Abstract
Many cell types in the retina are coupled via gap junctions and so there is a pressing need for a potent and reversible gap junction antagonist. We screened a series of potential gap junction antagonists by evaluating their effects on dye coupling in the network of A-type horizontal cells. We evaluated the following compounds: meclofenamic acid (MFA), mefloquine, 2-aminoethyldiphenyl borate (2-APB), 18-α-glycyrrhetinic acid, 18-β-glycyrrhetinic acid (18-β-GA), retinoic acid, flufenamic acid, niflumic acid, and carbenoxolone. The efficacy of each drug was determined by measuring the diffusion coefficient for Neurobiotin (Mills & Massey, 1998). MFA, 18-β-GA, 2-APB and mefloquine were the most effective antagonists, completely eliminating A-type horizontal cell coupling at a concentration of 200 μM. Niflumic acid, flufenamic acid, and carbenoxolone were less potent. Additionally, carbenoxolone was difficult to wash out and also may be harmful, as the retina became opaque and swollen. MFA, 18-β-GA, 2-APB and mefloquine also blocked coupling in B-type horizontal cells and AII amacrine cells. Because these cell types express different connexins, this suggests that the antagonists were relatively non-selective across several different types of gap junction. It should be emphasized that MFA was water-soluble and its effects on dye coupling were easily reversible. In contrast, the other gap junction antagonists, except carbenoxolone, required DMSO to make stock solutions and were difficult to wash out of the preparation at the doses required to block coupling in A-type HCs. The combination of potency, water solubility and reversibility suggest that MFA may be a useful compound to manipulate gap junction coupling.
Keywords: Horizontal cells, Retina, Gap junction antagonist
Introduction
In the retina, both chemical synapses and electrical synapses, known as gap junctions, play important roles in neuronal communication (Rodieck, 1998; Goodenough & Paul, 2003). Gap junction channels are composed of proteins called connexins. Six connexin proteins form a hemichannel, or connexon, and two docked hemichannels form a gap junction channel (Goodenough & Paul, 2003). The central pore in a gap junction allows the passage of small ions and molecules up to a molecular weight around 1 kDa (Guldenagel et al., 2000). Approximately 20 connexin genes have been identified and this diversity is thought to provide gap junctions with distinct functional properties for different populations of neurons (Willecke et al., 2002). In mammals, four neuronal connexins have been identified from this gene family. Cx36 appears to be the dominant neuronal connexin, but horizontal cells (HCs) express Cx50 or Cx57 and other inner retinal neurons express Cx45 (Massey et al., 2003; Hombach et al., 2004; Sohl et al., 2005; O'Brien et al., 2006).
Gap junctions are abundant throughout the retina. Horizontal cells are laterally-extensive neurons which make feedback connections with photoreceptors (Boycott et al., 1978; Wässle et al., 1978). They are exceptionally well-coupled by gap junctions which dramatically increase the size of the receptive field relative to the dendritic field (Dacheux & Raviola, 1982; Mills & Massey, 2000). Most mammals, except for rodents, contain two different kinds of horizontal cell (Boycott et al., 1978; Peichl & Gonzalez-Soriano, 1994). In the rabbit retina, A-type and B-type horizontal cells have distinctly different coupling properties and appear to contain gap junctions composed of different connexins (Dacheux & Raviola, 1982). A-type HCs are the best coupled, approximately 20× better than B-type HCs (Mills & Massey, 1998) and express Cx50 gap junctions, including giant plaques between major dendrites (O'Brien et al., 2006). B-type HCs in the rabbit retina are axon bearing (Dacheux & Raviola, 1982) and the homologous cell type in the mouse retina expresses Cx57 (Hombach et al., 2004). Furthermore in the Cx57 knockout mouse, there is no HC coupling and the size of the receptive field is reduced (Hombach et al., 2004). The size of horizontal cells, their extensive coupling and the ease with which HCs can be dye injected makes them suitable to test gap junction antagonists.
In the inner retina, AII amacrine cells of the rod pathway are coupled to other AII amacrine cells via Cx36 gap junctions (Feigenspan et al., 2001; Mills et al., 2001) but they also make heterotypic Cx36/Cx45 gap junctions with ON cone bipolar cells (Han & Massey, 2005; Lin et al., 2005; Maxeiner et al., 2005; Dedek et al., 2006). This pathway has been shown to be essential for the transmission of dark-adapted rod signals through the retinal network (Deans et al., 2002). In addition, photoreceptor coupling is mediated via Cx36-gap junctions (Feigenspan et al., 2004; O'Brien et al., 2004; Zhang & Wu, 2004). Finally, some amacrine cells and ganglion cells are also coupled by either Cx36 or Cx45 gap junctions (Vaney, 1994; Feigenspan et al., 2001; Maxeiner et al., 2005; Schubert et al., 2005; Volgyi et al., 2005). These differently coupled networks make it possible to test the effectiveness of potential gap junction antagonists on a variety of neuronal connexins.
In contrast to the highly specific antagonists for voltage- and ligand-gated channels, there are no good antagonists for gap junctional channels. Long-chain alcohols such as heptanol or octanol are known to block gap junctions non-specifically at relatively high concentrations, usually in the millimolar range (Lu et al., 1999; Mills & Massey, 2000). Carbenoxolone has been widely used to block gap junctions, but there is evidence that it also blocks voltage-dependent calcium channels and it is difficult to wash out (Vaney et al., 1994, 1998; Trexler et al., 2005). A related compound 18-β-glycyrrhetinic acid (18-β-GA, also known as enoxolone) was used successfully at a low concentration to block ganglion cell coupling (Ackert et al., 2006). Several other drug groups, such as the fenamates and certain antimalarials, including quinine and mefloquine, have also been reported to block gap junctions, but they have not been widely used or tested in the retina (Srinivas et al., 2001, 2005; Srinivas & Spray, 2003; Cruikshank et al., 2004).
In this paper, we used dye coupling in several retinal networks as a tool to evaluate a series of potential gap junction antagonists. Primarily using A-type HCs, we tested different concentrations of each drug to determine relative potency. Rather than counting the number of cells in a coupled patch, which may vary uncontrollably with variation in dye concentration and electrode resistance, we determined the diffusion coefficient for the spread of Neurobiotin with different drug concentrations (Mills & Massey, 1998). Our results showed that meclofenamic acid (MFA), mefloquine, 18-β-GA and 2-aminoethyldiphenyl borate (2-APB) are the most potent gap junction antagonists. MFA may be more convenient to use because it is water-soluble and readily reversible. MFA also blocked dye coupling in B-type HCs and AII amacrine cells. This suggests that MFA blocks several different types of neuronal gap junctions.
Materials and methods
Preparation of isolated retina
The preparation has previously been described in detail (Massey & Mills, 1999). A brief summary is provided here. Under a protocol approved by the Institutional Animal Welfare Committee, adult New Zealand Albino white rabbits of either sex (2–3 kg) were deeply anesthetized with urethane (loading dose, 1.5 g/kg, i.p.) and the orbit was infused with 2% lidocaine hydrochloride before enucleation. The eye was then removed and hemisected. The retina was isolated from the inverted eyecup while immersed in oxygenated Ames medium. Retinal cells were prelabeled with 4,6-diamino-2-phenylindole (DAPI) by incubating in Ames medium with 5μM DAPI for 30 min.
Injection of Neurobiotin
Pieces of retina prelabeled with DAPI were visualized on an Olympus BX-50WI microscope (Tokyo, Japan) equipped with epifluorescence. The retina was bathed in the drugs 10 min before injection. Cells were impaled under visual control by using pipettes tip-filled with 4% Neurobiotin (Vector Laboratories, Burlingame, CA) and 0.5% Lucifer Yellow-CH (Molecular Probes, Eugene, OR) in ddH2O, then back-filled with 3M LiCl. The electrode resistance was ∼100 MΩ. We filled horizontal cells with 4% Neurobiotin for 10 min (A-type: 2.5 nA; B-type: 1 nA) and allowed 10 min additional diffusion time before fixation. AII amacrine cells were filled for 5 min (1 nA) with 15 min additional diffusion before fixation. After the last injection, the retinal pieces were fixed in 4% paraformaldehyde for 10 min before further experiments. For visualizing Lucifer Yellow, a 4% solution was used for dye injections and images were acquired with an Orca digital camera (Hamamatsu). Drugs used were: meclofenamic acid (Sigma, M-4531); niflumic acid (Sigma, N0630); flufenamic acid (Sigma, F9005); 18-α-glycyrrhetinic acid (Sigma, G8503); 18-β-glycyrrhetinic acid (Sigma, 50510); carbenoxolone (Sigma, C4790); retinoic acid (Sigma, R2625); 2-aminoethyldiphenyl borate (Sigma, D9754); mefloquine was provided by the Drug Synthesis and Chemistry Branch, National Cancer Institute.
Drug solutions
All drugs were made in stock solution first, and then diluted to different concentrations of working solution (Table 1). Meclofenamic acid and carbenoxolone are water soluble. Retinoic acid, 18-α-glycyrrhetinic acid, mefloquine, flufenamic acid, and niflumic acid were dissolved in dimethyl sulfoxide (DMSO) as stock solutions. Stock solutions were prepared daily. Final concentrations of DMSO were less than 0.5% (see Table 1). The minimum concentration of DMSO was used for each antagonist and control experiments with 0.5% DMSO alone showed no effect on coupling.
Table 1.
Drug solutions
| Drug | Stock Solution (mM) |
Maximum Dose (μM) |
Solvent |
|---|---|---|---|
| Retinoic Acid | 10 | 50 | DMSO (0.5%) |
| Carbenoxolone | 100 | 400 | Water |
| Meclofenamic Acid | 100 | 200 | Water |
| Niflumic Acid | 200 | 500 | DMSO (0.25%) |
| Flufenamic Acid | 200 | 500 | DMSO (0.25%) |
| Mefloquine | 100 | 200 | DMSO (0.2%) |
| 18-β-Glycyrrhetinic Acid | 100 | 200 | DMSO (0.2%) |
| 18-α-Glycyrrhetinic Acid | 10 | 50 | DMSO (0.5%) |
Immunocytochemistry
After fixation, the tissues were washed extensively with 0.1M phosphate buffer (PB, pH 7.4) and blocked with 3% donkey serum in 0.1M PB with 0.5% Triton-X 100 and 0.1% NaN3 overnight. The antibodies were diluted in 0.1M PB with 0.5% Triton-X 100 and 0.1% NaN3 containing 1% donkey serum. The tissues were incubated for 3–7 days at 4°C, and after extensive washing, incubated in secondary antibodies overnight at 4°C. After washing with 0.1M PB, the tissues were mounted in Vectashield (Vector Laboratories) for observation.
A mouse monoclonal antibody against Calbindin was purchased from Abcam, (CL-300, product ab9481, Cambridge, MA; 1:100) that recognizes purified calbindin-D from chicken. This antibody is specific to a protein of 28 kDa and provided as mouse ascites fluid. The distribution pattern for the calbindin antibodies in the rabbit retina is well known and has been previously reported (Massey & Mills, 1996).
The secondary antibodies used were donkey anti-mouse 1:200 conjugated to Alexa 488 (Invitrogen, Carlsbad, CA). Neurobiotin was visualized with Alexa-488 conjugated streptavidin (Invitrogen, Carlsbad, CA) or Cy3-conjugated streptavidin (Jackson Immuno-Research Laboratories, West Grove, PA) 1:200 overnight.
Confocal microscopy
Images were acquired on a Zeiss LSM-410 or Zeiss LSM-510 (Zeiss, Thornwood, NY) confocal microscope. Alignment for all three channels and resolution were checked at 8× zoom using 1 μm fluorescent spheres (Invitrogen, Carlsbad, CA). The XY resolution of the instrument was 200–300 nm and all three channels were superimposed. Z-axis steps were usually 0.5 μm and the resulting images are presented as short stacks of 4–6 optical sections (2–3 μm) to compensate for slight ripples across the tissue and present an even plane of focus. Z-axis reconstructions were over sampled in 0.3 μm steps and reconstructed in Zeiss software. For image stacks, the brightest value in the Z dimension is presented for any XY pixel. Brightness, contrast, and color balance of digital images were adjusted in Adobe PhotoShop (Adobe Systems, San Jose, CA), but no filtering or region specific adjustments were made to any images.
Diffusion coefficient
Tracer movement via diffusion through gap junctions is related to diffusion time. There are several factors governing tracer movement: the junctional resistance, cytoplasmic diffusion rates, and internal binding and sequestration. When a bolus of fluorescent tracer is first introduced into a cell, it produces a single intense fluorescent cell, with no fluorescence in the neighboring cells. As time elapses, dye moves into adjoining cells at a rate proportional to the coupling rate constant and distance from the injected cell. The injected cell and its near neighbor peak rapidly, and then decline in intensity due to the loss of tracer to more distant cells. The distant cells accumulate the tracer slowly. Due to limited amounts of dye and a threshold for detection, cells beyond some distance from the site of injection become undetectable. Junctional flux is much smaller than cytoplasmic diffusion rates and is strongly rate limiting. Tracer loss due to membrane permeability and compartmental sequestration (Zimmerman & Rose, 1985) was not found to be significant in these coupled networks (Mills & Massey, 1998) and was therefore not considered further. In retina, different eccentricities show variation in size, dendritic-field area, and sometimes amount of overlap. For this reason, all horizontal cells were injected in the superior retina. The density of the injected patch was also recorded. This number was used to correct the changes in radial diffusion that reflected changes in size and density of cells rather than changes in junctional diffusion rates.
A-type horizontal cells are well coupled and pass both Lucifer Yellow and Neurobiotin. The diffusion of dye was measured after fixation and the rate constants were determined. In practice, the amount of dye injected and diffusion coefficient (kj) determine the height of the initial curve and the rate of drop off, respectively.
where C is the difference in concentration between coupled cells (Mills & Massey, 1998). By calculating the diffusion coefficient, different gap junction antagonists can be compared.
Measurement of absolute Neurobiotin concentrations within cells
To estimate Neurobiotin concentrations within cells, HeLa cells were stained via diffusion from patch electrodes that contained specific concentrations of Neurobiotin. Then, the cell was incubated in medium containing carbenoxolone (10 μM) to prevent the spread of Neurobiotin to neighboring cells. Next, we processed and imaged these cells identically to retinal preparations. These measurements converted image pixel intensity to Neurobiotin concentration in a lookup table. Both HeLa and retinal cells were imaged at confocal settings that positioned them in the range 50–200 by adjusting the laser brightness. In order to compare different concentrations of Neurobiotin, a single function could be moved along the abscissa to fit HeLa cells containing different Neurobiotin concentrations; this was predictable using the laser attenuation settings which use neutral density filters. We used these functions to calculate the Neurobiotin concentrations of all stained retinal cells by making use of the lookup table in the program SigmaScanPro (SPSS Science, Inc., Chicago, IL), which also converted the distance metric of the image from pixels to microns. Intensity was measured using the average of a 5 × 5 pixel array placed over the soma. The area occupied by the nucleus was excluded, as it was less intensely stained than the cytoplasm. The output of the Sigma Scan program was a table of distances from the injected cell and Neurobiotin concentrations for each cell in a coupled group. We normalized the actual distance in microns by the spacing density of the group of A-type horizontal cells. The diffusion rate therefore reflects the number of cells the tracer traverses rather than the actual distance. The unit measure of the diffusion rate is then (cell separations)2/s. Differential equations that related diffusion rates to concentration differences in the various compartments were solved using second- and third-order Runge-Kutta ordinary differential equations in MatLab (The Math-Works, Natick, MA) (Mills & Massey, 1998).
Results
Type-A horizontal cells
Type-A HCs are extensively coupled and therefore provide a useful model to test the effectiveness of potential gap junction antagonists. A control injection of Neurobiotin resulted in a large patch of A-type horizontal cells (Fig. 1A). The injected cell, marked by an arrow, is shown at the top of the field so we can illustrate the extent of dye coupling. A-type horizontal cells have prominent asymmetrical dendrites with a coverage factor of 5–6 so the Neurobiotin injection provides a dense matrix of large dendrites (Mills & Massey, 1994). In fact, coupling is so extensive in the A-type matrix that many fine processes, such as those that contact individual cone pedicles are not visible because the dye diffuses away through the network instead of into the terminal dendrites. Away from the injection site, the intensity decreases until, at the edge of the patch, only the somas are visible, brightly at first, then fading to the background. We took the distance from the injection site to the last stained soma as the radius of the injected patch. Then, the diffusion coefficient was calculated, based on the distance from the injection site.
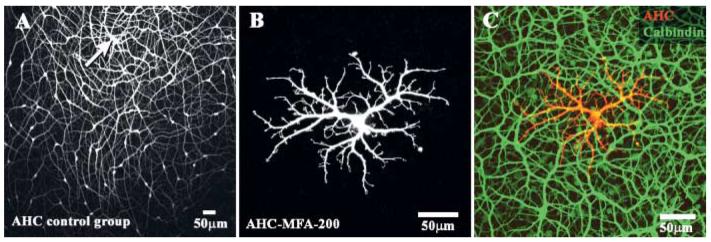
Fig. 1.
The effect of MFA on dye coupling in A-type horizontal cells. (A) A control experiment showing extensive coupling after injecting Neurobiotin into a single A-type HC (arrow). (B) After perfusion with 200 μM MFA, a single highly detailed A-type HC was recovered. (C) Same field, staining with an antibody against calbindin (green), showed the presence of a dense matrix of additional A-type HCs which are uncoupled from the dye injected cell (red). The injected cell is distinctly orange due to double labeling with Neurobiotin and calbindin.
Potential gap junction antagonists were added to the perfusion medium and a typical example for meclofenamic acid (MFA) is shown in Fig. 1B. At 200 μM, MFA completely abolished dye coupling in A-type HCs. As a result, only one A-type HC is visible. No other stained somas can be seen. In addition, because the Neurobiotin is restricted to this single cell, the entire cell is stained, down to the finest dendritic processes. The very fine branches which leave the major dendrites contact cone pedicles and the site of each cone pedicle is shown by a cluster of dendritic terminals. A single A-type HC may contact up to 200 cones (Raviola & Dacheux, 1990). The presence of such fine details is diagnostic for the block of gap junction coupling. This single A-type HC was not an individual stray cell. Thus, labeling with an antibody against calbindin stained all the other A-type HCs in the background (Fig. 1C). Many other A-types HCs overlapped the dendritic field of the single dye filled cell but there was no evidence of coupling. The level of detail available in this micrograph speaks to the absence of cellular swelling, which produces a reduction in resolution. In summary, this is a very clear example that dye coupling in A-type HCs can be completely blocked by MFA.
Intermediate concentrations of each antagonist produced a less than total block of gap junction coupling. We digitized confocal images of these patches of coupled cells and from the gradient of filled somas calculated the diffusion coefficient in the presence of varying concentrations for each antagonist. The diffusion coefficient was plotted against the drug concentration; each data point was the mean of 3–6 experiments. Some of the antagonists were water soluble and could be dissolved in the perfusate. For the others, a stock solution was first prepared in DMSO and aliquots were added to the perfusate such that the final concentration of DMSO was less than 0.5%. In control experiments, perfusion with 0.5% DMSO alone had no effect on dye-coupling in A-type HCs.
In A-type HCs, MFA had a threshold dose of approximately 10 μM and a total block of dye coupling was obtained at 100 or 200 μM. From a series of similar experiments with varying concentrations, a family of curves was obtained for all the drug candidates (Fig. 2). MFA was among the most potent gap junction antagonists with an EC50 of 21 μM, slightly lower than the EC50 for 18-β-GA (25 μM), mefloquine (34 μM), or flufenamic acid (41 μM). 2-APB was slightly more potent with a complete block of coupling in A-type HCs at a concentration of 100 μM. However, we did not evaluate 2-APB in further detail because it is known to block store-operated calcium channels. We were unable to obtain a complete block with retinoic acid and 18-α-glycyrrhetinic acid because we could not dissolve them at high enough concentrations. Carbenoxolone and niflumic acid were the least potent drugs with EC50 values of 118 μM and 173 μM, respectively. For these two drugs, a few coupled somas were found even at the highest concentrations, 400 μM, indicating that a total block of A-type HCs was not obtained. Finally, when perfusing with carbenoxolone, we frequently noticed that the retina took on an unhealthy appearance. It should be noted that carbenoxolone is water soluble so no DMSO was present in these experiments. After 10 min with carbenoxolone, the DAPI-stained nuclei appeared fuzzy or out of focus and after 30 min, the retina lost transparency. In contrast, after prolonged perfusion with MFA, sufficient to block coupling in A-type HCs, the retina was clear and the cells appeared in sharp focus.
Fig. 2.
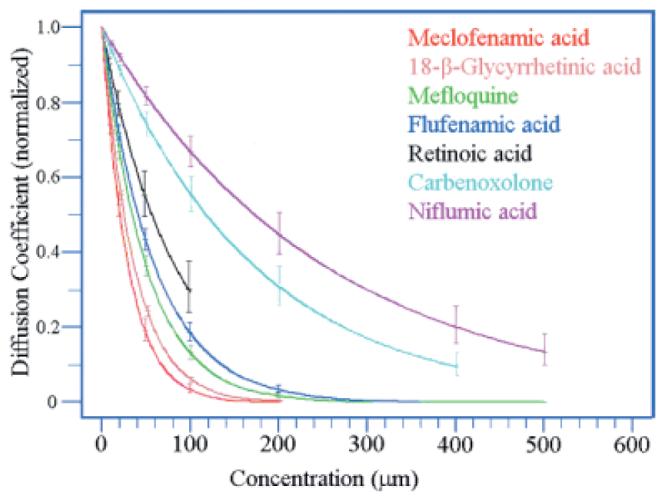
Change in diffusion coefficient for Neurobiotin in A-type HCs against the concentration of different gap junction antagonists. All curves were normalized to 1 for the control experiments. Vertical bars show standard deviations. MFA, 18-β-GA and mefloquine were the most potent antagonists. Insufficient data was collected to plot a curve for 2-APB because of its well known effect on store-operated calcium channels. A complete block of dye coupling in A-type HCs could not be obtained with carbenoxolone (400 μM) or niflumic acid (500 μM). Retinoic acid could not be dissolved at a concentration greater than 100 μM without exceeding the limit of 0.5% for DMSO.
Reversibility
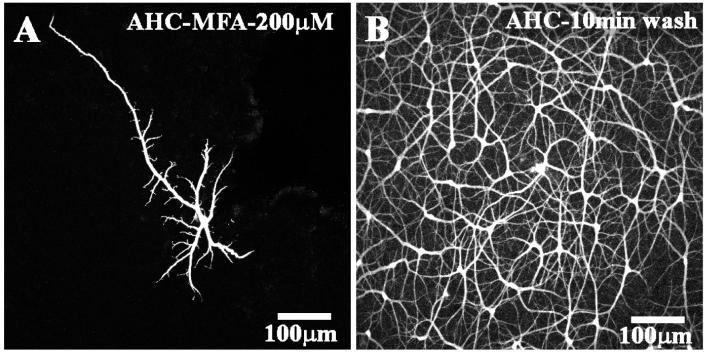
Experimentally, it would be extremely useful if a potential gap junction antagonist were easily reversible. In general, it has been difficult or impossible to reverse the effects of carbenoxolone. To test for reversibility, we took a single piece of retina and cut it in half. With the first piece, we blocked gap junctions by perfusing for 10 min with 200 μM MFA. Then a A-type HC was impaled and dye-injected for 5 min, the retina was fixed and processed to visualize the Neurobiotin injection. As expected, under these conditions, a single A-type HC was obtained (Fig. 3A). This confirms that dye coupling in A-type HCs was completely blocked. With the second piece of retina, we perfused with MFA in the same way and impaled a single A-type HC during the drug perfusion. Then, the electrode was removed and the perfusate switched back to control solution. After a 10 min wash, the retina was fixed and processed for Neurobiotin. Under these conditions, a large patch of A-type HCs was obtained (Fig. 3B). This indicates that the block of gap junction coupling by MFA was reversible with a time course of less than 10 min. None of the DMSO-soluble compounds we evaluated, or carbenoxolone, were reversible in this time frame.
Fig. 3.
Meclofenamic acid is a reversible gap junction antagonist. (A) Dye injection during perfusion with MFA (200 μM for 5 min) produced a detailed fill of a single A-type HC. (B) After 5 min dye injection during perfusion with MFA (200 μM), the tissue was washed for 10 min. Then, dye injection produced a large patch of coupled A-type HCs
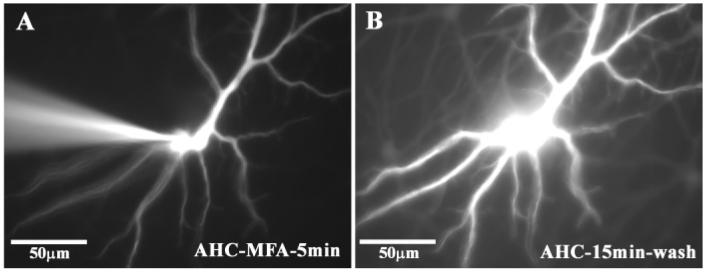
A-type HCs are the only neurons in the retina which allow the passage of Lucifer Yellow through the network, presumably because they express Cx50 (O'Brien et al., 2006). Lucifer Yellow is less permeable than Neurobiotin but it has the experimental advantage that it can be observed with epifluorescence. We took advantage of this property to follow dye coupling in A-type HCs in real time. A single A-type HC was dye injected with Lucifer Yellow in the presence of 100 μM MFA. After a 5-min fill, a single brightly-filled cell was observed (Fig. 4A). Then, the electrode was removed and the perfusate switched to control medium. After a 15 min wash, a network of Lucifer Yellow coupled A-type HCs was observed (Fig. 4B). This again indicates that the action of MFA was reversible. The patch of Lucifer Yellow coupled cells is not as large as the Neurobiotin filled patch but this is due to the reduced mobility of Lucifer Yellow compared with Neurobiotin. Carbenoxolone is also water soluble but we were not able to reverse the block of Lucifer Yellow coupling in A-type HCs. In addition, we could not reverse the block of Lucifer Yellow coupling caused by the DMSO-soluble gap junction antagonists such as mefloquine, 18-β-GA, and 2-APB within 15 min.
Fig. 4.
Reversal of Lucifer Yellow coupling in real time after the block of gap junctions with meclofenamic acid. (A) shows the electrode impaling a single A-type HC after 5 min of dye injection with 4% Lucifer Yellow during perfusion with 100 μM MFA. (B) The electrode was withdrawn and after a 15 min wash, Lucifer Yellow spread to other A-type HCs. Same field as A, as shown by identical morphology of filled cell. This indicates that coupling was reversible after the washout of meclofenamic acid.
Other coupled networks
Many cell types in the retina are coupled via gap junctions and there is evidence that different neuronal types express different gap junctions. For example, A-type and B-type horizontal cells have distinct coupling properties, probably because they express different connexins. A-type HCs are coupled via Cx50 gap junctions while B-type HCs may be coupled by Cx57 (Hombach et al., 2004). In the inner retina, AII amacrine cells are coupled by Cx36 gap junctions (Feigenspan et al., 2001; Mills et al., 2001). To test if the potential gap junction antagonists are effective on different connexins, we tested their effects on coupling in different neural networks.
B-type HCs are well-coupled and, following dye-injection with Neurobiotin, an extensive patch of coupled cells is recovered (Fig. 5A). Perfusion with 50 μM MFA dramatically reduced the dye coupling in B-type HCs, such that only a small number of filled cells were visible (Fig. 5B). Furthermore, the dendrites of the filled cell are very brightly labeled and the long axon is prominently labeled. These are the unmistakable signs of a block of gap junctions. When the dose of MFA was increased to 100 μM, fewer cell bodies were visible but the dendrites and axon were better filled. In this case, the extensive branches of the axon terminal are clearly visible (Fig. 5C). At 200 μM MFA, only a single B-type HC was obtained. These experiments indicate that MFA blocks gap junctions in B-type HCs.
Fig. 5.
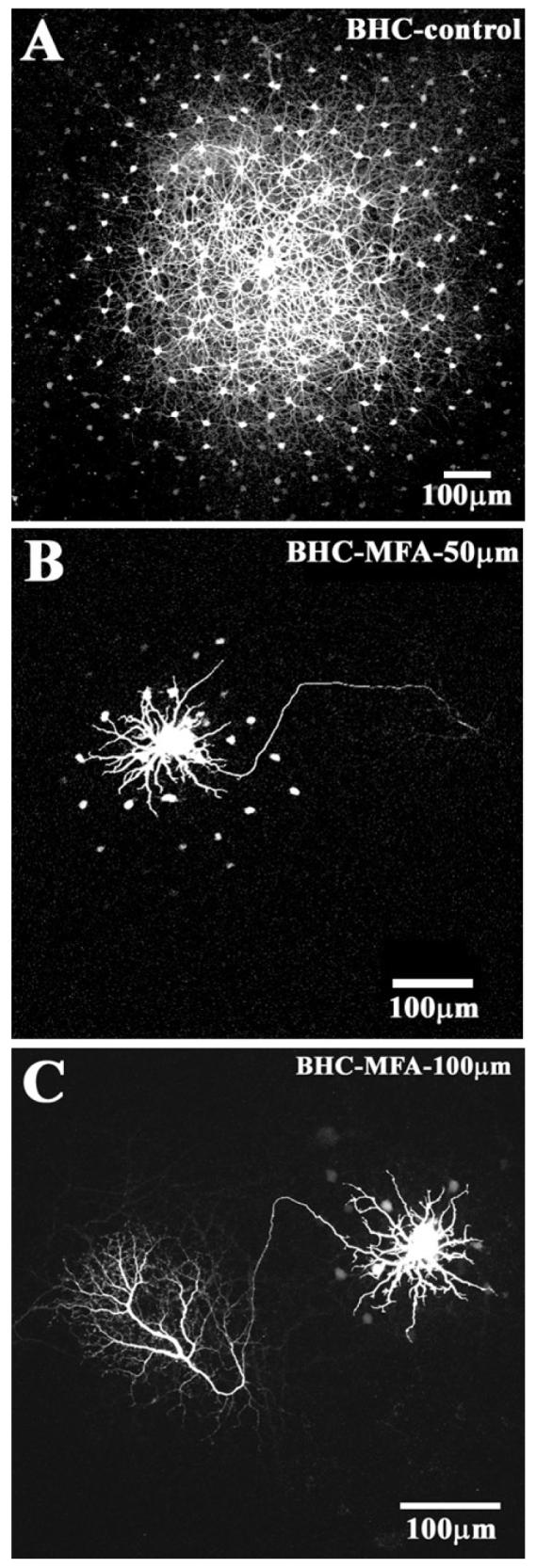
The block of gap junction coupling in B-type HCs. (A) Control experiment shows a large patch of coupled B-type HCs after 10 min of dye injection followed by 10 min of diffusion. (B) The same protocol in the presence of 50 μM MFA produced a dramatic reduction in coupling. A single prominent B-type HC was surrounded by a few coupled somas and the axon was brightly stained. (C) Increasing the dose of MFA to 100 μM further reduced the coupling of B-type HCs and produced axon and axonal terminal.
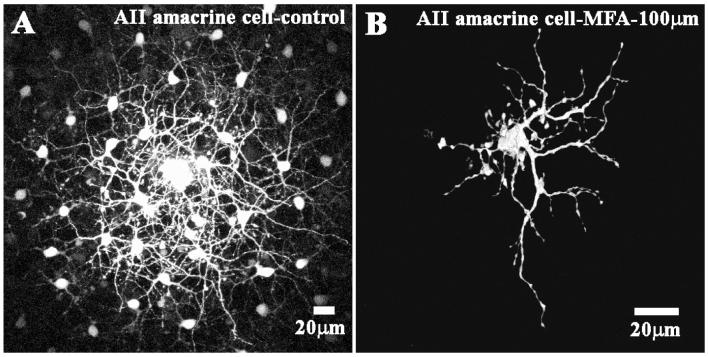
AII amacrine cells and ON cone bipolar cells form a complex heterocellular network in the inner retina (Mills & Massey, 1995; Bloomfield et al., 1997; Vaney et al., 1998). AII/AII coupling is mediated by Cx36 gap junctions (Feigenspan et al., 2001; Mills et al., 2001) while the coupling between AII amacrine cells and ON cone bipolar cells may rely on heterotypic Cx36/Cx45 gap junctions (Dedek et al., 2006). After dye injecting an AII amacrine cell, a large coupled patch of AII amacrine cells was recovered with an overlying set of cone bipolar cells (Fig. 6A). Perfusion with MFA reduced dye coupling to a single AII amacrine cell and the fine details of the dendrites and lobules are clearly visible (Fig. 6B). Dye coupling to the overlying cone bipolar cells was also eliminated. These results suggest that MFA is able to block a variety of gap junction channels in different coupled networks throughout the retina. Similar results were obtained with mefloquine, 18-β-GA and 2-APB. We found no evidence for the selectivity of gap junction antagonists among the different coupled networks in the retina.
Fig. 6.
Block of dye coupling in AII amacrine cells by meclofenamic acid. (A) The control panel shows a patch of coupled AII amacrine cells with an overlapping matrix of fine dendrites after 5 min of dye injection followed by 15 min of diffusion. Some coupled cone bipolar cells were also present in a deeper focal plane but they are obscured by the AII matrix. (B) After perfusion with 100 μM MFA, only one AII amacrine cell was filled in great detail with the fine dendrites and lobules clearly visible.
Discussion
The diffusion of Neurobiotin throughout a coupled network depends on the presence of gap junction coupling. Some potential gap junction antagonists were evaluated according to their ability to block dye coupling in the network of A-type HCs in the rabbit retina. The main findings can be summarized as follows: (1) 2-APB, MFA, Mefloquine and 18-βGA were the most potent at reducing the spread of Neurobiotin in A-type HCs. (2) All antagonists blocked coupling in several different neuronal networks. This suggests non-selective activity on several types of gap junction. (3) Of the compounds we screened, only MFA was water-soluble and easily reversible.
Meclofenamic acid is a potent, water-soluble, reversible gap junction antagonist
Among the compounds that we tested, in rank order of potency, 2-APB, MFA, mefloquine, and 18-β-GA blocked coupling in A-type HCs most effectively. At a concentration of 200 μM, all these compounds completely suppressed Neurobiotin coupling in A-type HCs. The other compounds, including NFA, FFA, and carbenoxolone, all fell into a low potency group. In particular, we were unable to obtain a complete block of A-type HC coupling with carbenoxolone: a few coupled somas remained. This probably reflects a combination of low potency compared to the other compounds and the extremely robust coupling of the A-type HC network. Two additional compounds, 18α-GA and retinoic acid had low solubility and a complete block of A-type HC coupling was not obtained.
Only carbenoxolone and MFA were water soluble. The remaining compounds required the use of DMSO to make a stock solution. However, the water-insoluble compounds were not easily reversible. Compounds requiring DMSO as a solvent are likely to be more lipid soluble and this may contribute to the difficulty in washing out the drug. Even though carbenoxolone is water soluble, it is notoriously difficult to reverse. The block of coupling with MFA was easily reversible and large patches of A-type HCs were obtained after 10–15 min of washing. Of the other compounds tested, 2-APB was irreversible and only a single labeled cell was obtained after a 10 min wash. For 18-β-GA, mefloquine and carbenoxolone, a few A-types HCs were observed around the dye injected cell after a 10 min wash. Thus, these compounds should be regarded as partially reversible following the relatively high dose required to block coupling in A-type HCs.
The dose of MFA and other compounds is substantially higher than those reported for cultured cells. This may be partly due to the diffusion barrier presented by the use of intact retina. However, we should remember that A-type HCs have exceptional dye coupling properties. They are coupled via Cx50 gap junctions which have a much higher unitary conductance (220pS) (Srinivas et al., 1999a) compared to 15pS for Cx36 (Srinivas et al., 1999b). Furthermore, A-type HCs are coupled, at least in part, via giant plaques which may occupy 50 μm2 and contain hundreds of thousands of gap junctions channels (O'Brien et al., 2006). In summary, A-type HCs are the best coupled of all retinal cell types and it should therefore be expected that a higher dose of gap junction antagonist is required. By comparison, slightly lower doses of MFA, in the range of 50–100 μM, were sufficient to abolish coupling in AII amacrine cells or B-type HCs. For example, filling B-type HCs in the presence of 100 μM MFA produced isolated cells with brightly filled axon terminals.
Meclofenamic acid has no selective effect on different gap junctions
There is evidence that A-type HCs, B-type HCs, and AII amacrine cells all express different connexins. AII amacrine cells express Cx36 (Feigenspan et al., 2001; Mills et al., 2001), A-type HCs express Cx50 (O'Brien et al., 2006) and the mouse homolog of the rabbit B-type HC may use Cx57 (Hombach et al., 2004). MFA, 18-β-GA, mefloquine and 2-APB blocked coupling in all these networks, and so we conclude they have little selectivity between different connexins, at least within the group of neuronal connexins such as Cx36, Cx50 and Cx57 in the retina.
In contrast, some specificity against different connexins has previously been reported for mefloquine, 2-APB and quinine (Srinivas et al., 2001; Cruikshank et al., 2004; Bai et al., 2006). For example, mefloquine preferentially blocked Cx36 and Cx50 gap junctions compared to Cx26, Cx32, Cx43, and Cx46 gap junctions (Srinivas et al., 2001). A similar profile was reported for 2-APB. Thus, these compounds seem to differentiate between the neuronal connexins (Cx36 and Cx50) and the other connexins. However, MFA also blocked Cx43 gap junctions expressed in monolayer (Harks et al., 2001). This also suggests that MFA shows poor selectivity between different connexin types.
The block of dye coupling in B-type horizontal cells
The blockade of gap junctions in B-type HCs had one additional effect which may be generally useful. Normally, dye injections of B-type HCs produce a cluster of coupled cells and sometimes the axons from individual cells are visible, but not in their entirety (Reitsamer et al., 2006). The reason for this labeling pattern is because the injected dye diffuses through the coupled network in preference to traveling a long distance down the fine axon. However, when the gap junctions are blocked, the axon of the injected cell and the entire axon terminal are labeled. Axon terminals form a major component of the OPL and this is a convenient way to label them with detail comparable to Golgi staining or HRP injections (Boycott et al., 1978; Dacheux & Raviola, 1982). We conclude that dramatically increased detail can be obtained for individual examples of a coupled population when gap junctions are blocked. An alternative method to obtain bright labeling of a single cell is by injecting an intracellular dye of a higher molecular weight such as biotin-xx-cadaverine (BXXC) or Alexa-568. For example, BXXC (MW 555) also produced increased axonal labeling in B-type HCs compared with Neurobiotin (MW 286) (Mills & Massey, 2000). In this case, the reason was because the gap junctions impeded the passage of the larger tracer and so the axon structure was labeled instead. Biocytin-x-hydrazide has also been used as a membrane impermeable tracer to obtain detailed fills of single horizontal cells (Packer & Dacey, 2002).
Comparative pharmacology of gap junction antagonists
The ability of the fenamates, some anti-malarials, and several other compounds to block gap junctions has been reported in several expression studies. The potency in inhibiting electrical coupling decreases in the order: meclofenamic acid > niflumic acid > flufenamic acid (Harks et al., 2001). MFA is among the most potent of the gap junction antagonists in comparative studies. This work has also suggested some limited selectivity against a variety of gap junctions constructed from particular connexins. However, there was little selectivity among the most common neuronal connexins identified in the retina, namely, Cx36 and Cx50.
The fenamates all have non-steroidal anti-inflammatory drug (NSAID) activity but this is not thought to contribute to their ability to block gap junctions (Harks et al., 2001). Rather, they appear to be relatively general channel antagonists. We were unable to block coupling in the impaled cell by including MFA in the pipette, even at concentrations as high as 100 mM, attainable due to the water solubility of MFA. Likewise, when the fenamates were included in patch pipettes, they did not block gap junction coupling and it was concluded that these compounds do not interact with the inside of the open pore (Eskandari et al., 2002; Srinivas & Spray, 2003). Rather, it has been suggested that the fenamates have a direct interaction with connexins or the membrane protein interface that could affect connexin conformation (Harks et al., 2001).
Niflumic acid (NFA) is also a potent blocker of calcium-activated chloride channels and 2-APB is well known as an antagonist of storage operated calcium channels (Dobrydneva & Blackmore, 2001). Given the dependence of sustained rod transmission on calcium induced calcium release from intracellular stores (Suryanarayanan & Slaughter, 2006), 2-APB may be unsuitable for physiological studies in the retina. Carbenoxolone is commonly used as a gap junction antagonist but it also blocks calcium channels (Vessey et al., 2004) and has a direct inhibitory effect on light responses (Verweij et al., 2003; Xia & Nawy, 2003). In addition, the comparative toxicity of carbenoxolone and the difficulty in washing it out complicate the interpretation of experiments based on the use of this gap junction antagonist.
18-β-GA has been used successfully to block coupling between amacrine cells and ON directionally selective (DS) ganglion cells at a relatively low dose, 25 μM (Ackert et al., 2006). These gap junctions are undoubtedly smaller, compared to HC gap junctions, as shown by the restricted pattern of tracer coupling, and their position in the inner retina makes them relatively accessible. Furthermore, the ON DS cells still produced normal directional responses, albeit with desynchronized spikes, which suggests that 18-β-GA is a relatively selective gap junction antagonist with minimal side effects (Ackert et al., 2006). However, it has also been reported that the same dose of 18-β-GA caused swelling of retinal cells (Vaney et al., 1998) and at a dose of 50 μM, ganglion cell firing rate was reduced (Xia & Nawy, 2003). In comparison, the extensive coupling of A-type HCs combined with their depth in the retina require a much higher dose of antagonist to block gap junctions. To produce a single uncoupled A-type HC, it was necessary to use MFA or 18-β-GA at a dose of 200 μM (Figs. 1 and 2). At these high doses, it was easier to reverse the water soluble MFA.
In summary, we found that MFA had many desirable properties as a gap junction antagonist: it is potent, water soluble and reversible. However, the effects of MFA on other channels, especially calcium channels, are unknown and will be the subject of additional experiments. MFA has been reported to open KCNQ2/Q3 potassium channels which underlie the M-current and may lead to membrane hyper-polarization and a reduction in the firing rate of spiking cells (Peretz et al., 2005). In addition, at high doses, such as 1 mM, MFA had a protective effect against swelling caused by retinal ischemia, due to the block of NMDA receptors (Chen et al., 1998). Thus, the suitability of MFA for use in physiological experiments has not yet been established. Nevertheless, it should be useful to block dye transfer in coupled networks and to produce well-stained examples of uncoupled cells.
Acknowledgments
This research was supported by the National Eye Institute (NEI); Grant Numbers: EY 06515 (to S.C.M.), EY10121 (to S.L.M.) and EY 10608 (Vision Core Grant). Additional support was provided by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Science. S.C.M. is the Elizabeth Morford Professor of Ophthalmology and Visual Science. The authors wish to thank Dr. John O'Brien for helpful discussion and Dr. Alice Z. Chuang for statistic help. This paper is dedicated to the memory of our colleage Ray Dacheux.
References
- Ackert JM, Wu SH, Lee JC, Abrams J, Hu EH, Perlman I, Bloomfield SA. Light-induced changes in spike synchronization between coupled ON direction selective ganglion cells in the mammalian retina. Journal of Neuroscience. 2006;26:4206–4215. doi: 10.1523/JNEUROSCI.0496-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB) Journal of Pharmacology and Experimental Therapeutics. 2006;319:1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Visual Neuroscience. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Peichl L, Wässle H. Morphological types of horizontal cell in the retina of the domestic cat. Proceeding of the Royal Society London B: Biological Science. 1978;203:229–245. doi: 10.1098/rspb.1978.0103. [DOI] [PubMed] [Google Scholar]
- Chen Q, Olney JW, Lukasiewicz PD, Almli T, Romano C. Fenamates protect neurons against ischemic and excitotoxic injury in chick embryo retina. Neuroscience Letters. 1998;242:163–166. doi: 10.1016/s0304-3940(98)00081-0. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of C×36 and C×50 gap junction channels by mefloquine. Proceedings of the National Academy of Sciences. 2004 doi: 10.1073/pnas.0402044101. 0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Horizontal cells in the retina of the rabbit. Journal of Neuroscience. 1982;2:1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Schultz K, Pieper M, Dirks P, Maxeiner S, Willecke K, Weiler R, Janssen-Bienhold U. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. European Journal of Neuroscience. 2006;24:1675–1686. doi: 10.1111/j.1460-9568.2006.05052.x. [DOI] [PubMed] [Google Scholar]
- Dobrydneva Y, Blackmore P. 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Molecular Pharmacology. 2001;60:541–552. [PubMed] [Google Scholar]
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. Journal of Membrane Biology. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Janssen-Bienhold U, Hormuzdi S, Monyer H, Degen J, Sohl G, Willecke K, Ammermuller J, Weiler R. Expression of connexin36 in cone pedicles and OFF-cone bipolar cells of the mouse retina. Journal of Neuroscience. 2004;24:3325–3334. doi: 10.1523/JNEUROSCI.5598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Teubner B, Willecke K, Weiler R. Expression of Neuronal Connexin36 in AII Amacrine Cells of the Mammalian Retina. Journal of Neuroscience. 2001;21:230–239. doi: 10.1523/JNEUROSCI.21-01-00230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: Functions of unpaired connexon channels. Nature Reviews Molecular Cell Biology. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Guldenagel M, Sohl G, Plum A, Traub O, Teubner B, Weiler R, Willecke K. Expression patterns of connexin genes in mouse retina. Journal of Comparative Neurology. 2000;425:193–201. [PubMed] [Google Scholar]
- Han Y, Massey SC. Electrical synapses in retinal ON cone bipolar cells: Subtype-specific expression of connexins. Proceedings of the National Academy of Sciences. 2005;102:13313–13318. doi: 10.1073/pnas.0505067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, van Zoelen EJ, Theuvenet AP. Fenamates: A novel class of reversible gap junction blockers. Journal of Pharmacology and Experimental Therapeutics. 2001;298:1033–1041. [PubMed] [Google Scholar]
- Hombach S, Janssen-Bienhold U, Sohl G, Schubert T, Bussow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. European Journal of Neuroscience. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- Lin B, Jakobs TC, Masland RH. Different functional types of bipolar cells use different gap-junctional proteins. Journal of Neuroscience. 2005;25:6696–6701. doi: 10.1523/JNEUROSCI.1894-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Zhang DQ, McMahon DG. Electrical coupling of retinal horizontal cells mediated by distinct voltage-independent junctions. Visual Neuroscience. 1999;16:811–818. doi: 10.1017/s0952523899165015. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. Journal of Comparative Neurology. 1996;366:15–33. doi: 10.1002/(SICI)1096-9861(19960226)366:1<15::AID-CNE2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL. Gap junctions between AII amacrine cells and calbindin-positive bipolar cells in the rabbit retina. Visual Neuroscience. 1999;16:1181–1189. doi: 10.1017/s0952523899166173. [DOI] [PubMed] [Google Scholar]
- Massey SC, O'Brien JJ, Trexler EB, Li W, Keung JW, Mills SL, O'Brien J. Multiple neuronal connexins in the mammalian retina. Cell Communication and Adhesion. 2003;10:425–430. doi: 10.1080/cac.10.4-6.425.430. [DOI] [PubMed] [Google Scholar]
- Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermuller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K, Weiler R. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. Journal of Neuroscience. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Distribution and coverage of A- and B-type horizontal cells stained with Neurobiotin in the rabbit retina. Visual Neuroscience. 1994;11:549–560. doi: 10.1017/s0952523800002455. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. The kinetics of tracer movement through homologous gap junctions in the rabbit retina. Visual Neuroscience. 1998;15:765–777. doi: 10.1017/s0952523898154159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. A series of biotinylated tracers distinguishes three types of gap junction in retina. Journal of Neuroscience. 2000;20:8629–8636. doi: 10.1523/JNEUROSCI.20-22-08629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, O'Brien JJ, Li W, O'Brien J, Massey SC. Rod pathways in the mammalian retina use connexin36. Journal of Comparative Neurology. 2001;436:336–350. [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Nguyen HB, Mills SL. Cone photoreceptors in bass retina use two connexins to mediate electrical coupling. Journal of Neuroscience. 2004;24:5632–5642. doi: 10.1523/JNEUROSCI.1248-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. Journal of Neuroscience. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer OS, Dacey DM. Receptive field structure of H1 horizontal cells in macaque monkey retina. Journal of Vision. 2002;2:272–292. doi: 10.1167/2.4.1. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Visual Neuroscience. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Peretz A, Degani N, Nachman R, Uziyel Y, Gibor G, Shabat D, Attali B. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Molecular Pharmacology. 2005;67:1053–1066. doi: 10.1124/mol.104.007112. [DOI] [PubMed] [Google Scholar]
- Raviola E, Dacheux RF. Axonless horizontal cells of the rabbit retina: Synaptic connections and origin of the rod aftereffect. Journal of Neurocytology. 1990;19:731–736. doi: 10.1007/BF01188041. [DOI] [PubMed] [Google Scholar]
- Reitsamer HA, Pflug R, Franz M, Huber S. Dopaminergic modulation of horizontal-cell-axon-terminal receptive field size in the mammalian retina. Vision Research. 2006;46:467–474. doi: 10.1016/j.visres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Rodieck R. The First Steps in Seeing. Sinauer Associates, Inc.; Sunderland, MA: 1998. [Google Scholar]
- Schubert T, Maxeiner S, Kruger O, Willecke K, Weiler R. Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. Journal of Comparative Neurology. 2005;490:29–39. doi: 10.1002/cne.20621. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nature Reviews Neuroscience. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. Journal of Physiology. 1999a;517(3):673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proceedings of the National Academy of Sciences. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophysics Journal. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. Journal of Neuroscience. 1999b;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Molecular Pharmacology. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- Suryanarayanan A, Slaughter MM. Synaptic transmission mediated by internal calcium stores in rod photoreceptors. Journal of Neuroscience. 2006;26:1759–1766. doi: 10.1523/JNEUROSCI.3895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. Journal of Neurophysiology. 2005;93:1476–1485. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Patterns of neuronal coupling in the retina. Progress in Retinal and Eye Research. 1994;13:301–355. [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. Journal of Neuroscience. 1998;18:10594–10602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. Journal of Neuroscience. 2003;23:10249–10257. doi: 10.1523/JNEUROSCI.23-32-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. Journal of Neurophysiology. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Volgyi B, Abrams J, Paul DL, Bloomfield SA. Morphology and tracer coupling pattern of alpha ganglion cells in the mouse retina. Journal of Comparative Neurology. 2005;492:66–77. doi: 10.1002/cne.20700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Boycott BB, Peichl L. Receptor contacts of horizontal cells in the retina of the domestic cat. Proceeding of the Royal Society London B: Biological Science. 1978;203:247–267. doi: 10.1098/rspb.1978.0104. [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Journal of Biological Chemistry. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Xia Y, Nawy S. The gap junction blockers carbenoxolone and 18beta-glycyrrhetinic acid antagonize cone-driven light responses in the mouse retina. Visual Neuroscience. 2003;20:429–435. doi: 10.1017/s0952523803204089. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Connexin35/36 gap junction proteins are expressed in photoreceptors of the tiger salamander retina. Journal of Compartive Neurology. 2004;470:1–12. doi: 10.1002/cne.10967. [DOI] [PubMed] [Google Scholar]
- Zimmerman AL, Rose B. Permeability properties of cell-to-cell channels: Kinetics of fluorescent tracer diffusion through a cell junction. Journal of Membrane Biology. 1985;84:269–283. doi: 10.1007/BF01871390. [DOI] [PubMed] [Google Scholar]