Abstract
Background
Gender disparities in the incidence of torsade de pointes (TdP) ventricular tachycardia exist, but the mechanisms in humans are unresolved. We addressed this issue using a mathematical model of a human ventricular cell.
Methods
We implemented gender differences in the Priebe-Beuckelmann model cell by modifying the amplitudes of the L-type Ca2+ current (ICa,L), transient outward K+ current (Ito), and rapid component of the delayed rectifier K+current (IKr), according to experimental data from animal male and female hearts. Gender disparities in electrical heterogeneity between transmural layers (subepicardium, midmyocardium, subendocardium) were implemented by modifying various ion currents according to experimental data.
Results
Action potentials in female cells have longer durations and steeper duration versus frequency relationships than male cells. In the female cells, electrical heterogeneity between transmural layers is larger and the susceptibility to early afterdepolarisations is higher than in male cells.
Conclusion
Gender-related differences in ICa,L, Ito, and IKr may explain the gender disparities in human cardiac electrophysiology. Female cells have an increased susceptibility to early afterdepolarisations following mild reductions in net repolarising forces. Combined with their greater electrical heterogeneity, this renders them more vulnerable to TdP. (Neth Heart J 2007;15:405-11.)
Keywords: gender, arrhythmias, torsade de pointes, electrophysiology, computer simulations
Gender differences in the incidence of cardiac arrhythmias exist (for a review, see James et al.)1 Most notably, women are more likely to sustain torsade de pointes (TdP) ventricular tachycardia than men in inherited2,3 and acquired (e.g., secondary to drug use) long-QT syndrome (LQTS).4 Clinical observations suggest that the gender disparity in the incidence of TdP is largely due to male sex hormones. While boys and girls under the age of 15 years have similar incidences of TdP in inherited LQTS, from puberty through to adulthood, men have a lower incidence of TdP.2
Clinical5 and experimental6-8 studies have produced two theories regarding the electrophysiological basis of TdP. One theory holds that TdP arises from triggered activity in competing ventricular foci. Evidence for this hypothesis stems from experimental observations6,7 and computer models9 which demonstrate an enhanced susceptibility of cardiac myocytes to early afterdepolarisations (EADs) in response to factors that prolong action potential duration (APD). The other theory emphasises transmural dispersion of repolarisation, suggesting an involvement of reentrant excitation.7,8
To date, data in healthy subjects about sexdependent differences in the occurrence of EADs or transmural dispersion, which may explain gender differences in TdP, are not available. We aimed to investigate whether gender disparities in the density of sarcolemmal ion currents may account for the gender difference in the incidence of TdP. Accordingly, we modified ion current conductances in the Priebe and Beuckelmann (PB) human ventricular cell model,10 based on experimental data obtained from healthy male and female hearts of various species. We studied action potential characteristics, transmural electrophysiological heterogeneity, and susceptibility to EAD development.
Methods
Priebe-Beuckelmann model
The PB mathematical model of a human ventricular cell10 is based on the Luo and Rudy guinea pig ventricular cell model11 with modified time constants of Ca2+ release and new equations for L-type Ca2+ current (ICa,L), inward rectifier K+ current (IK1), rapid (IKr) and slow (IKs) components of the delayed rectifier K+ current, and transient outward K+ current (Ito). These new equations in the PB model were based on experimental data obtained from single ventricular cells isolated from explanted human hearts. As these cells were generally isolated from midmyocardial areas of the left ventricle of male patients (see Priebe & Beuckelmann,10 and the primary references cited therein), the PB model is one of a typical male midmyocardial ventricular cell.
To study gender disparities in action potential properties, transmural electrophysiological heterogeneity, and susceptibility to EAD development, we incorporated the disparities in ion current densities between genders and myocardial layers, as reported in experimental studies, into the PB model. These changes are discussed below and summarised in table 1. All values are expressed as conductance relative to the conductance of the PB model. All figures show action potential properties determined in steady-state conditions, two minutes after the onset of stimulation (stimulus pulse: 2 ms, 3 nA).
Table 1.
Relative magnitude of sarcolemmal ion currents in models of male and female myocytes.
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Epi | Mid | Endo | Epi | Mid | Endo | |
| INa | 1 | 1 | 1 | 1 | 1 | 1 |
| ICa,L | 1 | 1 | 1 | 1.32 | 1.32 | 1 |
| Ito | 1 | 1 | 0.5 | 0.75 | 0.75 | 0.375 |
| IKr | 1 | 1 | 1 | 0.83 | 0.83 | 0.83 |
| IKs | 1.42 | 1 | 1.42 | 1.42 | 1 | 1.42 |
| IK1 | 1 | 1 | 1 | 1 | 1 | 1 |
| INa,b | 1 | 1 | 1 | 1 | 1 | 1 |
| ICa,b | 1 | 1 | 1 | 1 | 1 | 1 |
| INaK | 1 | 1 | 1 | 1 | 1 | 1 |
| INaCa | 1 | 1 | 0.69 | 1 | 1 | 0.69 |
Values for subepicardial (epi), midmyocardial (mid), and subendocardial (endo) myocytes are relative to the current magnitude in the original Priebe-Beuckelmann model of a human ventricular myocyte.10
INa=fast sodium current, ICa,L=L-type calcium current, Ito=transient outward current, IKr=rapid delayed rectifier potassium current, IKs=slow delayed rectifier potassium current, IK1=inward rectifier potassium current, INa,b=background sodium current, ICa,b=background calcium current, INaK=sodium-potassium pump current, INaCa=sodiumcalcium exchange current.
Gender disparities in ion current densities
We reviewed all studies into gender disparities in ion current densities, conducted in single ventricular myocytes. These cells were obtained from dog, rabbit, guinea pig, and mouse hearts, but not from human hearts. ICa,L in female subepicardium and midmyocardium is 1.32 times that of males.12 Ito in females is 0.75 times that of males in all cell layers,13,14 although this is not a consistent finding.15,16 IKr in females is 0.83 times that of males.17 These gender disparities were incorporated into the PB model.
Although the ultrarapid component of the delayed rectifier K+ current (IKur) and the ATP-regulated K+ current (IK,ATP) also exhibit gender disparities (57% larger15 and 60% smaller18 in males than in females, respectively), these disparities were not incorporated into the PB model, because IKur is not present in human ventricular cells,19 while IK,ATP is not functional under normal conditions. Similarly, the Na+ current (INa),15 IKs,20 and IK115-17 were left unchanged, because they are similar in male and female hearts, as are the intracellular concentrations of Na+and Ca2+ at baseline.18,21
Transmural heterogeneities in ion current densities
To study gender disparity in transmural heterogeneity, we incorporated the differences in ion current densities between subepicardium, midmyocardium, and subendocardium according to quantitative measurements in isolated ventricular myocytes. Data from dog hearts were used, because those from human hearts are limited. ICa,L in subepicardium and midmyocardium is 1.32 times that in subendocardium in females, but not in males.12 In both genders, Ito in subepicardium and midmyocardium is twice that in subendocardium,20,22 while IKs in subepicardium and subendocardium is 1.42 times that in midmyocardium.20 The Na+-Ca2+ exchange current (INaCa) in subendocardium is 0.69 times that in the other layers.23 Although a late component of INa (INa,late) is 27% smaller in subepicardium and subendocardium than in midmyocardium,24 this transmural heterogeneity was not incorporated, because INa,late is not present in the original PB model. Similarly, no transmural heterogeneities in INa, IKr, and IK1 were reported.20,22,24
Results
We used our ‘male’ and ‘female’ models of subepicardial, midmyocardial, and subendocardial cells to assess gender disparities in APD restitution, transmural dispersion in APD, and susceptibility to EADs.
Effects of gender on action potential duration
Simulations at different stimulation frequencies (0.1 to 2 Hz) revealed significant APD differences between both genders. Figure 1, left panels, shows superimposed action potentials at 1 Hz in the female and male model of subepicardial (figure 1A), midmyocardial (figure 1B), and subendocardial (figure 1C) cells. In all cell types, the action potentials were significantly longer in females than males. Figure 1, right panels, summarises the APD at 90% repolarisation (APD90) of males and females at various stimulation frequencies.
At all frequencies, APD90 of subepicardial (figure 1A), midmyocardial (figure 1B), and subendocardial (figure 1C) model cells were longer in females than males. Moreover, APD90 was longest at low stimulus frequencies and decreased at higher frequencies in all cell types. This APD-frequency relationship was steeper in females than in males. Linear fits25 of the APD-frequency relationships (restitution curves, fitting correlation coefficients >0.92) indicated that APD-frequency relationships were steeper in females, except in subendocardium (figures 1A-C).
Figure 1.
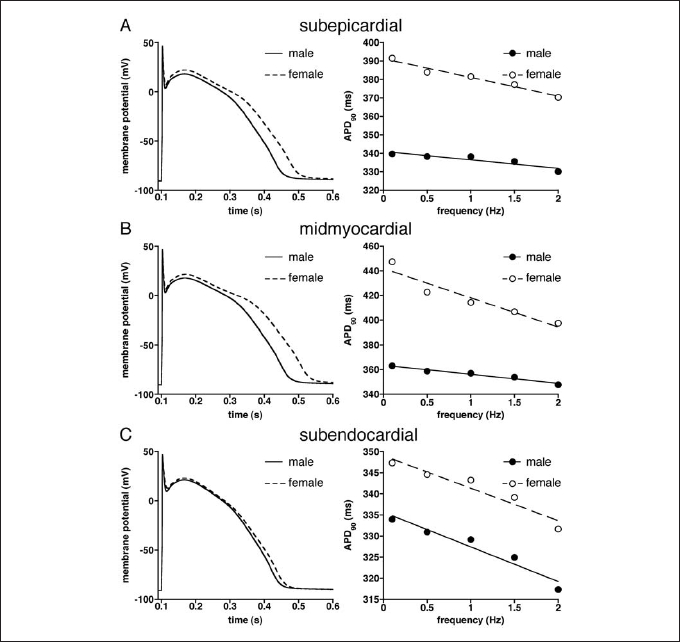
Superimposed action potentials elicited at 1 Hz (left) and action potential duration at 90% repolarisation (APD90) vs. stimulus frequency (right) of (A) subepicardial, (B) midmyocardial, and (C) subendocardial model cells of male and female.
Effects of gender on transmural electrical heterogeneity
There were significant differences in APD90 between subepicardial, midmyocardial, and subendocardial cells in both genders at all stimulation frequencies (0.1 to 2 Hz). This is illustrated in figure 2, which shows superimposed action potentials at 0.5 Hz in male (A) and female (B) subepicardial, midmyocardial, and subendocardial model cells. In both genders, action potential was shortest in subepicardial cells and longest in midmyocardial cells, but with a considerably larger difference between shortest and longest action potential in the female. This APD90 heterogeneity (ΔAPD90) was larger in females than in males at all frequencies, as summarised in figures 2C and 2D. Of note, ΔAPD90 in females was particularly large at slow stimulation frequencies.
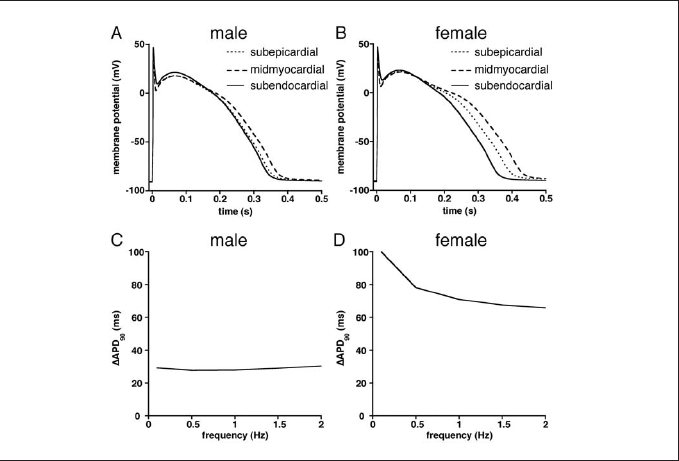
Figure 2. (A,B) Superimposed action potentials (0.5 Hz) of subepicardial, midmyocardial, and subendocardial model cells of male (A) and female (B). (C,D) Differences between longest (midmyocardial) and shortest (subendocardial) action potentials (ΔAPD90) at various stimulus frequencies in male (C) and female (D) model cells.
Effects of gender on early afterdepolarisations
EADs typically occur at slow heart rates (for a review, see Tan et al.26). In isolated ventricular myocytes, EADs may result from moderately enhanced ICa,L or reduced IKr.27,28 Simulations with 25% increased ICa,L or 50% decreased IKr densities revealed significant gender differences in the incidence of EADs. Figure 3 shows superimposed action potentials at 0.1 Hz in the female and male midmyocardial model cells resulting from increased ICa,L (A, top panel) or reduced IKr (B, top panel). In both genders, the applied changes in ICa,L and IKr conductances resulted in action potential prolongation. The excessive prolongation that was observed in females (top panels, arrows), but not in males, appeared to be due to reactivation of ICa,L (bottom panels, arrows), consistent with previous findings.29
Figure 3.
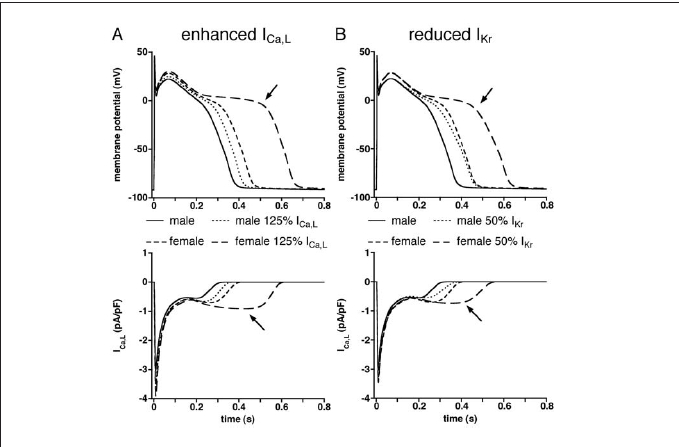
(A) Action potentials (top) and L-type calcium current (ICa,L, bottom) of midmyocardial cells at 0.1 Hz under control conditions and enhanced ICa,L(125% of control). (B) Action potentials (top) and ICa,L(bottom) of midmyocardial cells at 0.1 Hz under control conditions and reduced rapid delayed rectifier potassium current (IKr, 50% of control). Arrows indicate excessive action potential prolongation (top) due to reactivation of ICa,L(bottom).
This prolongation prolongs the plateau phase of the action potential and thus increases the susceptibility
to EADs.
Discussion
We studied whether gender disparities in the densities of sarcolemmal ion currents may explain the gender disparity in the incidence of TdP in humans. We incorporated gender differences in a human ventricular model cell by modifying ICa,L, Ito, and IKr conductances, according to experimental data obtained from animal studies. We demonstrated that human cardiac electrophysiology exhibits clear gender disparities in action potential repolarisation, transmural dispersion, and susceptibility to EADs.
Gender effects on action potential characteristics
We found that female model cells had longer action potentials than their male counterparts (figure 1). This agrees with experimental findings of gender disparity in APD in left ventricular midmyocardial myocytes isolated from explanted hearts of patients with endstage heart failure who underwent cardiac transplantation. 30 In addition, our results are in agreement with experimental findings in animal studies. In mouse subepicardial myocytes13,15 and rabbit subendocardial myocytes,31 action potentials were significantly longer in females than in males. Our results are also in agreement with the clinical observations in healthy subjects that women have longer QTc intervals.32
The female model cells had steeper APD-frequency relationships than their male counterparts (figure 1). Although not studied in detail, experimental findings in animals provide supportive results. In rabbits, APD at 20, 50 and 90% repolarisation (APD20, APD00, and APD90, respectively) are similar in male and female at a cycle length of 300 ms, but different at longer cycle lengths.31 Our results are in keeping with previously reported ECG observations that women have a steeper QT-heart rate relationship than men.25
Gender effects on transmural heterogeneity and incidence of EADs
We found that the female model cells exhibit greater differences in APD90 between subendocardium, midmyocardium, and subepicardium, i.e., greater transmural dispersion, particularly at slow heart rates (figure 2). This agrees with findings in left ventricular myocytes of dog which also demonstrated a greater transmural APD heterogeneity in females.33 These observed gender disparities may contribute to the clinically observed sex-related differences in the slopes of the ascending and descending limbs of the T wave,34,35 suggesting larger transmural heterogeneity in the final repolarisation phase. We also found that increasing ICa,L or reducing IKr prolonged action potentials in both genders (figure 3), but significantly more so in females than in males. Furthermore, these interventions resulted in excessive action potential prolongation, thus favouring EAD formation, in the female, but not the male, model cell. These findings are in agreement with experimental findings in rabbits.28
Two theories regarding the electrophysiological basis of TdP exist, i.e., triggered activity in competing ventricular foci and dispersion of transmural repolarisation. Either way, female cells have an increased susceptibility to EADs following mild reductions in net repolarising forces. Combined with their greater electrical heterogeneity, particularly at slow heart rates, this renders them more vulnerable to TdP.
Limitations
While the clear differences in action potential properties between models of subepicardial, midmyocardial, and subendocardial cells, as reported here, may explain clinical observations, it is conceivable that normal cellto- cell coupling in the intact heart would attenuate these inherent differences.36,37 This has been demonstrated in various studies (see Akar et al. and the primary references cited therein).8 Nevertheless, it has been found that the specific electrophysiological properties of M cells, in conjunction with their topographical distribution (midmyocardium), may create spatial gradients of repolarisation of sufficient magnitude to cause unidirectional block and reentrant excitation underlying TdP in LQTS type 2.8
Conclusion
We provide insights into the cellular and ionic basis for the sex-related distinction in the incidence of TdP. Gender disparities in Ito, IKr, and IKs conductance result in an increased susceptibility to EADs in females. Combined with a larger electrical transmural heterogeneity in female, this renders females more vulnerable to TdP.
Acknowledgement
Dr Tan was supported by a fellowship of the Royal Netherlands Academy of Arts and Sciences (KNAW) and by the Netherlands Heart Foundation (NHS grants 2002B191 and 2005B180).
References
- 1.James AF, Choisy SCM, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol 2007;94:265-319. [DOI] [PubMed] [Google Scholar]
- 2.Zareba W, Moss AJ, le Cessie S, Locati EH, Robinson JL, Hall WJ, et al. Risk of cardiac events in family members of patients with long QT syndrome. J Am Coll Cardiol 1995;26:1685-91. [DOI] [PubMed] [Google Scholar]
- 3.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation 1998;97:2237-44. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann MH, Hardy S, Archibald D, Quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation 1996;94:2535-41. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Periti M, Malliani A. Fundamentals of clinical cardiology: the long Q-T syndrome. Am Heart J 1975;89:378-90. [DOI] [PubMed] [Google Scholar]
- 6.Antzelevitch C, Sicouri S. Clinical relevance of cardiac arrhythmias generated by afterdepolarizations: role of M cells in the generation of U waves, triggered activity and Torsade de Pointes. J Am Coll Cardiol 1994;23:259-77. [DOI] [PubMed] [Google Scholar]
- 7.Verduyn SC, Vos MA, van der Zande J, van der Hulst FF, Wellens HJ. Role of interventricular dispersion of repolarization in acquired torsade-de-pointes arrhythmias: reversal by magnesium. Cardiovasc Res 1997;34:453-63. [DOI] [PubMed] [Google Scholar]
- 8.Akar FG, Yan G-X, Antzelevitch C, Rosenbaum DS. Unique topographical distribution of M cells underlies reentrant mechanism of Torsade de Pointes in the long-QT syndrome. Circulation 2002;105:1247-53. [DOI] [PubMed] [Google Scholar]
- 9.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res 1994;74:1097-113. [DOI] [PubMed] [Google Scholar]
- 10.Priebe L, Beuckelmann DJ. Simulation study of cellular electric properties in heart failure. Circ Res 1998;82:1206-23. [DOI] [PubMed] [Google Scholar]
- 11.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res 1994;74:1071-96. [DOI] [PubMed] [Google Scholar]
- 12.Pham TV, Robinson RB, Danilo P Jr, Rosen MR. Effects of gonadal steroids on gender-related differences in transmural dispersion of L-type calcium current. Cardiovasc Res 2002;53:752-62. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Anderson ME. Reduced repolarization reserve in ventricular myocytes from female mice. Cardiovasc Res 2002;53:763-9. [DOI] [PubMed] [Google Scholar]
- 14.Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Perez GJ, et al. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 2002;106: 2004-11. [DOI] [PubMed] [Google Scholar]
- 15.Trepanier-Boulay V, St-Michel C, Tremblay A, Fiset C. Genderbased differences in cardiac repolarization in mouse ventricle. Circ Res 2001;89:437-44. [DOI] [PubMed] [Google Scholar]
- 16.Leblanc N, Chartier D, Gosselin H, Rouleau JL. Age and gender differences in excitation-contraction coupling of the rat ventricle. J Physiol 1998;511:533-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XK, Katchman A, Drici MD, Ebert SN, Ducic I, Morad M, et al. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther 1998;285: 672-9. [PubMed] [Google Scholar]
- 18.Ranki HJ, Budas GR, Crawford RM, Jovanovic A. Genderspecific difference in cardiac ATP-sensitive K+ channels. J Am Coll Cardiol 2001;38:906-15. [DOI] [PubMed] [Google Scholar]
- 19.Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 1996;78:689-96. [DOI] [PubMed] [Google Scholar]
- 20.Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol 2002;283: H1031-41. [DOI] [PubMed] [Google Scholar]
- 21.Sugishita K, Su Z, Li F, Philipson KD, Barry WH. Gender influences [Ca2+]i during metabolic inhibition in myocytes overexpressing the Na+-Ca2+ exchanger. Circulation 2001;104:2101-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res 1993;72:671-87. [DOI] [PubMed] [Google Scholar]
- 23.Zygmunt AC, Goodrow RJ, Antzelevitch C. INaCa contributes to electrical heterogeneity within the canine ventricle. Am J Physiol Heart Circ Physiol 2000;278:H1671-8. [DOI] [PubMed] [Google Scholar]
- 24.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol Heart Circ Physiol 2001;281:H689-97. [DOI] [PubMed] [Google Scholar]
- 25.Kligfield P, Lax KG, Okin PM. QT interval-heart rate relation during exercise in normal men and women: definition by linear regression analysis. J Am Coll Cardiol 1996;28:1547-55. [DOI] [PubMed] [Google Scholar]
- 26.Tan HL, Hou CJY, Lauer MR, Sung RJ. Electrophysiologic mechanisms of the long QT interval syndromes and torsade de pointes. Ann Intern Med 1995;122:701-14. [DOI] [PubMed] [Google Scholar]
- 27.Veldkamp MW, Verkerk AO, van Ginneken ACG, Baartscheer A, Schumacher C, de Jonge N, et al. Norepinephrine induces action potential prolongation and early afterdepolarizations in ventricular myocytes isolated from human end-stage failing hearts. Eur Heart J 2001;22:955-63. [DOI] [PubMed] [Google Scholar]
- 28.Pham TV, Sosunov EA, Gainullin RZ, Danilo P Jr, Rosen MR. Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by IK-blocking drugs. Circulation 2001;103:2207-12. [DOI] [PubMed] [Google Scholar]
- 29.Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J 1995;68:949-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verkerk AO, Wilders R, Veldkamp MW, de Geringel W, Kirkels JH, Tan HL. Gender disparities in cardiac cellular electrophysiology and arrhythmia susceptibility in human failing ventricular myocytes. Int Heart J 2005;46:1105-18. [DOI] [PubMed] [Google Scholar]
- 31.Valverde ER, Biagetti MO, Bertran GR, Arini PD, Bidoggia H, Quinteiro RA. Developmental changes of cardiac repolarization in rabbits: implications for the role of sex hormones. Cardiovasc Res 2003:57:625-31. [DOI] [PubMed] [Google Scholar]
- 32.Bazett HC. An analysis of the time relations of electrocardiograms. Heart 1920;7:353-67. [Google Scholar]
- 33.Xiao L, Zhang L, Han W, Wang Z, Nattel S. Sex-based transmural differences in cardiac repolarization and ionic-current properties in canine left ventricles. Am J Physiol Heart Circ Physiol 2006;291: H570-80. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Elko P, Fromm BS, Baga JJ, Pires LA, Schuger CD, et al. Maximal ascending and descending slopes of the T wave in men and women. J Electrocardiol 1997;30:267-76. [DOI] [PubMed] [Google Scholar]
- 35.Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, et al. Sex-dependent electrocardiographic pattern of cardiac repolarization. Am Heart J 2000;140:430-6. [DOI] [PubMed] [Google Scholar]
- 36.Lesh MD, Pring M, Spear JF. Cellular uncoupling can unmask dispersion of action potential duration in ventricular myocardium: a computer modeling study. Circ Res 1989;65:1426-40. [DOI] [PubMed] [Google Scholar]
- 37.Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation 1999;99:2466-74. [DOI] [PubMed] [Google Scholar]


