Abstract
Early olfactory experience with a specific odorant enhances the subsequent response of the glomerular layer of the rat olfactory bulb to that same odorant. Because different odorants activate different glomerular layer regions, it seemed plausible that experience with a large number of odorants might result in enhanced glomerular activation during subsequent exposure to both the previously experienced odorants, as well as to novel odorants evoking activity in regions that overlapped with those previously stimulated by different odorants. To this end, seven odorants were selected using our glomerular response data archive that together stimulated much of the glomerular layer (alpha-phellandrene, benzaldehyde, L-carvone, decanal, pentanol, santalol, and valeric acid). Young rats were exposed to a different odorant each day for seven days, and this cycle was repeated three times from postnatal days 1-21. The [14C] 2-deoxyglucose technique was used to measure neural activity in response to both previously experienced and novel odorants. The two novel odorants (alpha-ionone and L-menthone) activate regions of the glomerular layer that overlap with those stimulated by the seven enrichment odorants. Our results indicate that early experience with multiple odorants results in increased responsiveness both to previously experienced odorants, and to novel odorants that stimulate previously activated regions of the bulb.
Keywords: Olfaction, deoxyglucose, mapping, enriched environment
Introduction
Olfactory experiences during the early postnatal period can have a large impact on the development of the olfactory system in rodents. For example, enriched odor experience during the first three postnatal weeks with a specific odorant results in a significant increase in [14C] 2-deoxyglucose (2-DG) uptake in focal regions of the glomerular layer in response to that same odorant (Coopersmith and Leon, 1989; Johnson et al., 1995; Johnson and Leon, 1996). In another series of studies of glomerular responses, we have supported the idea of a combinatorial code for olfactory processing, in which different odorants can share glomerular representations by possessing common molecular features (Johnson et al., 1998; 1999; 2002; Johnson and Leon, 2000a, b). It therefore seemed possible that if rats experienced a number of odorants that together evoked responses over a wide area of the bulb, novel odorants may also be able to evoke an enhanced response if they had overlapping responses with previously experienced odorants.
To ensure that a sufficient portion of the glomerular layer was activated by our panel of odorants, we selected seven odorants from our glomerular response data archive (http://leonserver.bio.uci.edu) that we knew together would stimulate virtually the entire glomerular layer: alpha-phellandrene, benzaldehyde, L-carvone, decanal, pentanol, santalol, and valeric acid. Each of these enrichment exposure odorants stimulated a different region of the bulb, but collectively the odorants stimulated the entire bulb (Johnson et al., 2002). Groups of young rats were exposed to different odorants over the first three postnatal weeks. A variety of chemical classes were presented in this odorant series: alcohols, acids, ketones, and aldehydes. Animals experienced three cycles of a seven-day rotation of odorants, with odorants changed daily for postnatal days 1-21. Two alicyclic ketone test odorants (alpha-ionone and L-menthone) were selected from the odorant archive that shared molecular features with some of the enrichment odorants. These two odorants activated regions that overlapped with the glomerular regions activated by the enrichment odorants and were used as novel test odorants in the study. The 2-DG method was used to determine the bulbar neural response to both previously experienced odorants and the two novel odorants.
Materials and methods
Odor enrichment for odor coding analysis
To ensure that a sufficient portion of the glomerular layer was activated by the odorants, we used our glomerular response data archive to select seven odorants that together would activate the vast majority of the glomerular layer. These seven odorants were alpha-phellandrene, benzaldehyde, L-carvone, decanal, 1-pentanol, santalol, and valeric acid (Figure 1). Beginning on postnatal day 1, 6-9 drops of undiluted odorant were applied on a clean cotton ball that was put in a metal tea ball suspended from the top of the housing cage. Two such metal tea balls were suspended in different areas of each cage. Litters in the enriched group (n=10) were exposed to one odorant per day, and the odorant was different for each of seven days. This odorant schedule was repeated over the course of three weeks. Control litters (n=10) were exposed to two tea balls each containing a clean cotton ball that was changed daily. Control and enriched litters were housed in separate rooms to avoid odor contamination. All procedures used in this study were approved by the Irvine Animal Care and Use Committee.
Figure 1.
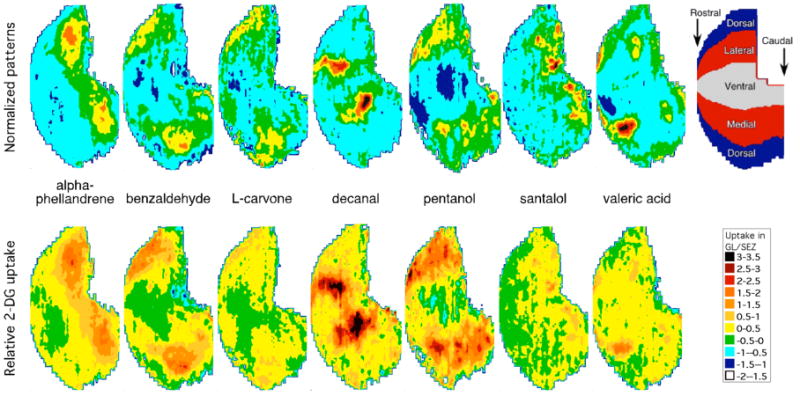
Averaged z-score normalized (top) and relative 2-DG uptake (bottom) contour charts for the seven odorants used for early olfactory enrichment (alpha-phellandrene, benzaldehyde, L-carvone, decanal, pentanol, santalol, and valeric acid). Data from the glomerular response archive (http://leonserver.bio.uci.edu) were transformed to create relative 2-DG uptake charts. An orientation schematic diagram (top, right) and a color key for relative uptake (top, left) are included for reference. GL, glomerular layer; SEZ, subependymal zone.
[14C]2-deoxyglucose procedure
Odorants were volatilized by bubbling high-purity nitrogen gas through the liquid in a gas-washing bottle. Volatilized odorants then were diluted by mixing the vapor with ultra-zero grade air at a final flow rate of 2 L/min. Flow rates were regulated and measured with Gilmont flow meters. On postnatal day 22, using our standard technique (Johnson et al., 1998), male rats (n=9-10 per group in a split-litter design) were injected subcutaneously with [14C]-2-deoxyglucose (0.2 mCi/kg; Sigma Chemicals) and immediately exposed to a previously experienced odorant (L-carvone or valeric acid), or to a novel odorant (alpha-ionone or L-menthone). Animals were freely breathing and remained in Mason jars during the 45-minute odor exposure. Animals were decapitated, and brains were rapidly removed and frozen in −45° isopentane. Frozen, coronal sections were cut at 20μm through the entire bulb, and every sixth section was used for autoradiography.
Mapping procedure
The activity in the glomerular layer was mapped as previously described (Johnson et al., 1999; 2002; 2004), using customized image analysis software. Briefly, the glomerular layer was located in regularly spaced, coronal, autoradiographic images of 2-DG uptake by using overlaid images of adjacent sections that had been stained with cresyl violet. Discrete measurements of radioactivity were taken at intervals specified by the intersections of the glomerular layer with standard grid lines chosen on the basis of each section's relative anterior-posterior position between anatomical landmarks. The resulting data arrays were transformed to correct for the different sizes of individual bulbs, and the matrices corresponding to left and right bulbs for each animal were averaged to produce anatomically standardized matrices corresponding to the entire extent of the glomerular layer. The average uptake array for animals exposed to air vehicle was subtracted from each odorant-exposed animal's array. The distribution of uptake across the layer was plotted as color-coded contour charts. To examine amounts of uptake, we expressed glomerular layer radioactivity as a ratio to that measured in the subependymal zone of the same brain. Statistical analysis of these subependymal zone values revealed no significant differences across experimental groups (F[1, 4] = 1.989; P = 0.162). To examine patterns of uptake independently of the amount of uptake, we expressed individual measurements as z scores of relative activity across the same bulb.
Modules and quantitative comparisons of odorant-evoked activity
Statistical comparisons of the patterns and amounts of uptake across the bulb were made after reducing the complexity of the data matrices. To accomplish this reduction, we obtained a single mean value for each of 27 non-overlapping regions of the bulb that were determined in previous studies to display common responses to particular odorant molecular features (Linster et al., 2001; Johnson et al., 2002). For comparisons of uptake patterns, we used the maximum z-score value within each of these response regions (Linster et al., 2001; Johnson et al., 2004; 2005).
Results
Odor enrichment increases neural activity in the glomerular layer
Early olfactory enrichment using multiple odorants increased the uptake of 2-DG both to previously experienced odorants and to novel odorants (Figure 2, top). Increased levels of uptake were apparent across the entire layer encompassing most of the bulb, both within high-uptake foci and across regions of the layer not displaying high levels of uptake (Figure 2, bottom, yellow, orange, and red). Using a 3-way ANOVA, neural activity (average uptake divided by subependymal zone) patterns were compared across group condition, odorants, and 27 glomerular response modules that we had identified previously. A statistically significant increase in activity was observed across groups (F[1, 26] = 225.59; P < 0.0001), with enriched animals exhibiting increased activity in response to the test odorants relative to controls for both previously experienced (L-carvone and valeric acid) and novel odorants (alpha ionone and L-menthone). In addition, statistically significant differences also were observed across odorants (F[1,1890]=311.162; P < 0.0001), and across glomerular response modules (F[26,1890]=35.230; P < 0.0001).
Figure 2.
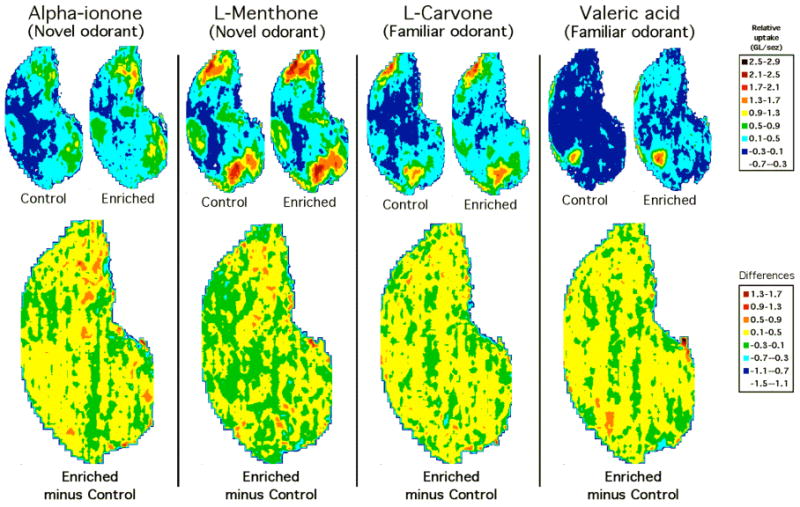
Olfactory responses following early olfactory enrichment. Normalized contour charts of averaged glomerular responses from control and enriched animals to novel (alpha-ionone and l-menthone) and familiar (l-carvone and valeric acid) odorants (top) and difference charts showing Enriched minus Control values (bottom). For the latter, the colors yellow, orange, and red are assigned difference values of 0.1 and greater. Note for each of the odorants, the very similar patterns of activity for the control and enriched groups exposed to the same odorant, with a statistically significant increase in activity present in the enriched group. GL, glomerular layer; SEZ, subependymal zone.
Odor enrichment does not change the normalized spatial pattern of activity in the glomerular layer
The spatial pattern of activity evoked by the various odorants was examined separately, using the calculated z-score values. Z-score analyses allow one to examine patterns of activity by normalizing the data matrices thereby de-emphasizing absolute activity values. A 3-way ANOVA of maximum z-score values across group condition, odorants, and glomerular modules revealed no difference between enriched and control groups (F[1,1890]=2.731; P > 0.05), although the expected difference was observed across odorants (F[3,1890]=108.96; P < 0.0001), as well as the expected difference across the glomerular response modules (F[26,1890]=73.976; P < 0.0001).
Discussion
Early olfactory enrichment using multiple odorants significantly increased the intensity of neural activity in response to both familiar odorants and unfamiliar odorants that were structurally similar. The spatial patterns of activity evoked by each of these odorants, however, remained unchanged. It appears, therefore, that olfactory enrichment during the first three postnatal weeks does not change the spatial representation of the odorants within the bulb, but rather, affects the intensity of the response of the bulb to these odorants. The two novel odorants stimulated regions of the bulb that overlapped with the previously experienced odorants used during the enrichment procedure, demonstrating that responses evoked by one odorant can lead to enhanced responsiveness to other odorants that stimulate spatially similar regions of the bulb.
One hypothesis for why pre-exposure to one odorant would increase the level of response to another is that these odorants might stimulate the same receptors, sensory neurons, and glomeruli. Individual odorants typically activate local clusters involving multiple glomeruli (Johnson et al., 1998; 1999), suggesting that these glomeruli receive projections from sensory neurons of similar specificity (Johnson et al., 1999; 2004). The particular combination of glomeruli activated is thought to convey information about the distinctive odor of the stimulus (Leon and Johnson, 2003). Repeated activation of a set of glomeruli by an odorant under conditions related to those causing early odor preference learning (Coopersmith and Leon, 1984; Sullivan and Leon, 1986) appear to be effective in priming these glomeruli for responding to other odorants bearing overlapping molecular features.
As can be seen in the z-score transformation of the glomerular activity arrays (Figure 1, top), focal areas of peak activity as well as regions of lower activity can be observed across the bulb for each of the seven enrichment exposure odorants. These odor-specific, global patterns of activity may be used by the animal to represent the individual odorants experienced by the animal (Cleland et al., 2005). From the relative 2-DG uptake arrays (Figure 1, bottom), however, it is clear that each odorant individually stimulated a large proportion of the bulb, and together, stimulated the entire bulb. Because the entire bulb was stimulated during the enrichment procedure, we cannot determine precisely whether the subsequent enhanced responsiveness to the novel odorants was related to the structural similarities of the novel odorants to the enrichment odorants, or whether a global, generalized increase in bulb responsiveness resulted from the enrichment procedure. Global brain or behavioral changes following environmental enrichment have been observed elsewhere (van Praag et al., 2000; Scotto Lomassese et al, 2000; Polley et al, 2004; Sandeman and Sandeman, 2000) including increased levels of neurotrophins (Pham et al., 1999; Pinaud, 2004; Branchi et al., 2004), increased expression of immediate early genes (Pinaud, 2004), and altered responsiveness to stress (Mlynarik, et al., 2004; Benaroya-Mishtein, et al., 2004).
Sensory enrichment enhances neural activity and behavioral performance in other sensory systems, including motor cortex in mice (Turner et al., 2002), the auditory system of adult rats (Engineer et al., 2004), the visual system of mice (Cancedda et al., 2004), and the mushroom body of the cricket (Scotto Lomassese et al., 2000). There are also reports of improved olfactory performance in adult mice and rats following diverse early olfactory experiences (Rochefort et al., 2002, Mandairon et al., 2006). The increase in glomerular activity following early olfactory enrichment observed here with multiple odorants is also consistent with data showing increased neural activity in the bulb following early olfactory training with a single odorant (Coopersmith and Leon, 1984; Johnson et al., 1995; Johnson and Leon, 1996; Sullivan and Leon, 1986; Yuan et al., 2003). Because experience with one odorant does not increase responsiveness to another odorant with a very different pattern of spatial activity in the glomerular layer (Coopersmith, et al., 1986), it will be interesting to determine exactly how much overlap is required for one odorant to facilitate the subsequent response of another.
References
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Branchi I, Francia N, Alleva E. Epigenetic control of neurobehavioural plasticity: the role of neurotrophins. Behav Pharmacol. 2004;15:353–362. doi: 10.1097/00008877-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland T, Johnson B, Leon M, Linster C. Glomerular computations in the olfactory bulb can normalize neural response patterns to a range of odor concentrations. Soc Neurosci Abstr. 2005;614:12. [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;4664:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Dev Brain Res. 1986;27:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Spatial distribution of [14C]2-deoxyglucose uptake in the glomerular layer of the rat olfactory bulb following early odor preference learning. J Comp Neurol. 1996;376:557–566. doi: 10.1002/(SICI)1096-9861(19961223)376:4<557::AID-CNE5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic and acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9827–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Enrichment to odors improves olfactory discrimination in adult rats. Behav Neurosci. 2006;120:173–179. doi: 10.1037/0735-7044.120.1.173. [DOI] [PubMed] [Google Scholar]
- Mlynarik M, Johansson BB, Jezova D. Enriched environment influences adrenocortical response to immune challenge and glutamate receptor gene expression in rat hippocampus. Ann N Y Acad Sci. 2004;1018:273–280. doi: 10.1196/annals.1296.032. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Pinaud R. Experience-dependent immediate early gene expression in the adult central nervous system: evidence from enriched-environment studies. Int J Pharm. 2004;272:109–119. doi: 10.1080/00207450490264142. [DOI] [PubMed] [Google Scholar]
- Polley DB, Kvasnak E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. “Impoverished” and “enriched” living conditions influence the proliferation and survival of neurons in crayfish brain. J Neurobiol. 2000;45:215–226. [PubMed] [Google Scholar]
- Scotto Lomassese S, Strambi C, Strambi A, Charpin P, Augier R, Aouane A, Cayre M. Influence of environmental stimulation on neurogenesis in the adult insect brain. J Neurobiol. 2000;45:162–71. [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Dev Brain Res. 1986;27:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. Early odor preference learning in the rat: bidirectional effects of camp response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. J Neurosci. 2003;23:4760–4765. doi: 10.1523/JNEUROSCI.23-11-04760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


