Abstract
Objective
To determine effects in late gestation of U. parvum serovar 3 colonization, and effects, preterm, of U. parvum serovar 6.
Study design
Ewes received an intra-amniotic (IA) injection of U. parvum serovar 6 (20x106 cfu; n=9), U. parvum serovar 3 (20x103 cfu; n=6), vehicle (n=10) or saline (n=4) on day 80 of pregnancy (d). Lambs were delivered at 125d (ureaplasma, n=9; saline or media controls, n=9) or 145d (ureaplasma, n=6; media controls, n=5) for assessment of inflammation and lung maturation.
Results
IA ureaplasmas caused histologic chorioamnionitis but not preterm delivery. Fetal lung epithelium was colonized with ureaplasmas at both gestational ages and pulmonary IL-8 levels had doubled in the ureaplasma-colonized animals compared to controls at 145d. Surfactant levels in bronchoalveolar lavage fluid had increased 8 fold and 2.5 fold at 125 and 145d, respectively after ureaplasma injection.
Conclusions
Fetal lung inflammation and altered development accompanies ureaplasma colonization regardless of age at delivery.
Keywords: intrauterine infection, fetal inflammation, ureaplasmas
Introduction
Ureaplasmas are the microorganisms most frequently associated with preterm birth (1–3), and their presence in amniotic fluid or the neonatal lungs influences an infant’s risk of pulmonary disease (4, 5). We demonstrated previously that intra-amniotic injection of Ureaplasma parvum serovar 3 results in amniotic and fetal pulmonary colonization, and induces functional ‘maturation’ of the preterm lungs in sheep (6). For those experiments, preterm lambs were delivered 1–10 weeks after injection, with the magnitude of effect greatest in those animals exposed to ureaplasmas for a long period (6). The effects of intra-amniotic ureaplasmas on the fetal lungs at term are unknown. Such information would be relevant to many human pregnancies, in which ureaplasmas are likely to be present but in which preterm birth does not occur (2, 7).
There are 14 distinct ureaplasma serovars in the Ureaplasma urealyticum and Ureaplasma parvum species, with different prevalence rates and different degrees of pathogenicity. Ureaplasma parvum serovar 6 was the most prevalent serovar isolated from a group of pregnant women who delivered preterm (8) and was the most common serovar identified in semen samples after a standard washing procedure used during assisted reproduction (9). Since U. parvum serovar 6 is more adherent to host cells than other ureaplasma serovars, it is potentially more pathogenic (9).
We hypothesised that exposure of the fetus to intra-amniotic ureaplasmas from mid-gestation until term would result in chronic fetal and intrauterine inflammation, with persisting effects on lung development. Further, we hypothesised that effects of intra-amniotic injection of U. parvum serovar 6 would be greater than those observed by us previously in response to intra-amniotic U. parvum serovar 3.
Methods
The Animal Ethics Committee of the Western Australian Department of Agriculture approved all animal experimentation. The ureaplasmas used were isolated from semen samples collected from patients attending the Wesley IVF Service (Brisbane, Australia); these patients gave informed consent for these isolates to be used for such studies. A PCR assay, using three primer pairs, was developed for the detection of Ureaplasma urealyticum serovars 3 and 6 in cultured clinical specimens (8).
Pregnant ewes bearing single fetuses were enrolled randomly into one of two distinct experiments: (i) to investigate the effect of amniotic fluid colonization of low titre Ureaplasma parvum serovar 3 in the late gestation sheep fetus (145 days of gestation); at 80 days of gestation, a single ultrasound guided intra-amniotic injection of either live Ureaplasma parvum serovar 3 (20 x 103 colony forming units; n=11) or media (n=5) was given.
(ii) to investigate the effect of amniotic fluid colonization of Ureaplasma parvum serovar 6 in the preterm sheep fetus at 125 days of gestation; at 80 days of gestation, a single injection of live Ureaplasma parvum serovar 6 (20 x 106 colony forming units; n=9), media (n=5) and saline (n=4) was given into the amniotic fluid under ultrasound guidance (6).
To prepare ureaplasmas for injection, 2 aliquots of ureaplasma-positive semen samples were serially diluted in 10B broth and, after incubation, serial dilutions in exponential growth phase were pooled and inoculated (20–30ml volume) into 1 litre of 10B broth. After further culture (until alkaline color shift was observed), ureaplasmas were pelleted by centrifugation at 4°C for 1 hour (37000 x g: Beckman J2-21 M/E), resuspended and washed in 2ml cold sterile phosphate-buffered saline (PBS), and centrifuged again at 4°C for 15 minutes (Beckman Microfuge M). After washing, the pellets were resuspended in 10B broth and pooled. The number of colony forming units (cfu)/ml was determined by the serial dilution and drop plate method and aliquots containing known cfu/ml were stored at −80°C until required for inoculation. Concentrated 10B broth was prepared using these same methods for control (vehicle) injections. Prior to injection, aliquots were thawed, diluted in sterile cold PBS to achieve desired concentrations and then mixed. Injection volumes of 2ml were kept on ice until warming immediately prior to injection. After intra-amniotic ureaplasma, media or saline injection ewes were returned to pasture, where they remained until the time of tissue collection.
At 125 days of gestation, fetuses exposed to amniotic fluid Ureaplasma parvum serovar 6 (n=9), media (n=5) or saline (n=4) were delivered by Caesarean section for assessment of inflammation and lung development. At 145 days of gestation, other fetuses (Ureaplasma parvum serovar 3, n=6; media, n=5) were delivered for identical assessments. Five ewes that received amniotic fluid injection of Ureaplasma parvum serovar 3 were left to deliver their lambs spontaneously. These lambs were euthanized (IV pentobarbitone) 2–3 days after birth. Samples of lung tissue were collected for culture but no other measurements were made on samples from these animals.
Caesarean delivery and tissue sampling
Ewes were killed by penetrating captive bolt and a laparotomy was performed to locate the fetal head. The uterus was incised without cutting the fetal membranes. A sample of amniotic fluid was collected, using a sterile needle and syringe, for subsequent assessment of ureaplasma colonization. The fetus was delivered, the umbilical cord clamped, and pentobarbitone (100mg/kg) was injected into an umbilical vein. The cord was cut and fetuses were weighed. Umbilical arterial blood was collected for blood gas and pH analysis (Rapidlab 865; Bayer Diagnostics), and for total and differential white blood cell counts. Delivery of the fetus and aspiration of blood for cord gas measurements and the time of death of the pregnant ewe takes between 3 to 5 minutes and is very consistent from animal to animal. Plasma was collected after centrifugation for subsequent measurement of plasma cortisol concentration (10). Samples of chorioamnion were frozen for subsequent biochemical measurements and immersion fixed in 10% buffered formalin for subsequent histological analysis (11).
Each lamb was exsanguinated, the chest opened, and a deflation pressure-volume curve was measured after air inflation of the lungs to a pressure of 40 cm H2O (6). After the removal of the lungs from the chest, each lung was weighed, and the left lung was lavaged 3 times by infusion and withdrawal of a sufficient volume of 4°C physiologic saline solution to fully fill the lung (brochoalveolar lavage fluid). Pieces of the lower lobe of the right lung were rapidly frozen in liquid nitrogen and stored (−80°C) for subsequent analysis. The upper lobe of the right lung was fixed by airway instillation of 10% buffered formalin at 30 cm H2O pressure.
Cells recovered from bronchoalveolar lavage fluid and amniotic fluid after centrifugation were resuspended in 0.1M phosphate-buffered saline solution (pH 7.4), stained with Trypan blue, and counted with a hemocytometer. Differential cell counts were performed on cytospin preparations that were stained with Diff Quick (Scientific Products, McGraw Park, Ind). Saturated phosphatidylcholine was isolated from chloroform-methanol (2:1) extracts of alveolar lavage fluid or homogenized lung tissue (right lower lobe) by neutral alumina column chromatography after exposure of lipid extracts to osmium tetroxide (12). Saturated phosphatidylcholine was quantified by phosphorus assay (13). Surfactant protein B (SP-B) protein was measured by Western Blot as reported previously by our group (14).
Total RNA was extracted from lung tissue (right lower lobe) and chorioamnion using a modified Chomzynski method; 10 μg of total RNA was used for IL-1β, IL-6, and IL-8 mRNA quantification using RNase protection analysis (11). IL-8 protein in amniotic fluid, bronchoalveolar lavage fluid and homogenated lung tissue (right lower lobe) was measured by ELISA (15). The minimum detection of the assay was 12.5 pg/ml.
Samples of chorioamnion, immersion fixed in 10% buffered formalin, were embedded in paraffin; 3 x 5μm sections, 100μm apart were stained with hematoxylin and eosin. Inflammatory cell influx into the chorioamnion was graded (in each 5μm section) by the assignment of a score of 0 (no inflammatory cells), 1 (few cells), 2 (moderate influx), or 3 (severe influx of inflammatory cells) (11).
The location of IL-8 protein in lung tissue (right upper lobe) was determined by immunohistochemistry as described previously (16).
For quantification of ureaplasma colonization, lung tissue samples (0.2g) were homogenized in 1.8ml of 10B broth (Mini beadbeater 8-cell disrupter, Daintree Scientific, Australia). Lung homogenates and amniotic fluid samples were serially diluted (9 x 10-fold dilutions) in 10B broth and incubated at 37°C. Ureaplasma growth (measured in color changing units; ccu) was detected by an alkaline shift and subsequent color change using phenol red indicator in 10B broth.
Sections of lung tissue (5μm) from each fetal lamb were used for identification of ureaplasmas by Indirect Fluorescence Assay (IFA) staining. Antigen retrieval was achieved by immersion of mounted sections in 0.1M Tris HCl-5% urea buffer and heating in a microwave (output 800W) for 5 minutes, cooling for 5 minutes, further heating for 5 minutes and cooling for 20 minutes. Sections were then blocked using 10% skim milk, 70% horse serum (CSL, Brisbane, Australia), 10% bovine serum albumin (BSA) and 10% gelatin. Sections were then incubated (4°C) overnight with primary antibody (serovar-specific for U. parvum serovar 3 or -6 as appropriate), rabbit anti-ureaplasma antiserum (1/500 dilution in PBS; kindly provided by Dr Patricia Quinn, Department of Bacteriology, Hospital for Sick Children, Toronto, Canada). Slides were subsequently washed in PBS (3 x 5 minutes), incubated with secondary antibody (goat anti-rabbit Alex Fluor 488, Molecular Probes, Eugene, OR, USA), washed in PBS again (3 x 5 minutes), and viewed using a confocal laser scanning microscope (TCS 4D, Leica Microsystems, Wetzlar, Germany). Samples incubated with secondary antibody only and lung tissue from a control animal were used as negative controls.
Statistical analysis
Data are presented as mean ± SEM. Ureaplasma-exposed and control (media - and/or saline-exposed at corresponding gestational age) groups were compared by t-test. If a normal distribution could not be achieved after data transformation then non-parametric statistical tests were used (rank sum test). A two way repeated measures ANOVA was used to compare the Pressure-Volume curves of control and Ureaplasma-treated groups.
Results
Late gestation effects of Ureaplasma parvum serovar 3
Intra-amniotic injections of Ureaplasma parvum serovar 3 did not cause preterm deliveries, nor maternal losses and lambs were born spontaneously at term. Ureaplasmas were cultured from amniotic fluid and lung tissue samples from all animals delivered at 145 days gestation in which Ureaplasmas were injected but not from any of the controls. Amniotic fluid ureaplasma levels ranged from 5x103 to over 5x106 colour changing units/ml. Ureaplasmas could not be cultured from lung tissue collected 2–3 days after birth from lambs that had been delivered spontaneously.
Intra-amniotic injection of U. parvum serovar 3 at 80 days of gestation did not alter fetal body weight, umbilical arterial pH or blood gases, or plasma cortisol concentrations at 145 days of gestation (Table I). Total white blood cell counts were higher than control (p=0.039) in lambs exposed to ureaplasmas, due to an increase in neutrophils (p=0.034) and monocytes (p=0.006; Table I).
Table I.
Observations from lambs delivered at 145 days of gestation after intra-amniotic injection of U parvum serovar 3 or control (media) at 80 days.
| Control (n=5) | U parvum serovar 3 (n=6) | |
|---|---|---|
| Body weight (kg) | 4.4±0.3 | 4.9±0.2 |
| Lung weight (g/kg) | 32.5±2.1 | 36.5±2.2 |
| Umbilical arterial pH | 7.27±0.03 | 7.27±0.03 |
| Total WBC (109/l) | 3.02±0.43 | 5.55±0.83* |
| Neutrophils (109/l) | 0.66±0.20 | 2.25±0.55* |
| Lymphocytes (109/l) | 1.98±0.29 | 2.35±0.43 |
| Monocytes (109/l) | 0.04±0.02 | 0.25±0.05* |
| Plasma cortisol (μg/dl) | 3.29±0.81 | 3.47±0.36 |
p<0.05 versus Control. WBC, white blood cell count.
Intra-amniotic injection of U. parvum serovar 3 at 80 days of gestation resulted in histologic chorioamnionitis, as reflected by higher chorioamnion inflammatory scores in the ureaplasma-exposed group (p=0.003; Figure 1A). Expression of IL-1β, IL-6 and IL-8 mRNA in chorioamnion averaged 5- to 10-fold higher than control after exposure to ureaplasmas but expression was variable and not significantly different from control (Figure 1D). IL-8 was undetectable in amniotic fluid of controls; the IL-8 concentration in amniotic fluid after intra-amniotic ureaplasma injection was 0.98±0.12μg/ml (p<0.001).
Figure 1.
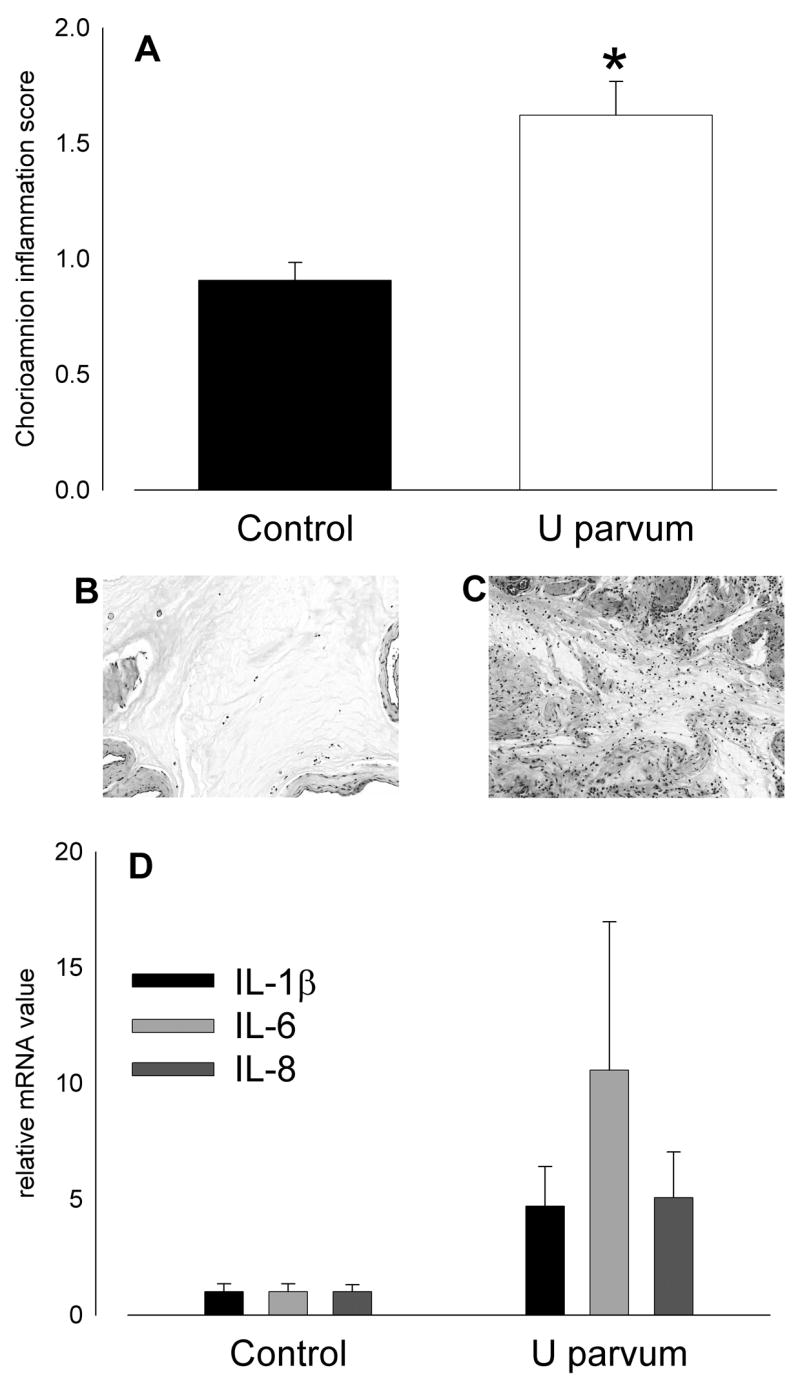
Intra-amniotic injection of U. parvum serovar 3 (20x103 cfu) on day 80 of pregnancy caused intrauterine inflammation at 145 days. (A) Chorioamnion inflammation scores were higher than control after ureaplasma injection (*p=0.003 versus Control). Hematoxylin and eosin stained sections of chorioamnion show few inflammatory cells in tissue from a control animal (B; score = 1) and extensive infiltration in a sample from a ureaplasma-exposed animal (C; score = 3). (D) Expression of mRNA for proinflammatory cytokines, IL-1β, IL-6 and IL-8 was elevated after exposure to ureaplasmas but levels were variable and not significantly higher than control.
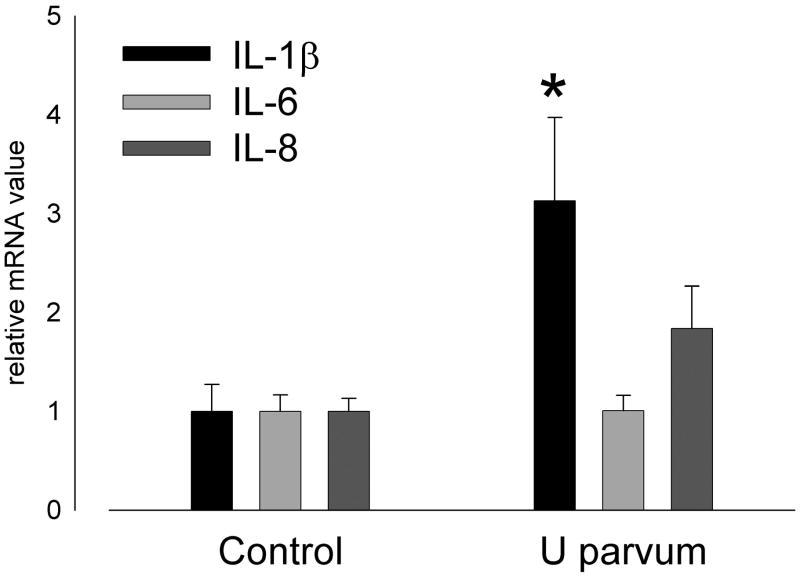
Lung weights of lambs exposed to U. parvum serovar 3 were not different to control (Table I). Bronchoalveolar lavage fluid protein content and inflammatory cell counts were not affected by exposure to ureaplasmas, possibly because the increased numbers of resident macrophages marked a moderate inflammatory response. IL-8 content in lung tissue from ureaplasma-exposed fetal lambs (43.9±8.2 μg/kg) was higher than control (4.4±0.9 μg/kg; p<0.001). IL-8 immunostaining was present in the lung parenchyma, mainly in cells lining the air spaces, in animals exposed to ureaplasmas but not in controls (Figure 2). The concentration of IL-8 in bronchoalveolar lavage fluid from ureaplasma-exposed fetal lambs was 42.6±10.1 μg/kg; levels were undetectable in controls. Expression of IL-1β mRNA in lung tissue was higher than control after exposure to ureaplasmas (p=0.036; Figure 3); expression of IL-6 and IL-8 mRNA was not different between groups (Figure 3).
Figure 2.
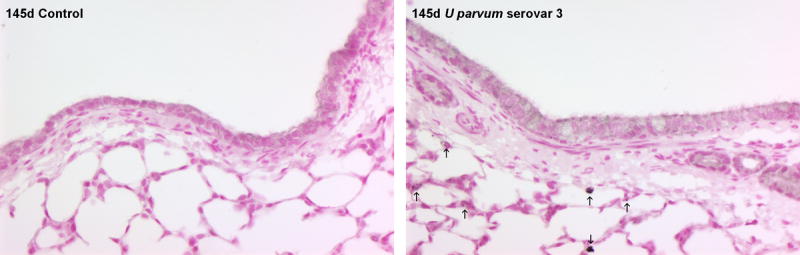
IL-8 immunostaining in lung tissue was elevated at 145 days of gestation after injection of U. parvum serovar 3 at 80 days. Arrows show IL-8 immunostaining in cells lining the airspaces within the lung parenchyma. Images captured at x200 magnification.
Figure 3.
Pulmonary expression of IL-1β mRNA was higher than control at 145 days of gestation after intra-amniotic U. parvum serovar 3 injection at 80 days (*p=0.036) but IL-6 and IL-8 mRNA expression was not increased.
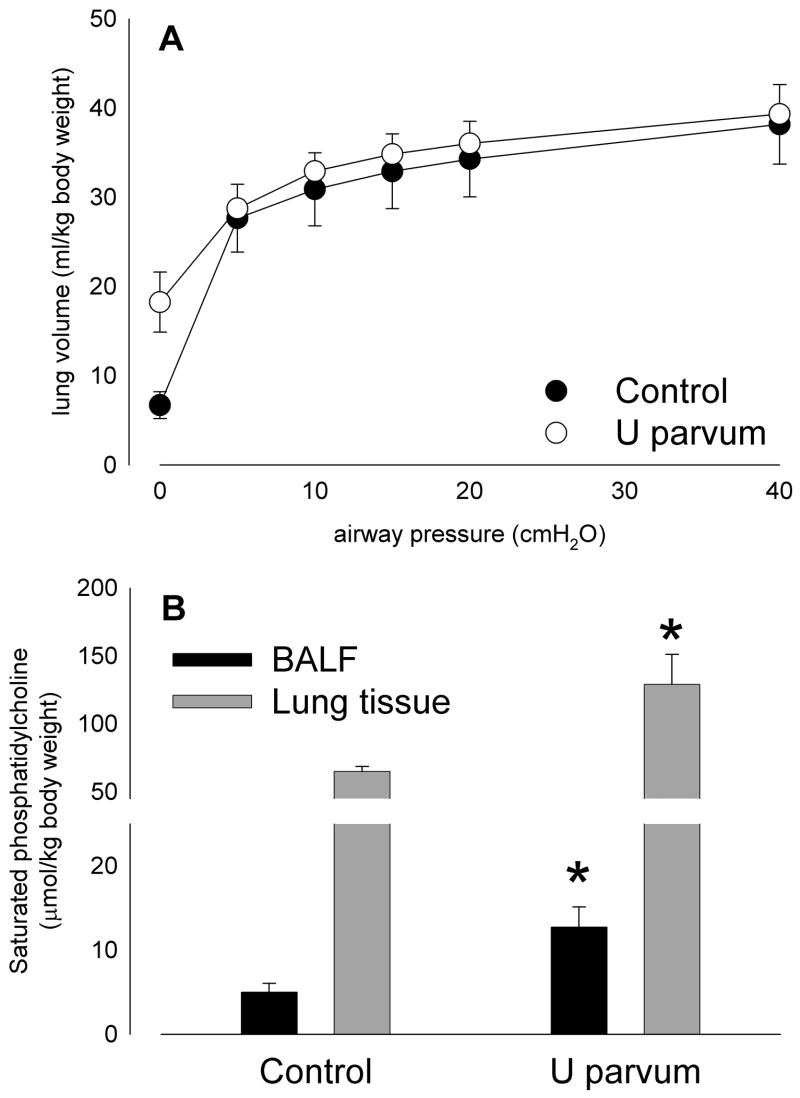
Lung compliance, as indicated by pressure-volume curve (Figure 4A), was not different between groups, despite greater surfactant levels in bronchoalveolar lavage fluid (p=0.023) and lung tissue (p=0.008) after exposure to ureasplasmas (Figure 4B). Lung volumes at zero airway pressure tended higher in the ureaplasma-exposed group likely due to higher surfactant levels preventing airspace collapse.
Figure 4.
Lung compliance in lambs aged 145 days of gestation was not significantly different from control after intra-amniotic U. parvum serovar 3 injection at 80 days (A), despite elevated levels of surfactant lipid (saturated phosphatidylcholine; B) in bronchoalveolar lavage fluid and lung tissue. (*p<0.05 versus Control)
Effects of Ureaplasma parvum serovar 6
A single intra-amniotic injection of Ureaplasma parvum serovar 6 did not cause preterm deliveries or maternal losses before the caesarean deliveries at 125 days of gestation. Media (n=5) and saline (n=4) controls did not show inflammatory changes in the chorioamnion or altered fetal lung development and therefore the results (media and saline) were combined for analysis. Ureaplasmas were cultured from amniotic fluid and lung tissue samples from all animals in which ureaplasmas were injected but not from any of the controls. Amniotic fluid ureaplasma levels were greater than 5x106 colour changing units/ml in all ureaplasma-injected animals. Lung tissue ureaplasma counts ranged from 3.7x107 to 3.4x1010 ccu/g in ureaplasma-injected animals. Ureaplasmas were located, using IFA staining, within cells lining the distal airspaces (Figure 5).
Figure 5.
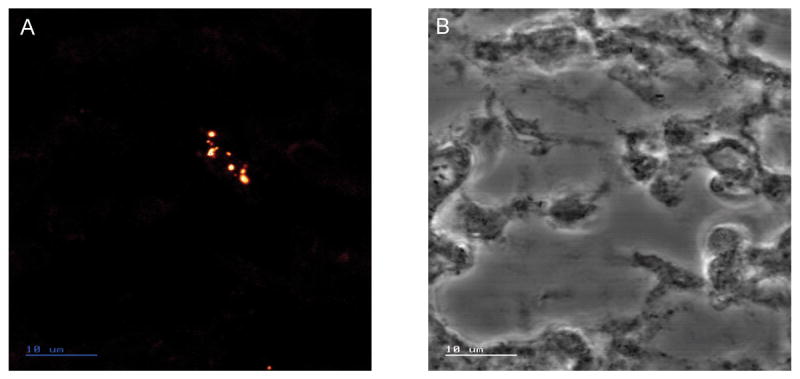
Indirect Fluorescence Assay staining, of ureplasmas, viewed by confocal laser scanning microscope, in lung tissue from a fetal sheep aged 125 days of gestation, after amniotic fluid injection of 2 x 107 colony forming units of U. parvum serovar 6 at 80 days of gestation. (A) An aggregation of ureaplasmas in the cells lining the airspaces. The ureaplasmas are bounded by only a plasma membrane and appear as pleomorphic cells ranging in size from 0.6–1.3μm. (B) Transmitted light image of the same section. Scale bar is 10μm.
Fetal body weight at 125 days of gestation after intra-amniotic U. parvum serovar 6 injection at 80 days was not different to control (Table II). Umbilical arterial pH and blood gases, and plasma cortisol concentrations, were not different between ureaplasma-exposed fetal lambs and controls (Table II). Total white blood cell counts were lower in ureaplasma-exposed fetal lambs than in controls (p=0.032; Table II).
Table II.
Observations from lambs delivered at 125 days of gestation after intra-amniotic injection of U parvum serovar 6 or control (media or saline) at 80 days.
| Control (n=9) | U parvum serovar 6 (n=9) | |
|---|---|---|
| Body weight (kg) | 2.7±0.1 | 2.7±0.2 |
| Lung weight (g/kg) | 33.0±1.5 | 35.0±1.6 |
| Umbilical arterial pH | 7.32±0.03 | 7.29±0.02 |
| Total WBC (109/l) | 4.06±0.47 | 2.67±0.36* |
| Neutrophils (109/l) | 1.48±0.37 | 0.81±0.27 |
| Lymphocytes (109/l) | 1.83±0.42 | 1.32±0.14 |
| Monocytes (109/l) | 0.13±0.06 | 0.14±0.04 |
| Plasma cortisol (μg/dl) | 1.04±0.16 | 0.89±0.09 |
p<0.05 versus Control. WBC, white blood cell count.
Intra-amniotic injection of U. parvum serovar 6 at 80 days of gestation resulted in histologic chorioamnionitis at 125 days (Figure 6); chorioamnion inflammation scores were higher than control after exposure to ureaplasmas (p<0.001; Figure 6A). Expression of IL-1β, IL-6 and IL-8 mRNA in chorioamnion was 3- to 20-fold higher than control after exposure to ureaplasmas (Figure 6D). IL-8 was undetectable in amniotic fluid from controls but after injection of ureaplasmas the amniotic fluid IL-8 concentration was 0.94±0.14 μg/ml (p<0.001).
Figure 6.
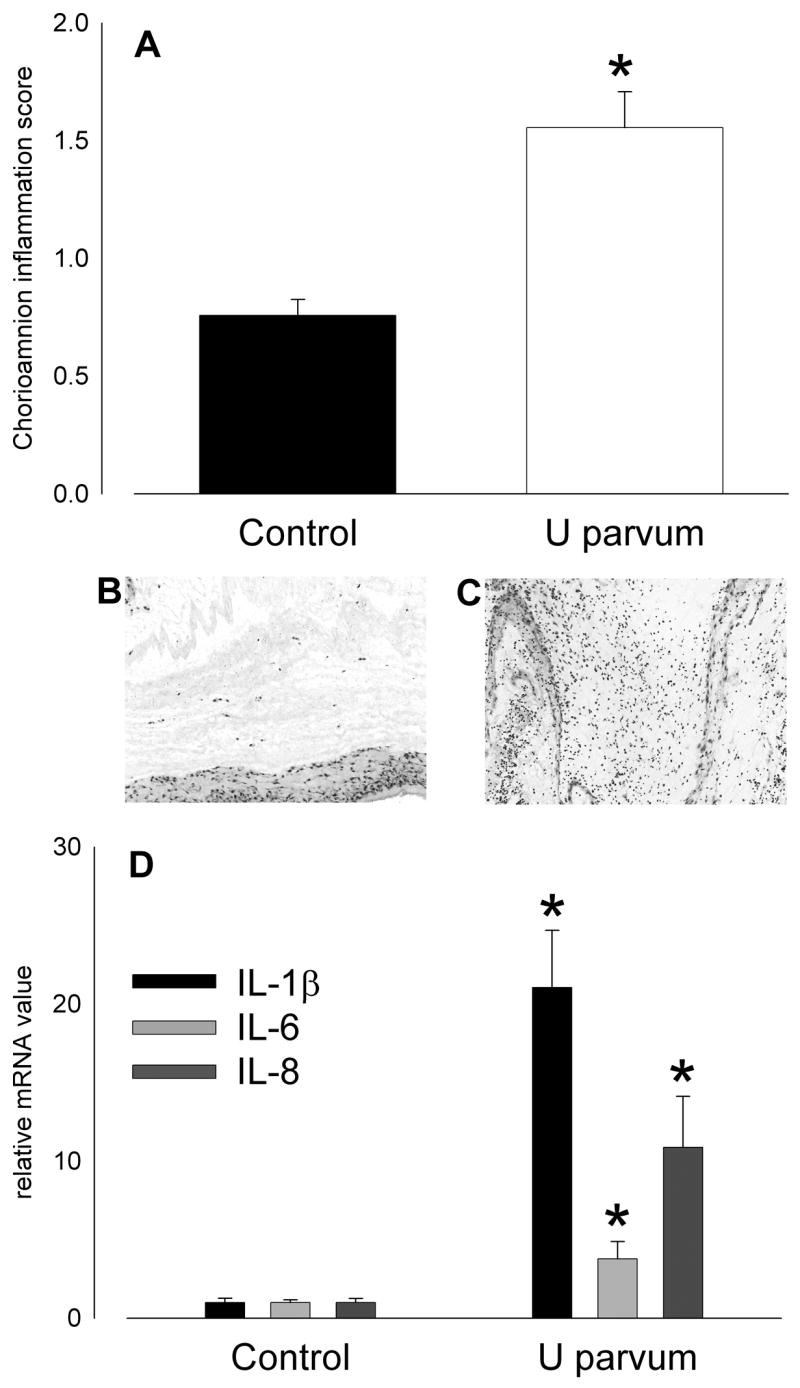
Intra-amniotic injection of U parvum serovar 6 (20x106 cfu) on day 80 of pregnancy caused intrauterine inflammation at 125 days. (A) Chorioamnion inflammation scores were higher than control after ureaplasma injection. Hematoxylin and eosin stained sections of chorioamnion show few inflammatory cells in tissue from a control animal (B; score = 1) and extensive infiltration in a sample from a ureaplasma-exposed animal (C; score = 3). (D) Expression of mRNA for proinflammatory cytokines, IL-1β, IL-6 and IL-8 in chorioamnion at 125 days of gestation was higher than control after intra-amniotic injection of U parvum serovar 6 at 80 days. (*p<0.05 versus Control)
Intra-amniotic injection of U. parvum serovar 6 at 80 days of gestation did not affect lung weight at 125 days. Bronchoalveolar lavage fluid from fetal lambs exposed to ureaplasmas was usually bloody, and contained more neutrophils (p=0.007) and monocytes (p=0.011; Figure 7A), and more protein (p=0.009; Figure 7B) than control. IL-8 protein content of lung tissue was higher than control (3.2±0.7 μg/kg) after exposure to ureaplasmas (48.1±8.0 μg/kg; p<0.001). IL-8 immunostaining was present in infiltrating inflammatory cells and cells lining the airspace of the lungs of animals exposed to ureaplasmas; there was minimal staining in controls (Figure 8). Pulmonary expression of IL-1β mRNA was 5-fold higher than control after exposure to ureaplasmas (p<0.001) but IL-6 and IL-8 mRNA expression was not different between groups (Figure 7C).
Figure 7.
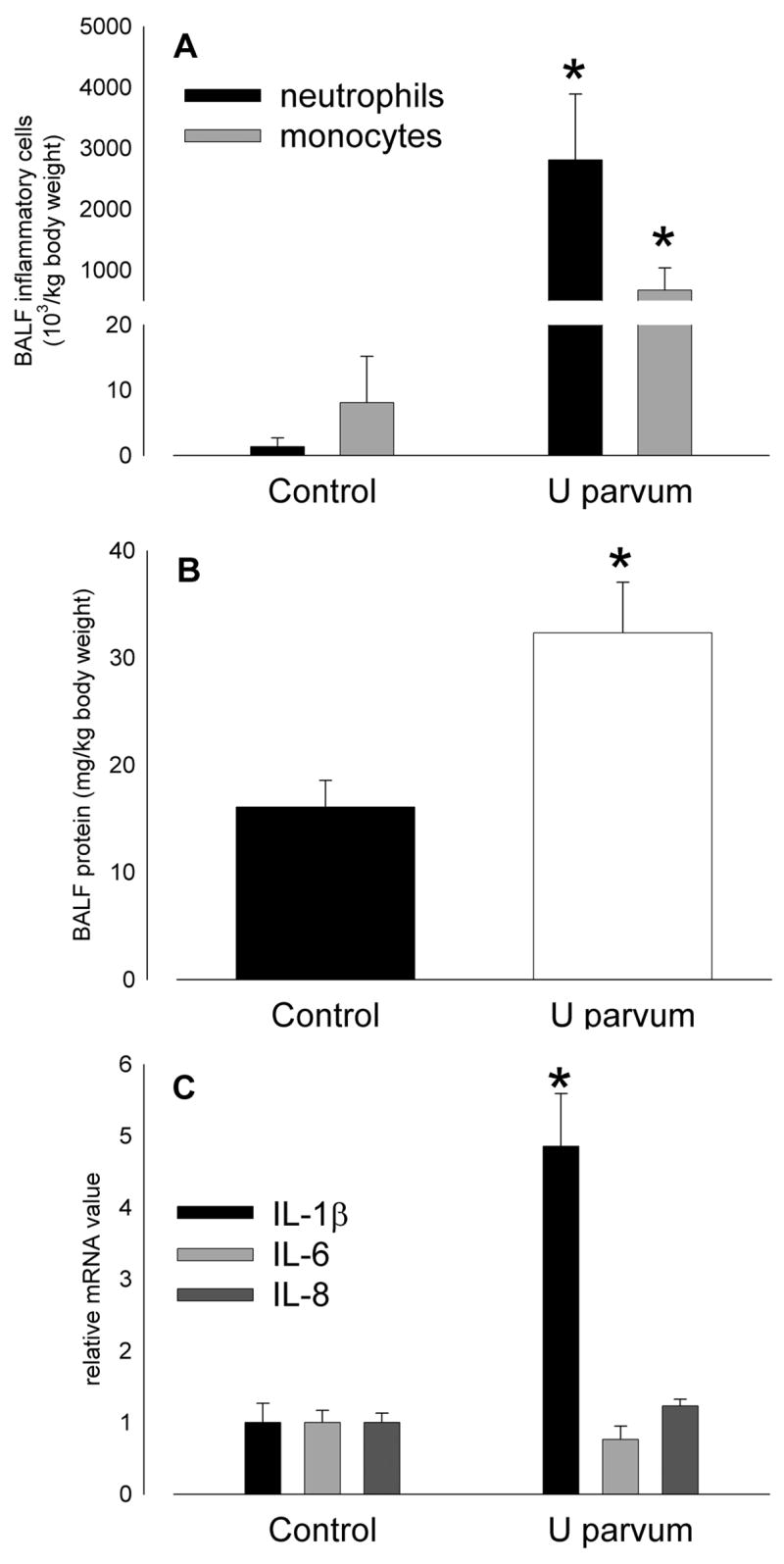
Inflammatory cell infiltration (A) and protein content of bronchoalveolar lavage fluid (B) at 125 days of gestation was higher than control after intra-amniotic U parvum serovar 6 injection at 80 days. (C) Pulmonary expression of IL-1β mRNA was 5-fold higher than control at 125 days of gestation after intra-amniotic U parvum serovar 6 injection at 80 days but IL-6 and IL-8 mRNA expression was not increased. (*p<0.05 versus Control)
Figure 8.
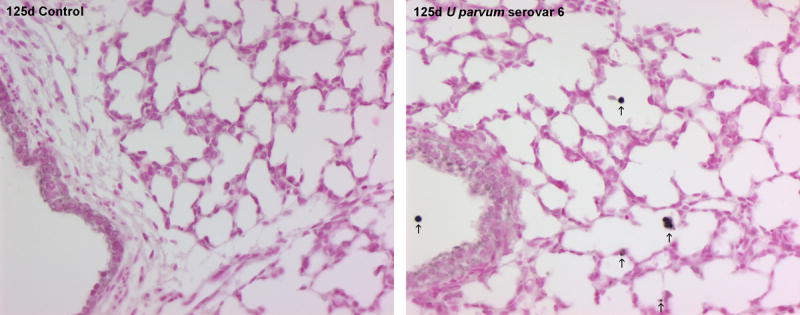
IL-8 immunostaining in lung tissue was elevated at 125 days of gestation after injection of U parvum serovar 6 at 80 days. Arrows show IL-8 immunostaining in cells lining the airspaces within the lung parenchyma and in infiltrating inflammatory cells. Images captured at x200 magnification.
Lung compliance at 125 days of gestation, as indicated by pressure-volume curve, was statistically increased by intra-amniotic U parvum serovar 6 injection at 80 days (Figure 9A). Saturated phosphatidylcholine concentrations in bronchoalveolar lavage fluid were higher than control after exposure to ureaplasmas (p<0.001; Figure 9B). Saturated phosphatidylcholine in lung tissue also was elevated in fetal lambs exposed to ureaplasmas (p<0.001; Figure 9B), resulting in higher total lung saturated phosphatidylcholine content than control (p<0.001). The amount of SP-B protein also increased 19±3 times relative to the SP-B in the bronchoalveolar lavage fluid.
Figure 9.
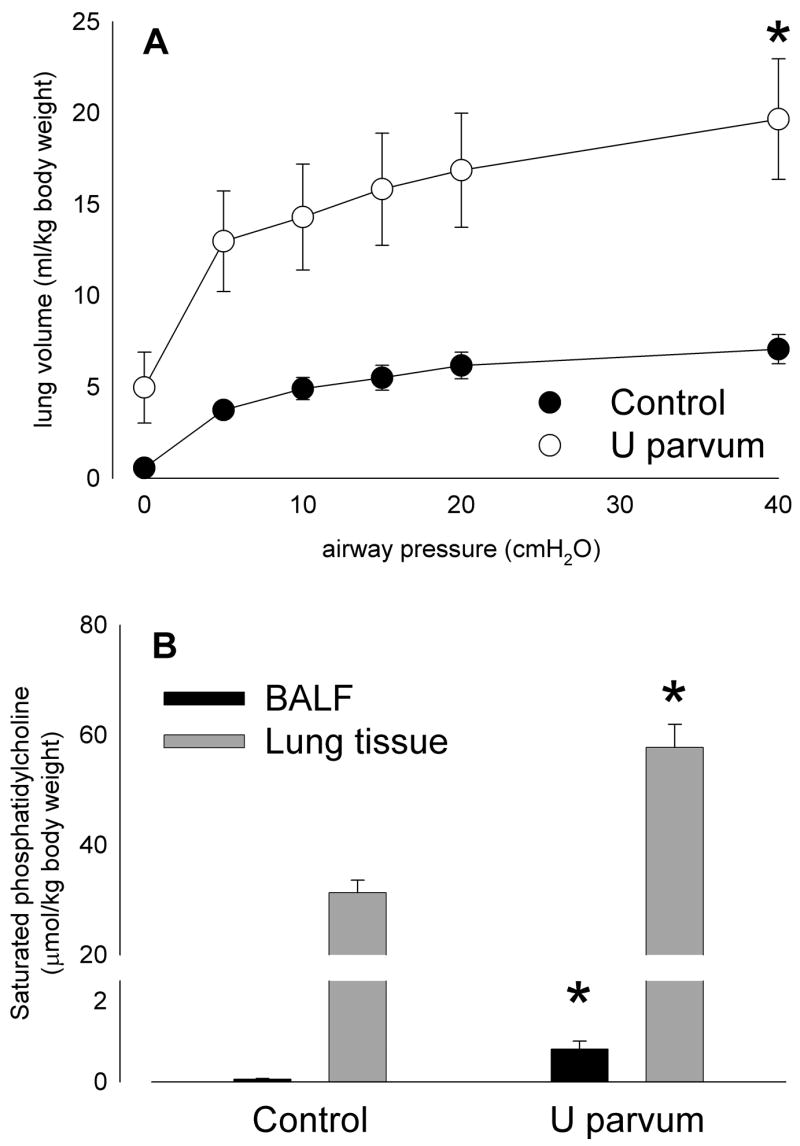
Lung compliance in lambs aged 125 days of gestation was statistically higher than control after intra-amniotic U parvum serovar 6 injection at 80 days (A), likely due to accompanying increases in surfactant lipid levels (saturated phosphatidylcholine; B) in bronchoalveolar lavage fluid and lung tissue. (*p<0.05 versus Control)
Discussion
We have extended our investigations of the effects of chronic amniotic fluid U. parvum serovar 3 colonization (6), to demonstrate that injection of a low titre in mid-gestation causes persisting colonization, with intrauterine inflammation and effects on the fetal lungs, until close to term. Preterm labour was not a consequence of amniotic fluid ureaplasma colonization. The majority of normal human pregnancies that had amniotic fluid that was PCR positive for Ureasplama at 15–18 weeks gestation also did not deliver prematurely (7, 17). Despite the presence of ureaplasmas in the lungs of late gestation lambs and persisting effects on lung development, as indicated by increased surfactant lipid content of 145d lambs, lung function in late gestation was not affected.
The intrauterine inflammatory effects and fetal responses to amniotic ureaplasma colonization are similar to those we have observed in response to intra-amniotic lipopolysaccharide, and are likely the consequences of a common underlying mechanism. Recently, it was demonstrated that activation of the fundamental cellular mediator of inflammation, nuclear factor κB, occurs in pulmonary leukocytes of infants with Ureaplasma spp. colonization of their airways (18). Our ongoing investigations with lipopolysaccharide demonstrate that preterm lung ‘maturation’ in sheep, apparently like that observed in response to ureaplasma colonization, is the consequence of pulmonary leukocyte-mediated lung inflammation (19).
Increased surfactant and lung compliance in preterm lambs exposed to ureaplasmas are consistent with data from human infants, which show a reduction in the risk of respiratory distress syndrome in the presence of airway (and likely antenatal) colonization (4, 20). The clinical course of a group of infants with tracheal aspirate samples positive for Ureaplasma spp. has recently been described (20). After few initial signs of respiratory distress syndrome and earlier weaning from ventilators than ureaplasma-negative infants, ureaplasma-positive infants have an increased risk of requiring reestablishment of ventilatory support, greater subsequent ventilatory requirements and radiological evidence of emphysematous change (20). Another finding of our study that is consistent with data from human patients is the elevated amniotic fluid IL-8 concentrations in the presence of amniotic fluid colonization (21).
We have shown that ‘maturational’ effects of inflammation on the preterm lungs are accompanied by other effects, characteristic of bronchopulmonary dysplasia (BPD) (22). BPD is a potential consequence of neonatal airway ureaplasma colonization (23); our studies suggest that initiation of pulmonary inflammation in such cases has an antenatal origin, consistent with limited available data from humans (5, 24). There are only limited data about which Ureaplasma spp. serovars are associated with BPD in human infants; colonization by U. parvum (serovars 1, 3, 6, 14) appears more common than by U. urealyticum (serovars 2, 4, 5, 7 through 13) but associations with BPD were similar for each species (25).
Our present study suggests that U. parvum serovar 6 causes fetal lung inflammation that is at least as great in magnitude as that in response to serovar 3 (6). The frequent observation of blood in bronchoalveolar lavage fluid, as we saw in animals exposed to U. parvum serovar 6, has not been a feature of our previous studies of fetal lung inflammation induced by intra-amniotic lipopolysaccharide or IL-1 (15, 22, 26, 27). We observed increases, significantly above control, in lung inflammatory cell counts and surfactant lipid levels after 6 weeks of exposure to U. parvum serovar 6 but not to serovar 3 (6). Effects of U. parvum serovar 6 were qualitatively similar to those of longer duration (10 weeks) exposure to serovar 3, as observed previously by us (6). The progression of the effects of ureaplasmas we observed previously with serovar 3 (6) might, therefore, be accelerated by serovar 6. Such a result is consistent with the concept that serovar 6, which appears particularly adherent to cells, is more pathogenic than other serovars (9). There is little microbiological information about ureaplasma tissue adherence and invasion, to relate to the progression of effects of ureaplasma colonization in pregnancy.
We observed growth restriction in 125d lambs exposed to U parvum serovar 3 for 10 weeks in a previous study (6). Reduced fetal body weight at 125d was not a consequence of exposure to U parvum serovar 6 for 6 weeks, despite apparently greater inflammatory effects. Similarly, reduced fetal body weight was not evident at 145d or at birth at term, after exposure to serovar 3 for 10 weeks after injection of a low titre at 80d. There are few studies of human populations investigating potential links between amniotic fluid ureaplasma colonization and IUGR; available data are inconclusive.
The lungs of all late gestation lambs exposed to ureaplasmas were positive for the microorganisms but ureaplasmas could not be cultured from the 5 post-partum lambs we studied. This observation is consistent with previous studies demonstrating clearance of ureaplasmas from the nasal cavities of lambs, colonized in utero, soon after birth (28) and an inability of ureaplasmas to colonize the respiratory tract of older lambs (29). Therefore the persisting colonization of the lungs by ureaplasmas, which we have observed in fetal sheep, may be a consequence of the unique fetal pulmonary environment. The time course of resolution of ureaplasma colonization of the airways of human neonates is not known, but it is possible that the immature immune systems of preterm neonates impairs their ability to clear ureaplasma infections and increases their vulnerability (30).
Our studies of the effects of intra-amniotic Ureaplasma spp. colonization in sheep demonstrate clearly the capacity of these microorganisms to cause intrauterine inflammation and alter fetal lung development. Given the common occurrence of amniotic ureaplasma colonization in human pregnancies, and its association with neonatal morbidity, further investigations are required to determine the optimal means of detection, antenatal treatment and management of exposed children before and after birth.
Acknowledgments
Funded by The National Institutes of Health, USA (HL-65397) and The National Health and Medical Research Council of Australia (303261).
This project was supported by funding from the National Institutes of Health, USA (HL-65397) and the National Health and Medical Research Council of Australia (303261). The authors are grateful to Mr Adrian Jonker, Ms Amanda Meyer, Mrs Mary Davis, Mr Shawn Grant, and the staff of the Agriculture WA Mt Barker and Medina Research Stations, for expert technical and research assistance. The authors are grateful also for the support of the Women and Infants Research Foundation, Perth, Western Australia.
Footnotes
Condensation
Colonization of amniotic fluid by ureaplasmas results in intrauterine and fetal pulmonary inflammation that persists until term, and has lasting effects on fetal lung development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 2.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 3.Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Worda C, Husslein P. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am J Obstet Gynecol. 2005;193:1663–9. doi: 10.1016/j.ajog.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 4.Hannaford K, Todd DA, Jeffery H, John E, Blyth K, Gilbert GL. Role of ureaplasma urealyticum in lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1999;81:F162–7. doi: 10.1136/fn.81.3.f162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kafetzis DA, Skevaki CL, Skouteri V, Gavrili S, Peppa K, Kostalos C, Petrochilou V, Michalas S. Maternal genital colonization with ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004;39:1113–22. doi: 10.1086/424505. [DOI] [PubMed] [Google Scholar]
- 6.Moss TJ, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192:1179–86. doi: 10.1016/j.ajog.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 7.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–6. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 8.Knox CL, Timms P. Comparison of PCR, nested PCR, and random amplified polymorphic DNA PCR for detection and typing of Ureaplasma urealyticum in specimens from pregnant women. J Clin Microbiol. 1998;36:3032–9. doi: 10.1128/jcm.36.10.3032-3039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knox CL, Allan JA, Allan JM, Edirisinghe WR, Stenzel D, Lawrence FA, Purdie DM, Timms P. Ureaplasma parvum and Ureaplasma urealyticum are detected in semen after washing before assisted reproductive technology procedures. Fertil Steril. 2003;80:921–9. doi: 10.1016/s0015-0282(03)01125-7. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore Ervin M, Padbury JF, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162:1656–61. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 11.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L527–36. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 12.Mason RJ, Nellenbogen J, Clements JA. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976;17:281–4. [PubMed] [Google Scholar]
- 13.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–8. [PubMed] [Google Scholar]
- 14.Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Cell Mol Physiol. 2001;280:L279–85. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- 15.Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164:982–8. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami M, Kallapur SG, Jobe AH. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am J Physiol Lung Cell Mol Physiol %R. 2004;286:L573–579. doi: 10.1152/ajplung.00211.2003. [DOI] [PubMed] [Google Scholar]
- 17.Gerber S, Vial Y, Hohfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reation correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 18.Cheah FC, Winterbourn CC, Darlow BA, Mocatta TJ, Vissers MC. Nuclear factor kappaB activation in pulmonary leukocytes from infants with hyaline membrane disease: associations with chorioamnionitis and Ureaplasma urealyticum colonization. Pediatr Res. 2005;57:616–23. doi: 10.1203/01.PDR.0000156209.37627.82. [DOI] [PubMed] [Google Scholar]
- 19.Kallapur SG, Moss TJ, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited Inflammatory Cells Mediate Endotoxin-induced Lung Maturation in Preterm Fetal Lambs. Am J Respir Crit Care Med. 2005;172:1315–21. doi: 10.1164/rccm.200506-1007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theilen U, Lyon AJ, Fitzgerald T, Hendry GM, Keeling JW. Infection with Ureaplasma urealyticum: is there a specific clinical and radiological course in the preterm infant? Arch Dis Child Fetal Neonatal Ed. 2004;89:F163–7. doi: 10.1136/adc.2003.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Husslein P. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med. 2005;33:22–6. doi: 10.1515/JPM.2005.003. [DOI] [PubMed] [Google Scholar]
- 22.Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002;165:805–11. doi: 10.1164/ajrccm.165.6.2108053. [DOI] [PubMed] [Google Scholar]
- 23.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–89. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger A, Witt A, Haiden N, Kretzer V, Heinze G, Kohlhauser C. Microbial invasion of the amniotic cavity at birth is associated with adverse short-term outcome of preterm infants. J Perinat Med. 2003;31:115–21. doi: 10.1515/JPM.2003.016. [DOI] [PubMed] [Google Scholar]
- 25.Katz B, Patel P, Duffy L, Schelonka RL, Dimmitt RA, Waites KB. Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J Clin Microbiol. 2005;43:4852–4. doi: 10.1128/JCM.43.9.4852-4854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, Possmayer F, Hallman M, Ikegami M. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182:401–8. doi: 10.1016/s0002-9378(00)70231-6. [DOI] [PubMed] [Google Scholar]
- 27.Willet KE, Kramer BW, Kallapur SG, Ikegami M, Newnham JP, Moss TJ, Sly PD, Jobe AH. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L411–20. doi: 10.1152/ajplung.00097.2001. [DOI] [PubMed] [Google Scholar]
- 28.Ball H, McCaughey W, Kennedy S, McLoughlin M. Experimental intrauterine inoculation of pregnant ewes with ureaplasmas. Vet Res Comm. 1985;9:35–43. doi: 10.1007/BF02215126. [DOI] [PubMed] [Google Scholar]
- 29.Ball H, McLoughlin M. Experimental inoculation of an ovine ureaplasma strain into the respiratory tract of lambs. Vet Record. 1984;115:626. doi: 10.1136/vr.115.24.626. [DOI] [PubMed] [Google Scholar]
- 30.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24:1033–9. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]