Abstract
This article addresses psychoneuroimmunology (PNI) as an integrative paradigm for advancing both theoretical and empirical knowledge of physiological patterns that contribute to the dynamics of health. We depict relationships among relevant psychobehavioral and physiological components in a PNI-based framework. We then provide examples of how this framework guided 2 clinical trials designed to assess the effectiveness of selected nursing interventions to reduce stress and enhance coping, one in persons with human immunodeficiency viral disease and the other in persons with cancer. The examples address disease-specific measures for assessing the components of the PNI-based framework.
Keywords: cancer, health, HIV, physiology, psychoneuroimmunology, theory development
This article addresses psychoneuroimmunology (PNI) as an integrating paradigm for advancing both theoretical and empirical knowledge of physiological patterns that contribute to the dynamics of health. We broadly define health to include the entire spectrum of wellness-illness phenomena. We concentrate here on application of the PNI paradigm as the foundation for a theoretical framework to discern patterns, processes, and consequences of stress and coping as they relate to health dynamics. Using the PNI-based framework to outline relationships among relevant psychosocial and physiological components, we provide a description of the underlying mechanisms. We then illustrate specific applications of the framework for the study of persons with human immunodeficiency viral (HIV) infection, followed by a theoretical model applicable to research in women with breast cancer. The applications address disease-specific measures for assessing variables within the PNI-based framework and provide specific justification for the inclusion of these measures. It is our intent to encourage nurse researchers to apply the PNI framework to studies of various populations in order to further develop comprehensive views of health dynamics.
OVERVIEW OF THE PNI PARADIGM
PNI is concerned with the mechanisms of multidimensional psychobehavioral-neuroendocrine-immune system interactions. The emphasis in PNI is on developing an understanding of how the immune system is influenced by both sociobehavioral (psychosocial-spiritual) and physiological (neuroendocrine) interactions.1 Within the PNI framework, behavioral aspects are viewed as moderators; that is, their effect on the immune system is thought to be moderated through the neuroendocrine system. Neuroendocrine influences within this framework are viewed as mediators; that is, they are thought to have direct effects on and multidimensional interactions with the immune system. We consider the PNI paradigm to be comprehensive in that it provides for inclusion of individual as well as social/collective/environmental phenomena and accommodates a range of research methods, from quantitative measurement of biological variables to qualitative approaches focused on subjective experience.
PNI FRAMEWORK FOR THE STUDY OF STRESS AND COPING
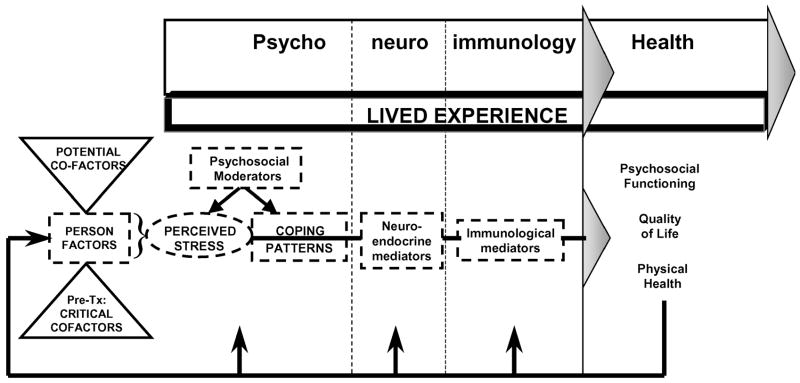
PNI provides a comprehensive approach for integrating the Lazarus and Folkman2 cognitive-transactional model of stress with the psychobehavioral and pathophysiological processes affecting health dynamics in numerous stress-disease relationships (see Fig 1). Lazarus and Folkman2 described the stress process as a dynamic transaction among person factors, social-environmental factors, and illness-related stress factors that influences both cognitive appraisal (thereby defining perceived stress) and coping patterns. The generic model depicted in Figure 1 focuses on the impact of illness-related chronic stressors. It makes explicit the role of coping strategies in altering stress-related PNI responses and improving the adaptational outcomes of psychosocial functioning, quality of life, and physical health. In addition, the PNI-based model incorporates neuroendocrine-immune processes underlying biological adaptation and physical health. The model presented as Figure 1 allows cause and effect relationships among variables of interest to be made explicit. This model was designed to depict general relationships among relevant components rather than to detail the complex physiological and pathophysiological interactions involved in the underlying processes.
Figure 1.
Generic model of the PNI-based theoretical framework.
The major components in Figure 1 include cofactors, psychological components (broadly defined to include psychosocial and spiritual aspects and including perceived stress and coping), neurological components (defined to include both neurological and endocrine elements), immunology (focusing on components of the immune system as classically defined), health (indicated by adaptational outcomes, including relevant disease-related indicators), and the lived experience of relevant health dynamics.
Cofactors are those components that have the potential to predispose an individual to certain stress, coping, and health patterns. They include relevant personal characteristics of the individual, such as gender, age, and nutritional status. Cofactors also include health-related features of one’s life, such as the severity of one’s illness, adherence to treatment regimens, and side effects of treatments. They may affect how an individual’s transactional processes proceed and thus affect what is perceived as stressful, as well as how an individual is able to cope psychologically and respond physiologically to perceived stressors.
The “psycho” component of the model addresses sociobehavioral aspects, including various psychological “states” or emotions that can be broadly classified as negative affect, or psychological distress (eg, depressed mood, grief, perceived loss of personal control, and illness-related uncertainty), which have been shown to have reliable immunosuppressive effects.3,4 Chronic stress and the associated psychological distress can activate the hypothalamic-pituitary-adrenocortical and sympathetic-adrenomedullary systems, thereby inducing immunosuppression. Thus, chronic or severe psychological stress associated with living with serious illness may further compromise immune functioning over the illness trajectory, thereby increasing risks for morbidity and mortality. Measures for the “psycho” component of the model include illness-specific psychosocial measures as well as qualitative interviews; the specific measures are determined on the basis of the disease condition and population being studied.
Biobehavioral interventions are directed at the “psycho” component of the model. They are designed to reduce stress or enhance coping and thus have the potential to moderate or diminish the neuroendocrine and immunosuppressive effects of stress over the illness trajectory. If interventions are effective, illness-related psychological and physical well-being may be improved, particularly in diseases in which immunosuppression is a problem, such as HIV disease and cancer.
The “neuro” component of the model addresses the classic understanding of physiological responses to psychosocial stressors. This involves activation of the sympathetic-adrenomedullary system, resulting in the release of epinephrine, norepinephrine, and enkephalins. Simultaneously, the hypothalamic-pituitary-adrenocortical system is stimulated, leading to elaboration of corticotropin (ACTH), endorphins, and cortisol. “Neuro” components of the model are measured using physiological indicators, such as cortisol.
Ongoing research in PNI continues to elaborate the multidimensional nature and complexity of the stress response, particularly as it involves the immune system. These mechanisms are incorporated in the “immunology” component of the model. A number of bidirectional relationships between the neuroendocrine and immune systems are known to exist. These include direct sympathetic nervous system innervation of lymphoid tissues (including the spleen, thymus, and lymph nodes, where sympathetic fibers are concentrated in zones of maturing T lymphocytes). Second, receptors for neurotransmitters, neurohormones, and neuropeptides exist on cells of the immune system (such as corticotropin, cortisol, and β2-adrenergic receptors of lymphocytes). Third, leukocytes produce neurohormones and neurotransmitters (including lymphocyte production of corticotropin and macrophage production of interleukin [IL]-1 and β-endorphin).5 Researchers have documented that the stress response involves both direct and indirect effects on the immune system, such that numerous cytokines, neurohormones, and neuropeptides are elaborated by cells of the immune system and function in physiological regulation and adaptation.6,7 The “immuno” component of the model is measured using numerous general and disease-specific physiological indicators of immune functioning.
The interrelationships between the “neuro” and “immuno” components of the model are measured by indicators of physiological function in the context of the disease or population being studied. The best known and perhaps most important immunosuppressive effects in the context of PNI are due to elevated levels of cortisol and associated changes in cytokine immunoregulation processes. It is widely accepted that high levels or prolonged elevations of cortisol inhibit virtually all components of the immune response.8 Cortisol inhibits almost all known cytokines, at least in part through its potent inhibition of nuclear factor kappa B (NF-κB), an activator of many immunoregulatory genes.9 Elevated cortisol causes, for example, (a) decreased IL-1, resulting in decreased T-lymphocyte costimulation and decreased macrophage function; (b) decreased IL-2 and interferon-gamma (IFN-γ), reducing CD8+ lymphocyte and macrophage functioning and T-lymphocyte proliferation; and (c) inhibited production of IL-12 by monocytes, which further decreases the production of IFN-γ and increases the production of IL-4. The resultant change in cross-regulation suppresses type 1 cytokines and stimulates type 2 cytokine responses.10,11 The roles of cytokines, particularly the proinflammatory cytokines involved in acute phase responses (IL-1, IL-6, and tumor necrosis factor-alpha [TNF-α]), have been of considerable interest in recent research on psychobehavioral changes in illness, commonly termed “sickness behaviors” and including fatigue and depressed mood.12,13 Furthermore, each of these cytokines can stimulate the hypothalamic-pituitary-adrenocortical axis, resulting in inhibition of the inflammatory response by elevated cortisol.14
Endorphins and enkephalins also are stress-related neuroendocrine mediators, but little clinical or biobehavioral research examining these endogenous opioids has yet been conducted. Opioid peptides are widely distributed throughout the central, peripheral, and autonomic nervous systems as well as multiple endocrine and target tissues. There is mounting evidence that opioids downregulate neuroendocrine and autonomic stress responses and may counteract some aspects of cortisol-induced immunosuppression.15 Opioids have been shown to affect in vitro function of virtually all cells of the immune system and generally have dose-dependent effects such that low doses enhance and high doses suppress immune function.16 Given the available evidence and increasing interest in positive responses to stressors, quantification of opioid peptides is indicated in clinical studies to explore their potential roles in neuroendocrine mediation of the stress process.
Finally, the model incorporates a variety of health outcomes, termed “adaptational outcomes.” As classically defined by Lazarus and Folkman,2 these include psychosocial functioning, quality of life, and physical health. As with other components of the model, indicators of adaptational outcomes are specifically selected on the basis of the particular research question, target population, putative pathophysiological processes, and other health dynamics of interest. “Feedback” arrows from adaptational outcomes to the other components of the model reflect the complex interactions addressed within this model.
The “lived experience” component of the model provides an integrated and comprehensive emphasis on the significance of one’s day-to-day life and its influence on all aspects of the model. A variety of sociocultural and economic forces that influence one’s life, as well as the influences of the larger community in which individuals live, can be explored as deemed important by the study participants. In addition to the quantification of the psychosocial-spiritual components, we believe that a qualitative component is essential to clarify and contextualize the quantification of other variables and to appreciate new and emerging phenomena, thereby generating new hypotheses for further study.
Strengths and limitations
Contributions to nursing empirics are enhanced through the use of a framework that includes defined variables and specifies relationships among those variables. Use of the PNI framework allows nurse researchers to make explicit the physiological basis for effectiveness of psychobehavioral nursing interventions designed to affect stress and coping, and ultimately, health outcomes and quality of life. The comprehensiveness of the model also provides a basis for nurse researchers to acknowledge nursings commitment to individuals as complex, multidimensional, or holistic beings. Thus, the use of the PNI framework is another avenue for nurses to participate in the mainstream scientific community while maintaining a holistic focus on the individual person.
As PNI-based research has grown, scientific evidence of associations among psychosocial, neuroendocrine, immunological, and disease-specific outcomes has continued to amass, contributing to an ongoing need to evaluate the relationships among the components of the model. In addition, the state-of-the-science is such that many PNI mechanisms remain unclear, particularly in the context of such complexity as is presented by HIV disease and human cancer. Valid and precise biological measures, including surrogate biomarkers that can be evaluated as intermediate outcomes among persons with life-threatening or chronic diseases, will be required to discern the mechanisms of any potential PNI-based effects of psychobehavioral interventions on health outcomes. Evaluating such intermediate outcomes and neuroendocrine and immunological biomarkers is a major challenge to be addressed in PNI-based nursing research.
Significance to nursing
The PNI-based framework is congruent with nursing commitments to a holistic view of human beings and to health dynamics. It provides for the inclusion of data regarding the full spectrum of human experiences as understood via both qualitative and quantitative approaches. The model provides a logical and scientifically sound framework for investigating stress-related nursing interventions and facilitates the development of knowledge regarding the underlying mechanisms of such interventions.
APPLICATIONS OF THE PNI-BASED THEORETICAL FRAMEWORK
While researchers have generated compelling evidence for neuroendocrine and behavioral interactions with the immune system, especially within the context of stress effects, the influence of these interactions on health outcomes is only beginning to be examined.6,7,17 The PNI paradigm accounts for the negative impact of perceived stress on health outcomes, primarily as a function of immunosuppression mediated by elevated cortisol. However, the underlying mechanisms for such downregulation of immune function are neither simplistic nor entirely clear.
We are studying these mechanisms, along with their influences on health outcomes, in 2 populations: people who have HIV disease and women who are undergoing chemotherapy for the treatment of breast cancer. We have approached the development of knowledge about underlying PNI mechanisms within the context of clinical trials to evaluate stress management interventions aimed at attenuating the negative consequences of stress. We selected particular interventions because they represent strategies that provide avenues for holistic change that may reduce perceived stress, enhance coping, and contribute to an enhanced quality of life. Each intervention focuses differentially on mind-body-spirit dimensions of human experience and has some evidence of effectiveness in either our previous work or that of others.18–22 In the following section, we provide an overview of the PNI-based models that have guided our research in persons with HIV disease and breast cancer. We offer these as exemplars of applications of the generic PNI-based framework to the study of specific populations and to the evaluation of stress management interventions.
PNI in HIV disease
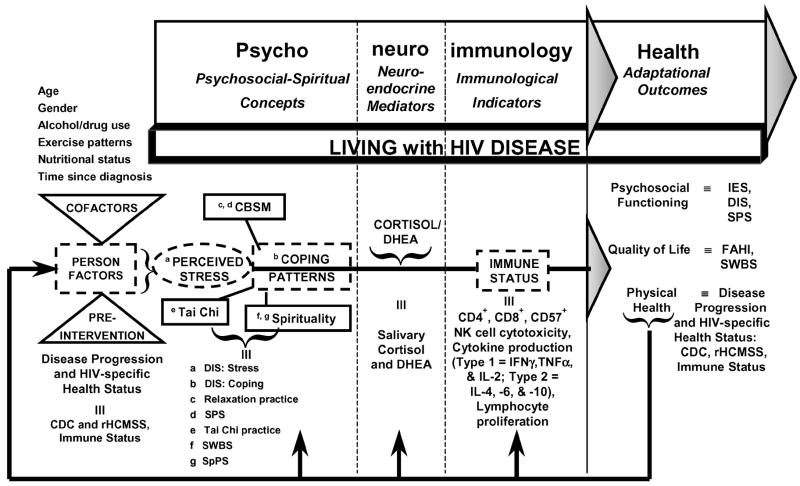
Our research group is continuing a series of studies assessing the effectiveness of alternative stress management approaches (cognitive-behavioral stress management, spiritual growth groups, and tai chi training) on PNI-based outcomes among persons with HIV disease at varying stages of progression (see Fig 2).21,22 This is a particularly important area of study, given the multiple relationships among stress and HIV infection that may influence disease progression. For example, physiological responses to psychological factors may reactivate other latent viral infections and thereby activate immune cells infected with HIV. Also, changes in neuropeptide or hormone levels (particularly cortisol) due to perceived stress could alter the distribution of lymphocyte subsets and exacerbate immune impairment due to HIV. In addition, stress may influence the host immune response and containment of HIV infection through such mechanisms as reducing natural killer (NK) cell or T-lymphocyte cytotoxicity. Cortisol may directly increase HIV activation and expression by way of a glucocorticoid receptor within the long terminal repeat of the virus.23,24 Finally, numerous mechanisms for the negative influence of elevated cortisol on HIV disease progression involve its effects on cytokines. Because IFN-γ inhibits HIV replication,25 reductions in IFN-γ levels could disinhibit viral activity. In addition, and of critical importance, enhanced production of IL-10 directly inhibits the anti-HIV response of CD8+ T lymphocytes,26 and IL-6 directly induces HIV expression and synergizes with TNF-α,27 all of which permit enhanced HIV expression.
Figure 2.
PNI-based model in HIV disease. DIS: Stress indicates Dealing with Illness Scale: Stress subscale; DIS: Coping, Dealing with Illness Scale: Coping subscale; SPS, Social Perspectives Scale; SWBS, Spiritual Well-being Scale; SpPS, Spiritual Perspectives Scale; DHEA, dehydroepiandrosterone; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor-alpha; IES, Impact of Event Scale; FAHI, Functional Assessment of HIV Infection Scale; CDC, Centers for Disease Control and Prevention, HIV Disease Classification; and rHCMSS, Revised HIV Center Medical Staging Scale.
In terms of psychological correlates, Evans et al28 reported significant relationships between severe stress levels and reduced numbers of CD8+ and/or CD57+ cytotoxic T lymphocytes as well as NK cells (both CD16+ and CD56+). The 99 HIV-infected men in Evans’ study were entirely asymptomatic and were not taking antiretroviral or other immunoreactive drugs at the time of the study. Thus, there is preliminary evidence that stress-associated changes in the CD57+ subset of non-antigen–specific cytotoxic cells may occur relatively early in the HIV disease trajectory.
The theoretical framework for our research is presented schematically as Figure 2. This PNI-based theoretical framework synthesizes psychosocial, spiritual, neuroendocrine, immunological, and physical health constructs as well as lived experience within the context of HIV disease, and the research design includes multiple indicators of study constructs measured over time.
Study constructs are measured using disease-specific instruments as indicated. In the realm of psychological factors, the psychosocial-spiritual concepts of interest include perceived stress associated with living with HIV disease, coping patterns, social support, and spiritual well-being. Physiological health dynamics are integrated within this model by measuring (a) the classic neuroendocrine mediators of cortisol and dehydroepiandrosterone and (b) immunological indicators related to HIV pathogenesis, namely CD4+, CD8+, and CD57+ lymphocyte subsets, NK cell cytotoxicity, selected type 1 and type 2 cytokine production levels, and lymphocyte proliferative function. Health dynamics specific to HIV disease are reflected in the adaptational outcome of psychosocial functioning by indicators of psychological distress, perceived stress level, and social support, and in the quality of life outcome by both general and HIV-specific measures of quality of life and spiritual well-being. Physical health is directly reflected by clinical indicators of HIV disease progression status and HIV-specific health status. Specific research hypotheses are included in Table 1.
Table 1.
Selected hypotheses from HIV study
|
The qualitative component is implemented through interviews that include both a general focus on the experiences of living with HIV disease and a more focused exploration of HIV-specific and non–HIV-related stressors and coping strategies used by participants. These data have been used in several ways. First, they provided the basis for continued development of an understanding of the day-to-day experiences of living with HIV disease,29,30 thus providing systematic input into creating relevant interventions for future studies. Second, they contributed to our ability to expand our understanding of stressors and coping strategies, including and beyond those addressed in the stress and coping instruments, and may lead to empirically based revision of the stress and coping instruments. Third, the qualitative data permit clarification and contextualization of the quantitative findings.
Significance to nursing
As noted above, a growing body of research suggests that immunosuppression associated with perceived stress may adversely affect the clinical course of HIV disease.31 We assume that perceived stress and coping are modifiable constructs, and that nursing interventions are an appropriate means for such modification. Modification of perceived stress should be seen as a significant priority by nurse researchers, given the number of biological pathways that could be activated by high levels of perceived stress, thus explaining the negative impact of psychological stress on HIV disease progression. Interventions aimed at stress management not only may reduce psychological distress, but also may attenuate HIV disease progression. The still-limited but rapidly growing research literature provides a foundation for pursuing potential modulation of the stress-disease relationship through traditional as well as alternative approaches to stress management. Such interventions are clearly within the scope of nursing practice and, by being grounded in an explicit theoretical model that allows for multimethod inquiry, their study contributes to the ongoing development of the PNI model.
PNI in cancer
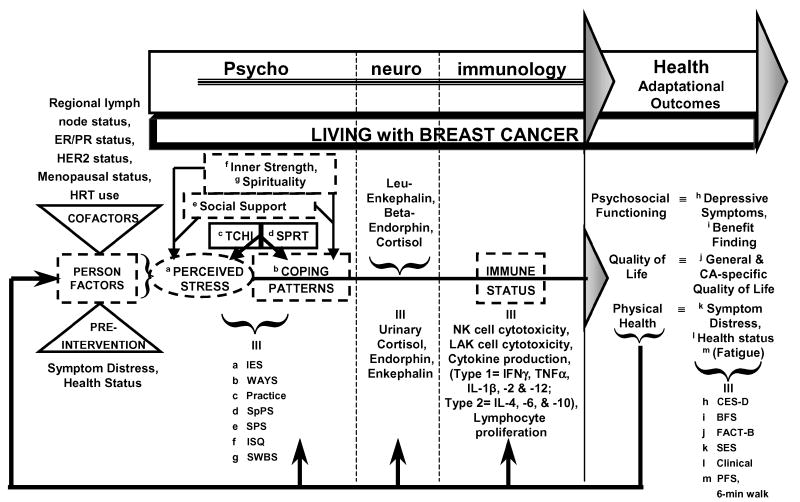
We also are studying the effects of 2 mind-body-sprit strategies for stress management (tai chi training and spiritual growth groups) on PNI-based outcomes among women with early breast cancer (see Fig 3). The theoretical model for this study was derived from the generic PNI model and modified to incorporate specific variables of interest into the context of living with breast cancer. Further, the model was enhanced on the basis of our growing appreciation of the importance of positive psychological states, such as inner strength32 and benefit finding,18,30 as components of coping.
Figure 3.
PNI-based model in cancer. IES indicates Impact of Event Scale; WAYS, Ways of Coping Questionnaire; SpPS, Spiritual Perspectives Scale; SPS, Social Perspectives Scale; ISQ, Inner Strength Questionnaire; SWBS, Spiritual Well-being Scale; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor-alpha; CES-D, Center for Epidemiological Studies-Depression Scale; BFS, Benefit Finding Scale; FACT-B, Functional Assessment of Cancer Therapy-Breast Scale; SES, Symptom Experience Scale; and PFS, Piper Fatigue Scale.
Immune surveillance theory in cancer holds that an intact immune system has the capacity to destroy tumor cells and protect against tumor growth. Fundamentally, the immune system recognizes “foreign” cells and continuously monitors the body to eradicate “foreignness” through the mobilization of internal defenses, such as macrophages and NK cells.33 Although the theory still has relevance, it is now known that there are a variety of complex mechanisms that affect cancer cell proliferation, including angiogenesis, apoptosis, and a host of immune escape mechanisms.34,35 Nonetheless, NK cells have particular relevance for immune function in persons with cancer, in that they are able to spontaneously lyse tumor cells (ie, without prior sensitization). When activated by certain cytokines (including IFN-γ, IL-2, and IL-12) NK cells, then termed lymphokine-activated killer (LAK) cells, have even greater cytotoxic capacity.36,37 Further support for stress-related immunosuppression is emerging from recent studies documenting the association of higher psychological distress with reduced NK cell cytotoxicity as well as reduced in vitro augmentation of NK activity by type 1 cytokines (ie, reduced LAK cell cytotoxicity).36,38 In another recent report, incubation of NK cells with cortisol resulted in a 67% reduction in NK cell cytotoxicity in normal women, suggesting that elevated cortisol levels accompanying perceived stress may be directly associated with reduced NK cell function.38,39 Given the critical role of NK cells, enhancement of NK/LAK cell cytotoxicity has been recognized as a clinical approach to eliminating micrometastases.40 To the extent that mind-body-spirit interventions attenuate stress-related suppression of NK/LAK cell activity, such strategies may directly contribute to cancer control. Further studies with PNI-based explanatory power are needed to confirm such associations and putative mechanisms.
Psychological stress has been directly linked with increased depressive mood and other indicators of psychological distress or negative affect in several studies among persons with cancer.41,42 There also is evidence of associations between psychological distress and immunosuppression or disease progression in persons with cancer.43,44 On the other hand, the relationship between psychological well-being and creating positive meaning also has been documented in a number of stress-related studies,45 including study of the influence of psychobehavioral interventions on enhancing positive affect among women with breast cancer.46 In the context of dealing with stressful experiences, Folkman and Moskowitz47 suggested that creating positive meaning is involved in coping processes that generate and sustain positive affect. In the research that resulted in reconceptualization of the transactional model of stress and coping to include positive psychological states, Folkman identified 4 types of positive coping processes. These processes were grounded in the theme of “searching for and finding positive meaning.”45(p1212) Stress management through strategies such as relaxation, exercise/movement, spiritual contemplation, and meditation may determine the individual’s perception of stressors and subsequent psychological adaptation, at least in part by their enhancement of positive coping strategies such as finding meaning or benefit in the experience,4,45 and enhancing inner strength32 and spirituality.48 In the theoretical context of our current work for women with breast cancer, benefit finding is conceptualized as a primary outcome, and spirituality, inner strength, and social support are considered to be moderators of the stress-coping process.
Spirituality and inner strength are closely related constructs that may enhance or support benefit finding in the context of stressful experiences. Spirituality is considered to be a “life force” and an integral dimension of all persons, characterized by (a) “unfolding mystery” (concerning meaning and purpose), (b) “harmonious interconnectedness” (involving relationships with others and/or God), and (c) “inner strength” (relating to one’s personal resources and a sense of the sacred).49 Burkhardt and Nagai-Jacobson50 described inner strength as a component of spirituality that gives one the ability, energy, and resources to express or experience spirituality. In a phenomenological study of inner strength in women with breast cancer,32 participants expressed and experienced spirituality as either attention to stillness and being in a quiet place or connectedness to God or Higher Being, the environment, nature, other people and loved ones, and the self. Experiencing this synchrony assisted the women in attuning to their sources of inner strength. On the basis of the conclusions from these studies, we conceptualize inner strength as a central human resource that enhances psychological well-being and quality of life. In our work, spirituality and inner strength are being investigated as psychospiritual dimensions integral to psychosocial well-being, quality of life, and physical health.
Social support is a third factor that is widely considered to be a moderator of the stress process. For example, in a study of 61 women with early breast cancer, Levy et al51 found that, in addition to estrogen-receptor–negative tumor status, higher NK cell cytotoxicity was significantly predicted by the psychobehavioral variables of perception of high-quality emotional support from a spouse or an intimate other, as well as perceived social support from the individual’s physician, and actively seeking social support. Social support factors accounted for the largest percentage of variance explained by the set of variables (R2 = 0.18).
The schematic model presented as Figure 3 is grounded in the PNI paradigm, but the model is specific to the study of women with breast cancer. Similar to Figures 1 and 2, the transactional model of person-environment-stressor interactions is depicted, leading to perceptions of stress. The left side of the model presents key baseline factors (cofactors) that are known to affect the stress process in the context of breast cancer. Within the psychobehavioral domain, the key modifiers of inner strength, spirituality, and social support may influence the processes of stress appraisal and coping independently, that is, outside of or in addition to the interventions of tai chi training and spiritual growth groups. The interventions being tested are thought to modify both perceptions of stress and coping patterns.
In the neuroendocrine domain, the mediators of leuenkephalin, β-endorphin, and cortisol are being evaluated as potential correlates of psychobehavioral factors, and these neuroendocrine mediators may impact immune status. Neuroendocrine mediation is being evaluated using 24-hour measures of urinary cortisol, along with β-endorphin and leuenkephalin, neuropeptides that may be associated with more positive psychological factors, such as inner strength and benefit finding.
Numerous indicators of immune function in the context of chemotherapeutic treatment for breast cancer are being tested as mediators that may be affected by psychobehavioral and intervention factors and, in turn, may affect health outcomes. Selected adaptational health outcomes in the domains of psychosocial functioning, quality of life, and physical health may reflect the influence of cofactors, moderators (including the mind-body-spirit interventions), and mediators in this PNI-based framework. The specific research hypotheses are included in Table 2.
Table 2.
Selected hypotheses from cancer study
|
Qualitative data include a general focus on the meaning of being diagnosed with and having breast cancer. We designed the qualitative component of the study to provide an opportunity to explore women’s subjective experiences and understandings related to the variables of interest in the study, including stress, coping, spirituality, inner strength, and benefit finding.
Significance to nursing
As summarized by Turner-Cobb et al,52 stress has been found to be associated with certain immunosuppressive changes involved in tumor defense, but evidence regarding the magnitude of such changes and their ability to meaningfully affect immune resistance in cancer is inconclusive. Measures of multiple, state-of-the-science indicators of immune functioning will contribute to evolving knowledge related to health dynamics, such as the enhancement of immune responses in cancer. Further, the comprehensive PNI-based theoretical framework enables a more holistic approach for testing effects of both traditional and alternative nursing interventions. By incorporating positive psychological states along with neuroendocrine biomarkers that may be associated with such positive indicators of well-being, nurse researchers can greatly enhance the knowledge base related to “positive” adaptation and potentially promote healthier recovery from cancer.
CONCLUSION
The illustrated theoretical models demonstrate disease-specific conceptions derived from the PNI paradigm. Use of such models provides holistic views of complex health dynamics, including multiple physiological indicators. Depending on the purpose and design of a study, one may consider the concepts of the model from different temporal or causal conceptions. For example, cofactors may be conceptualized as antecedents and they may be baseline variables in repeated measures designs. Concepts may be viewed as mediators, moderators, disease-specific indicators, or intermediate outcomes affecting health dynamics. Finally, health outcomes may be long-term or primary outcomes specific to the purpose of the study.
Research grounded in PNI, along with integration of quantitative and qualitative methods, synthesizes numerous psychobehavioral and physiological concepts underlying health and illness within a comprehensive model. Applications of PNI-based frameworks, as illustrated by these theoretical-empirical models, will enable more comprehensive views of physiological phenomena affecting health dynamics.
Acknowledgments
The studies referenced herein were supported by grants (N. L. McCain, PI) from the National Institute of Nursing Research (R01 NR04395), the National Center for Complementary and Alternative Medicine (R01 AT000331), and the National Cancer Institute (R01 CA114718).
References
- 1.McCain NL, Zeller JM. Psychoneuroimmunological studies in HIV disease. Ann Rev Nurs Research. 1996;14:23–55. [PubMed] [Google Scholar]
- 2.Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 3.Byrnes DM, Antoni MH, Goodkin K, et al. Stressful events, pessimism, natural killer cell cytotoxicity, and cytotoxic/suppressor T cells in HIV+ black women at risk for cervical cancer. Psychosom Med. 1998;60:714–722. doi: 10.1097/00006842-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Folkman S. Psychosocial effects of HIV infection. In: Goldberger L, Breznitz S, editors. Handbook of Stress: Theoretical and Clinical Aspects. 2. New York: Free Press; 1993. pp. 658–681. [Google Scholar]
- 5.Besedovsky HO, Del Rey A. Cytokines as mediators of central and peripheral immune-neuroendocrine interactions. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3. Vol. 1. San Diego: Academic Press; 2001. pp. 1–17. [Google Scholar]
- 6.Ader R. Historical perspectives on psychoneuroimmunology. In: Friedman H, Klein T, Friedman AL, editors. Psychoneuroimmunology, Stress and Infection. Boca Raton, Fla: CRC Press; 1996. pp. 1–24. [Google Scholar]
- 7.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 8.Dhabhar FS, McEwen BS. Bi-directional effects of stress and glucocorticoid hormones on immune function: possible explanations for paradoxical observations. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3. Vol. 2. San Diego, Calif: Academic Press; 2001. pp. 301–338. [Google Scholar]
- 9.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 10.Clerici M, Trabattoni D, Piconi S, et al. A possible role for the cortisol/anticortisols imbalance in the progression of human immunodeficiency virus. Psychoneuroendocrinology. 1997;22(suppl 1):S27–S31. doi: 10.1016/s0306-4530(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 11.DeKruyff J, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- 12.Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behave Immun. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 14.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 15.Drolet G, Dumont EC, Gosselin I, Kindead R, LaForst S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 16.Heijnen CJ, Kavelaars K. Opioid peptide production by the immune system. In: Schedlowski M, Tewes U, editors. Psychoneuroimmunology: An Interdisciplinary Introduction. New York: Plenum; 1999. pp. 209–222. [Google Scholar]
- 17.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuck I. Development of a spirituality intervention to promote healing. J Theory Constr Test. 2004;8:67–71. [Google Scholar]
- 19.Wolf SL, Coogler C, Xu I. Exploring the basis for Tai Chi Chuan as a therapeutic exercise approach. Arch Phys Med Rehabil. 1997;78:886–892. doi: 10.1016/s0003-9993(97)90206-9. [DOI] [PubMed] [Google Scholar]
- 20.Astin JA. Stress reduction through mindfulness meditation: effects on psychological symptomatology, sense of control and spiritual experiences. Psychother Psychosom. 1997;66:97–106. doi: 10.1159/000289116. [DOI] [PubMed] [Google Scholar]
- 21.McCain NL, Munjas BA, Munro CL, et al. Effects of stress management on PNI-based outcomes in persons with HIV disease. Res Nurs Health. 2003;26:102–117. doi: 10.1002/nur.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCain NL, Tuck I, Robins J, et al. Alternative stress management intervention reduces distress and symptomatology and enhances quality of life in persons with HIV disease [abstract] Int J Behav Med. 2004;11(suppl):331. [Google Scholar]
- 23.Pitzalis C, Pipitone N, Bajocchi G, et al. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: an additional mechanism for their anti-inflammatory and immunosuppressive effect. J Immunol. 1997;158:5007–5016. [PubMed] [Google Scholar]
- 24.Rosenburg YJ, Anderson AO, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunol Today. 1998;19:10–17. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani S, DelPrete G, Manetti R, et al. Role of TH1/TH2 cytokines in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 26.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans DL, Leserman J, Perkins DO, et al. Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am J Psychiatry. 1995;152:543–550. doi: 10.1176/ajp.152.4.543. [DOI] [PubMed] [Google Scholar]
- 29.Gray DP, Robins JW, McCain NL. Viewing PNI from a qualitative perspective: stress and coping in HIV disease [abstract] Brain Behav Immun. 2001;15:152–153. [Google Scholar]
- 30.Gray DP, McCain N, Tuck I, et al. Qualitative data contextualize findings of spirituality intervention designed to reduce stress and enhance coping in people with HIV infection [abstract published electronically] Brain Behav Immun. 2004;18(3) [Google Scholar]
- 31.Cole SW, Kemeny ME. Psychobiology of HIV infection. Crit Rev Neurobiol. 1997;11:289–321. doi: 10.1615/critrevneurobiol.v11.i4.30. [DOI] [PubMed] [Google Scholar]
- 32.Roux G, Bush H, Dingley C. Inner strength in women with breast cancer. J Theory Constr Test. 2001;5:19–27. [Google Scholar]
- 33.Whiteside TL, Herberman RB. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995;7:704–710. doi: 10.1016/0952-7915(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 34.Igney FH, Behrens CK, Krammer PH. Tumor counterattack—Concept and reality. Eur J Immunol. 2000;30:725–731. doi: 10.1002/1521-4141(200003)30:3<725::AID-IMMU725>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Salih HR, Nussler V. Immune escape versus tumor tolerance: how do tumors evade immune surveillance? Eur J Med Res. 2001;6:323–332. [PubMed] [Google Scholar]
- 36.Cohen M, Klein E, Kuten A, Fried G, Zinder O, Pollack S. Increased emotional distress in daughters of breast cancer patients is associated with decreased natural cytotoxic activity, elevated levels of stress hormones and decreased secretion of Th1 cytokines. Int J Cancer. 2002;100:347–354. doi: 10.1002/ijc.10488. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside TL. Measurement of NK-cell activity in humans. In: Rose NR, Hamilton RG, Detrick B, editors. Manual of Clinical Laboratory Immunology. 6. Washington: ASM Press; 2002. pp. 296–300. [Google Scholar]
- 38.Anderson BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garland M, Doherty D, Golden-Mason L, Fitzpatrick P, Walsh N, O’Farrelly C. Stress-related hormonal suppression of natural killer activity does not show menstrual cycle variations: implications for timing of surgery for breast cancer. Anticancer Res. 2003;3B:2531–2535. [PubMed] [Google Scholar]
- 40.Block KI, Boyd DB, Gonzalez N, Vojdani A. The immune system in cancer. Integr Cancer Ther. 2002;1:294–316. doi: 10.1177/153473540200100314. [DOI] [PubMed] [Google Scholar]
- 41.Larson MR, Duberstein PR, Talbot NL, Caldwell C, Moynihan JA. A presurgical psychosocial intervention for breast cancer patients. J Psychosom Res. 2000;2:187–194. doi: 10.1016/s0022-3999(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 42.Matthews A, Ridgeway V, Warren R, Britton P. Predicting worry following a diagnosis of breast cancer. Psychooncology. 2002;5:415–418. doi: 10.1002/pon.600. [DOI] [PubMed] [Google Scholar]
- 43.Kogon MM, Biswas A, Pearl D, Carlson RW, Spiegel D. Effects of medical and psychotherapeutic treatment on the survival of women with metastatic breast carcinoma. Cancer. 1997;80:225–230. [PubMed] [Google Scholar]
- 44.Levy SM, Herberman RB, Lippman M, D’Angelo T, Lee J. Immunological and psychological predictors of disease recurrence in women with early-stage breast cancer. Behav Med. 1991;2:67–75. doi: 10.1080/08964289.1991.9935161. [DOI] [PubMed] [Google Scholar]
- 45.Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med. 1997;45:1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 46.Antoni MH, Lehman JM, Kilbourn, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20:20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 47.Folkman S, Moskowitz JT. Positive affect and the other side of coping. Am Psychol. 2000;55:647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- 48.Ironson G, Solomon G, Balbin E, et al. The Ironson-Woods Spirituality/Religiousness Index is associated with long survival, health behaviors, less distress, and low cortisol in people with HIV/AIDS. Ann Behav Med. 2002;24:34–48. doi: 10.1207/S15324796ABM2401_05. [DOI] [PubMed] [Google Scholar]
- 49.Burkhardt MA. Spirituality: an analysis of the concept. Holist Nurs Pract. 1989;3:69–77. doi: 10.1097/00004650-198905000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Burkhardt MA, Nagai-Jacobson MG. Spirituality and health. In: Dossey BM, Keagan L, Gauzzetta CE, editors. Holistic Nursing: A Handbook for Practice. Gaithersburg, Md: Aspen; 2000. pp. 91–122. [Google Scholar]
- 51.Levy SM, Herberman RB, Whiteside T, Sanzo K, Lee J, Kirkwood J. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med. 1990;52:73–85. doi: 10.1097/00006842-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Turner-Cobb JM, Sephton SE, Speigel D. Psychosocial effects on immune function and disease progression in cancer: human studies. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3. Vol. 1. San Diego, Calif: Academic Press; 2001. pp. 565–582. [Google Scholar]