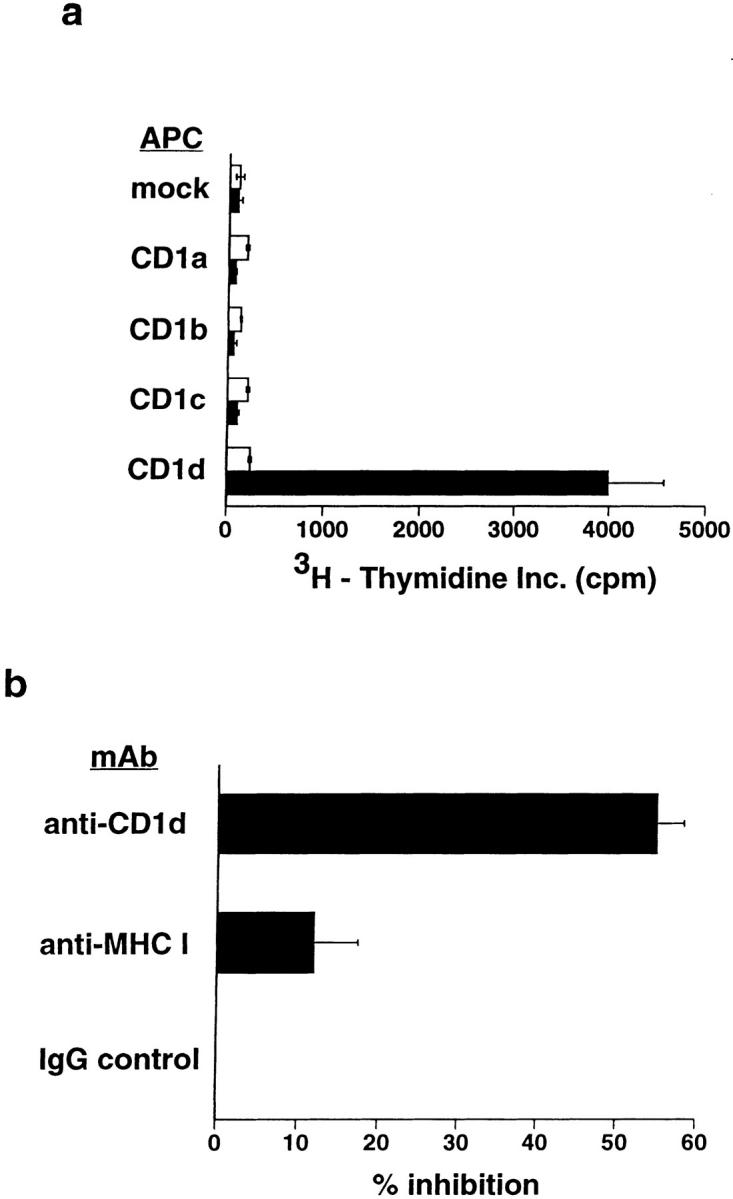
CD1d dependence of synthetic glycolipid antigen recognition by human NK T cells. (a) Response of human NK T cell clone DN2.C7 to αGalAPS (100 ng/ml) in the presence of HeLa cells not expressing CD1 (transfected with vector alone; mock) or expressing each of the known protein isoforms of human CD1. T cell proliferation in the absence of αGalAPS is shown by the white bars, and in the presence of αGalAPS by the black bars. Identical results were obtained with two other human NK T cell clones, DN2.B9 and DN2.D5 (data not shown). (b) Inhibition of αGalAPS-stimulated proliferation of human NK T cell clone DN2.B9 by anti-CD1d mAb. Results are shown as the percentage of inhibition of the αGalAPS-dependent proliferation in the presence of CD1d+ HeLa cells. With no antibody present, proliferation in the presence of the αGalAPS was 3,900 cpm, and in the absence of αGalAPS was <200 cpm in the experiment shown. Marginal blocking was observed with anti–MHC class I mAb W6/32 in some experiments. This was also seen in parallel experiments using MHC class II–restricted T cell clones (data not shown), and thus most likely represents a nonspecific effect of this antibody.
Requirements for Uptake and Endosomal Loading of Synthetic Glyclolipid Antigens.
To determine if antigen internalization by CD1d+ APCs was required for presentation, we examined the effects of blocking plasma membrane trafficking by light chemical fixation of APCs. Because aldehyde fixatives caused strong augmentation of the autoreactivity of human NK T cells to CD1d+ APCs (4), we instead used the chemical cross-linker ECDI as a fixative (25). CD1d+ HeLa cells were surface cross-linked by ECDI either before or after a 12-h pulse with αGalAPS (100 ng/ ml), and then tested for their ability to stimulate proliferation of clone DN2.C7. Whereas APCs treated with ECDI after the antigen pulse stimulated strong proliferation of DN2.C7, fixation with ECDI before antigen pulsing completely abrogated the response to αGalAPS (Fig. 3 a). This strongly suggested a requirement for antigen uptake by APCs in the response to this synthetic glycolipid.
Figure 3.
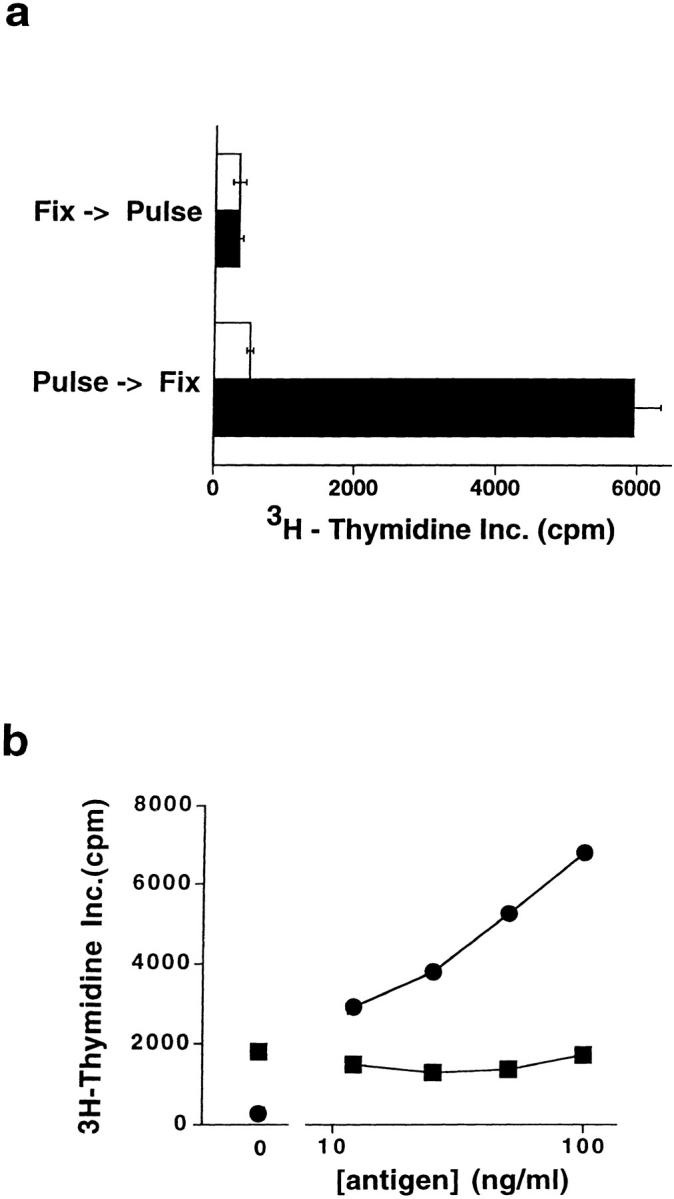
Requirement for antigen uptake and endosomal delivery of CD1d in recognition of synthetic glycolipids by human NK T cells. (a) Proliferative responses of clone DN2.C7 to ECDI-fixed APCs. CD1d+ HeLa cells either were pulsed for 12 h with αGalAPS (100 ng/ml) and subsequently fixed by ECDI treatment, or were fixed first followed by αGalAPS pulsing. White bars represent APCs pulsed with vehicle (DMSO) alone, and black bars represent APCs pulsed with αGalAPS. (b) Proliferation of clone DN2.C7 to αGalAPS presented by HeLa cell transfectants expressing wild-type CD1d (circles) versus HeLa transfectants expressing the CD1d/a chimeric protein that lacks an endosomal targeting signal (squares). Both transfectants expressed comparable levels of immunoreactive CD1d on the cell surface. Proliferation in the absence of APCs was <200 cpm in this experiment. Note that DN2.C7 showed a weak but significant response to the CD1d/a transfectant in the absence of added antigen, but no augmentation of this response at any concentration of αGalAPS tested.
Consistent with this requirement for antigen uptake, studies of αGalAPS presentation to murine NK T cells have demonstrated that this process is inhibited by chloroquine, and thus may require endosomal acidification (18). To further examine this possibility, we assessed the ability of a modified form of human CD1d that is deficient in endosomal localization to present αGalAPS. Human CD1d, like CD1b and CD1c but not CD1a, has a short cytoplasmic tail containing a tyrosine-based four-amino acid motif (24). For CD1b, this motif has been shown to be critical for endosomal localization and efficient microbial lipid antigen presentation by this molecule (20). Previously, we generated a chimeric form of the human CD1d protein in which the CD1d ectodomains are fused to transmembrane and cytoplasmic domains of CD1a, eliminating the endosomal targeting signal normally present in CD1d. Previous studies with this construct expressed in B lymphoblastoid cells showed that the chimeric CD1d/a protein was defective in endosomal localization, but was well expressed on the cell surface (4). To assess the importance of endosomal delivery of CD1d, we examined the dose response of clone DN2.C7 to αGalAPS presented by HeLa cells expressing either wild-type CD1d or the CD1d/a chimera. This revealed a striking effect of the deletion of the targeting motif, essentially abolishing the ability of CD1d/a to present αGalAPS (Fig. 3 b). This finding, along with the requirement for antigen internalization by the APCs, strongly suggested that synthetic glycolipid antigens colocalize with CD1d in the endocytic system, and that this is required for the generation of a complex that is recognized by NK T cells on the APC surface.
Functional Consequences of CD1d-restricted Presentation of Synthetic Glycolipids.
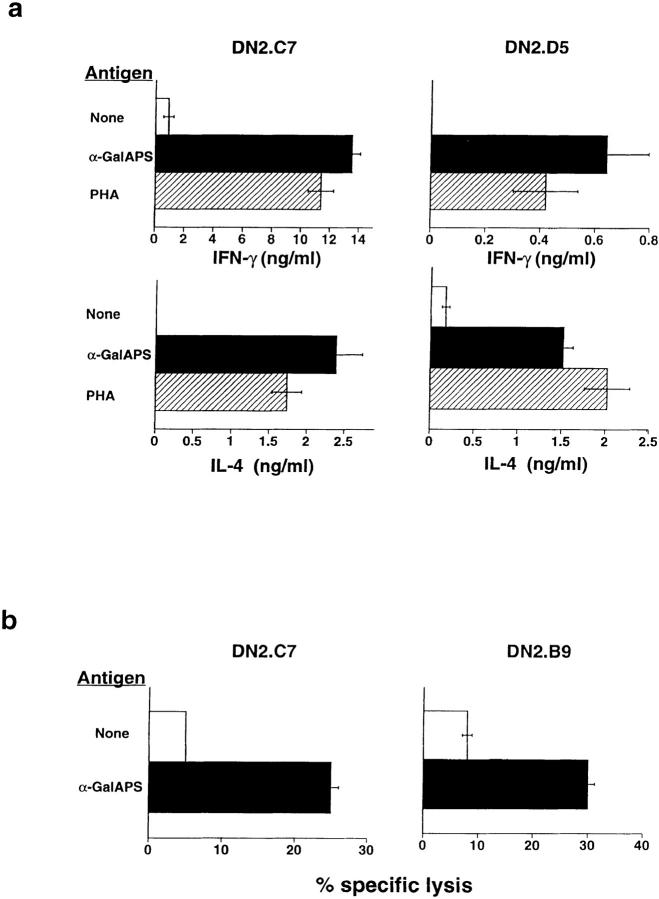
A hallmark of both murine and human NK T cells has been their ability to rapidly produce cytokines associated with both Th1 (IFN-γ) and Th2 (IL-4) responses upon TCR engagement (1, 4, 11). Interestingly, however, it has been noted that several conditions exist in which this apparent Th0 profile of cytokine production may be skewed either toward a predominance of IFN-γ (11, 26) or of IL-4 (14, 27). We found that stimulation with αGalAPS generated substantial levels of both IFN-γ and IL-4 by the three human NK T cell clones tested (Fig. 4 a, and data not shown). Some variation between clones in the relative amounts of IFN-γ and IL-4 production was observed, but in all cases the cytokine production mirrored that observed with activation by PHA. Human NK T cell clones also showed enhanced cytotoxic activity against CD1d+ HeLa cells pulsed with αGalAPS (Fig. 4 b). Thus, CD1d-restricted recognition of αGalAPS augmented the known effector functions of human NK T cell clones, and preserved their Th0 pattern of cytokine secretion.
Figure 4.
Activation of effector functions of human NK T cells by synthetic glycolipid antigens. (a) Stimulation of IL-4 and IFN-γ secretion by αGalAPS. Human NK T cell clones were cultured with CD1d-expressing HeLa cells in the presence (white bars) or absence (black bars) of αGalAPS (100 ng/ml) or with PHA (hatched bars), and supernatants were harvested after 48 h and assayed for cytokines by ELISA. No cytokine production was detected in cultures containing T cells only or CD1d-transfected HeLa cells only (data not shown). (b) Human NK T cell clones specifically lysed CD1d-transfected HeLa cells pulsed for 12 h with αGalAPS (200 ng/ml). No lysis was observed using mock-transfected HeLa cells as targets (data not shown).
Our results demonstrated CD1d-restricted recognition of synthetic glycosyl-APS by human NK T cells, and confirmed the striking conservation of this T cell subset between humans and mice in both phenotype and function. The presentation of synthetic glycolipids by CD1d also supports the growing body of data demonstrating that the main function of CD1 proteins may be the presentation of foreign microbial lipid and glycolipid antigens (15–17, 21). In spite of this substantial progress, the main immunological functions of NK T cells remain unclear. The synthetic glycolipids used in this study are not yet known to have precise counterparts in relevant infectious agents, and the prototype natural compounds in this family were originally isolated not from microbial pathogens but from marine sponges (18). Nevertheless, given the impressive antitumor effects of NK T cells and a variety of studies indicating that they may play a role in regulating autoimmune inflammatory diseases, the discovery of exogenous ligands that can modulate the activity of this unique T cell subset is of great potential importance. Our results demonstrating that synthetic glycosyl-APS derivatives are active modulators of the human NK T cell system suggests that these compounds may be promising candidates for immunotherapy of human diseases.
Acknowledgments
The authors thank Drs. Branch Moody and Brian Wilson for thoughtful suggestions, and Drs. Steven Balk and Mark Exley (Beth Israel-Deaconess Medical Center, Boston, MA) for many helpful discussions and for providing the CD1d/a plasmid DNA construct.
This work was supported by National Institutes of Health grant R29 AI-40135, a grant from the American Cancer Society, and an Arthritis Foundation Investigator Award to S. Porcelli.
References
- 1.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8−T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koseki H, Asano H, Inaba T, Miyashita N, Moriwaki K, Lindahl KF, Mizutani Y, Imai K, Taniguchi M. Dominant expression of a distinctive V14+T-cell antigen receptor α chain in mice. Proc Natl Acad Sci USA. 1991;88:7518–7522. doi: 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8−T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prussin C, Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862–5870. [PubMed] [Google Scholar]
- 6.Davodeau F, Peyrat M-A, Necker A, Dominici R, Blanchard F, Leget C, Gaschet J, Costa P, Jacques Y, Godard A, et al. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J Immunol. 1997;158:5603–5611. [PubMed] [Google Scholar]
- 7.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8−T cells. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porcelli S, Gerdes D, Fertig A, Balk SP. Human T cells expressing an invariant Vα24-JαQ TCRα are CD4−and heterogeneous with respect to TCRβ expression. Hum Immunol. 1996;48:63–67. doi: 10.1016/0198-8859(96)00090-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Paul WE. Cultured NK1.1+ CD4+T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 10.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 12.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. Selective reduction of Vα14+NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 13.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 15.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted αβ+T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 16.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 17.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 18.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson, K.-A. 1982. Glycosphingolipids and surface membranes. In Biological Membranes. D. Chapman, editor. Academic Press, London. 1–74.
- 20.Jackman RM, Stenger S, Lee A, Moody DB, Rogers RA, Niazi KR, Sugita M, Modlin RL, Peters PJ, Porcelli SA. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity. 1998;8:341–351. doi: 10.1016/s1074-7613(00)80539-7. [DOI] [PubMed] [Google Scholar]
- 21.Beckman EM, Melian A, Behar SM, Sieling PA, Chatterjee D, Furlong ST, Matsumoto R, Rosat JP, Modlin RL, Porcelli SA. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. 1996;157:2795–2803. [PubMed] [Google Scholar]
- 22.Brodsky FM, Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982;128:129–135. [PubMed] [Google Scholar]
- 23.Panchamoorthy G, McClean J, Modlin RL, Morita CT, Ishikawa S, Brenner MB, Band H. A predominance of the T cell receptor Vγ2/Vδ2 subset in human mycobacteria-responsive T cells suggests germline encoded recognition. J Immunol. 1991;147:3360–3369. [PubMed] [Google Scholar]
- 24.Porcelli S. The CD1 family: a third lineage of antigen presenting molecules. Adv Immunol. 1995;59:1–98. doi: 10.1016/s0065-2776(08)60629-x. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1998;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gombert JM, Tancrede-Bohin E, Hameg A, Leite-de-Moraes MC, Vicari A, Bach JF, Herbelin A. IL-7 reverses NK1+T cell-defective IL-4 production in the non-obese diabetic mouse. Int Immunol. 1996;8:1751–1758. doi: 10.1093/intimm/8.11.1751. [DOI] [PubMed] [Google Scholar]