Abstract
Systematic mapping studies involving 365 odorant chemicals have shown that glomerular responses in the rat olfactory bulb are organized spatially in patterns that are related to the chemistry of the odorant stimuli. This organization involves the spatial clustering of principal responses to numerous odorants that share key aspects of chemistry such as functional groups, hydrocarbon structural elements, and/or overall molecular properties related to water solubility. In several of the clusters, responses shift progressively in position according to odorant carbon chain length. These response domains appear to be constructed from orderly projections of sensory neurons in the olfactory epithelium and may also involve chromatography across the nasal mucosa. The spatial clustering of glomerular responses may serve to “tune” the principal responses of bulbar projection neurons by way of inhibitory interneuronal networks, allowing the projection neurons to respond to a narrower range of stimuli than their associated sensory neurons. When glomerular activity patterns are viewed relative to the overall level of glomerular activation, the patterns accurately predict the perception of odor quality, thereby supporting the notion that spatial patterns of activity are the key factors underlying that aspect of the olfactory code. A critical analysis suggests that alternative coding mechanisms for odor quality, such as those based on temporal patterns of responses, enjoy little experimental support.
Keywords: Sensory coding, rat, 2-deoxyglucose, imaging techniques, mapping
Olfactory stimuli
Olfactory stimuli are typically vaporous chemicals that bind to odorant receptors on olfactory sensory neurons in the nasal epithelium (Buck and Axel, 1991; Axel, 1995; Buck, 1996). The odorant molecules are thought to bind and activate receptors through mechanisms similar to those dictating other receptor-ligand interactions (Araneda et al., 2000; Kajiya et al., 2001; Katada et al., 2005). To begin to understand these interactions, it seems reasonable to study pure odorant chemicals of known structure, just as early studies of visual coding used controlled spots of light (Kuffler, 1953; Hubel and Wiesel, 1959) and studies of auditory coding used pure tones of controlled frequency and volume (e.g., Scheich and Zuschratter, 1995).
Because pure chemicals differ from one another incrementally rather than continuously, even small differences in odorant structure can change multiple chemical dimensions that might be relevant to receptor interactions. Many incremental changes can be made to a single molecule, as shown in Figure 1, and each change can result in alterations in associated molecular properties such as length, hydrophobicity, polarity, and flexibility that might affect the ability of different parts of the molecule to associate with any given receptor (Ho et al., 2006b). Therefore, one needs to study a large number of odorant chemicals varying along these different dimensions to be able to understand stimulus coding by the olfactory system.
Figure 1.
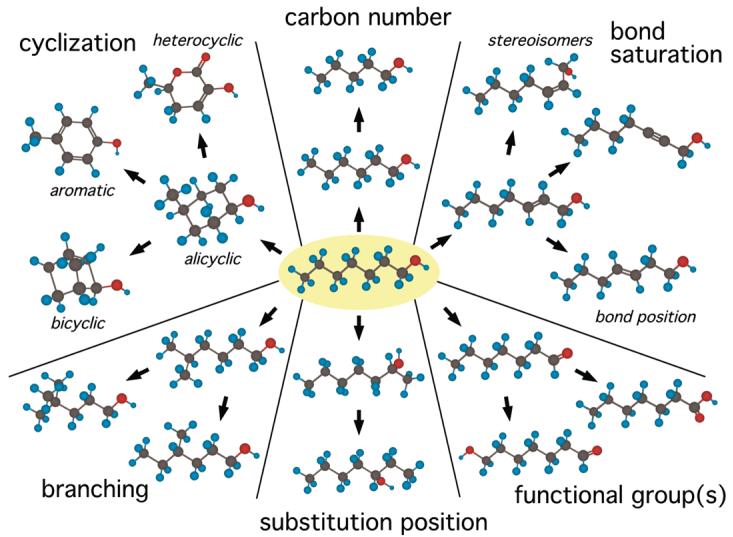
Odorants can differ along many chemical dimensions. The odorant chemical in the center (pale yellow background) is 1-heptanol. Arrows indicate various incremental differences in structure, each of which produces a chemical of distinct steric and electronic properties. Although some dimensions, such as those involving changed or additional functional groups, result in greater differences in the molecular properties of the odorants than do other dimensions, almost all distinct chemicals evoke odors that can be discriminated by animals that are trained to do so. Studies of olfactory coding must account for both the breadth of odorant chemical structures that are detected by animals and the subtle discriminations that are possible between closely related compounds. Because of this dual challenge, the research must involve a great number of odorants.
Anatomical foundation for the early stages of olfactory processing in rodents
There are about 1,000 different odorant receptor genes in rats and mice (Zhang and Firestein, 2002), and most sensory neurons probably express only one of these receptors (Serizawa et al., 2004). Sensory neurons expressing the same receptor are organized in many overlapping but distinct zones stretching anterior to posterior across the olfactory epithelium (Ressler et al., 1993; Vassar et al., 1993; Miyamichi et al., 2005). During passive breathing, an odorized air stream is drawn at low flow rate along the central channel of the nasal epithelium and then across the remaining receptor expression zones, with odorant molecules absorbing differentially into the chemically complex, aqueous mucosa along the way (Mozell, 1964; Hornung and Mozell, 1977; Hornung et al., 1987; Mozell et al., 1987; Figure 2). During active sniffing, such as occurs when rodents are exploring their environment, or performing learned olfactory-guided behaviors, animals alter the airflow dramatically (Youngentob et al., 1987).
Figure 2.
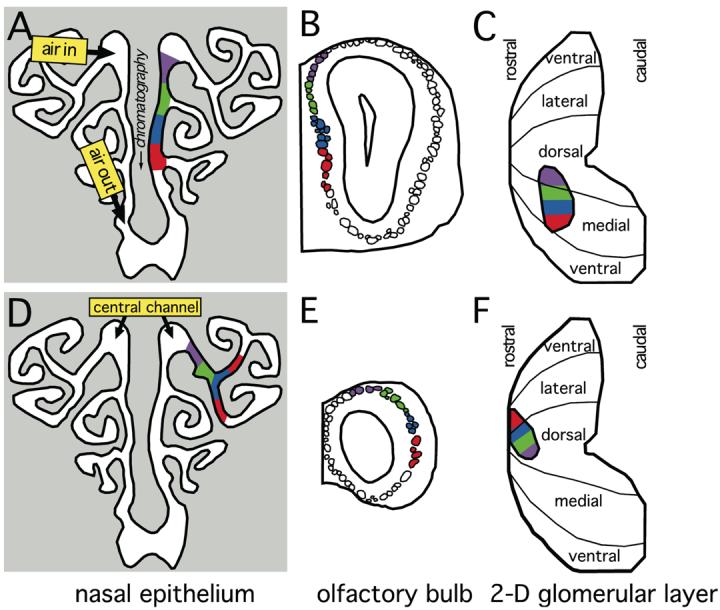
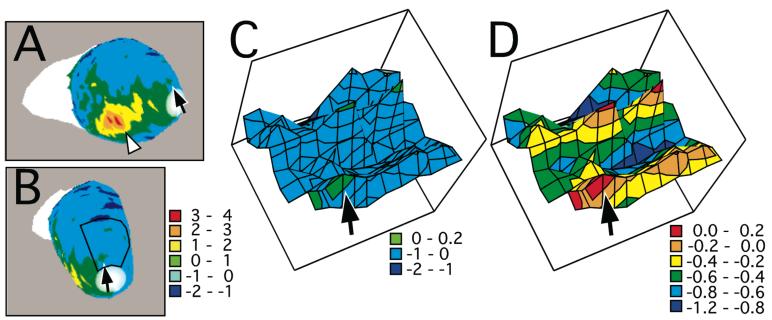
Epithelial chromatography may be related to chemotopic progressions in the olfactory bulb. A, D: The structure of the nasal cavity is shown as a diagram of a coronal section taken just anterior to the caudal end of the internal naris. Odorant representations in the medial aspect of the bulb involve septal and ventral portions of the nose (A), whereas odorant representations in the lateral aspect of the bulb involve lateral portions of the nose (D). Because air exits ventrally from the rat nose, the prevailing flow of odorants is from dorsal to ventral across the olfactory epithelium. Odorants that are more soluble in mucosa (e.g., smaller molecules in a homologous series) therefore will tend to be absorbed more strongly to dorsal parts of the epithelium (magenta and green shading), while odorants that are less strongly absorbed (e.g., larger molecules in a homologous series) will be free to associate with more ventral epithelial regions (blue and red shading). The illustration of air flow in A is highly simplified; for example, in posterior and ventral regions of the nose, air actually flows from posterior to anterior, and under any given respiration condition, there are places in the nose where air velocity is zero (Kimbell et al., 1997). B, E: The structure of the bulb is shown as diagrams of coronal sections. The coloring of the glomeruli outlined in B shows the expected projection zones of similarly colored regions in A, whereas the coloring in E shows the expected projection of similarly colored regions in D. The topography of the epithelium-to-bulb projection is such that more dorsal epithelial regions are associated with more dorsal bulbar regions. C, F: The entire glomerular layer is represented as dorsal-centered charts. The medial acid-preferring response domain is outlined in C and colored to indicate the locations of glomeruli shaded in B. The corresponding lateral domain is outlined in F and shaded to show the locations of glomeruli in E. The relative absorption of odorants differing in carbon number together with the topography of the epithelium-to-bulb projection therefore could explain observed chemotopic progressions of glomerular responses from dorsal to ventral across the glomerular layer.
Olfactory sensory neurons expressing the same odorant receptor gene converge in their projection into only a few glomeruli in the olfactory bulb, and these glomeruli are consistently located across individuals (Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996). Sensory neurons located along the nasal septum or in the ventral part of the nasal turbinates project to glomeruli in the medial half of the bulb, whereas homologous sensory neurons located in the lateral turbinates project to lateral glomeruli (Astic and Saucier, 1986; Clancy et al., 1994; Lèvai et al., 2003; Figure 2). Sensory neurons located in the central channel of the nose project to glomeruli in the dorsal half of the bulb, while sensory neurons located in progressively peripheral or ventral parts of the nose project more ventrally (Saucier and Astic, 1986; Schoenfeld et al., 1994; Schoenfeld and Cleland, 2005; Figure 2). These projections form two mirror-image maps of odorant receptor input, one on the lateral aspect and one on the medial aspect of the bulb (Miyamichi et al., 2005; Tsuboi et al., 2006). In addition, a distinct set of sensory neurons expressing characteristic odorant receptor genes is clustered at the tips of certain turbinates rather than along the anterior-posterior expression zones (Strotmann et al., 1992; 1999), and sets of these neurons expressing the same gene project to unpaired glomeruli along the ventral extremity of the bulb (Strotmann et al., 2000). Most evidence suggests that each glomerulus receives input only from sensory neurons homologous with respect to the expression of a single odorant receptor gene (Treloar et al., 2002; Wachowiak et al., 2004).
The convergence of homologous sensory neurons to produce receptor-based maps in the glomerular layer of the main olfactory bulb has provided an obvious target for imaging studies in olfaction. If one could monitor differential odorant-evoked activity across all of the glomeruli in an olfactory bulb, one would obtain a read-out of the differential activation of odorant receptors. By using a large number of systematically chosen odorant stimuli and monitoring the response of the entire glomerular layer, one could determine (1) the features of an odorant stimulus that are responsible for activation of each glomerulus, (2) the aspects of each odorant stimulus that are most relevant to its representation, (3) the features that are ignored by the receptors altogether, and (4) the spatial arrangements of different responses in the glomerular layer that may suggest a specific kind of processing of particular odorant-related information.
We first will review the early work establishing the fact that different odorants and different aspects of odorant chemistry are associated with the activation of neurons in different parts of the olfactory bulb, and then we will discuss the current state of understanding of the relationships between odorant chemistry and activity of various bulbar locations. We will address criticisms of different approaches to study this system, as well as the relevance of the measured spatial patterns to odor perception. We also offer a brief, critical discussion of temporal coding hypotheses that consider spatially distinct activity patterns to be largely irrelevant to olfaction.
“Odotopic” organization of bulbar responses
Adrian concluded from evoked potential studies that different odorants activate different parts of the olfactory bulb, a concept termed “odotopy” (e.g., Adrian, 1950a). A consideration of the anatomy of the olfactory nerve projections led Le Gros Clark (1957) to suggest that different glomeruli might contain converging input from sensory neurons of similar specificity, and that different glomeruli might respond differentially to different odorant chemicals. Indeed, evoked potentials in particular glomeruli then were shown to differ in sensitivity to particular odorants (Levetau and MacLeod, 1966).
Possible relationships between particular odorants and particular bulbar locations were later mapped onto “representative” coronal sections by studying differential degeneration following long-term exposures to an impressively large array of single odorants (e.g., Pinching and Døving, 1974). These degeneration studies also showed a consistency in location across different animals exposed to the same single odorant (Døving and Pinching, 1973; Pinching and Døving, 1974). Despite these early indications of a relationship between particular odorants and particular bulbar locations, the specificity of this relationship was not recognized until the work of Gordon Shepherd and colleagues using the 2DG method (Sharp et al., 1975; 1977; Stewart et al., 1979). They identified foci of 2DG uptake and made two-dimensional activity maps of individual olfactory bulbs. They showed that individual odorant chemicals stimulated a number of segregated areas within the glomerular layer that were consistently located in different individuals, but that were also to some extent overlapping for different odorants (Stewart et al., 1979). These responses were seen in deeper bulbar layers as well (Sharp et al., 1977; Lancet et al., 1982). Other researchers extended the mapping of 2DG uptake to other odorants (Skeen, 1977; Jourdan et al., 1980; Teicher et al., 1980; Coopersmith and Leon, 1984; Coopersmith et al., 1986; Bell et al., 1987; Wilson and Leon, 1988; Sicard et al., 1989; Slotnick et al., 1989), and the collection of 2DG data was systematized and subjected to statistical analysis that showed that spatial patterns of uptake differed significantly for different odorants (Royet et al., 1987).
The odotopic activation of particular parts of the bulb by particular odorants now has been confirmed by many techniques including the mapping of field potentials (Mori et al., 1992), unit recordings of mitral cells (Mori et al., 1992; Imamura et al., 1992; Katoh et al., 1993), up-regulation of immediate early gene products in the glomerular and granule cell layers (Onoda, 1992; Guthrie et al., 1993; 2000; Sallaz and Jourdan, 1993; Schellink et al., 1993; Schaefer et al., 2001a; 2002; Inaki et al., 2002; Montag-Sallaz and Buonviso, 2002; Salcedo et al., 2005), optical imaging of either endogenous responses (Rubin and Katz, 1999; Uchida et al., 2000; Meister and Bonhoeffer, 2001; Takahashi et al., 2004a,b; Igarashi and Mori, 2005) or voltage- and calcium-sensitive dye responses (Wachowiak and Cohen, 2001; 2003; Fried et al., 2002; Spors and Grinvald, 2002; Spors et al., 2006), and functional magnetic resonance imaging (Yang et al., 1998; Xu et al., 2000; 2003; 2005; Schafer et al., 2006).
Odotopy has been extended to other classes of vertebrates (e.g., zebrafish: Friedrich and Korsching, 1997; 1998; Fuss and Korsching, 2001, catfish: Nikonov and Caprio, 2001; Nikonov et al., 2005, salamanders: Cinelli et al., 1995), as well as to insects (e.g., honeybees: Joerges et al., 1997; Galizia et al., 1999a; Sachse et al., 1999, moths: Galizia et al., 2000; Carlsson et al., 2002; Collmann et al., 2004; Skiri et al., 2004; Lei et al., 2004, ants: Galizia et al., 1999b, and Drosophila: Rodrigues, 1988; Wang et al., 2003; Kreher et al., 2005). Given this evidence, it would appear that odorant-specific spatial patterning of glomerular activation is a basic characteristic of olfactory systems.
Odotopy is in fact an inevitable consequence of the fact that sensory neurons expressing different odorant receptors have differential responses to different odorants and project to different locations in the bulb. This fact does not by itself indicate that the brain uses information about the location of the activated glomeruli in olfactory processing, because glomeruli must be located somewhere in space. However, the fact that different individuals exposed to the same odorant have the same pattern of activity would not be predicted by any hypothesis that considers spatial location to be unimportant for olfactory coding.
Chemotopic organization of bulbar responses
Adrian (1950a, 1953) reported that spatial patterns of bulbar activity might be related to odorant chemical features such as functional groups or lipid solubility, but this idea of chemotopy was not pursued until Korsching and coworkers showed that amino acids with similar side chains activated similar sets of glomerular clusters in zebrafish (Friedrich and Korsching, 1997; Fuss and Korsching, 2001). Other odorant classes such as bile acids and pheromones stimulated other parts of the bulb, whereas nucleic acids stimulated characteristic glomerular activity patterns in regions partly overlapping with amino acid-sensitive zones (Friedrich and Korsching, 1998). Segregation of glomeruli responding to some of these same odorant classes also has been clearly demonstrated in catfish (Nikonov and Caprio, 2001), where chemotopic organization extends into the forebrain (Nikonov et al., 2005).
Fish live in an aquatic environment where individual odorant chemicals are water-soluble, often charged molecules of limited diversity; they also have a more limited repertoire of odorant receptors and many fewer glomeruli than do rodents (Alioto and Ngai, 2005), which are exposed to a wider variety of meaningful odorant chemistries through the air. Despite its greater complexity, the rat olfactory system also has a chemotopic organization. We have performed a series of studies focused on systematic sets of odorants organized with respect to their chemical structures (Table 1), and we have mapped 2DG uptake across the entire glomerular layer in response to each odorant (Johnson et al., 1998; 1999; 2002; 2004; 2005a,b; 2006; 2007a,b; Johnson and Leon, 2000a,b; Farahbod et al., 2006; Ho et al., 2006a,b). We average our data across a group of individuals exposed to the same odorant to obtain spatial maps that can be used for statistical analyses to establish where responses are significantly different among odorants. By using the same methods across our different experiments, we have been able to construct an archive of odorant responses that allows relationships between these patterns to be visualized and compared in different orientations and formats (http://leonserver.bio.uci.edu).
Table 1.
Systematic differences in odorant chemistry studied using the 2DG technique
| Chemical difference | Reference |
|---|---|
| Functional group in aliphatic compounds |
Johnson and Leon, 2000a Johnson et al., 2002 |
| Carbon number in straight-chained aliphatic compounds |
Johnson et al., 1999 Johnson et al., 2004 Ho et al., 2006a |
| Functional group position in aliphatic compounds | Johnson et al., 2005a |
| Branching in hydrocarbon chains |
Johnson and Leon, 2000b Ho et al., 2006b |
| Bond saturation |
Johnson and Leon, 2000b Ho et al., 2006b Johnson et al., 2007a |
| Cis-trans isomerism at double bonds |
Ho et al., 2006b Johnson et al., 2007a |
| Multiple functional groups | Johnson et al., 2007b |
| Cyclization |
Johnson and Leon, 2000b Johnson et al., 2006 |
| Enantiomers | Linster et al., 2001 |
| Functional group on aromatic compounds |
Johnson et al., 2005b Farahbod et al., 2006 |
| Substitution position in aromatic compounds | Farahbod et al., 2006 |
| Interactions between functional group and hydrocarbon structure |
Johnson and Leon, 2000b Johnson et al., 2005b |
Three types of chemotopic organization have emerged from these studies. In the first, a cluster of glomeruli responds similarly to odorants with similar structural features and/or with similar overall molecular properties such as water solubility. In the second type of chemotopy, activated glomeruli within a cluster are arranged systematically in space in relation to a molecular property of the odorant. In the third type, which we have termed “global chemotopy”, the degree of similarity in overall spatial patterns of activity across the glomerular layer is proportional to the degree of similarity in odorant chemistry. We shall discuss each level of chemotopic organization in turn.
Local clusters of glomeruli respond to odorants of similar chemistry
An early piece of evidence that clusters of adjacent glomeruli have similar chemical response specificity came from studies of glomerular responses to increasing concentrations of a single odorant. Imaging methods including 2DG (Stewart et al., 1979; Johnson and Leon, 2000a), in situ hybridization for c-fos mRNA (Guthrie and Gall, 1995), and optical imaging of either voltage-dependent dyes (Cinelli et al., 1995) or intrinsic signals (Meister and Bonhoeffer, 2001), showed that low concentrations of an odorant tend to stimulate very few glomeruli in any given location, consistent with the activation of the highest affinity receptors for the odorant ligand. Increasing concentrations of the same odorant recruit responses in glomeruli located nearby the originally activated ones, with the overall effect of increasing the area of the response roughly in proportion to odorant concentration. The newly activated neighboring glomeruli are likely associated with odorant receptors possessing a lesser affinity for the odorant ligand, perhaps because their best stimuli are closely related odorant chemicals.
The stimulation of a large cluster of neighboring glomeruli at high concentrations of some odorants may be related to the ability of those odorants to assume multiple conformations that satisfy the binding requirements for multiple, related receptors. In general, we have found that flexible, straight-chained aliphatic odorants such as valeric acid and methyl valerate are the ones that tend to activate large clusters of glomeruli at high concentrations (Figure 3A), whereas odorant molecules with less flexibility (fewer rotatable bonds) activate fewer glomeruli (Johnson et al., 1999; 2006). These more rigid odorants include shorter aliphatic molecules such as propionic acid and methyl acetate (Figure 3B), as well as various cyclic odorants such as cyclobutanecarboxylic acid and oxyoctaline formate (Figure 3C). The focal 2DG responses shown in Figure 3 also serve to illustrate that the 2DG method is capable of single-glomerular resolution when it is applied to individual olfactory bulbs.
Figure 3.
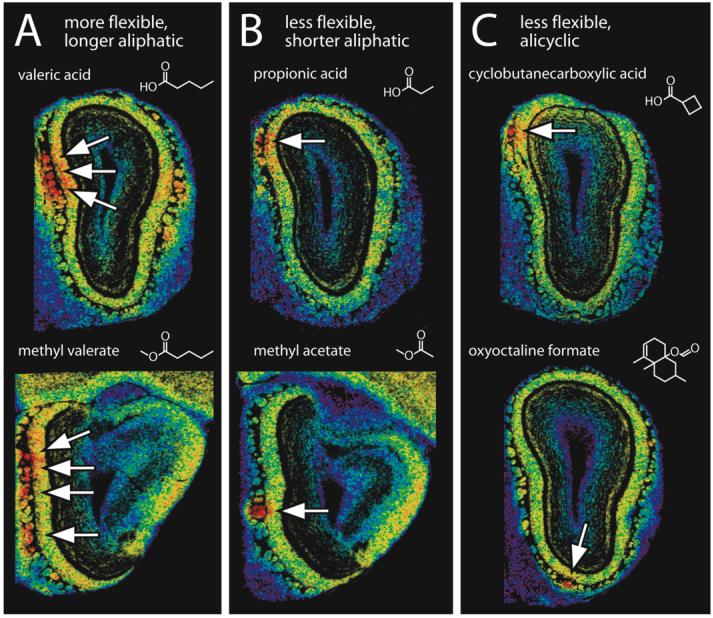
Flexible odorants often activate clusters of glomeruli at high concentrations, whereas small or rigid odorants often activate isolated glomeruli in the same response regions. False-colored images of autoradiography sections indicate the relative distributions of [14C]2-deoxyglucose uptake within regions exhibiting primary responses (arrows) to each of the illustrated odorants, and enhanced-contrast images of adjacent cresyl violet-stained sections are overlaid to indicate the relationships between foci of uptake and individual glomeruli. A: Valeric acid and methyl valerate, which are flexible odorants capable of assuming a variety of conformations, evoke 2DG uptake over clustered sets of glomeruli. B: Smaller aliphatic compounds with the same functional groups, such as propionic acid and methyl acetate, cannot assume as many conformations and appear to activate isolated glomeruli in the same parts of the bulb. C: Rigid, cyclic structures such as cyclobutanecarboxylic acid and oxyoctaline formate also cannot assume multiple conformations and also appear to stimulate isolated glomeruli.
The best evidence for spatial clustering of glomeruli with similar specificity comes from experiments where responses are mapped to systematic sets of odorants differing by small increments in structure. Typically, different odorants sharing certain aspects of chemistry are found to stimulate overlapping, but distinct sets of glomeruli in the same general area of the bulb. Figure 4 summarizes our understanding of the modular representations of these odorant chemical features across the glomerular layer of the rat olfactory bulb. Some of these glomerular modules have specificities characterized by odorant functional groups, others are associated with elements of odorant hydrocarbon structure, and still others are associated with overall molecular properties of the odorants, independent of their specific structural features. The maps of odorant chemistry appear in duplicate, with one copy on the lateral aspect and one copy on the medial aspect of the bulb (Johnson et al., 1998), an organization that parallels the paired lateral and medial projection of sensory neurons expressing the same odorant receptor gene (Ressler et al., 1994; Vassar et al., 1994; Miyamichi et al., 2005). Our previously published diagrams of odorant response modules (Johnson and Leon, 2000a; Johnson et al., 2002) have obvious relationships to the present summary figure (Figure 4). However, the mapping of hundreds of additional responses predictably has led to adjustments in boundaries, and in some cases adjacent modules that were previously defined as separate entities now have been fused, with the recognition that they actually contain responses to similar compounds.
Figure 4.
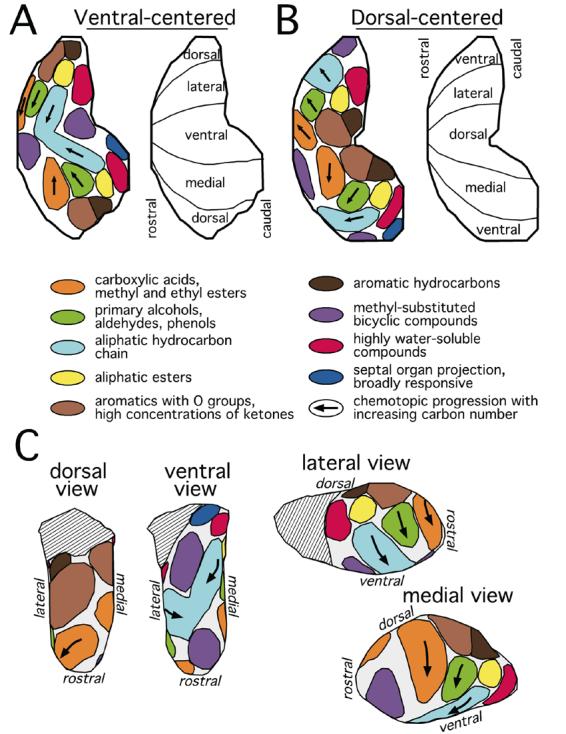
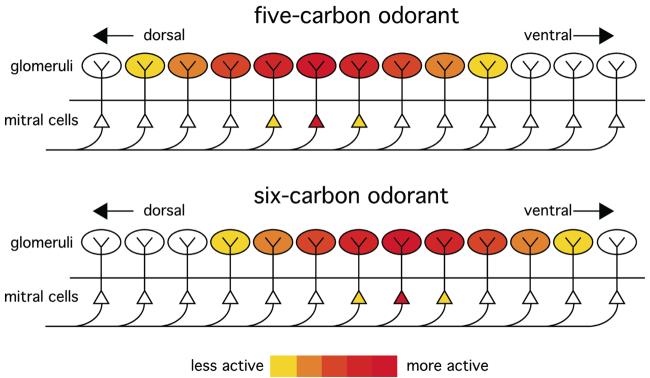
Responses to odorants are organized chemotopically in the rat olfactory bulb. Distinct odorant chemicals sharing certain molecular features (e.g., functional groups or aspects of hydrocarbon structure) or overall molecular properties (e.g., water solubility) stimulate overlapping, but distinct sets of glomeruli that are clustered within functionally defined domains distributed across the glomerular layer. These domains are illustrated as colored areas within a ventral-centered 2D plot of the glomerular layer in A, a dorsal-centered 2D plot in B, and various views of a 3D model of the surface of the glomerular layer in C. Chemotopically defined response domains are usually present as pairs, one on the medial aspect and one on the lateral aspect of the bulb. Arrows indicate the directions of systematic shifts in the location of responses that are observed for odorants of increasing carbon number along homologous series of straight-chained, saturated, aliphatic odorants.
The specificities of these modules are described in detail in the final section of this review, which both summarizes our own 2DG mapping studies and relates our findings to those from other labs that have used other methods to monitor odorant-evoked glomerular activity. In most cases, our results using 2DG are complementary to observations from optical imaging studies, especially when these are conducted across large portions of the glomerular layer (see Mori et al., 2006).
All possible chemical features are not represented as unique modules
As described above, glomeruli responding to odorants with similar functional groups, hydrocarbon structures, or overall molecular properties are often clustered together in the bulb. However, there are some chemical features that are not represented by their own glomerular modules. For example, although double and triple bonds have unique partial charge distributions and steric configurations that presumably could serve as distinctive binding sites for sets of odorant receptors, odorants sharing such features do not specifically overlap in their stimulation of particular glomeruli (Ho et al., 2006b; Johnson et al., 2007a). Instead, unsaturated bonds seem either to disrupt the recognition of certain odorant ligands by certain receptors or to change the glomeruli that are activated within a module (Ho et al., 2006b; Johnson et al., 2007a). Another example is certain cyclic structures, which would seem to provide enough specific chemical “information” to support recognition by a class of odorant receptors, but which do not activate common glomeruli (Johnson et al., 2006).
More generally, some chemical attributes appear to have greater importance in determining responses than others, just as some odorants seem to evoke more overall activity than others (Johnson and Leon, 2000b; Ho et al., 2006b; Johnson et al., 2006; 2007a). The olfactory system evolved to solve biologically relevant problems under certain constraints, and an all-purpose qualitative analysis of odorant chemistry would not be expected of the system, which is more likely to be focused on sets of odorant chemicals that contribute important information relevant to survival and reproduction. We will revisit this theme in the discussion of chemotopic progressions below.
Chemotopic progressions within glomerular modules
Different odorants activating the same response module typically stimulate overlapping, but distinct, sets of glomeruli within the module. This phenomenon is apparent even after the averaging of patterns of 2DG uptake across both bulbs of several different animals (Johnson et al., 1998; 1999; 2004; Johnson and Leon, 2000b; Farahbod et al., 2006), and we take it as evidence that sensory neurons of distinct, but related specificity project to nearby, quite consistently situated glomeruli in these modules. For three pairs of modules, the arrangement of glomeruli is systematic with respect to odorant chemistry, establishing another of level of chemotopic organization in the system. In all three pairs (shaded orange, green, and light blue in Figure 4), straight-chained, unsaturated, aliphatic odorants of greater carbon number stimulate progressively ventral glomeruli (arrows in Figure 4).
Ventral progressions with increasing carbon number in homologous series have been shown for carboxylic acids and ethyl esters in the anterior domains responding to these compounds (Figure 4, orange shading). These progressions have been seen in studies of 2DG uptake (Johnson et al., 1999; 2004), optical imaging (Uchida et al., 2000), and immediate early gene expression (Inaki et al., 2002). In the lateral member of this pair of domains, the shift also involves a progressive movement rostrally with increasing carbon number, such that aliphatic acids of eight or more carbons stimulate glomeruli located on the ventral part of the rostral pole of the bulb (Johnson et al., 1999; unpublished data). Shifts in the same areas are found in many studies using aldehyde odorants and either 2DG uptake (Johnson et al., 2004) or optical imaging (Rubin and Katz, 1999; Uchida et al., 2000; Meister and Bonhoeffer, 2001), although we interpret these shifts as being related to carboxylic acids present as oxidized contaminants in the aldehyde preparations (Johnson et al., 2004). One study using a recombinant fluorescent marker of synaptic vesicle fusion did not detect chemotopic progressions in this lateral, anterior module in response to a series of aldehydes (Bozza et al., 2004), which may indicate either a variable oxidation state of the odorants used in that study, or a different imaged area.
In any homologous series of straight-chained odorants, a greater carbon number is associated with systematic differences in numerous related chemical properties, including molecular length, molecular volume, and hydrophobicity. Carboxylic acid odorants of different hydrocarbon structures (double bonded, branched, and cyclic) do not show as much covariance in these related properties. We exploited this situation and found that the chemotopic progression of responses in the medial domain was more correlated with molecular length than with the other properties (Johnson and Leon, 2000b).
Increasing carbon number in alcohols and aldehydes is associated with ventral chemotopic progressions in the module preferring these functional groups (green shading in Figure 4), as determined by 2DG uptake (Johnson et al., 2004) and immediate early gene expression (Inaki et al., 2002). The phenomenon is seen for both primary alcohols and secondary alcohols with the hydroxyl group in the 2-position (Johnson et al., 2004). The hydrocarbon chain-related domains (light blue in Figure 4) also show chemotopic progressions for all effective stimuli including esters, acids, alcohols, aldehydes, and alkanes (Johnson et al., 1998; 1999; 2004; Inaki et al., 2002; Igarashi and Mori, 2005; Ho et al., 2006a).
In summary, chemotopic progressions with increasing odorant carbon number have been detected in multiple response modules using multiple odorant series and multiple imaging methods. In many experiments characterizing these progressions, odorants are presented at fixed vapor phase concentrations (Johnson et al., 1998; 1999; 2004) or across a range of concentrations (Meister and Bonhoeffer, 2001), although similar progressions can be found without controlling odorant concentration (Rubin and Katz, 1999; Uchida et al., 2000; Johnson and Leon, 2000b; Inaki et al., 2002; Igarishi and Mori, 2005; Ho et al., 2006a). The suggestion that chemotopic progressions arise as an artifact of different odorant concentrations across a series (Wilson and Mainen, 2006) is therefore unfounded.
Gaps in chemotopic progressions involving homologous series
In our analyses of 2DG uptake, we average data matrices first across the two bulbs of an individual animal and then across different animals. Due to a small amount of biological variance in glomerular location (Royal and Key, 1999; Strotmann et al., 2000; Schaefer et al., 2001b), as well as to experimental variation in dissection and tissue sectioning, the detailed spatial arrangement of activated glomeruli that is evident in original autoradiograms (e.g., Figure 3) often is not apparent in our averaged matrices. Similarly, statistical analyses of the shift in location of responses within a glomerular module are affected by such variance, so that the chemotopic progressions in 2DG uptake often appear to be smooth and continuous (Johnson et al., 1999; 2004). However, when the contrast between differentially active individual glomeruli was enhanced by mathematical filtering following optical imaging studies of a homologous series of straight-chained odorants, it was evident that glomeruli activated by these odorants often are interrupted by glomeruli that do not respond as well to any members of the series (Meister and Bonhoeffer, 2001). What do these gaps indicate about chemotopic progressions?
We have found that odorants of a wide variety of hydrocarbon structure can stimulate modules that also are activated by straight-chained compounds (Johnson and Leon, 2000b). It seems likely that some of these odorants would stimulate individual glomeruli that are distinct from those activated by straight-chained compounds. In the medial acid-preferring domain, the responses to branched, double-bonded and alicyclic acids are laid out chemotopically along with the straight-chained compounds in an arrangement proportional to molecular length (Johnson and Leon, 2000b). Therefore, we hypothesize that some of the less active glomeruli in the optical imaging studies might be activated by related odorants of distinct hydrocarbon structure that were not tested in those optical imaging studies. We cannot exclude the possibility, however, that glomeruli of unrelated specificity might also be present in these domains.
Possible causes of chemotopic progressions
We have considered two explanations for how chemotopic progressions might arise within glomerular domains. The first idea is that the progressions are actually laid out by chromatography of odorants in the olfactory epithelium (Mozell, 1964; Hornung and Mozell, 1977; Hornung et al., 1987; Mozell et al., 1987; Scott et al., 2000), and then are fed forward to the bulb by way of the topographical relationships involved in that projection (Saucier and Astic, 1986; Clancy et al., 1994; Schoenfeld et al., 1994; Schoenfeld and Cleland, 2005). Relative absorption into the olfactory mucosa depends on the air-mucosa partition coefficient. Although some odorants may absorb to the mucosa through interactions with macromolecules (Pelosi, 1996; Johnson et al., 2005b), much of the absorption is thought to be related to water solubility such that more water-soluble members of any given series should absorb earlier in the air path, which projects to more dorsal bulbar locations, while less water soluble odorants can diffuse further through the air to reach parts of the nose projecting to more ventral bulbar locations (Schoenfeld and Cleland, 2005; Zhao et al., 2006). Within any given homologous series of odorants, the odorants with fewer carbons would be more water-soluble and thereby more prone to early absorption and stimulation of more dorsal bulbar locations (Figure 2).
The second explanation is that sensory neurons bearing receptors specific for shorter molecules project to the dorsal-most glomeruli in the domains, while sensory neurons bearing receptors specific for the longer molecules project more ventrally. The molecular processes that cause axons of sensory neurons expressing the same receptor to bundle together to converge onto single glomeruli also might operate to insure that adjacent glomeruli receive projections from sensory neurons of the most similar specificity. The odorant receptor itself is involved in axonal path finding (Wang et al., 1998), and sensory neurons containing receptors of similar sequence indeed project to nearby bulbar areas (Tsuboi et al., 1999; Strotmann et al., 2000). Therefore, if receptors of similar specificity with respect to odorant molecular length also have similar amino acid sequences, then orderly glomerular clustering would be expected. This receptor-based hypothesis for the origin of chemotopic progressions is consistent with our finding that odorant molecular length is more predictive of the location of response within the medial acid-preferring module than is odorant hydrophobicity (Johnson and Leon, 2000b). Similar experiments have not yet been conducted for other odorant-module combinations.
Chromatographic separation of odorants in the olfactory epithelium and orderly, differential bulbar projections of sensory neurons responding to odorants of different length are not mutually exclusive. These two processes could work together to establish chemotopic progressions within glomerular response modules (Scott-Johnson et al., 2000), especially considering that rats probably can optimize the location of epithelial stimulation by modulating various attributes of their respiratory behavior (Youngentob et al., 1987).
Not all modules show chemotopic progressions
The very dorsal, ketone-responsive domain does not show chemotopic progressions of activity proportional to the number of carbons in straight-chained ketone odorants (Johnson et al., 2004); nor have we been able to identify any other molecular feature or property of ketones that is organized chemotopically within that domain (e.g., Johnson et al., 2005a). One possible explanation is that the most dorsal bulbar domains are associated with the central channel (zone 1) of the epithelium (Schoenfeld and Cleland, 2005). Initial airflow through the central channel should be in the anterior-to-posterior direction across zone 1 (Kimbell et al., 1997). Any chromatography of strongly absorbed odorants occurring along this axis would not result in the stimulation of a distinct set of odorant receptors, so that chromatography could not contribute to a chemotopic progression. The domains preferring aliphatic esters (yellow shading in Figure 4) or aromatic hydrocarbons (dark brown shading), both of which also may involve sensory neurons in epithelial zone 1, also do not show evidence for chemotopic progressions (Farahbod et al., 2006).
We also have found no evidence for chemotopic progressions within the ventral domains that respond to bicyclic and camphoraceous odorants (Johnson et al., 2006). These molecules overlap heavily in the areas they stimulate in the ventral domains, and the complexity of the structure of these odorants makes them difficult to classify along any single dimension. The ventral region of the bulb receives projections from sensory neurons expressing receptors in a non-zonal epithelial distribution (Strotmann et al., 1992), which may also explain the absence of chemotopic progression. Finally, we have not seen evidence for chemotopic progressions corresponding to any attribute of the water-soluble odorants stimulating posterior bulbar domains (Figure 4, red shading), although this topic was not the subject of any direct study (Johnson et al., 2007b).
Possible consequences of chemotopic progressions
Mitral and tufted cell projection neurons located in the dorsomedial part of the rabbit olfactory bulb respond to acid and aldehyde odorants possessing a narrow range of carbon number along a homologous series (Mori et al., 1992; Imamura et al., 1992; Yokoi et al., 1995). Yokoi et al. (1995) showed that when inhibition by interneurons was blocked using the GABA receptor antagonist picrotoxin, these mitral cells responded with robust sequences of action potentials to a broader range of straight-chained odorants, suggesting that lateral inhibition among neighboring glomeruli had served to narrow the molecular receptive range of the mitral cells in this region. Such “tuning” by center-surround lateral inhibition should occur preferentially in areas where related odorants directly activate glomeruli organized in a chemotopic progression (Figure 5). Studies of 2DG uptake (Johnson et al., 1999; 2004), optical imaging (Uchida et al., 2000; Meister and Bonhoeffer, 2001; Takahashi et al., 2004a), and expression of immediate-early genes (Inaki et al., 2002) all showed that the area from which Yokoi et al. (1995) recorded is indeed chemotopically organized with respect to the dimension of carbon chain length that was investigated in that study. Therefore, one consequence of chemotopic organization may be to insure that relative responses of nearby glomeruli can be “compared” by way of mutual inhibition to produce a pattern of mitral cell output that is more distinct with respect to these similar odorants (Figure 5).
Figure 5.
Center-surround lateral inhibition can narrow the molecular receptive range of mitral cells within chemotopically arranged glomerular response domains. Flexible aliphatic odorants often stimulate clusters of adjacent glomeruli (Figure 3). Although odorants differing by a single carbon in length activate overlapping sets of glomeruli, the strongest activation shifts systematically with carbon number in many domains (Figure 4). Because the most strongly activated mitral cells suppress the activity of their neighbors in a center-surround arrangement involving inhibitory periglomerular and granule cell interneurons, the systematic shifts in activity insure that mitral cells within a principal response domain are more selective for particular odorants within such a series than are the corresponding odorant receptors, sensory neurons, and glomeruli (Yokoi et al., 1985).
Given the chemotopic organization of the olfactory bulb, we would not expect mitral cells that are randomly recorded across other parts of the olfactory bulb to show this kind of tuning along any randomly chosen chemical dimension, especially if the definition of “response” were broadened to include inhibition and delayed action potentials. Therefore, the failure of these spatially (and temporally) unconstrained approaches to find evidence for tuning (c.f.: Motokizawa, 1996) cannot be taken as evidence that tuning of odorant responses does not occur.
The rabbits used in these studies of mitral cell tuning (Mori et al., 1992; Imamura et al., 1992; Yokoi et al., 1995) were under urethane anesthesia, and spontaneous mitral cell activity was greatly reduced during the experiments. It has been argued that this condition may have obscured a broader responsiveness of these cells than would have been detected if the animals were differently anesthetized (Motokizawa, 1996) or entirely awake (Bhalla and Bower, 1997; Kay and Laurent, 1999; Rinberg et al., 2006a). Awake animals, such as those we study using 2DG, show complex spontaneous mitral cell activity against which it appears to be easier to detect information about the animal's behavioral state (e.g., hunger, expectation of odor, alertness, or responses to any odor independent of its identity) than to detect information directly relevant to odor quality (Pager, 1974a,b; Bhalla and Bower, 1997; Kay and Laurent, 1999; Rinberg et al., 2006a), at least when the recordings are made in arbitrary locations in response to arbitrarily chosen odorants. We predict that recording from mitral cells associated with the peak glomerular responses in awake rats might reveal selective, high levels of responsiveness to specific odorants against a lower level of background activity, although these mitral cells are also likely to be highly responsive to top-down control of their activity.
Because mitral cell activity ultimately must be responsible for carrying information about odor identity, downstream processing must somehow extract odorant-specific activity from the background of other “information” that is related to behavioral state. The possibility should be considered that the use of anesthetics simply reveals the most relevant odorant-specific information in mitral cells independent of the influences of behavioral state, information that olfactory cortex would extract in some other way. When we mapped 2DG uptake in the superficial granule cell layer and the external plexiform layer of awake rats, where all activity would be secondary to the activation of mitral cells, we found local areas of increased uptake directly beneath the foci of glomerular uptake, which suggests that a predictable subpopulation of mitral cells is more responsive to a given odorant than are the other mitral cells in the bulb (Johnson et al., 1999). Similar relationships between activity in the glomerular layer and deeper bulbar lamina were noted in earlier 2DG (Sharp et al., 1977) as well as in c-fos studies (Guthrie et al., 1993). Moreover, the largest optically imaged glomerular responses are good predictors of the largest projection neuron responses in both rats (Luo and Katz, 2001) and honeybees (Sachse and Galizia, 2002; 2003). We further found that the granule cell activity shifted progressively in location with increasing carbon chain length, just as it did in the glomerular layer (Johnson et al., 1999), thereby confirming the anatomical foundation for the type of tuning detected in the Yokoi et al. (1995) study.
To date, the Yokoi et al. (1995) study appears to be the only one to have reported direct evidence for tuning by way of lateral inhibition in mammals, although Sachse and Galizia (2002) found that principal neurons in honeybees respond more broadly to odorants in the absence of inhibition by local interneurons, and Luo and Katz (2001) found that rat mitral cells located beneath strongly activated glomeruli show a pattern of excitation while surrounding mitral cells are inhibited, a pattern of response that would be expected if the output of the bulb were subject to lateral inhibition. It would be good to have additional examples of tuning in this system before the phenomenon is completely accepted. One benefit of mapping responses to multiple odorants across the entire bulb is to find areas and candidate odorant series to test such hypotheses, and on the basis of our mapping studies, a reasonable choice would be to use homologous series of carbon chain length and to study any of the six (three pairs of) chemotopically organized domains that responds to the odorants with the chosen functional group (Figure 4).
It also should be noted that simpler olfactory systems with fewer glomeruli might not use center-surround, nearest-neighbor relationships to tune output neurons. For example, inhibitory interneurons can connect nearly all glomeruli in an antennal lobe of a honeybee, and spatial proximity is not as meaningful as similarity of response profile in predicting which glomeruli are functionally involved in inhibitory networks in this species (Linster et al., 2005). Lateral inhibition and spatial arrangements of glomeruli likely would be more important in a larger structure such as a rodent olfactory bulb, where periglomerular and granule cell neurons are expected to exert their inhibitory influences over only a fraction of the total bulbar area (Shepherd, 1972). Differences in anatomical organization may explain why there is more evidence of spatial clustering of responses to similar odorant chemicals in vertebrates than in most invertebrates, although chemotopic organization is apparent in Drosophila larva (Kreher et al., 2005).
Not all continuously varying molecular properties are coded by progressions
A criticism of the hypothesis that center-surround lateral inhibition is used to tune responses in the olfactory system is that odorants vary in a highly dimensional space (Figure 1), whereas center-surround architecture is only two-dimensional (Laurent, 1999; Cleland and Sethupathy, 2006; Wilson and Mainen, 2006). The implication is that any two-dimensional organization would be useful only for a sensory system in which a two-dimensional stimulus space is being represented (e.g., the retina). There are at least four important problems with this criticism. First, as will be discussed in another section, the representation of any odorant usually involves a combination of multiple modules involving independent molecular features of the odorant, and tuning to different features in different modules clearly adds dimensionality to the representation. Second, tuning of some responses does not prevent individual receptors and mitral cells from being represented independently to construct a higher-dimensional combinatorial code. Third, differences along some of the “dimensions” of chemistry that are implied by the criticism, such as the nature of functional groups and certain variations in hydrocarbon structure, actually involve such large changes in the odorant stimulus that incremental differences along these “dimensions” cause the activation of entirely distinct sets of receptors, glomerular modules, and mitral cells, so that no center-surround tuning is required. Fourth, we have evidence from glomerular responses and behavioral analyses that not all chemical dimensions of an odorant stimulus are coded at the same level of detail, reducing the number of dimensions that need to be represented.
We have found only one chemical dimension that is mapped by chemotopic progressions across any given glomerular module, namely carbon number (or a correlated property such as molecular length or hydrophobicity). Other systematic changes in odorant chemistry either do not have much impact on the patterns at all, or are represented by differential activity across different response modules. For example, changes involving the presence, number, position, or stereochemistry of double or triple bonds in hydrocarbons have little impact on 2DG uptake patterns (Ho et al., 2006b). Behavioral studies showed that these differences also did not have significant effects on perception using an assay capable of showing odorant generalization (Ho et al., 2006b). The presence, position and number of methyl group branches in hydrocarbon odorants also do not greatly impact 2DG uptake patterns or perception (Ho et al., 2006b). Functional group position has little impact on patterns evoked by ketones or esters, but completely different glomerular modules respond to alcohols differing in substitution position (Johnson et al., 2005a). Position of substitution has little effect on the representation of aromatic hydrocarbons, whereas aromatic odorants with alkyl substituents activate distinct glomeruli from aromatic odorants with oxygenic substituents (Farahbod et al., 2006).
Data from these individual 2DG mapping studies exemplify how an empirical approach to coding in the olfactory system is more productive than considerations about stimulus dimensionality that are not meaningfully constrained by definitions of those dimensions. The olfactory system is not likely to have evolved into an all-purpose analysis system for identification and relative quantification of each and every chemical that might be synthesized by an organic chemist. Rather, natural selection has more likely led to an olfactory system focused on chemical detection and analysis problems relevant to survival and reproduction. By studying many odorants, we can both discover the factors that are of greatest importance to the animal and identify factors that are not as important to them.
Animals appear to be able to learn to discriminate between virtually any pair of odorant chemicals, even those that do not differ along the dimensions that are encoded by chemotopic progressions or by activity in different modules (Linster et al., 2002). Indeed, optical imaging never fails to show a small difference in the relative activation of individual glomeruli even by odorants of very similar structure (Uchida et al., 2000; Takahashi et al., 2004a), indicating that there may always be sufficient information for any olfactory discrimination if the animal is highly motivated and is trained to identify such differences. However, it also is not clear whether animals in nature would receive the dozens (or hundreds) of reinforced experiences necessary for learning these subtle discriminations (Linster et al., 2002).
In a related issue, it may not be the case that all inhibitory interactions between glomeruli or their underlying mitral cells will have uniform center-surround architecture. Rather, it is possible that certain response units will exert a disproportionate influence on some regions of the bulb compared to others (Willhite et al., 2006).
Global chemotopy
Quantitative mapping allows precise evaluation of the relatedness between different patterns, and it has revealed that overall spatial patterns of activity across the bulb are chemotopically organized. We compare pairs of data matrices of 2DG uptake and express the overall relatedness between odorant-evoked spatial patterns using various indices such as Pearson correlation coefficients and principal components analysis. The similarities and differences found in these comparisons reflect both the relative modular representations of odorant features and the chemotopic progressions within the modules, without the need for a priori definitions of modular boundaries. Overall similarities between patterns then can be used to test hypotheses concerning predicted perceptual similarities between odorants.
We very often find that overall pattern similarity is greater for odorant stimuli that share similarity along a single chemical dimension. For example, patterns are more similar for odorants that have a comparable number of carbons along a homologous series of straight-chain aliphatic odorants (carboxylic acids: Johnson et al., 1999; aldehydes, esters, primary alcohols, secondary alcohols, ethyl esters, and acetates: Johnson et al., 2004; alkanes: Ho et al., 2006a). Overall pattern similarities are proportional to similarities in the molecular length of hydrocarbons that differ in branching and bond saturation (Ho et al., 2006b). Patterns are more similar for odorants with more similar functional group positions in aliphatic alcohols and esters (Johnson et al., 2005a), and patterns are more similar for aromatic hydrocarbons with similar numbers of methyl group substituents (Farahbod et al., 2006). Finally, when viewed across large numbers of odorant pairs, the greatest similarities involve odorants that resemble each other in both functional group and hydrocarbon structure, such as odorant enantiomers and positional isomers of aromatic compounds (Johnson et al., 2002).
Combinatorial coding of odorant molecular features
There are more olfactory perceptions than there are receptors, indicating that the identity of the stimulus must be coded using a combination of responses. Moreover, multiple distinct odorant receptor types and multiple glomeruli are activated by most individual odorants (Polak, 1973; Kauer and Cinelli, 1993; Friedrich and Korsching, 1997; Malnic et al., 1999). While many of the glomeruli activated by a single odorant are located in clusters, there also can be stimulation of glomeruli in domains located in very different parts of the bulb (Stewart et al., 1979; Johnson et al., 1998; 1999; 2002; 2004; 2005b; 2006; Inaki et al., 2002). Such clusters often have a different set of chemical determinants for their activation. For example, aliphatic odorants often activate both glomerular domains directly related to their functional groups and domains related to their hydrocarbon chains (Johnson et al., 1998; 2004; Johnson and Leon, 2000a,b; Ho et al., 2006a).
In our experiments using either simple, straight-chained aliphatic compounds with different functional groups (Johnson and Leon, 2000a; Johnson et al., 2002; 2004) or carboxylic acids and esters differing dramatically in hydrocarbon structure (Johnson et al, 1998; 1999; Johnson and Leon, 2000b), it appeared that responses to functional groups and hydrocarbon elements occurred independently of one another, as if these distinct chemical features were separately detected by subsets of odorant receptors. Encouraged by these results, we formulated a hypothesis involving the combinatorial coding of discrete odorant molecular features by discrete glomerular modules (Johnson et al., 2002; Leon and Johnson, 2003). While this simple model maintains its power to predict accurately the responses to other simple aliphatic odorants with single functional groups, further studies involving more complex odorant structures have shown that there are important interactions between some chemical features, resulting in activity patterns that are not initially predictable from responses to the individual features.
Complex hydrocarbon structural features can prevent modular responses to functional group features that were identified using simple aliphatic compounds. For example, when a benzene ring is substituted with an oxygenic functional group such as a methyl ester, responses are not observed in the anterior domain responding to that functional group in aliphatic odorants. Instead, the aromatic feature trumps that response and activity is confined to the posterior, dorsal domain responding to benzyl odorants with oxygenic substituents (Johnson et al., 2005b; Farahbod et al., 2006). Another example is that glomerular responses related to oxygenic functional groups can be hindered if alicyclic structures (Johnson et al., 2007b) or triple bonds (Johnson et al., 2007a) are located near the functional group in the odorant molecule, perhaps because the hydrocarbon feature interferes with the proper positioning of the odorant functional group at the receptor binding site (Araneda et al., 2000).
When two oxygenic functional groups are present in a single odorant molecule, one rarely sees the modular responses that are related to each of the functional groups separately (Johnson et al., 2007b). Instead, such an odorant stimulates glomeruli in the region responding to highly water-soluble molecules (Johnson et al., 2007b). Responses associated with certain functional groups also are conditional on their position within a molecule (Johnson et al., 2005a).
These observations of interactions between separate odorant structural features in complex molecules indicate that the whole odorant structure must be considered in predicting the response pattern. Odorant receptors do not recognize molecular features independently of one another, and therefore an earlier, simple notion of modular coding of discrete features that still apparently holds for simple odorant structures is, as might have been expected, not applicable to more complex odorants.
The complete data involving isolated odorant chemicals perhaps better fit a model wherein intact odorants are represented by combinations of active glomeruli rather than molecular features being represented by combinations of glomerular modules. In this model, the modules would represent the locations where responses to intact molecules are used to tune or otherwise decorrelate mitral cell responses along different stimulus dimensions. The specificities of both modules and individual glomeruli are still best defined in terms of odorant molecular features given the large number of intact odorants capable of stimulating them. In this newer model, the definition of a molecular feature both for a module and for a glomerulus would be narrower than was envisioned in our earlier model (e.g., instead of the feature “carboxylic acid”, the feature relevant to the anterior-dorsal module might be “aliphatic or alicyclic carboxylic acid, methyl ester, or ethyl ester without additional oxygenic functional groups and without triple bonds or cyclopropyl structures within one carbon atom of the single permissive functional group,” and the feature for a particular glomerulus in the module might be constrained further by a range of overall molecular length.)
Odorant concentration
As measured using various imaging techniques, absolute levels of glomerular activity generally increase with increasing odorant concentration, sometimes approaching plateau values that would be predicted from saturation of odorant receptors across the various sensory neurons projecting to a given glomerulus (Stewart et al., 1979; Cinelli et al., 1995; Guthrie and Gall, 1995; Friedrich and Korsching, 1997; Johnson and Leon, 2000a; Xu et al., 2000; Fried et al., 2001; Meister and Bonhoeffer, 2001; Sachse and Galizia, 2003). In addition, at any given arbitrary level for defining the presence of a “response,” increasing odorant concentrations also are associated with an increased number of responding glomeruli. Typically, the glomeruli recruited at higher concentrations are located near the originally activated glomeruli, a consequence of the chemotopic clustering of glomeruli with similar odorant specificities.
When activity is measured across the entire glomerular layer using the 2DG method, it is possible to express the activity of each glomerulus relative to the activity of all other glomeruli in the bulb. In our experiments, we typically express this relative activity as a z score, where the uptake at each location is calculated as the number of standard deviations above or below the mean glomerular layer uptake (Johnson et al., 1998; 1999). For most odorants, there is little concentration-dependent change in this relative z-score pattern (Figure 6; Johnson and Leon, 2000a; Johnson et al., 2002; 2006), despite clear increases in absolute levels of 2DG uptake (Figure 6; Johnson and Leon, 2000a). Because most odorants are perceived to have the same odor quality at different concentrations, we reasoned that the olfactory system might use some similar relational code to transform bulbar activity patterns into perceptions (Johnson and Leon, 2000a).
Figure 6.
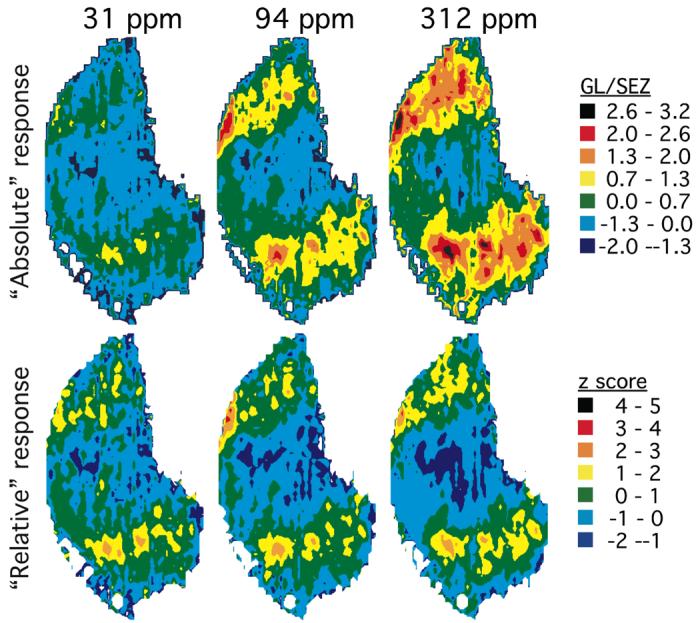
Absolute measures of glomerular metabolic activity show increases in both intensity and area with increasing odorant concentration (top row), whereas responses relative to other responses in the glomerular layer are constant across odorant concentration (bottom row). Shown are contour charts averaged across three animals exposed to each of three concentrations of the odorant 1-pentanol (Johnson and Leon, 2000a). In the top row, 2DG uptake is expressed as a ratio of glomerular layer (GL) uptake to uptake occurring in a portion of the subependymal zone (SEZ), a region containing immature neurons that do not respond to odorants. Green and warmer colors indicate uptake that is greater than that detected in the same locations in animals exposed to air vehicle. In the bottom row, glomerular layer uptake at each location is expressed as a z score relative to the mean and standard deviation of all measurements across the glomerular layer. Green and warmer colors indicate uptake that is greater than the mean uptake across the glomerular layer in the same bulbs. Each color bin corresponds to one standard deviation.
Indeed, z-score patterns of uptake were found to be better predictors of odorant discriminations involving different odorants and concentrations than were patterns of absolute uptake (Cleland et al., 2007). Computational modeling further indicated that the olfactory bulb itself has circuitry that is capable of normalizing the input to generate a relational code in a manner similar to how we calculate a z score from the raw data (Cleland et al., 2007). Short-axon cells (Aungst et al., 2003) can relay information about overall levels of glomerular input across the entire olfactory bulb by way of excitatory synapses, and we calculated that they can suppress mitral cells throughout the bulb by way of their connections to inhibitory periglomerular interneurons, thereby insuring that the intensity of output signals are adjusted relative to the overall glomerular activity (Cleland et al., 2007). Indeed, there is evidence that mitral cells do not show monotonic increases in firing rates with increases in odorant concentration (Chalansonnet and Chaput, 1998). These findings suggest that all responses that are recorded in the olfactory system do not necessarily contain the coded odorant information, but may be background responses that are normalized by glomerular-layer networks. Moreover, the ability of these normalized patterns to predict perception accurately (detailed in subsequent sections), raises the possibility that such patterns actually represent the output activity of the bulb, rather than simply the input of the olfactory sensory neurons.
There are a few odorants that evoke different z score-standardized patterns of uptake at different concentrations, and also evoke different odor perceptions at different concentrations (Johnson and Leon, 2000a). For example, at low concentrations, pentanal does not stimulate activity in the anterior parts of the olfactory bulb that prefer carboxylic acid odorants, while at high concentrations anterior activity becomes prominent (Johnson and Leon, 2000a). Others using optical imaging methods also have found that different concentrations of an aldehyde can evoke unique relative activity patterns in the dorsal aspect of the bulb (Fried et al., 2001; Meister and Bonhoeffer, 2001). We now think that a likely cause of this change is the presence of acid contaminants (1-5%) in many preparations of aldehydes (Johnson et al., 2004).
Ketone odorants also evoke different z-score patterns at different concentrations (Johnson and Leon, 2000a; Johnson et al., 2004). Oddly, higher 2-hexanone concentrations cause decreases in the amount of uptake in ventrally located glomeruli while causing new responses in dorsal glomeruli (Johnson and Leon, 2000a). Rats can change the nasal distribution of odorants by adjusting their respiration patterns, and deeper sniffs may be required for full access of odorants to the most ventral and peripheral culs-de-sac of the nasal turbinates (Youngentob et al., 1987; Kimbell et al., 1997; Scott et al., 2006). Therefore, one explanation for the changes in relative patterns evoked by 2-hexanone is that the rats withhold deep sniffs at higher concentrations, a phenomenon known to occur for various other odorants (Alarie, 1973; Youngentob et al., 1987).
Odorant mixtures
Although the use of isolated odorant chemicals of defined structure has been invaluable for our understanding of the relationships between stimulus and response in the olfactory system, the fact remains that natural odor stimuli are not pure chemicals, but rather are mixtures of chemicals emitted from various objects signifying attractive or unattractive food sources, environmental cues, predators, and kin. Any natural selection that may have operated during the formation of the olfactory system would have acted in the context of these natural mixtures as opposed to individual odorant chemicals, raising the possibility that special mechanisms may have arisen either to insure the robust detection and identification of particular biologically relevant odorant mixtures or, more generally, to process information about the kinds of mixtures that often characterize natural odor objects.
Studies of invertebrate olfactory systems have shown that the presence of multiple odorants in a mixture can result in interactions at many levels. In an impressive series of systematic studies reconstructing the responses of the lobster olfactory system to natural food stimuli, individual odorants were found to interact by way of competition for receptor binding sites, synergistic stimulation of distinct excitatory receptors on the same sensory neurons, and inhibitory responses to some odorants in the face of excitatory responses to other odorants by the same sensory neurons (reviewed in Derby, 2000). Similar results for food-related mixtures have been obtained for fish (Kang and Caprio, 1997). Inhibitory interactions between odorants in arbitrary mixtures also were observed during optical imaging of calcium responses in honeybee glomeruli (Joerges et al., 1997). The situation may be somewhat different for mammals, which are generally thought to express only a single odorant receptor gene in a given sensory neuron (Serizawa et al., 2004). However, chemically related odorants indeed compete for binding to individual rodent odorant receptors (Araneda, 2000; Oka et al., 2004; Sanz et al., 2005), and there is some evidence that different odorants can excite and inhibit the same sensory neuron (Sanhueza et al., 2000; DuChamp-Viret et al., 2003), that synergistic responses can occur in single sensory neurons (DuChamp-Viret et al., 2003), that multiple receptor genes can be expressed in certain sensory neurons (Rawson et al., 2000), and that mixtures of chemically unrelated odorants can result in suppression of some responses by way of peripheral mechanisms (Bell et al., 1987). These findings suggest that mammals also may possess a foundation for mixture interactions.
In contrast with the choices of food-related stimuli in experiments on odorant mixtures using aquatic species, studies on mammals typically have used binary mixtures of arbitrary odorant pairs. The typical result has been that the mixture response is well predicted from the responses to the individual odorants (Belluscio and Katz, 2001; Lin et al., 2006). However, if natural selection has shaped mixture interactions, it would be more likely to discover their existence using naturally occurring odorant mixtures rather than arbitrary odorant pairs. Responses to urine, a natural odor mixture, have been detected in remarkably well-confined portions of the ventral part of the mouse bulb through the use of both Fos immunohistochemistry (Schaefer et al., 2001a; 2002) and electrophysiology (Lin et al., 2005). Although a particular chemical detected in urine activates similar bulbar regions and elicits similar behaviors as urine (Lin et al., 2005), the effect of other urine components on the overall mixture-evoked activity has not been investigated.
Limitations of mapping methods
Various methods can be used to measure the differential activity at each anatomical level of the olfactory system. Some of these methods are capable of tracking responses to numerous stimuli in individual animals across small increments of time, but cannot access responses across the entire structure, whereas other methods require between-animal comparisons and long odorant exposure times, but can monitor activity across an entire anatomical level. A complete understanding of olfactory processing probably will require an analysis of every level at all resolutions because each analysis technique captures a different aspect of the neural response, some of which may be more closely related to the information that the brain actually uses to build an olfactory perception. To identify which information is used, behavioral studies are required in order to identify the aspects of the neural response that are critical for perception.
Much of the evidence for chemotopic odorant representations has come from our own studies of 2DG uptake. Louis Sokoloff and coworkers originally developed the 2DG technique to measure real values of glucose utilization in the brain (Kennedy et al., 1975), but now the method is typically used in a semiquantitative manner to compare relative levels of activity in different brain regions. After being taken up through the glucose transporter in proportion to a neuron's demand for glucose, 2DG becomes phosphorylated. Because the charged product cannot pass back through the cell membrane and because most neurons lack the enzymes capable of further catabolizing the product, the 2DG becomes trapped in the active neuron (Sokoloff et al., 1977). Most evidence suggests that 2DG uptake primarily reflects synaptic rather than somatic activity in numerous brain regions (Schwartz et al., 1979; Nudo and Masterson, 1986), although short-term experiments on radiolabeled glucose uptake indicated the possibility of uptake by both terminals and active cell bodies (Duncan and Stumpf, 1991). In the olfactory bulb, the vast majority of odorant-evoked 2DG uptake occurs in glomerular neuropil, although some labeling is detected in cell bodies (Benson et al., 1985). Because many postsynaptic dendrites in glomeruli are themselves presynaptic to other dendrites (Shepherd, 1972; Wachowiak and Shipley, 2006), the general presumption of presynaptic labeling by 2DG is not definitive with respect to which elements are active. Electron microscopic analysis of labeled glomeruli revealed very small patches of labeling surrounded by larger unlabeled regions, which suggested that olfactory nerve terminals might contribute more to the signal than postsynaptic structures (Benson et al., 1985). As discussed below, uncertainty in the exact nature of the elements being labeled is considered by some to be a disadvantage of the 2DG technique.
Another clear disadvantage of the 2DG method is that only one odorant condition is used for each animal. Because glomeruli associated with the same odorant receptor vary slightly in their position from bulb to bulb (Royal and Key, 1999; Strotmann et al., 2000; Schaefer et al., 2001b), and because of experimental variation in the preparation and sectioning of tissue, the 2DG method does not allow a comparison of the activation of a single glomerulus by multiple odorants. Rather, across-odorant comparisons in 2DG data pertain to the average and variance in activity over an area somewhat broader than a glomerulus. Optical imaging and electrophysiological approaches to measuring odorant-evoked neural activity do not have this same disadvantage. Interestingly, however, the necessity of a statistical approach in studies of 2DG uptake also may be a strength of this technique. The evident details in individual images and electrophysiological time series from single animals and neurons appear to have encouraged an almost anecdotal presentation of data in studies using these techniques, so that it is often not clear to what extent the conclusions are valid for the entire population of animals.
Measurements of 2DG uptake through autoradiography of multiple sections taken throughout the bulb provide access to the entire glomerular layer, allowing the identification of the principal responses to odorants in awake, behaving animals. Many parts of the olfactory system respond in a minor way to many odorants, probably due to the presence of both multiple, low-level contaminants and low-affinity responses to the principal compound. Any small set of cells or glomeruli therefore probably will show some kind of response to every odorant. If there is no knowledge of the presence of much larger responses to those odorants elsewhere in the system, one might come to the conclusion that the system is broadly and not differentially responsive, a very different conclusion than would be made after observing the enormously differential responsiveness that is apparent when one can view the entire bulb (Figure 7). Techniques limited in their spatial scope such as mapping responses only on the dorsal surface or sampling from only several cells randomly located through the bulb risk analyzing only the background responses. Also, if one were to use few odorant stimuli when looking at a limited set of cells or glomeruli, then one might not be aware that those same cells or glomeruli display much larger responses to other odorants, and one might come to incorrect conclusions regarding the breadth of tuning in the system. If, for these technical reasons, there appeared to be no differential spatial responses, one would be forced to look for mechanisms other than a spatial or identity code to represent odorant stimuli.
Figure 7.
Weak, background responses to odorants can receive undue attention if the principal responses to the same odorants go unobserved. A: A color-coded contour chart of 2DG uptake (z scores) in response to the odorant octanal has been used to texture a 3-D model of the glomerular layer, the lateral aspect of which is illustrated here. The white arrowhead indicates a principal, ventrally located, response domain, where the uptake is more than 3 standard deviations above the mean uptake calculated across the entire glomerular layer. The black arrow indicates a relatively minor patch of uptake (green shading) that barely exceeds the average uptake across the glomerular layer. B: The 3-D model is rotated to emphasize the dorsal aspect of the bulb, which is the aspect that typically is studied in experiments using optical imaging techniques. C: Relative uptake within the region outlined in B is re-plotted as a surface chart with the same contour shading as in A and B. D: The surface plot in C is re-colorized to emphasize the largest responses visible in this region. This plot illustrates that weak “responses,” which may be due to odorant contaminants, can dominate the data collected using any method that either systematically samples from only a limited portion of the bulb or randomly samples only a few areas using only a few odorants. “Information” collected by such techniques can lead to incorrect conclusions involving broad response tuning and either broad or chaotic spatial distributions of odorant responses.
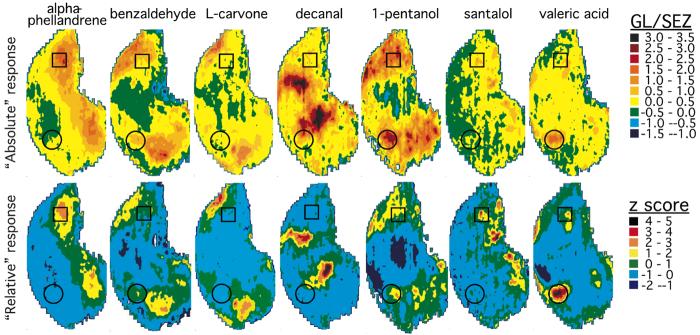
The problem of defining odorant responses is illustrated in a different way in Figure 8, which shows 2DG uptake in both “absolute” and “relative” scales (Woo et al., 2007). The absolute uptake (Figure 8, upper row) shows responses above background (yellow or warmer colors) to almost all odorants in almost all bulbar locations, which might be taken as “broad tuning” or a “highly distributed response.” Any arbitrary location in the bulb would likely show an above-background response to all odorants, with no clear systematic relationship to either odorant chemistry or perception, similar to results of imaging a small number of glomeruli or a few mitral cells. However, when each data point is expressed relative to the average response in the entire glomerular layer (z-score normalization; Figure 8, lower row), the differential responses of some bulbar locations relative to others are much easier to appreciate.
Figure 8.
Although individual odorants can stimulate much of the glomerular layer, relative levels of activity are very distinctive for different odorants. Color-coded contour charts are used to illustrate uptake evoked by seven different odorants. In the top row, responses are scaled as a ratio of uptake in each glomerular layer location to uptake measured in a consistent part of the subependymal zone, which is not expected to display odorant-dependent activity. Yellow or warmer colors indicate uptake that was above what was measured when rats were exposed to air vehicle only. In the bottom row, the same set of patterns are shown in a z score color scale, where yellow or warmer colors indicate uptake that is more than one standard deviation above the average uptake calculated across the glomerular layer. These patterns are very distinctive for different odorants. Outlined regions highlight consistent locations in the different charts. Whereas responses to different odorants in a given location do not seem as distinctive in the top row, the same locations are very differently active when viewed as relative (z score) responses in the bottom row.
The fact that glomerular circuitry involving the short-axon cell network may accomplish something very similar to z score standardization (Cleland et al., 2007) suggests that our typical maps of 2DG uptake may in fact be modeling the spatial pattern of input to mitral cells instead of the pattern of input to glomeruli. The mitral cell input pattern would be expected to show a closer correlation with behavior than the glomerular input pattern, which is exactly what is observed across odorant concentrations (Cleland et al., 2007).
Animals in 2DG experiments are not anesthetized, which contrasts with the use of general anesthetics in all optical imaging and functional magnetic resonance imaging studies used to map glomerular activity. In our hands, the use of urethane anesthesia almost entirely abolishes glomerular uptake evoked by the powerful odorant stimuli decanal and methyl benzoate (Johnson, Ong, and Leon, unpublished observations), which suggests the possibility that the responses measured by the other methods may also have been greatly affected by anesthesia. We have not yet determined whether the suppression of 2DG responses by the anesthetic involves direct actions on sensory neurons or whether it arises indirectly from the suppression of odorant inspiration. It is interesting that wakefulness has been reported to result in a sparsening of odorant-evoked mitral cell responses despite an increase in spontaneous activity (Rinberg et al., 2006a).
A criticism that has been leveled against 2DG and certain optical imaging methods is that they do not distinguish between presynaptic and postsynaptic activity, thereby failing to provide information regarding “computations” performed at that level (e.g., Laurent et al., 2001). While it is true that the 2DG method cannot definitively distinguish olfactory nerve terminal activity from other activity in the glomerular neuropil, the strong predictive relationship between 2DG uptake patterns and perception that we will describe later in this review should obviate these concerns. A related, but more vague, criticism of the 2DG technique is that it is “indirect,” with the unstated implication that focal responses somehow arise artifactually from the use of a metabolic marker of activity (e.g., Bhalla and Bower, 1997), but a mechanism by which this artifactual result could arise has not been proposed, or shown to exist. One might reason instead that both the use of glucose for biochemical processes unrelated to activity and the potential equal weighting of secondary excitation and inhibition would cause a metabolic tracking method to show less spatial specificity than actually exists, rather than more.
The 2DG method in its typical form involves exposing animals to an odorant for 45 minutes, whereas odor perception can occur in hundreds of milliseconds (Uchida and Mainen, 2003; Ditzen et al., 2003; Abraham et al., 2004; Rinberg et al., 2006b). Thus, there may be concern that 2DG uptake shows late metabolic events unrelated to perceptual processing. We have found that exposures in 2DG studies can be as short as two minutes and yet result in similar relative patterns of uptake, although the fainter signal against the higher background of unutilized radiolabel is generally undesirable for routine mapping experiments (Woo et al., 2004). Moreover, animals trained to sniff an odorant intermittently for less than a second at a time over the course of a 45-minute period (1-2 minutes total exposure) also show a pattern of 2DG uptake similar to animals exposed continuously to the odorant for 45 minutes (Slotnick et al., 1989). Finally, optical imaging experiments and electrophysiological studies that measure rapid responses in the same areas of the bulb as the 2DG measurements (Mori, et al., 1992; Uchida et al., 2000; Takahashi et al., 2004a,b; Igarashi et al., 2005; Lin, et a., 2005) show patterns of responses that are quite comparable to those from 2DG studies.
Indeed, in addition to the close agreement between 2DG and optical imaging for numerous odorants, almost all methods that map activity across the entire olfactory bulb have come to similar conclusions regarding the locations best stimulated by any given odorant, regardless of the temporal resolution of the techniques. For example, principal responses to carboxylic acids have been localized to the dorsal part of the bulb using 2DG (Bell et al., 1987; Royet et al., 1987; Sallaz and Jourdan, 1993; Johnson et al., 1999; 2004; Johnson and Leon, 2000a,b), immediate-early gene expression (c-fos: Sallaz and Jourdan, 1993; Guthrie et al., 2000; arc: Guthrie et al., 2000; zif/268: Inaki et al., 2002), evoked field potentials (Mori et al., 1992), and recordings of individual mitral cells (Mori et al., 1992). Chemotopic progressions in representations of homologous series are found in the same locations using 2DG (Johnson et al., 1999; 2004), optical imaging, and zif268 expression (Inaki et al., 2002). Posterior and ventral responses to sulfides are found using both mitral cell unit recordings (Lin et al., 2005) and 2DG (Johnson and Leon, unpublished data). Finally, responses to isoamyl acetate are similar for 2DG (Johnson et al., 1998) and fMRI, provided that the larger variance between individual animals in the fMRI method is accounted for by averaging over many rats (Schafer et al., 2006).
In comparing the results from different imaging methods, it also is important to consider the use of different species by different labs. For example, responses to carboxylic acids appear to involve the dorsomedial region of the bulb of rabbits (Mori et al., 1992), rats (Bell et al., 1987), and mice (Royet et al., 1987), suggesting a similarity in spatial areas of activation. However, 2DG experiments show primarily posterior activation by ethyl acetate in rats (Johnson et al., 1998), while c-fos studies show primarily ventral activation by ethyl acetate in mice (Salcedo et al., 2005). To determine if this sort of difference could be due to the use of different species, we have begun an analysis of 2DG uptake in mice using odorants previously characterized in rats, and we are finding that differences in spatial patterns between these species are actually quite common (Johnson, Xu, Ali, and Leon, unpublished data).
The final test of the ability of any method to collect data relevant to odor coding is to test how well olfactory system responses predict odor perceptions. As detailed in the next section, there has been considerable success in relating odor perceptions to spatial activity patterns obtained using the 2DG method, suggesting that any real or imagined shortcomings of the technique have not compromised its usefulness in understanding principles of odor coding.
Correlations between spatial patterns of glomerular activity and behavior
Cross-habituation
Rats initially investigate novel odorants in their environment, but upon repeated presentations, their interest diminishes. If an unrelated odorant then is presented to a rat that has become habituated to the first odorant, the rat will investigate that new odorant. However, if the second odorant is perceived as being the same as the habituated odorant, then there is less investigation. This phenomenon provides a simple assay to determine whether naïve rats, such as are used in our mapping studies, distinguish between odorants. The advantage of using such a technique is that the behavior is spontaneous, does not require hundreds or thousands of rewarded exposures to the odorants and does not raise the possibility of learning-related changes in the system.
The degree of behavioral cross-habituation between odorant pairs has been quantitatively related to the similarity in overall patterns of 2DG uptake in numerous experiments. For example, habituation among members of a homologous series of aliphatic acids or alkanes was proportional to the degree of similarity in their 2DG activity patterns (Cleland et al., 2002; Ho et al., 2006a). Quantitative similarities between 2DG patterns evoked by octane and/or by eight-carbon branched alkanes or eight-carbon alkenes and alkynes also predicted the amount of cross-habituation between the odorant pairs (Ho et al., 2006b). Differences in 2DG uptake patterns evoked by pentadecane obtained from different sources (with a likelihood of different impurities) also correctly predicted that the odorants would be perceived differently in a cross-habituation experiment (Ho et al., 2006a). Finally, the enantiomers D- and L-carvone, which evoked statistically distinct 2DG uptake patterns, were perceived as being different in a cross-habituation assay, while the chemically related enantiomer pairs D- and L-limonene and D- and L-terpinen-4-ol, which were not statistically different in their evoked 2DG patterns, were not distinguished in the cross-habituation assay (Linster et al., 2001).
Rate of acquiring a learned discrimination
Although rats can learn to discriminate between even very similar pairs of odorants with extensive reinforcement, it is sometimes possible to distinguish differences in the rate of acquisition of the behaviors that are used to monitor this kind of learning. Odorant pairs that are perceived to be similar yield slower acquisition than odorants that are perceived to be different. Indeed, rats required additional reinforced trials to learn to discriminate between pairs of odorants giving more similar 2DG patterns along a homologous series of acids, affirming the results of cross-habituation assays (Cleland et al., 2002). In female mice exposed to urine from donor male mice of different genetic backgrounds, relative similarities in bulbar patterns of c-fos in situ hybridization were related to the number of trials required by mice to learn a discrimination between the odors of the urine samples (Schaefer et al., 2002).
Similar to the results from cross-habituation studies, rats required additional reinforced trials to discriminate limonene and terpinen-4-ol enantiomers compared to carvone enantiomers (Linster et al., 2002). Carvone discriminations in these rewarded digging tasks were evident before the tenth trial, whereas discriminations involving the other two enantiomers were evident before the 15th trial (Linster et al., 2002). Using operant procedures involving water deprivation and water reward, McBride and Slotnick (2006) trained rats to discriminate between 10% dilutions of carvone enantiomers, followed by eight additional steps of dilution, using between 60 and 400 training trials at each concentration. After this extensive experience with discriminating carvone enantiomers, rats were shown to learn to discriminate between terpinen-4-ol enantiomers before the 20th trial (McBride and Slotnick, 2006). Although the authors interpreted these data otherwise, this result is fully consistent with those of the Linster et al. study (2002), where inexperienced rats could discriminate between terpinen-4-ol enantiomers before the 15th trial in a simple digging task. In general, the use of many trials of operant conditioning appears to result in successful discriminations of all odorants when they are tested at detectable concentrations (Slotnick et al., 1987; 1997; Lu and Slotnick, 1994; 1998; Bisulco and Slotnick, 2003; Slotnick and Bisulco, 2003; McBride and Slotnick, 2006), suggesting that the method is poorly suited for the investigation of odor similarities.
Odorant confusion matrix
All of the above behavioral tests of perception rely on the comparison between two odorants, and relationships among a group of odorants can be deduced only from binary comparisons. The correlation between odorant-evoked glomerular responses and odorant-evoked perceptions would be more compelling if one could demonstrate that a quantitative relationship among the patterns evoked by a larger group of unrelated odorants was paralleled by a similar quantitative relationship across their evoked perceptions. To address the comparison of multiple odors in a single experimental protocol, Steven Youngentob developed a five-odorant confusion matrix task in which rats learn to associate each of five different odorants with one of five different tunnels (Youngentob et al., 1990). Rats are given extensive training to identify the correct response tunnels with very few errors. Over thousands of trials, however, enough errors are committed to permit a rigorous statistical analysis. Individual rats tend to commit the same types of errors, revealing that some odorants are more likely to be misidentified than are others (Youngentob et al., 1990).
This experimental design was exploited to test the predictive power of spatial patterns of 2DG uptake, using five odorants of very distinct chemical structure that could not be characterized along any single chemical dimension (Youngentob et al., 2006). Both the 2DG patterns and the behavioral data then were subjected to independent multidimensional scaling analyses. Remarkably, the perceived similarity among the odorants closely matched the pattern of similarity for the 2DG response (Youngentob et al., 2006). Because the peak modular 2DG responses for some pairs of odorants did not overlap, and yet the information regarding their relative similarity was revealed in the behavior, it suggested that the overall activity pattern could be used for odor comparisons under at least some circumstances (Youngentob et al., 2006).
Multidimensional relationships between perception and epithelial activity patterns had been identified previously using these procedures (Kent et al., 1995; 2003), suggesting that bulbar patterns may further develop receptor-dependent information already present in the first neurons to respond to the odorants. Recent work also has shown a similar multidimensional correlation between antennal lobe activity patterns and perception in honeybees (Guerrieri et al., 2005). Behavioral methods that can test the perceived similarities of different odorants, such as the methods mentioned above, as well as certain others (Kay et al., 2006), are indispensable tools in identifying the neural activity that actually carries information relevant to odor perception.
Correlations between rat glomerular 2DG patterns and human odor descriptions
There is a great deal of information about odor quality perception in humans, who can name and verbally describe odors, and who can offer numerous other measures of olfactory performance not easily obtained for experimental animals (Zelano and Sobel, 2005). Given evidence that odor perception might be similar for different species (Laska et al., 1999; Laska and Galizia, 2001), relationships between rat activity patterns and human odor descriptors have been explored. A machine learning technique (support vector machine) was used to extract modules from a database of 172 of our 2DG uptake images on the basis of these modules being able to predict ten human odor descriptors (Yamanaka and Gutierrez-Osuna, 2006). The maps of 2DG uptake were able to predict these odor descriptors at well above chance levels.
As mentioned previously, most odorants evoke the same perceived odor at all concentrations, but certain odorants change in perception with intensity. We found that two odorants, pentanal and 2-hexanone, which are reported by humans to change in odor quality with concentration (Arctander, 1994) also exhibited changes in 2DG uptake patterns with concentration, while three other odorants not reported to change in odor evoked consistent uptake patterns at different concentrations (Johnson and Leon, 2000a).
Although there appears to be at least an overall similarity in olfactory coding between humans and rodents, there also appear to be species-specific differences in the responses of the two olfactory systems to particular classes of odorants. For example, rats exhibit a more focal and intense glomerular response to 2-methylbutyric acid than to other acids, perhaps due to its natural occurrence in rat feces (Johnson and Leon, 2000b). The differential patterns evoked by carboxylic acids of different hydrocarbon structure were not associated with proportionate differences in our own perceptions of these compounds. On the other hand, differences in bond saturation and branching changed our impressions of the odor of hydrocarbons, but did not change either the evoked 2DG pattern or odor perception in rats (Ho et al., 2006b). Heterocyclic compounds containing either oxygen, such as furans, or nitrogen, such as indole, have strong odors to humans, and yet evoked very little 2DG uptake in the rat olfactory bulb (Johnson et al., 2006). Many heterocyclic compounds are formed primarily by way of cyclization of other molecules as a result of heating, such as occurs during the cooking of sugars or amino acids. The odors of these heterocyclic compounds are likely to signify biologically relevant food sources to humans, and therefore, such receptors may have been positively selected. Rodents probably would have received less benefit from any selective detection of these compounds.
Interventive experiments testing the relevance of chemotopic representations
Patterned electrical micro-stimulation of the olfactory bulb
The most definitive interventive tests of bulbar spatial coding hypotheses appear in a series of experiments in which spatial patterns of activity were imposed on the rat olfactory bulb using electrical stimulation (Monod et al., 1981; 1989; Mouly et al., 1985; Mouly and Holley, 1986). Based on observations that humans report sensations of odor when their olfactory bulbs are electrically stimulated, Monod and coworkers (1981) stimulated rat olfactory bulbs and found that the rats began sniffing and orienting their noses in the direction of the incoming air, a typical reaction to the presence of a novel odorant. They further found that such bulbar stimulation could take the place of an odor stimulus in toxicosis conditioning (Monod et al., 1981).
By pairing one site of bulbar stimulation with water that was made palatable with sucrose, and pairing another site with water that was made unpalatable with quinine, rats could be taught to discriminate between different spatial locations of activity as if the different locations were equivalent to different odors (Mouly et al., 1985). The rats also could discriminate between pairs of activity patterns established using sets of four electrodes, with the two sets of electrodes being interdigitated with one another (Mouly et al., 1985). The rats required more training trials to discriminate these patterns as the distance decreased between the positively and negatively associated electrodes (Mouly et al., 1985). This finding is predicted by spatial coding hypotheses and recalls the greater difficulty that rats have in learning to distinguish odorants evoking more similar spatial activity patterns (Linster et al., 2002; Cleland et al., 2002; Schaefer et al., 2002). Similarly, rats could discriminate between a pair of three-site stimulation patterns involving two of the same electrodes, but when a set of four electrodes was stimulated with different intensities at two of the positions, more trials were required to learn this task (Mouly et al., 1985). Again, these data strongly support the existence of an identity code that is related to locations of activity.
On the other hand, rats were not able to learn a discrimination involving stimulation of the same sets of electrodes at different times during the respiration cycle (Monod et al., 1989). Thus, although different spatial locations of stimulation contained information recalling different perceived odors, different temporal aspects of stimulation evoked behavior consistent with a single odor. This observation favors spatial or “identity” coding hypotheses over temporal coding hypotheses, as will be discussed at greater length below.
Effects of odorant enrichment on subsequent habituation
Rats that have undergone daily exposure to individual odorants or odorant pairs spontaneously distinguish novel and familiar odorant pairs that are not discriminated by naïve rats in a cross-habituation assay (Mandairon et al., 2006a,b). The effectiveness of the enrichment procedure was related to the overall similarity between the 2DG pattern evoked by the enrichment odorant and the patterns evoked by the test odorants (Mandairon et al., 2006a,b). For example, enrichment with odorants evoking 2DG patterns that overlapped a great deal with test odorant patterns enhanced spontaneous discrimination, while experience with patterns that did not overlap much did not enhance discrimination (Mandairon et al., 2006a,b).
Perceptual relevance of particular receptors and sensory neurons
There are a number of interventive experiments showing differential contributions of certain sets of glomeruli, receptors, or sensory neurons to the perception of particular odorants. The clearest cases involve invertebrate models such as the special involvement of the V glomerulus in carbon dioxide avoidance in Drosophila (Suh et al., 2004), the special involvement of the odr-10 receptor gene to diacetyl chemotaxis in C. elegans (Sengupta et al., 1996), and the special involvement of a single sensory neuron in chemotactic responses to several other odorants in C. elegans (Wes and Bargmann, 2001). In each of these cases, elimination of particular neurons or genes blocked the ability to perceive specific odors, findings consistent with the presence of an identity code in which specific neurons carry specific odorant information.
Evidence for the perceptual importance of particular odorant receptors is also emerging from studies on rats. Perfusion of the rat nasal cavity with the lectin concanavalin A, which selectively binds to specific carbohydrate moieties on a subset of glycoproteins, impaired the ability of rats to learn a response to low concentrations of the odorant D-carvone, although learning the response to the enantiomer L-carvone was unaffected (Kirner et al., 2003). Intranasal concanavalin A also impaired detection of low concentrations of dimethyl disulfide while not affecting detection of ethyl acetate (Apfelbach et al., 1998). These studies suggest that there are subsets of sensory neurons that are specially involved in the perception of particular odorants. Although animals can detect ethyl acetate even after concanavalin A application, the odor of the chemical apparently changes in quality, because rats do not recognize the compound if they are trained to the odorant in the absence of the lectin and then tested in its presence (Apfelbach, 2004). As measured by Fos-like immunoreactivity, the spatial activity pattern in the olfactory bulb in response to ethyl acetate was correspondingly changed by intranasal concanavalin A, while the pattern evoked by L-carvone was unaffected (Apfelbach, 2004).
In another set of studies, polyclonal antibodies to the rat I7 odorant receptor were applied to the olfactory mucosa with the intention of disrupting interactions between this receptor and one of its preferred odorant ligands, octanal (Araneda et al., 2000; Deutsch and Apfelbach, 2006). After application of the antibody, rats were impaired in their detection of octanal, but not in their detection of another aldehyde, citral (Deutsch and Apfelbach, 2006). The Fos-like immunoreactivity evoked by octanal in the glomerular layer of the olfactory bulb was accordingly reduced by the antibody (Deutsch and Apfelbach, 2006). Although it is possible that the polyclonal antibody to the I7 peptide sequence cross-reacted with a number of related odorant receptors, this result nevertheless appears to show a remarkable degree of importance of specific receptors to perception in rats. The specificity of this relationship is probably related to the focal 2DG response evoked by octanal and the close relationship between the location of this response and the target of the projection of sensory neurons expressing the I7 receptor (Johnson et al., 2004).
The toxin dichlobenil causes the death of sensory neurons in zone 1 of the mouse epithelium, and this observation was exploited to test the relative importance of this zone for the detection of different odorants (Vedin et al., 2004). The toxin increased the threshold of detection for certain odorants, while the detection of other odorants was unaffected, suggesting that different parts of the olfactory epithelium were involved in the perception of different odorants (Vedin et al., 2004). Also, destruction of large portions of the epithelium using methyl bromide gas disrupted odor perception as measured in a confusion matrix task, with differential effects on specific odorants (Youngentob and Schwob, 2006). Given the topography of the epithelium-to-bulb projection (Schoenfeld and Cleland, 2005), these results suggest that different parts of the olfactory bulb are involved in the perception of different odorants.
It is likely that the use of gene-altered mice soon will allow more specific tests of the contribution of particular odorant receptors to the perception of particular odors. Already, mice possessing genetic deletions of a subunit of the cyclic nucleotide-gated channel show reliable deficits in detecting certain odorants (Lin et al., 2004), and mice with deletions of the olfactory marker protein have altered odor quality perception as measured in a five-odorant confusion matrix task (Youngentob et al., 2001).
Bulbar ablation studies
While the above interventive studies strongly support the use of an identity code in olfactory perception, a number of bulbar lesion studies have come to the opposite conclusion. In these studies, varying amounts of olfactory bulb tissue were removed by ablation, starting from the dorsal surface of the bulb and extending ventrally (Hudson and Distel, 1987; Slotnick et al., 1987; 1997; Lu and Slotnick, 1994; 1998; Bisulco and Slotnick, 2003; Slotnick and Bisulco, 2003; McBride and Slotnick, 2006). These studies have shown that following even relatively large lesions, rats retain a remarkable capacity to detect odorants and to learn odorant discriminations. Similar to the work of Lashley, who showed that rats could learn and remember to run mazes following extensive ablations of the cerebral cortex (e.g., Lashley and Wiley, 1933), this work clearly has established that there is a spare capacity in the olfactory nervous system that can operate after trauma, although it does not establish that all areas are equally involved in processing all odors in an intact animal.
However, while such ablation studies could contribute to understanding the relationships between perception and activity patterns, they have failed to do so because they have not eliminated the major responses that appear to underlie the perception. For example, we found that enantiomers of carvone differed in evoked 2DG uptake primarily in a restricted part of the posterior, ventromedial glomerular layer, while the greatest similarities in uptake were in the dorsal part of the bulb (Linster et al., 2001). If the 2DG patterns were relevant to olfactory discrimination, we would predict that a lesion of the differentially activated posterior-ventral glomeruli would decrease the overall pattern differences between the odorants and therefore might make them more difficult to discriminate. On the other hand, we would predict that removal of the dorsal part of the bulb, where the activity evoked by carvone enantiomers is similar, would not make the odorants more difficult to discriminate (Figure 9). Indeed, a dorsal lesion could conceivably make the overall bulbar patterns more different from each other, and the animals would still retain the key response that differentiates them. Fully consistent with these predictions, McBride and Slotnick (2006) showed that dorsal lesions did not impair discriminations between carvone enantiomers. Unfortunately, the authors apparently did not recognize that their results would have been predicted from the 2DG patterns, and they made the opposite conclusion by incorrectly regarding any large lesion as being sufficient to eliminate the critical representation of the odorant.
Figure 9.
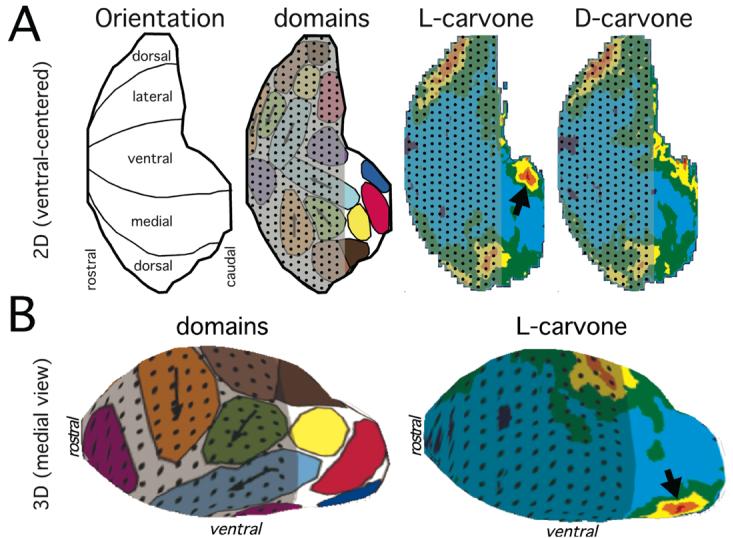
Very important odorant-evoked responses are spared following large bulbar lesions. A: Ventral-centered 2D charts show domains as in Figure 4 as well as color-coded z score responses to the odorants L-carvone and D-carvone averaged across six rats each (Linster et al., 2001). A semi-transparent, stippled overlay simulates a lesion removing 80% of the glomerular layer, including all of the lateral aspect. The area not covered by this overlay would be spared after such a lesion. The spared area includes at least a portion of five identified response domains as labeled in Figure 4. The spared area also includes the main differential response between L- and D-carvone (black arrow). B: This same spared region is shown on a 3D model of the glomerular layer viewed from a medial perspective. The representations of odorants that persist in this posterior part of the medial aspect of the bulb can explain the spared discriminatory powers of rats given such bulbar lesions.
The same kinds of concerns apply to all of the odorant discriminations tested following bulbar ablations. Even the most extensive lesions still leave unaffected about 40% of the medial aspect of the bulb, particularly the posterior and ventral parts (Figure 9). The posterior part of the bulb is quite important in the differential representations of the aliphatic and water-soluble odorants that were typically used in the ablation studies (Johnson et al., 1999; 2004; 2006a), so that the 2DG patterns predict continued detection and discrimination of these odorants. It should be further recognized that most of the animals in these studies were given smaller lesions (Lu and Slotnick, 1994; Slotnick et al., 1997), which would be predicted to spare more function. In addition, the animals were typically tested with many odorants (Lu and Slotnick, 1998), and any subsequent statistical analysis then collapsed responses across different odorants, or involved so many randomly selected odorants that the specific deficits that should have been predicted from the patterns of 2DG uptake could easily have been obscured. Analyses also were often collapsed across animals that had received lesions of various sizes and locations, potentially obscuring additional specific deficits. Early bulbar ablation studies were not guided by a complete knowledge of the activity patterns evoked by the odorants investigated, because these response maps were not available at that time (Slotnick et al., 1987; 1997; Lu and Slotnick, 1994; 1998). It is our hope that subsequent experiments will use lesions specifically targeted to areas showing differential activity in response to odorants in the study, now that the locations of the focal responses of these odorants are easily available, and that the work will not continue to use odorants that have already been shown to have differential activity in the spared parts of the bulb (Bisulco and Slotnick, 2003; McBride and Slotnick, 2006).
When spatial organization is and when it is not a spatial code
Spatial activity patterns are clearly related to odor perception, but this situation is not the same as saying that the olfactory system reads the spatial locations of responses to identify the odor, which might be defined as “true” spatial coding in the system. If organic acid responses in the epithelium could be re-routed to mitral cells located in the ventral part of the bulb during development, would acid odorants smell camphoraceous to the adult animals? We would not predict this outcome, in part because it does not appear as though cortical regions have sufficient information regarding the bulbar location of the mitral cells projecting to them for this hypothesis to be tenable (Zou et al., 2001; 2005; Illig and Haberly, 2003), although hard-wired, chemotopic cortical circuits apparently do exist in fish (Nikonov et al., 2005). Rather, the stereotyped position of glomeruli receiving input from sensory neurons expressing the same receptors allows us to detect in the spatial pattern an identity and relative intensity code in the rat – it is which mitral cells, and how active they are that is likely read out by olfactory cortex.
The meaning of activity in each mitral cell may be acquired by experience in the rat. Combinations of mitral cells that are reliably activated in association with some object, event, or context likely result in a combination of co-incidentally active cortical neurons that then signifies the presence of a particular odor. This odor perception may then later be retrieved upon exposure to some smaller proportion of the mitral cells that have previously participated in that combination (Wilson and Stevenson, 2003).
Spatial coding, however, may be important in determining how active certain mitral cells are, in the sense that the activation of neighboring glomeruli by closely related odorant chemicals might lead to stronger lateral inhibition between associated mitral cells (by way of periglomerular and granule cell interneurons), thereby tuning the responses of these mitral cells to a narrower range of stimuli (Yokoi et al., 1995). If the major responses to two different acids of nearly identical molecular length were separated from one another into separate glomerular modules, we would predict that these acids would not be as easily distinguished, because in the absence of local lateral inhibition by periglomerular and granule cells, there would be a greater overlap in the number of highly activated mitral cells responding to the two odorants (there would be less decorrelation of the mitral cell activity).
Temporal coding of odorant information
As we have seen, several techniques have revealed a very close relationship between the physical attributes of odorant stimuli and the spatial patterns of activity they evoke in the olfactory bulb. These relationships are consistent with both the known specificities of odorant receptors and the connectivity between the olfactory epithelium and the olfactory bulb. There also is a large number of both correlative and interventive behavioral experiments showing close relationships between spatial activity patterns and odor perception.
The possibility of the spatial code being transformed into a temporal code for olfactory information also has been raised many times, based on observations that the temporal pattern of activity of mitral cells differs for different odorants, potentially allowing such differences to carry the coded odorant information (Kauer, 1974; Mair, 1982; Meredith, 1986; Harrison and Scott, 1986). In addition to temporal patterns of spiking in individual mitral cells, widespread odorant-evoked oscillatory patterns of a variety of periodicities also have been demonstrated in various olfactory systems (Adrian, 1950b; Freeman and Skarda, 1985; Freeman and Viana Di Prisco, 1986; Laurent and Naraghi, 1994; Gelperin, 1999; Kay, 2003). More recently, optical imaging methods have shown that different odorants can activate the same glomeruli with different kinetics, raising the possibility of a temporal code for odorant quality that is embedded in these differences (Cinelli et al., 1995; Spors and Grinwald, 2002; Spors et al., 2006). Temporal coding schemes have been advocated in a number of recent reviews (Laurent et al., 1999; Wilson and Mainen, 2006; Friedrich, 2006; Wachowiak and Shipley, 2006; Kay and Stopfer, 2006; Gelperin, 2006), but a critical discussion of the experimental support for any of these ideas has been lacking. Given that some temporal coding hypotheses not only look for a transformation of spatially distinct responses into a temporal code, but also oppose or diminish the importance of spatially distinct activity patterns, it seems important to provide a counterargument to such proposals.
There certainly is sufficient complexity in temporal patterns of neural responses to carry a great deal of information about odor, as has been illustrated in a number of computer simulations (for example, Rabinovich et al., 2000; Bazhenov et al., 2001; Brody and Hopfield, 2003; Margrie and Schaefer, 2003; Lozowski et al., 2004). However, computer simulations also have shown that different temporal patterning of mitral cell responses to different odorants is a necessary consequence of different spatial patterns of incoming activity (Meredith, 1992), raising the possibility that these temporal patterns may be epiphenomenal. The complicated ripples of activity propagated across the bulb as a result of several points of focal glomerular activity can be mathematically deconvoluted to give the same information as a simple report of the relative activity in those few locations, but the important question for biologists is whether olfactory systems actually use complex temporal information to evoke olfactory perceptions. To address this possibility, it would be reasonable to hold the temporal coding hypotheses to the same standards as have been brought to bear on coding hypotheses that do not necessarily depend on temporal patterns. These tests would include at least: (1) a search for correlations between similarities in temporal patterns and similarities in perceived odors, and (2) a determination of whether specific interventions with the temporal pattern produce the expected consequences for odor-guided behavior.
Unfortunately, however, there are very few direct tests of the correlation between temporal patterns and olfactory behavior in the literature, although it has been shown that similarities in temporal patterns of antennal lobe neurons in Manduca moths correlate with similarities in functional groups and similarities in chain length in systematic series of odorants (Daly et al., 2004). These chemical similarities likely would be related to similarities in perceived odor, but differences between such odorants are also clearly evident directly from the spatial patterns of glomerular activity (Carlsson et al., 2002). That is, analysis of the complex temporal responses is not needed to predict the olfactory perceptions, raising the possibility that such complex temporal information may be epiphenomenal.
In the few experiments where temporal patterns have been disrupted or are absent, there is a remarkable sparing of olfactory function. When the GABA receptor antagonist picrotoxin, which completely eliminates oscillatory synchronization, was injected into honeybees, they did not become anosmic, as one would predict if this aspect of temporal patterning were the basis for the olfactory code. Rather, these animals continued to distinguish geraniol from both 1-hexanol and 1-octanol, and they continued to perceive all three odorants (Stopfer et al., 1997; Hosler et al., 2000). However, the discrimination between 1-hexanol and 1-octanol was impaired under these conditions (Stopfer et al., 1997; Hosler et al., 2000), and this result has been interpreted as supporting only temporal coding hypotheses for the performance of difficult odor discriminations (Stopfer et al., 1997; Laurent et al., 2001; Hosler et al., 2000; Kay and Stopfer, 2006; Gelperin, 2006; Wilson and Mainen, 2006). It is somewhat surprising that other effects of the GABA antagonist, which blocks inhibition by local interneurons in the antennal lobe, have not been considered in the discussion of this work by temporal coding advocates. As mentioned above, lateral inhibition involving such neurons is also an integral part of the circuit proposed to provide “tuning” as a result of the spatial clustering of responses to related odorants. Indeed the experiments supporting the existence of tuning by lateral inhibition used the same antagonist, picrotoxin, to abolish lateral inhibition in rabbits and they found that the mitral cells were less able to distinguish between closely related odorants (Yokoi et al., 1995). Similarly, Sachse and Galizia (2002) showed that picrotoxin caused projection neurons in most honeybee glomeruli to respond to additional odorants, a finding that is consistent with a reduction in response tuning.
Like the findings with GABA antagonists, blocking oscillations with the nitric oxide synthesis inhibitor L-NG-nitroarginine methyl ester (LNAME) also did not induce anosmia, but rather caused closely related odorants to evoke more similar behavioral responses in terrestrial mollusks and honeybees, although the animals continued to discriminate learned odorants from unrelated novel odorants after these treatments (Teyke and Gelperin, 1999; Hosler et al., 2000; Sakura et al., 2004). In mollusks, LNAME disrupts oscillations in certain olfactory areas (Gelperin et al., 2000), but other actions of nitric oxide have not been well investigated in these species. In the moth Manduca sexta, nitric oxide is produced in the axon terminals of olfactory sensory neurons, where it is released upon odorant stimulation in an odorant-dependent spatial pattern in glomeruli to act on postsynaptic neurons in the antennal lobe (Collmann et al., 2004). It therefore is clear that a spatial pattern of activity would have been affected upon disrupting nitric oxide signaling if such experiments had been performed in this species. In almost all cases, agents affecting nitric oxide signaling would be expected to alter activity within local neural circuits as well as altering general oscillatory activity, and this situation will complicate any simple interpretation of such experiments as supporting only temporal coding hypotheses.
Temporal patterns across different species
Almost all of the interventive tests purporting to support temporal coding hypotheses have been conducted on insects and mollusks, but it has been assumed that these results are directly relevant to sensory coding in mammals and other vertebrates (Laurent et al., 2001; Kay and Stopfer, 2006; Gelperin, 2006). Similarities between these very different animals are generally thought to have involved convergent evolution, which may or may not suggest adaptations involving similar mechanisms or functions (Eisthen, 1997; 2002). Independently arising characteristics of olfactory systems that are frequently mentioned include such features as the presence of glomeruli and oscillatory neural activity, as well as details of neurochemistry and network architecture (Hildebrand and Shepherd, 1997; Gelperin, 1999; Ache and Young, 2005; Kay and Stopfer, 2006; Gelperin, 2006). Although we do not contest the usefulness of simple model systems in the development of ideas for the functioning of more complex nervous systems, there is a danger in overlooking the equally obvious differences between the species that might limit our ability to extrapolate between them.
For example, the glomerular structures of locusts and mollusks, the principal experimental animals studied by advocates of temporal coding, are quite different from the glomerular structures of other species, including many other invertebrates. In locusts, there are about 1,000 microglomeruli that receive input from sensory neurons (Laurent and Naraghi, 1994), whereas in Drosophila, there are only about 60 glomeruli, each receiving convergent input from homologous sensory neurons (Vosshall et al., 2000). Although the odorant receptor gene repertoire is not known in locusts, it seems likely that each odorant receptor gene is associated with numerous glomeruli, a situation differing greatly from the general principal of convergence that appears to apply for Drosophila and rodents (Vosshall et al., 2000; Mombaerts, 2006). Similarly, each locust projection neuron extends dendrites into multiple glomeruli (Laurent and Naraghi, 1994), in contrast to the uniglomerular connection of mitral cells in rodents (Shepherd, 1972). It is not known whether the multiglomerular connections of these principal neurons represent an anatomical convergence from different glomeruli receiving input from homologous sensory neurons, such as occurs in the mammalian accessory bulb (Del Punta et al., 2002), or whether it represents an even greater dispersion of homologous activity.
In mollusks, although there are glomerular-like structures in the tentacle containing the olfactory epithelium, the olfactory sensory neurons also project outside of glomeruli in the tentacle ganglion as well as in the procerebrum, which does not contain any glomeruli (Chase and Tolloczko, 1993). It is in the procerebrum where the odor-dependent oscillatory activity of Limax is studied (Teyke and Gelperin, 1999; Gelperin et al., 2000). There clearly could arise different types of information processing in any olfactory system that does not involve convergence of receptor-based neural information as an early step of organization. Indeed, to our knowledge, terrestrial mollusks represent the only animals studied using the 2DG method that did not show evidence of odorant-dependent differences in spatial patterns of activity (Chase, 1985).
Another example of differences between rodents and either locusts or slugs is that the latter have antennae and tentacles, respectively, while the former has a snout. Active sniffing is itself a dynamic process that dramatically imposes temporal structure on the incoming sensory stimulus in rodents. During active sniffing in awake rats, much of the oscillatory behavior of individual mitral cells breaks down (Kay and Laurent, 1999). The waving of antennae or tentacles through the air may be only vaguely analogous to sniffing.
The many reports advocating temporal coding in locusts also have ignored the evidence that locust neural responses may be highly tuned to particular odorants. Early work on sensory responses demonstrated that most locust sensory neurons display preferential responses to “green” odorants evoking grassy odors, such as aliphatic acids, alcohols, and aldehydes possessing about six carbons (Kafka, 1970). Just as is found for olfactory neurons in mammals, responses diminish as the odorant chemistry progressively differs from the preferred stimuli (Kafka, 1970). Evidence for such tuning also is apparent from examples of responses of an individual Kenyon cell to octanol and related alcohols and aldehydes (Perez-Orive et al., 2002). However, the data in these temporal coding papers are not analyzed or illustrated in a way that elucidates these relationships between stimulus and response, and the more typical stimuli in such studies are extracts and unrelated individual odorant chemicals, making it difficult to assess tuning in this system (Laurent and Naraghi, 1994; Laurent et al., 1996; Wehr and Laurent, 1996; MacLeod and Laurent, 1996; Wilson et al., 2004). If characteristics of locust sensory neurons have in fact been naturally selected for detection and discrimination of “green” odorants, it is possible that the use of other odorants might not readily reveal the natural coding principles at work in the system. Further work on locust olfaction would greatly benefit from more traditional studies of odorant receptor gene expression, a description of the projections from antennae into the antennal lobe, and a characterization of the molecular specificity of neurons at progressive levels of olfactory processing.
To our knowledge, there are only a small handful of experiments that have correlated olfactory performance with variations in temporal responses in rodents. In one study, seven-day old rats have greatly diminished fast-gamma- and beta-oscillatory responses to odorants, yet these rats discriminate straight-chained ethyl ester odorants differing by a single carbon just as readily as older rats that have such oscillations (Fletcher et al., 2005). Because it is unlikely that young animals have a completely different olfactory coding mechanism than that used a couple of weeks later, these oscillations are probably not critical for olfactory perception or olfactory discrimination. Mice with genetic deletions of GABA receptors normally present on granule cells displayed increased gamma- and theta-oscillations and also performed slightly better in discriminating straight-chained primary alcohols differing by a single carbon (Nusser et al., 2001). The increase in overall granule cell responsiveness in these animals also would be predicted to enhance mitral cell tuning along a homologous series by way of enhanced spatially based, center-surround lateral inhibition (Yokoi et al., 1995). It therefore seems best to consider these data as neutral with respect to supporting one coding model over another.
In summary, there are no data that convincingly implicate neural oscillations as the basis of an olfactory code. As mentioned above, imposing different temporal patterns of activity through electrical microstimulation fails to produce distinct odor perceptions under conditions where spatially distinct electrode locations are consistently discriminated (Monod et al., 1989).
What does the speed of olfaction tell us about odor coding?
It seems obvious that any mechanism responsible for a particular odor-guided behavior could not occur after that behavior occurs. Recent experiments in rodents (Uchida and Mainen, 2003; Abraham et al., 2004; Rinberg et al., 2006b) and honeybees (Ditzen et al., 2003) have shown that a reasonable degree of accurate olfactory processing (perception, discrimination and response) has been completed within less than a hundred to a few hundreds of milliseconds after an animal encounters an odorant. Very rapid, single-sniff, processing of olfactory information also has been shown for humans (Laing, 1986; Johnson et al., 2003). Therefore, any physiological response that takes longer than this brief period to emerge, such as much of the “temporal declustering” reported in zebrafish (Friedrich and Laurent, 2004), or the emergence of slow differences in glomerular responses in rodents (Spors and Grinwald, 2002; Spors et al., 2006) clearly cannot be responsible for the rapid odorant discriminations if these slowly developing responses occur after the discrimination has occurred. Also, locust principal neurons do not display what are thought to be critical temporal patterns of response to repeated deliveries of odorants until about 1.5 seconds after odorant onset (Stopfer and Laurent, 1999), well after the time needed by rats or honeybees to discriminate most odorant pairs accurately. There also is some dispute about whether odor identity information can be carried by any temporal patterning of response that extends beyond the brief period during which an animal crosses and re-crosses an airborne odorant plume (Vickers et al., 2001; Christensen, 2005).
Encouraging or imposing additional processing time by experimental animals and humans improves the accuracy of odorant discriminations for difficult comparisons (Wise and Cain, 2000; Abraham et al., 2004; Rinberg et al., 2006b), but it is impossible to determine whether this increased response time is due to an increase in the amount of time needed for sensory processing, or to the amount of time needed for cognitive processing (Khan and Sobel, 2004). The brief period of time needed for olfactory perception therefore eliminates the consideration of slowly evolving temporal differences that occur after the perception has occurred either as a basis for an olfactory code or as a basis for olfactory discrimination.
The notions of temporal coding of odorant quality arose during a time when: 1) there seemed to be broad responsiveness in the olfactory system to a wide variety of odorants, making it unlikely that any spatial pattern would have sufficient specificity to carry the coded signal, and 2) olfactory perception appeared to involve a slow response system, slow enough to allow late-developing temporal responses to carry the critical information in the system. As more recent data have shown that olfactory systems have a high degree of rapid differential activity, the bases for these hypotheses have been obviated.
Temporal evolution of glomerular responses
Differences in the timing of glomerular responses to different odorants are to be expected from many physicochemical processes occurring in the epithelium, but their presence does not constitute a prima facie case for an olfactory code. The very nature of airflow from the external to internal naris in rodents dictates that not all stimuli will arrive simultaneously at receptors present in different expression zones within the olfactory epithelium. Enzymatic and non-enzymatic chemical conversions of odorants into other molecules in the nasal mucosa also would be likely to proceed at different rates for different substrates, while other odorants are likely not to be modified prior to interacting with receptors. Expected enzymatic conversions would involve substrates of broad-specificity monooxigenases and transferases, all of which are found in nasal mucosa and are thought to cooperate in the removal of odorants and toxins from perireceptor areas (Pelosi, 1996). Expected non-enzymatic conversions would include the oxidation of aldehydes and the hydrolysis of esters. Differential adherence to odorant binding proteins, as well as differential diffusion of these complexes (Pelosi, 1996), also might change the kinetics of the presentation of different odorants to their receptors.
The imaging of calcium- and voltage-dependent dye responses in glomeruli has indeed captured evidence that would be consistent with these predictable time-dependent processes (Spors and Grinwald, 2002; Spors et al., 2006). These imaging techniques also provide a potential tool to test the separate involvement of each of these physicochemical processes in determining the actual specificity and timing of each of the sensory neuron responses. Perhaps an understanding of these processes ultimately will lead to the identification of interventive tools (e.g., enzyme inhibitors or transgenic animals with deleted or altered enzymes or odorant binding proteins) that then can be used to test whether these processes are actually used by the olfactory system to generate different odor quality perceptions. To date, however, there has been no experimental support for the perceptual relevance of these differences in the timing of glomerular responses.
Uses for temporal patterning other than odor quality coding
Although we cannot entirely exclude the possibility that some kind of temporal information might contribute to the sensory code involved in the most difficult odor discriminations, the experimental evidence supporting such a hypothesis seems weak, especially for rodents. However, it does seem likely that temporal features of neuronal responses play some role in olfaction. There is, for example, convincing recent evidence showing that temporal differences in responses between the two olfactory bulbs can be used by rodents to localize odorants to one side of the head versus the other (Rajan et al., 2006). Temporal patterning of perceived odors apparently can assist humans in identifying components of certain mixtures (Laing et al., 1994). There also is evidence suggesting that temporal coincidence of the activity of mitral cells associated with the same glomerulus, or otherwise co-activated by the same odorant, might amplify weak patterns of activity initiated in the glomerular layer (Kashiwadani et al., 1999; Schoppa and Westbrook, 2001). The timing of these action potentials is made more precise by the presence of respiration-linked theta-oscillations in the olfactory bulb (Schaefer et al., 2006). Temporal patterns of response in moth principal neurons depend heavily on the temporal pattern of sensory stimulation, which may be used to determine the source of an odorant encountered intermittently across a plume (Christensen et al., 1998). Slugs may use differences in the timing of responses reflecting differences in sensory stimulation to determine if two odorants arise out of a stationary mixture from a single source, or if they are mixing in the air after arising from separate sources (Hopfield and Gelperin, 1989; Gelperin, 1999). It therefore seems possible that temporal information can be used to solve very real and important olfactory problems that are distinct from the encoding of odor identity.
Suggestions for further studies of temporal coding
What is needed to understand time-varying signals in mammals is research on mitral cell responses that are associated with peak glomerular responses in awake animals. Any temporal pattern of activity hypothesized to carry information in these cells must then be shown to accurately predict perceptual behavior. We suggest special attention to excitatory responses occurring shortly after the initiation of active sniffing, and thereby less likely to reflect slow or persistent modulatory influences arising from descending centrifugal inputs more related to behavioral state than to odor coding itself, or more related to feedback after perception has occurred. Recent work in Manduca moths (Lei et al., 2004) and in honeybees (Szyszka et al., 2005) exemplify the type of efforts that may provide truly useful model systems for the development of specific hypotheses of how temporal “information” might relate to the pattern of incoming glomerular activity.
Hypotheses involving temporal coding range from arguing that temporal patterns of firing are carrying all odor quality information to suggesting a more limited role in only the very most difficult odor discriminations (e.g., those involving two mixtures containing very similar ratios of very similar odorant pairs, a task that may not be as naturally relevant to rodents as it is in the detection of pheromonal signals by some moths). The aspects of the temporal patterns proposed to carry information also differ greatly from lab to lab and even from publication to publication from the same lab. Some of this temporal information ultimately may turn out to be important to odor quality coding, but each claim requires a behavioral test, preferably a critical test capable of distinguishing temporal coding from already existing non-temporal explanations. Considerations of the rapidity of olfactory perception, the persistence of most olfactory discrimination in the absence of certain oscillations, and the failure of rats to perceive certain temporal patterns of bulbar microstimulation clearly eliminate some of the temporal coding hypotheses from serious consideration. Certainly, the evidence that has established the relevance of spatial patterns to odor quality perception should not be glossed over or ignored in the formulation of temporal hypotheses.
Detailed descriptions of the specificities of glomerular modules
In this section we summarize the results of our mapping studies that have related the chemistry of odorant stimuli to the spatial locations of responses. Wherever available, we also compare our results to those of others who have used alternative methods of monitoring glomerular activity.
Carboxylic acids, methyl esters, and ethyl esters
Carboxylic acids possessing a great variety of hydrocarbon structures (straight-chained from 2-11 carbons, double-bonded, branched, and cyclic) all stimulate glomeruli in a pair of anterior domains that are shaded orange in Figure 4 (Johnson et al., 1999; 2002; 2007a; Johnson and Leon, 2000b; and unpublished observations). Examples of effective odorants include propionic acid, valeric acid, and undecylenic acid. The lateral member of this pair of domains is located very dorsally and therefore can be detected by optical imaging techniques accessing the dorsal surface of the bulb (Uchida et al., 2000; Takahashi et al., 2004a,b). The medial member of this pair was one of the best-studied responses in earlier 2DG work using the odorant propionic acid (Bell et al., 1987; Slotnick et al., 1989; Sallaz and Jourdan, 1993). Electrophysiological recordings of field potentials and single-cell recordings indicated that mitral cells in the same region were responsive to acids and that individual mitral cells preferred straight-chained carboxylic acids of a given carbon number, while responses fell off incrementally for odorants that were progressively different in carbon number (Mori et al., 1992). The glomeruli responding to longer carboxylic acids are located more ventrally within each module (Johnson et al., 1999; Johnson and Leon, 2000b).
The acid-preferring domains also are stimulated by numerous intact methyl and ethyl esters, which share the carboxylate structural element with the acids (Johnson et al., 1998; 2002; 2004; Johnson and Leon, 2000a). Although higher vapor-phase concentrations of the esters are required for these responses, the esters are more volatile than the corresponding acids, so that one obtains similar responses to equal dilutions of saturated vapor (Johnson and Leon, 2000a). Like the responses to acids, responses to straight-chained ethyl esters in this module shifted in a progressively ventral direction with increasing carbon number (Johnson et al., 2004). We considered the possibility that responses to methyl and ethyl ester odorants could be explained by their hydrolysis into corresponding carboxylic acids, a spontaneous reaction that we have in fact documented for some preparations. In other cases, however, we found stimulation of the anterior domains by methyl esters in the absence of detectable levels of acid (Johnson and Leon, unpublished observations). A subset of the glomeruli that are responsive to aliphatic acids also responds to certain primary amines as judged by optical imaging of endogenous signals (Takahashi et al., 2004b).
Many olfactory mapping studies involving only the dorsal aspect of the bulb have unfortunately focused on series of aliphatic aldehydes as odorants. In typical experiments, these odorants apparently stimulate glomeruli in the same area that responds to carboxylic acids (Imamura et al., 1992; Rubin and Katz, 1999; Johnson and Leon, 2000a; Uchida et al., 2000; Wachowiak and Cohen, 2001; Meister and Bonhoeffer, 2001). However, the activation of this area is probably due to contaminating acids formed by rapid oxidation of the aldehyde odorants, a reaction that is so extensive that many preparations of shorter-chained aldehydes contain easily detectable quantities of acid when their containers are first opened (Johnson et al., 2004). The acid concentrations in the aldehyde preparations showed further increases over the course of our experiments even when special precautions were exercised, such as the use of nitrogen for odorant vaporization (Johnson et al., 2004). The use of only a small number of odorants and the observation of only a small part of the bulb, such as typified the early optical imaging studies, neglected the larger responses to acids in this area and also missed larger responses to aldehydes occurring in other bulbar locations, as will be discussed in greater detail below.
Primary alcohols and aldehydes
A pair of glomerular domains located just posterior and slightly ventral relative to the acid-preferring modules (green-shaded areas in Figure 4) contains principal responses to primary alcohols with various hydrocarbon structures, including straight-chained, simple branched, and double-bonded molecules such as 1-pentanol, 4-methyl-1-pentanol, and trans-2-hexen-1-ol (Johnson and Leon, 2000a; Johnson et al., 2002; 2004; 2005a; 2007a), as well as relatively weaker responses to straight-chained aliphatic aldehydes and ketones (Johnson and Leon, 2000a; Johnson et al., 2002; 2004). These same areas contain responses to phenols, which are aromatic odorants substituted with hydroxyl groups, resembling alcohols in that respect, and including spicy odorants such as vanillin and eugenol as well as smoky odorants such as guaiacol (Johnson et al., 2002; Farahbod et al., 2006). Aromatic compounds with methoxy (ether) substituents such as m-anisaldehyde also activate this module (Johnson et al., 2002; Farahbod et al., 2006). Responses in this area have been studied using optical imaging of intrinsic signals, wherein individual glomeruli are found to respond to both alcohols and phenols, but to have distinct specificities for different odorants in each class (Uchida et al., 2000; Takahashi et al., 2004a).
Straight-chained secondary alcohols with the hydroxyl group in the number 2 position such as 2-octanol also are effective stimuli for glomeruli in this domain, but alcohols with even more internal substitution positions are not very effective stimuli for this response domain (Johnson et al., 2005a). With increasing carbon number in homologous series of either primary alcohols or aldehydes, responses shift ventrally within this domain, just as was seen for the acid-preferring domain (Johnson et al., 2004).
Aliphatic esters
Aliphatic esters such as amyl acetate, ethyl butyrate, and geranyl acetate stimulate glomeruli in a pair of domains shaded yellow in Figure 4, one located dorsolaterally and one located in the posterior part of the medial aspect of the bulb (Johnson et al., 1998; 2002; 2004; 2005a,b; 2006; Johnson and Leon, 2000a). Activity in this region is the predominant response to aliphatic esters possessing a wide range of molecular lengths and hydrocarbon structures (Johnson et al., 2002; 2004; 2005b), and it is largely independent of the location of the ester bond in the molecule (Johnson et al., 2005a). However, glomeruli in these domains make up part of the response to many other odorants, including carboxylic acids, aldehydes, ketones, and alkanes from six to eight carbons (Johnson et al., 1999; 2002; 2004; 2005a,b; 2006; 2007a,b; Johnson and Leon, 2000a; Ho et al., 2006a,b), as well as terpenes and some bicyclic compounds (Johnson et al., 2002; 2006). Indeed, glomeruli in this region appear to be the most broadly responsive in the bulb.
High concentrations of ketones and aromatic odorants with oxygenic substituents
The dorsal-most part of the bulb contains a pair of domains (light brown shading in Figure 4) that exhibit 2DG uptake in response to very high concentrations of volatile aliphatic ketones of various hydrocarbon structures, including straight-chained, branched, and cyclic molecules (Johnson and Leon, 2000a; Johnson et al., 2002; 2005a,b). Effective odorants include 2-hexanone, 5-methyl-2-hexanone, and acetylcyclohexane. Lower concentrations of the same ketones are not effective in stimulating these dorsal domains, although they do stimulate glomeruli in more ventral locations (Johnson and Leon, 2000a; Johnson et al., 2004). Larger ketones (10 carbons or more) do not evaporate to achieve high vapor-phase concentrations, a situation that probably explains why they do not stimulate the dorsal domains (Johnson et al., 2004).
The same domains responding to volatile ketones also contain glomeruli stimulated by aromatic compounds with oxygen-containing functional groups, such as ketones (acetophenone), aldehydes (benzaldehyde), and methyl esters (methyl benzoate and methyl salicylate), as well as compounds substituted by these functional groups in combinations with others (Johnson et al., 2002; 2005b; Farahbod et al., 2006). Responses to a number of alicyclic (cyclic but not aromatic) compounds also occur in this area (Johnson et al., 2005b). The effective aromatic compounds stimulate glomeruli in these areas at much lower vapor phase concentrations than do ketones, but the aromatic odorants also are less volatile, so that the saturated vapor from a ketone such as 2-hexanone causes more 2DG uptake than does the saturated vapor over any aromatic compound that we have investigated.
Because of the extreme dorsal location of the lateral domain, most optical imaging studies probably have focused on this most conveniently accessible region (Rubin and Katz, 1999; Belluscio and Katz, 2001; Meister and Bonhoeffer, 2001; Wachowiak and Cohen, 2001; Spors and Grinwald, 2002). In most of the early studies using these imaging techniques, aromatic odorants were not investigated, and responses were reported to odorants that far more strongly activate other regions of the bulb as judged by the 2DG method. Because these early studies used such a small sample of odorants while investigating a small portion of the olfactory bulb, they may have led to some confusion regarding the preferred odorants for glomeruli in this region. Those optical imaging studies that have used a wider range of odorant chemical structures and that have accessed a greater portion of the bulbar surface have come to conclusions similar to ours regarding the preference of this area for ketones and aromatic odorants (Uchida et al., 2000; Takahashi et al., 2004a). It seems likely that other studies may have been recording background responses to the stimuli rather than their peak responses.
In most cases, a given response module contains glomeruli activated by similar odorant chemicals. Indeed, the same glomeruli that respond to subsets of carboxylic acids also respond to subsets of aliphatic esters (Uchida et al., 2000), suggesting that the similarity in the structure of these odorants allows them to stimulate the same receptors. It is not as readily apparent why responses to aliphatic ketones would be found in the same domain as responses to a subset of aromatic compounds, given the absence of any overt similarity in either chemical structure or molecular properties (Farahbod et al., 2006). Although contamination of one odorant with another can explain isolated instances of unexpected overlaps in some activity patterns (Johnson et al., 2004; 2007a; Ho et al., 2006a), the overlap between ketones and aromatic compounds with oxygen-containing functional groups seems too robust to be explained in this way. One possibility we considered was that glomeruli responding preferentially to ketones might be interspersed with glomeruli responding preferentially to the subset of aromatic odorants (Farahbod et al., 2006). However, optical imaging reveals that these two classes of odorants can stimulate the very same glomeruli (Uchida et al., 2000; Takahashi et al., 2004a; Zheng Y, Johnson BA, Frostig R, Leon M, unpublished observations), and in at least one case, benzaldehyde and 2-hexanone stimulated the very same fibers within a glomerulus (Wachowiak et al., 2004). This level of overlap would presumably require that these apparently disparate chemicals stimulate the same sensory neurons, either due to the presence of multiple receptors, multiple binding sites of distinct specificity on one receptor, or a covert similarity in chemical structure allowing both types of ligand to be accommodated by a single receptor binding site. The functional consequences of a mixed specificity in a single glomerulus also are unclear and worthy of further investigation. It should be noted that this somewhat broader pattern of response is not typical of the other glomerular response domains.
Aromatic odorants with alkyl substituents
Aromatic (benzyl) odorants with alkyl substituents such as tert-butylbenzene and o-xylene evoke 2DG uptake in glomeruli located significantly more posterior and ventral than those activated by aromatic odorants with oxygenic substituents (Farahbod et al., 2006). The paired modules activated preferentially by these aromatic hydrocarbons are illustrated in Figure 4 using dark brown shading. During optical imaging of intrinsic signals (Igarashi et al., 2005) and in electrophysiological studies (Katoh et al., 1993), aromatic hydrocarbons have been reported to stimulate glomeruli in a more ventral region overlapping with responses to alkanes (light blue-shaded domains in Figure 4). This pattern of response may represent the only set of stimuli for which optical imaging and 2DG uptake disagree, as 2DG uptake clearly does not extend as far ventrally in response to these aromatic hydrocarbons (Farahbod et al., 2006).
Aliphatic hydrocarbon chains
Alkanes from eight to fifteen carbons in length such as octane and pentadecane stimulate 2DG uptake along a large pair of domains (light blue in Figure 4), beginning about halfway down the lateral or medial surface of the bulb and extending ventrally all the way to the ventral extreme of the bulb, where the lateral and medial domains meet (Ho et al., 2006a). Responses in these domains shift progressively ventrally with increasing carbon number up to about 14 or 15 carbons, when responses apparently fuse to form one patch of uptake on the extreme ventral surface of the bulb (Ho et al., 2006a). Any other aliphatic compound with an extensive (four or more carbons) hydrocarbon chain also stimulates this region, with responses that shift ventrally with carbon number such that an aliphatic ketone, for example, stimulates about the same location as does an alkane of the same carbon number (Johnson et al., 1998; 1999; 2004; Ho et al., 2006a). Optically imaged responses to long, straight-chained alkanes also have been detected in ventrally positioned glomeruli within the lateral member of these domains (Igarashi and Mori, 2005).
Bicyclic, camphoraceous odorants
A number of bicyclic compounds related in structure to camphor and/or sharing a camphoraceous odor such as eucalyptol and bornyl acetate all evoke glomerular 2DG uptake in two domains located along the ventral extremity of the bulb (Johnson et al., 2006). Instead of involving lateral and medial bulbar aspects, this pair of modules involves anterior and posterior regions on either side of the responses to long alkanes (magenta shading in Figure 4). These regions are similar in location to the targets of projections from sensory neurons that have an unusual clustered distribution in the olfactory epithelium, that express a distinctive subset of odorant receptor genes, and that send axons to unpaired glomeruli along the ventral aspect of the bulb (Strotmann et al., 1992; 1999; 2000). The most ventral glomeruli that have been accessed by optical imaging techniques following surgical exposure of the lateral bulbar surface also seem to respond to bicyclic odorants (Igarashi and Mori, 2005), and these glomeruli probably correspond to the dorsal part of the posterior 2DG uptake domain.
Highly water-soluble, volatile odorants
The most recent pair of modules deduced through studies of 2DG uptake contains glomeruli responding to volatile odorants with high water solubility, regardless of specific functional groups or hydrocarbon structure (Johnson et al., 2007b). Glomeruli in these posterior domains (red shading in Figure 4) were activated by aliphatic odorants with two oxygenic functional groups such as diacetyl, acetoin, and diethyl malonate, by small (≤ 4 carbon) odorants with single oxygenic functional groups such as acetone and propanol, and by some heterocyclic compounds such as delta-valerolactone and abhexone, all of which attain high water solubility by virtue of their low hydrocarbon hydrogen content while also possessing polar functional groups (Johnson et al., 2007b). When analyzed across our entire database, the relative amount of uptake in these posterior regions was significantly correlated with the water solubility of the odorants (Johnson et al., 2007b).
There has been some speculation that mammalian class I odorant receptors, which are evolutionarily related to “fish-like” receptors, would respond to water-soluble odorants because actual fish receptors bind substances such as amino acids that are soluble in water (Zhang and Firestein, 2002; Igarashi and Mori, 2005). Class I receptors are expressed by sensory neurons in the part of the olfactory epithelium that projects only to the dorsal part of the olfactory bulb (Mori et al., 1985; Schwob and Gottlieb, 1986; Schoenfeld and Knott, 2002; Tsuboi et al., 2006). The paired modules that respond to the most water-soluble odorants do not fall within this dorsal projection zone, indicating that the receptors that respond to water-soluble, volatile odorants are almost certainly not class I receptors (Johnson et al., 2007b). This fact should perhaps not be entirely surprising, given that fish receptors actually respond to charged substances that do not evaporate, and there is only a remote similarity between these molecules and the most water-soluble, volatile odorants.
Mori and coworkers (2006) have pointed out that carboxylic acid and amine odorants that stimulate the dorsal zone both possess functional groups that are present in amino acid odorants, which are the natural stimuli for many fish receptors (Kang and Caprio, 1997; Friedrich and Korsching, 1997; Fuss and Korsching, 2001). Therefore, this chemical similarity rather than a similarity in water solubility might better explain the activation of the fish-like class I receptors (Mori et al., 2006). Given that a population of fish receptors also responds to nucleic acids (Friedrich and Korsching, 1998), it is interesting that other classes of odorant chemicals that stimulate the dorsal part of the bulb, namely aromatic compounds including pyrazines and pyridines (Johnson et al., 2006), have superficial chemical similarities to nucleotide bases.
Septal organ projection
In studies where we used high concentrations of five- and six-carbon aliphatic odorants possessing different functional groups, we measured a large amount of 2DG uptake in a posterior, ventral area located on the medial aspect of the bulb (Johnson and Leon, 2000a). Unlike most of the other responses, there was no lateral equivalent to this module (dark blue area in Figure 4), and it corresponded in location to the target glomeruli of the septal organ of Masera, which projects only to the medial aspect of the bulb (Pederson and Benson, 1986; Giannetti et al., 1992; Lèvai and Strotmann, 2003; Ma et al., 2003). Over the course of our further studies, we have detected 2DG uptake in this region for a number of different odorants with no clear similarities in structure or perceived odor. The broad responsiveness of this glomerular region is in good agreement with electrophysiological studies showing both that the septal organ expresses numerous different odorant receptors and that the different sensory neurons in the structure respond to chemically distinct odorants (Marshall and Maruniak, 1986; Ma et al., 2003; Kaluza et al., 2004; Tian and Ma, 2004).
Conclusion
Responses to hundreds of pure odorants are arranged predictably and systematically in space across the olfactory bulb in a manner closely related both to odorant chemical structure and to the bilateral projections of homologous olfactory sensory neurons. Moreover, the spatially specific activity patterns successfully predict performance in a variety of behavioral tests of perception. Predictions bridging neural responses and perception are most accurate when the glomerular patterns are read as a relational code, where focal activity is assessed by the system relative to activity across the entire olfactory bulb. When spatial patterns are either imposed on the olfactory system or disrupted in well-reasoned ways, these manipulations have the predicted effects on perceptual behavior. In contrast to the strong evidence supporting an olfactory spatial code, there is no evidence for a similarly close relationship between temporal patterning of olfactory responses and perception or discrimination.
Acknowledgments
Supported by United States Public Health Service Grants DC03545, DC006391, and DC006516
Footnotes
Associate editor: Thomas E. Finger
LITERATURE CITED
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Ache BW, Young JB. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Adrian ED. Sensory discrimination with some recent evidence from the olfactory organ. Brit Med Bull. 1950a;6:330–333. doi: 10.1093/oxfordjournals.bmb.a073625. [DOI] [PubMed] [Google Scholar]
- Adrian ED. The electrical activity of the mammalian olfactory bulb. EEG Clin Neurophysiol. 1950b;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- Adrian ED. Sensory messages and sensation: the response of the olfactory organ to different smells. Acta Physiol Scand. 1953;29:5–14. doi: 10.1111/j.1748-1716.1953.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Alarie Y. Sensory irritation by airborne chemicals. CRC Crit Rev Toxicol. 1973;2:299–363. doi: 10.3109/10408447309082020. [DOI] [PubMed] [Google Scholar]
- Alioto TS, Ngai J. The odorant receptor repertoire of teleost fish. BMC Genomics. 2005;6:173. doi: 10.1186/1471-2164-6-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfelbach R, Weiler E, Asselbergs WF, Polak EH, Slotnick B. Selective and reversible reduction of odor sensitivity in the rat by concanavalin A. Physiol Behav. 1998;65:513–516. doi: 10.1016/s0031-9384(98)00197-8. [DOI] [PubMed] [Google Scholar]
- Apfelbach R. 2004 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. Lectin application to the olfactory epithelium modifies odor quality. (Program No. 139.1). [Google Scholar]
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Arctander S. Perfume and Flavor Chemicals (Aroma Chemicals) Allured Publishing Company; Carol Stream, IL: 1994. [Google Scholar]
- Astic L, Saucier D. Anatomical mapping of the neuroepithelial projection to the olfactory bulb in the rat. Brain Res Bull. 1986;16:445–454. doi: 10.1016/0361-9230(86)90172-3. [DOI] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Axel R. The molecular logic of smell. Sci Am. 1995;273:154–159. doi: 10.1038/scientificamerican1095-154. [DOI] [PubMed] [Google Scholar]
- Bazhenov M, Stopfer M, Rabinovich M, Huerta R, Abarbanel HD, Sejnowski TJ, Laurent G. Model of transient oscillatory synchronization in the locust antennal lobe. Neuron. 2001;30:553–67. doi: 10.1016/s0896-6273(01)00284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GA, Laing DG, Panhuber H. Odour mixture suppression: evidence for a peripheral mechanism in human and rat. Brain Res. 1987;426:8–18. doi: 10.1016/0006-8993(87)90419-7. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci. 2001;21:2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson TE, Burd GD, Greer CA, Landis DM, Shepherd GM. High-resolution 2-deoxyglucose autoradiography in quick-frozen slabs of neonatal rat olfactory bulb. Brain Res. 1985;339:67–78. doi: 10.1016/0006-8993(85)90622-5. [DOI] [PubMed] [Google Scholar]
- Bhalla US, Bower JM. Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties. J Comput Neurosci. 1997;4:221–256. doi: 10.1023/a:1008819818970. [DOI] [PubMed] [Google Scholar]
- Bisulco S, Slotnick B. Olfactory discrimination of short chain fatty acids in rats with large bilateral lesions of the olfactory bulbs. Chem Senses. 2003;28:361–370. doi: 10.1093/chemse/28.5.361. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Brody CD, Hopfield JJ. Simple networks for spike-timing-based computation, with application to olfactory processing. Neuron. 2003;37:843–852. doi: 10.1016/s0896-6273(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Buck LB. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1996;19:517–44. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Carlsson MA, Galizia CG, Hansson BS. Spatial representation of odours in the antennal lobe of the moth Spodoptera littoralis (Lepidoptera: Noctuidae) Chem Senses. 2002;27:231–244. doi: 10.1093/chemse/27.3.231. [DOI] [PubMed] [Google Scholar]
- Chalansonnet M, Chaput MA. Olfactory bulb output cell temporal response patterns to increasing odor concentrations in freely breathing rats. Chem Senses. 1998;23:1–9. doi: 10.1093/chemse/23.1.1. [DOI] [PubMed] [Google Scholar]
- Chase R. Responses to odors mapped in snail tentacle and brain by [14C]-2-deoxyglucose autoradiography. J Neurosci. 1985;5:2930–2939. doi: 10.1523/JNEUROSCI.05-11-02930.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase R, Tolloczko B. Tracing neural pathways in snail olfaction: from the tip of the tentacles to the brain and beyond. Microsc Res Tech. 1993;24:214–230. doi: 10.1002/jemt.1070240303. [DOI] [PubMed] [Google Scholar]
- Christensen TA. Making scents out of spatial and temporal codes in specialist and gneralist olfactory networks. Chem Senses. 2005;30:i283–i284. doi: 10.1093/chemse/bjh225. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Waldrop BR, Hildebrand JG. Multitasking in the olfactory system: context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons. J Neurosci. 1998;18:5999–6008. doi: 10.1523/JNEUROSCI.18-15-05999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli AR, Hamilton KA, Kauer JS. Salamander olfactory bulb neuronal activity observed by video rate, voltage-sensitive dye imaging. III. Spatial and temporal properties of responses evoked by odorant stimulation. J Neurophysiol. 1995;73:2053–2071. doi: 10.1152/jn.1995.73.5.2053. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Schoenfeld TA, Forbes WB, Macrides F. The spatial organization of the peripheral olfactory system of the hamster. Part II: Receptor surfaces and odorant passageways within the nasal cavity. Brain Res Bull. 1994;34:211–241. doi: 10.1016/0361-9230(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 2006;7:7. doi: 10.1186/1471-2202-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Johnson BA, Leon M, Linster C. Relational representation in the olfactory system. Proc Natl Acad Sci USA. 2007;104:1953–1958. doi: 10.1073/pnas.0608564104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmann C, Carlsson MA, Hansson BS, Nighorn A. Odorant-evoked nitric oxide signals in the antennal lobe of Manduca sexta. J Neurosci. 2004;24:6070–6077. doi: 10.1523/JNEUROSCI.0710-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopersmith RM, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Dev Brain Res. 1986;27:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Daly KC, Wright GA, Smith BH. Molecular features of odorants systematically influence slow temporal responses across clusters of coordinated antennal lobe units in the moth Manduca sexta. J Neurophysiol. 2004;92:236–254. doi: 10.1152/jn.01132.2003. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Puche A, Adams NC, Rodriguez I, Mombaerts P. A divergent pattern of sensory axonal projections is rendered convergent by second-order neurons in the accessory olfactory bulb. Neuron. 2002;35:1057–1066. doi: 10.1016/s0896-6273(02)00904-2. [DOI] [PubMed] [Google Scholar]
- Derby CD. Learning from spiny lobsters about chemosensory coding of mixtures. Physiol Behav. 2000;69:203–209. doi: 10.1016/s0031-9384(00)00202-x. [DOI] [PubMed] [Google Scholar]
- Deutsch S, Apfelbach R. The antibody OR-I7 selectively affects the detection of noctanal and the odor induced c-Fos expression pattern in the rat olfactory bulb. Chem Senses. 2006;31:A75. [Google Scholar]
- Ditzen M, Evers JF, Galizia CG. Odor similarity does not influence the time needed for odor processing. Chem Senses. 2003;28:781–789. doi: 10.1093/chemse/bjg070. [DOI] [PubMed] [Google Scholar]
- Døving KB, Pinching AJ. Selective degeneration of neurones in the olfactory bulb following prolonged odour exposure. Brain Res. 1973;52:115–129. doi: 10.1016/0006-8993(73)90653-7. [DOI] [PubMed] [Google Scholar]
- Duchamp-Viret P, Duchamp A, Chaput MA. Single olfactory sensory neurons simultaneously integrate the components of an odour mixture. Eur J Neurosci. 2003;18:2690–2696. doi: 10.1111/j.1460-9568.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Stumpf WE. Brain activity patterns: assessment by high resolution autoradiographic imaging of radiolabeled 2-deoxyglucose and glucose uptake. Prog Neurobiol. 1991;37:365–382. doi: 10.1016/0301-0082(91)90023-t. [DOI] [PubMed] [Google Scholar]
- Eisthen HL. Evolution of vertebrate olfactory systems. Brain Behav Evol. 1997;50:222–233. doi: 10.1159/000113336. [DOI] [PubMed] [Google Scholar]
- Eisthen HL. Why are olfactory systems of different animals so similar? Brain Behav Evol. 2002;59:273–293. doi: 10.1159/000063564. [DOI] [PubMed] [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006;496:350–366. doi: 10.1002/cne.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Smith AM, Best AR, Wilson DA. High-frequency oscillations are not necessary for simple olfactory discriminations in young rats. J Neurosci. 2005;25:792–798. doi: 10.1523/JNEUROSCI.4673-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, Skarda CA. Spatial EEG patterns, non-linear dynamics and perception: the neo-Sherringtonian view. Brain Res Rev. 1985;10:147–175. doi: 10.1016/0165-0173(85)90022-0. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Viana Di Prisco G. Relation of olfactory EEG to behavior: time series analysis. Behav Neurosci. 1986;100:753–763. doi: 10.1037//0735-7044.100.5.753. [DOI] [PubMed] [Google Scholar]
- Fried HU, Fuss SH, Korsching SI. Selective imaging of presynaptic activity in the mouse olfactory bulb shows concentration and structure dependence of odor responses in identified glomeruli. Proc Natl Acad Sci U S A. 2002;99:3222–3227. doi: 10.1073/pnas.052658399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW. Mechanisms of odor discrimination: neurophysiological and behavioral approaches. Trends Neurosci. 2006;29:40–47. doi: 10.1016/j.tins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci. 1998;18:9977–9988. doi: 10.1523/JNEUROSCI.18-23-09977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamics of olfactory bulb input and output activity during odor stimulation in zebrafish. J Neurophysiol. 2004;6:2658–2669. doi: 10.1152/jn.01143.2003. [DOI] [PubMed] [Google Scholar]
- Fuss SH, Korsching SI. Odorant feature detection: activity mapping of structure response relationships in the zebrafish olfactory bulb. J Neurosci. 2001;21:8396–8407. doi: 10.1523/JNEUROSCI.21-21-08396.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia CG, Sachse S, Rappert A, Menzel R. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat Neurosci. 1999a;2:473–478. doi: 10.1038/8144. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Menzel R, Holldobler B. Optical imaging of odor-evoked glomerular activity patterns in the antennal lobes of the ant camponotus rufipes. Naturwissenschaften. 1999b;86:533–537. doi: 10.1007/s001140050669. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Sachse S, Mustaparta H. Calcium responses to pheromones and plant odours in the antennal lobe of the male and female moth Heliothis virescens. J Comp Physiol [A] 2000;186:1049–1063. doi: 10.1007/s003590000156. [DOI] [PubMed] [Google Scholar]
- Gelperin A. Oscillatory dynamics and information processing in olfactory systems. J Exp Biol. 1999;202:1855–1864. doi: 10.1242/jeb.202.14.1855. [DOI] [PubMed] [Google Scholar]
- Gelperin A. Olfactory computations and network oscillations. J Neurosci. 2006;26:1663–1668. doi: 10.1523/JNEUROSCI.3737-05b.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperin A, Flores J, Raccuia-Behling F, Cooke IR. Nitric oxide and carbon monoxide modulate oscillations of olfactory interneurons in a terrestrial mollusk. J Neurophysiol. 2000;83:116–127. doi: 10.1152/jn.2000.83.1.116. [DOI] [PubMed] [Google Scholar]
- Giannetti N, Saucier D, Astic L. Organization of the septal organ projection to the main olfactory bulb in adult and newborn rats. J Comp Neurol. 1992;323:288–298. doi: 10.1002/cne.903230211. [DOI] [PubMed] [Google Scholar]
- Guerrieri F, Schubert M, Sandoz JC, Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3:e60. doi: 10.1371/journal.pbio.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Gall CM. Functional mapping of odor-activated neurons in the olfactory bulb. Chem Senses. 1995;20:271–282. doi: 10.1093/chemse/20.2.271. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993;903:329–333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie K, Rayhanabad J, Kuhl D, Gall C. Odors regulate Arc expression in neuronal ensembles engaged in odor processing. Neuroreport. 2000;11:1809–1813. doi: 10.1097/00001756-200006260-00003. [DOI] [PubMed] [Google Scholar]
- Harrison TA, Scott JW. Olfactory bulb responses to odor stimulation: analysis of response pattern and intensity relationships. J Neurophysiol. 1986;56:1571–1589. doi: 10.1152/jn.1986.56.6.1571. [DOI] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Leon M. Long hydrocarbon chains serve as unique molecular features recognized by ventral glomeruli of the rat olfactory bulb. J Comp Neurol. 2006a;498:16–30. doi: 10.1002/cne.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Chen AL, Leon M. Differential responses to branched and unsaturated aliphatic hydrocarbons in the rat olfactory system. J Comp Neurol. 2006b;499:519–532. doi: 10.1002/cne.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JF, Gelperin A. Differential conditioning to a compound stimulus and its components in the terrestrial mollusk Limax maximus. Behav Neurosci. 1989;103:329–333. [Google Scholar]
- Hornung DE, Mozell MM. Factors influencing the differential sorption of odorant molecules across the olfactory mucosa. J Gen Physiol. 1977;69:343–361. doi: 10.1085/jgp.69.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung DE, Youngentob SL, Mozell MM. Olfactory mucosa/air partitioning of odorants. Brain Res. 1987;413:147–154. doi: 10.1016/0006-8993(87)90163-6. [DOI] [PubMed] [Google Scholar]
- Hosler JS, Buxton KL, Smith BH. Impairment of olfactory discrimination by blockade of GABA and nitric oxide activity in the honey bee antennal lobes. Behav Neurosci. 2000;114:514–525. doi: 10.1037/0735-7044.114.3.514. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959;148:574–91. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R, Distel H. Regional autonomy in the peripheral processing of odor signals in newborn rabbits. Brain Res. 1987;421:85–94. doi: 10.1016/0006-8993(87)91278-9. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Mori K. Spatial representation of hydrocarbon odorants in the ventrolateral zones of the rat olfactory bulb. J Neurophysiol. 2005;93:1007–1019. doi: 10.1152/jn.00873.2004. [DOI] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Imamura K, Mataga N, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 1992;68:1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Inaki K, Takahashi YK, Nagayama S, Mori K. Molecular-feature domains with posterodorsal-anteroventral polarity in the symmetrical sensory maps of the mouse olfactory bulb: mapping of odourant-induced Zif268 expression. Eur J Neurosci. 2002;15:1563–1574. doi: 10.1046/j.1460-9568.2002.01991.x. [DOI] [PubMed] [Google Scholar]
- Joerges J, Küttner A, Galizia G, Menzel R. Internal representations for odours and combinatorial coding of odour mixtures visualized by optical imaging. Nature. 1997;387:285–288. [Google Scholar]
- Johnson BA, Leon M. Modular glomerular representations of odorants in the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000a;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: One aspect of the olfactory code. J Comp Neurol. 2000b;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Saber S, Leon M. Effects of functional group position on spatial representations of aliphatic odorants in the rat olfactory bulb. J Comp Neurol. 2005a;483:192–204. doi: 10.1002/cne.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Leon M. Interactions between odorant functional group and hydrocarbon structure influence activity in glomerular response modules in the rat olfactory bulb. J Comp Neurol. 2005b;483:205–216. doi: 10.1002/cne.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Kwok J, Pancoast P, Ong J, Leon M. Differential specificity in the glomerular response profiles for alicyclic, bicyclic and heterocyclic odorants. J Comp Neurol. 2006;499:1–16. doi: 10.1002/cne.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ong J, Lee K, Ho SL, Arguello S, Leon M. Effects of double and triple bonds on the spatial representations of odorants in the rat olfactory bulb. J Comp Neurol. 2007a;500:720–733. doi: 10.1002/cne.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Arguello S, Leon M. Odorants with multiple oxygen-containing functional groups and other odorants with high water solubility preferentially activate posterior olfactory bulb glomeruli. J Comp Neurol. 2007b doi: 10.1002/cne.21322. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90:1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- Jourdan F, Duveau A, Astic L, Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res. 1980;188:139–154. doi: 10.1016/0006-8993(80)90563-6. [DOI] [PubMed] [Google Scholar]
- Kafka WA. Molecular interaction leading to excitation of single olfactory receptor cells. Z Vergl Physiol. 1970;70:105–143. [Google Scholar]
- Kajiya K, Inaki K, Tanaka M, Haga T, Kataoka H, Touhara K. Molecular bases of odor discrimination: Reconstitution of olfactory receptors that recognize overlapping sets of odorants. J Neurosci. 2001;21:6018–6025. doi: 10.1523/JNEUROSCI.21-16-06018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza JF, Gussing F, Bohm S, Breer H, Strotmann J. Olfactory receptors in the mouse septal organ. J Neurosci Res. 2004;76:442–452. doi: 10.1002/jnr.20083. [DOI] [PubMed] [Google Scholar]
- Kang J, Caprio J. In vivo responses of single olfactory receptor neurons of channel catfish to binary mixtures of amino acids. J Neurophysiol. 1997;77:1–8. doi: 10.1152/jn.1997.77.1.1a. [DOI] [PubMed] [Google Scholar]
- Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Koshimoto H, Tani A, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. II. Aromatic compounds. J Neurophysiol. 1993;70:2161–2175. doi: 10.1152/jn.1993.70.5.2161. [DOI] [PubMed] [Google Scholar]
- Kauer JS. Response patterns of amphibian olfactory bulb neurones to odour stimulation. J Physiol Lond. 1974;272:695–715. doi: 10.1113/jphysiol.1974.sp010772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JS, Cinelli AR. Are there structural and functional modules in the vertebrate olfactory bulb? Microsc Res Tech. 1993;24:157–167. doi: 10.1002/jemt.1070240207. [DOI] [PubMed] [Google Scholar]
- Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kay LM, Stopfer M. Information processing in the olfactory systems of insects and vertebrates. Semin Cell Dev Biol. 2006;17:433–442. doi: 10.1016/j.semcdb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Kay LM, Krysiak M, Barlas L, Edgerton GB. Grading odor similarities in a Go/No-Go task. Physiol Behav. 2006;88:339–346. doi: 10.1016/j.physbeh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Des Rosiers MH, Jehle JW, Reivich M, Sharpe F, Sokoloff L. Mapping of functional neural pathways by autoradiographic survey of local metabolic rate with (14C)deoxyglucose. Science. 1975;187:850–3. doi: 10.1126/science.1114332. [DOI] [PubMed] [Google Scholar]
- Kent PF, Youngentob SL, Sheehe PR. Odorant-specific spatial patterns in mucosal activity predict perceptual differences among odorants. J Neurophysiol. 1995;74:1777–1781. doi: 10.1152/jn.1995.74.4.1777. [DOI] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Youngentob SL, Yurco P. Mucosal activity patterns as a basis for olfactory discrimination: comparing behavior and optical recordings. Brain Res. 2003;981:1–11. doi: 10.1016/s0006-8993(03)02512-5. [DOI] [PubMed] [Google Scholar]
- Khan RM, Sobel N. Neural processing at the speed of smell. Neuron. 2004;44:744–747. doi: 10.1016/j.neuron.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Kimbell JS, Godo MN, Gross EA, Joyner DR, Richardson RB, Morgan KT. Computer simulation of inspiratory airflow in all regions of the F344 rat nasal passages. Toxicol Appl Pharmacol. 1997;145:388–398. doi: 10.1006/taap.1997.8206. [DOI] [PubMed] [Google Scholar]
- Kirner A, Deutsch S, Weiler E, Polak EH, Apfelbach R. Concanavalin A application to the olfactory epithelium reveals different sensory neuron populations for the odour pair D- and L-carvone. Behav Brain Res. 2003;138:201–206. doi: 10.1016/s0166-4328(02)00242-5. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Laing DG. Identification of single dissimilar odors is achieved by humans with a single sniff. Physiol Behav. 1986;37:163–170. doi: 10.1016/0031-9384(86)90400-2. [DOI] [PubMed] [Google Scholar]
- Laing DG, Eddy A, Francis GW, Stephens L. Evidence for the temporal processing of odor mixtures in humans. Brain Res. 1994;651:317–328. doi: 10.1016/0006-8993(94)90712-9. [DOI] [PubMed] [Google Scholar]
- Lancet D, Greer CA, Kauer JS, Shepherd GM. Mapping of odor-related neuronal activity in the olfactory bulb by high-resolution 2-deoxyglucose autoradiography. Proc Natl Acad Sci U S A. 1982;79:670–674. doi: 10.1073/pnas.79.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS, Wiley LE. Studies of cerebral function in learning. IX. Mass action in relation to the number of elements in the problem to be learned. J Comp Neurol. 1933;57:3–55. [Google Scholar]
- Laska M, Galizia CG. Enantioselectivity of odor perception in honeybees (Apis mellifera carnica) Behav Neurosci. 2001;115:632–639. doi: 10.1037/0735-7044.115.3.632. [DOI] [PubMed] [Google Scholar]
- Laska M, Trolp S, Teubner P. Odor structure-activity relationships compared in human and nonhuman primates. Behav Neurosci. 1999;113:998–1007. doi: 10.1037//0735-7044.113.5.998. [DOI] [PubMed] [Google Scholar]
- Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- Laurent G, Naraghi M. Odorant-induced oscillations in the mushroom bodies of the locust. J Neurosci. 1994;14:2993–3004. doi: 10.1523/JNEUROSCI.14-05-02993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Wehr M, Davidowitz H. Temporal representations of odors in an olfactory network. J Neurosci. 1996;16:3837–3847. doi: 10.1523/JNEUROSCI.16-12-03837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark W. The Ferrier lecture: inquiries into the anatomical basis of olfactory discrimination. Proc R Soc Lond B Biol Sci. 1957;146:299–319. doi: 10.1098/rspb.1957.0013. [DOI] [PubMed] [Google Scholar]
- Lei H, Christensen TA, Hildebrand JG. Spatial and temporal organization of ensemble representations for different odor classes in the moth antennal lobe. J Neurosci. 2004;24:11108–11119. doi: 10.1523/JNEUROSCI.3677-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Lèvai O, Strotmann J. Projection pattern of nerve fibers from the septal organ: DiI tracing studies with transgenic OMP mice. Histochem Cell Biol. 2003;120:483–492. doi: 10.1007/s00418-003-0594-4. [DOI] [PubMed] [Google Scholar]
- Lèvai O, Breer H, Strotmann J. Subzonal organization of olfactory sensory neurons projecting to distinct glomeruli within the mouse olfactory bulb. J Comp Neurol. 2003;458:209–220. doi: 10.1002/cne.10559. [DOI] [PubMed] [Google Scholar]
- Leveteau J, MacLeod P. Olfactory discrimination in the rabbit olfactory glomerulus. Science. 1966;153:175–176. doi: 10.1126/science.153.3732.175. [DOI] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Lin da Y, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22:6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Sachse S, Galizia CG. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol. 2005;93:3410–3417. doi: 10.1152/jn.01285.2004. [DOI] [PubMed] [Google Scholar]
- Lozowski AG, Lysetskiy M, Zurada JM. Signal processing with temporal sequences in olfactory systems. IEEE Trans Neural Netw. 2004;15:1268–1275. doi: 10.1109/TNN.2004.832730. [DOI] [PubMed] [Google Scholar]
- Lu X-CM, Slotnick BM. Recognition of propionic acid vapor after removal of the olfactory bulb area associated with high 2-DG uptake. Brain Res. 1994;639:26–32. doi: 10.1016/0006-8993(94)91760-4. [DOI] [PubMed] [Google Scholar]
- Lu X-CM, Slotnick BM. Olfaction in rats with extensive lesions of the olfactory bulbs: Implications for odor coding. Neuroscience. 1998;84:849–866. doi: 10.1016/s0306-4522(97)00520-4. [DOI] [PubMed] [Google Scholar]
- Luo M, Katz LC. Response correlation maps of neurons in the mammalian olfactory bulb. Neuron. 2001;32:1165–1179. doi: 10.1016/s0896-6273(01)00537-2. [DOI] [PubMed] [Google Scholar]
- Ma M, Grosmaitre X, Iwema CL, Baker H, Greer CA, Shepherd GM. Olfactory signal transduction in the mouse septal organ. J Neurosci. 2003;23:317–324. doi: 10.1523/JNEUROSCI.23-01-00317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- Mair RG. Response properties of rat olfactory bulb neurones. J Physiol Lond. 1982;326:341–359. doi: 10.1113/jphysiol.1982.sp014197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Enrichment to odors improves olfactory discrimination in adult rats. Behav Neurosci. 2006a;120:173–179. doi: 10.1037/0735-7044.120.1.173. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci USA. 2006b;103:13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J Physiol. 2003;546:363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DA, Maruniak JA. Masera's organ responds to odorants. Brain Res. 1986;366:329–332. doi: 10.1016/0006-8993(86)91312-0. [DOI] [PubMed] [Google Scholar]
- McBride K, Slotnick B. Discrimination between the enantiomers of carvone and of terpinen-4-ol odorants in normal rats and those with lesions of the olfactory bulbs. J Neurosci. 2006;26:9892–9901. doi: 10.1523/JNEUROSCI.0504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurophysiol. 1986;56:572–597. doi: 10.1152/jn.1986.56.3.572. [DOI] [PubMed] [Google Scholar]
- Meredith M. Neural circuit computation: complex patterns in the olfactory bulb. Brain Res Bull. 1992;29:111–117. doi: 10.1016/0361-9230(92)90014-o. [DOI] [PubMed] [Google Scholar]
- Miyamichi K, Serizawa S, Kimura HM, Sakano H. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. J Neurosci. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmonson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Monod B, Mouly AM, Vigouroux M, Holley A. La stimulation électrique multipolaire du bulbe olfactif, modèle d'étude du codage spatial dans le système olfactif. C R Seances Acad Sci III. 1981;293:731–734. [PubMed] [Google Scholar]
- Monod B, Mouly AM, Vigouroux M, Holley A. An investigation of some temporal aspects of olfactory coding with the model of multi-site electrical stimulation of the olfactory bulb in the rat. Behav Brain Res. 1989;33:51–63. doi: 10.1016/s0166-4328(89)80018-x. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Buonviso N. Altered odor-induced expression of c-fos and arg 3.1 immediate early genes in the olfactory system after familiarization with an odor. J Neurobiol. 2002;52:61–72. doi: 10.1002/neu.10069. [DOI] [PubMed] [Google Scholar]
- Mori K, Mataga N, Imamura K. Differential specificities of single mitral cells in rabbit olfactory bulb for a homologous series of fatty acid odor molecules. J Neurophysiol. 1992;67:786–789. doi: 10.1152/jn.1992.67.3.786. [DOI] [PubMed] [Google Scholar]
- Mori K, Fujita SC, Imamura K, Obata K. Immunohistochemical study of subclasses of olfactory nerve fibers and their projections to the olfactory bulb in the rabbit. J Comp Neurol. 1985;242:214–229. doi: 10.1002/cne.902420205. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Motokizawa F. Odor representation and discrimination in mitral/tufted cells of the rat olfactory bulb. Exp Brain Res. 1996;12:24–34. doi: 10.1007/BF00227174. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Holley A. Perceptive properties of the multi-site electrical microstimulation of the olfactory bulb in the rat. Behav Brain Res. 1986;21:1–12. doi: 10.1016/0166-4328(86)90054-9. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Vigouroux M, Holley A. On the ability of rats to discriminate between microstimulations of the olfactory bulb in different locations. Behav Brain Res. 1985;17:45–58. doi: 10.1016/0166-4328(85)90006-3. [DOI] [PubMed] [Google Scholar]
- Mozell MM. Evidence for sorption as a mechanism of the olfactory analysis of vapors. Nature. 1964;203:1181–2. doi: 10.1038/2031181a0. [DOI] [PubMed] [Google Scholar]
- Mozell MM, Sheehe PR, Hornung DE, Kent PF, Youngentob SL, Murphy SJ. “Imposed” and “inherent” mucosal activity patterns. J Gen Physiol. 1987;90:625–650. doi: 10.1085/jgp.90.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov AA, Caprio J. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J Neurophysiol. 2001;86:1869–1876. doi: 10.1152/jn.2001.86.4.1869. [DOI] [PubMed] [Google Scholar]
- Nikonov AA, Finger TE, Caprio J. Beyond the olfactory bulb: an odotopic map in the forebrain. Proc Natl Acad Sci U S A. 2005;102:18688–18693. doi: 10.1073/pnas.0505241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol. 2001;86:2823–2833. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- Oka Y, Nakamura A, Watanabe H, Touhara K. An odorant derivative as an antagonist for an olfactory receptor. Chem Senses. 2004;29:815–822. doi: 10.1093/chemse/bjh247. [DOI] [PubMed] [Google Scholar]
- Onoda N. Odor-induced fos-like immunoreactivity in the rat olfactory bulb. Neurosci Lett. 1992;137:157–160. doi: 10.1016/0304-3940(92)90393-l. [DOI] [PubMed] [Google Scholar]
- Pager J. A selective modulation of the olfactory bulb electrical activity in relation to the learning of palatability in hungry and satiated rats. Physiol Behav. 1974a;12:189–195. doi: 10.1016/0031-9384(74)90172-3. [DOI] [PubMed] [Google Scholar]
- Pager J. A selective modulation of olfactory input suppressed by lesions of the anterior limb of the anterior commissure. Physiol Behav. 1974b;13:523–526. doi: 10.1016/0031-9384(74)90283-2. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Benson TE. Projection of septal organ receptor neurons to the main olfactory bulb in rats. J Comp Neurol. 1986;252:555–562. doi: 10.1002/cne.902520411. [DOI] [PubMed] [Google Scholar]
- Pelosi P. Perireceptor events in olfaction. J Neurobiol. 1996;30:3–19. doi: 10.1002/(SICI)1097-4695(199605)30:1<3::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Døving KB. Selective degeneration in the rat olfactory bulb following exposure to different odours. Brain Res. 1974;82:195–204. doi: 10.1016/0006-8993(74)90598-8. [DOI] [PubMed] [Google Scholar]
- Polak EH. Mutiple profile-multiple receptor site model for vertebrate olfaction. J Theor Biol. 1973;40:469–484. doi: 10.1016/0022-5193(73)90005-2. [DOI] [PubMed] [Google Scholar]
- Rabinovich MI, Huerta R, Volkovskii A, Abarbanel HD, Stopfer M, Laurent G. Dynamical coding of sensory information with competitive networks. J Physiol Paris. 2000;94:465–471. doi: 10.1016/s0928-4257(00)01092-5. [DOI] [PubMed] [Google Scholar]
- Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Eberwine J, Dotson R, Jackson J, Ulrich P, Restrepo D. Expression of mRNAs encoding for two different olfactory receptors in a subset of olfactory receptor neurons. J Neurochem. 2000;75:185–195. doi: 10.1046/j.1471-4159.2000.0750185.x. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006a;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006b;51:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Rodrigues V. Spatial coding of olfactory information in the antennal lobe of Drosophila melanogaster. Brain Res. 1988;453:299–307. doi: 10.1016/0006-8993(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Royal SJ, Key B. Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-LacZ transgenic mice. J Neurosci. 1999;19:9856–64. doi: 10.1523/JNEUROSCI.19-22-09856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Sicard G, Souchier C, Jourdan F. Specificity of spatial patterns of glomerular activation in the mouse olfactory bulb: computer-assisted image analysis of 2-deoxyglucose autoradiograms. Brain Res. 1987;417:1–11. doi: 10.1016/0006-8993(87)90173-9. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sachse S, Galizia CG. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol. 2002;87:1106–1117. doi: 10.1152/jn.00325.2001. [DOI] [PubMed] [Google Scholar]
- Sachse S, Galizia CG. The coding of odour-intensity in the honeybee antennal lobe: local computation optimizes odour representation. Eur J Neurosci. 2003;18:2119–2132. doi: 10.1046/j.1460-9568.2003.02931.x. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rappert A, Galizia CG. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci. 1999;11:3970–3982. doi: 10.1046/j.1460-9568.1999.00826.x. [DOI] [PubMed] [Google Scholar]
- Sakura M, Kabetani M, Watanabe S, Kirino Y. Impairment of olfactory discrimination by blockade of nitric oxide activity in the terrestrial slug Limax valentianus. Neurosci Lett. 2004;370:257–261. doi: 10.1016/j.neulet.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Sallaz M, Jourdan F. C-fos expression and 2-deoxyglucose uptake in the olfactory bulb of odour-stimulated awake rats. Neuroreport. 1993;4:55–58. doi: 10.1097/00001756-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, Schmachtenberg O, Bacigalupo J. Excitation, inhibition, and suppression by odors in isolated toad and rat olfactory receptor neurons. Am J Physiol Cell Physiol. 2000;279:C31–C39. doi: 10.1152/ajpcell.2000.279.1.C31. [DOI] [PubMed] [Google Scholar]
- Sanz G, Schlegel C, Pernollet JC, Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem Senses. 2005;30:69–80. doi: 10.1093/chemse/bji002. [DOI] [PubMed] [Google Scholar]
- Saucier D, Astic L. Analysis of the topographical organization of olfactory epithelium projections in the rat. Brain Res Bull. 1986;16:455–462. doi: 10.1016/0361-9230(86)90173-5. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Angelo K, Spors H, Margrie TW. Neuronal oscillations enhance stimulus discrimination by ensuring action potential precision. PLoS Biol. 2006;4:e163. doi: 10.1371/journal.pbio.0040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001a;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Finger TE, Restrepo D. Variability of position of the P2 glomerulus within a map of the mouse olfactory bulb. J Comp Neurol. 2001b;436:351–362. [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JR, Kida I, Xu F, Rothman DL, Hyder F. Reproducibility of odor maps by fMRI in rodents. Neuroimage. 2006;31:1238–1246. doi: 10.1016/j.neuroimage.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Scheich H, Zuschratter W. Mapping of stimulus features and meaning in gerbil auditory cortex with 2-deoxyglucose and c-Fos antibodies. Behav Brain Res. 1995;66:195–205. doi: 10.1016/0166-4328(94)00140-b. [DOI] [PubMed] [Google Scholar]
- Schellinck HM, Smyth C, Brown R, Wilkinson M. Odor-induced sexual maturation and expression of c-fos in the olfactory system of juvenile female mice. Brain Res Dev Brain Res. 1993;74:138–141. doi: 10.1016/0165-3806(93)90094-q. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends Neurosci. 2005;28:620–627. doi: 10.1016/j.tins.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Knott TK. NADPH diaphorase activity in olfactory receptor neurons and their axons conforms to a rhinotopically-distinct dorsal zone of the hamster nasal cavity and main olfactory bulb. J Chem Neuroanat. 2002;24:269–285. doi: 10.1016/s0891-0618(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Clancy AN, Forbes WB, Macrides F. The spatial organization of the peripheral olfactory system of the hamster. I. Receptor neuron projections to the main olfactory bulb. Brain Res Bull. 1994;34:183–210. doi: 10.1016/0361-9230(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31:639–651. doi: 10.1016/s0896-6273(01)00389-0. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Gottlieb DI. The primary olfactory projection has two chemically distinct zones. J Neurosci. 1986;6:3393–3404. doi: 10.1523/JNEUROSCI.06-11-03393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Brierley T, Schmidt FH. Chemical determinants of the rat electroolfactogram. J Neurosci. 2000;20:4721–4731. doi: 10.1523/JNEUROSCI.20-12-04721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Acevedo HP, Sherrill L. Effects of concentration and sniff flow rate on the rat electroolfactogram. Chem Senses. 2006;31:581–593. doi: 10.1093/chemse/bjj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Johnson PE, Blakley D, Scott JW. Effects of air flow on rat electroolfactogram. Chem Senses. 2000;25:761–768. doi: 10.1093/chemse/25.6.761. [DOI] [PubMed] [Google Scholar]
- Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20:648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol. 1977;40:800–813. doi: 10.1152/jn.1977.40.4.800. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972;52:864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- Sicard G, Royet J-P, Jourdan F. A comparative study of 2-deoxyglucose patterns of glomerular activation in the olfactory bulbs of C57 BL/6J and AKR/J mice. Brain Res. 1989;481:325–334. doi: 10.1016/0006-8993(89)90810-x. [DOI] [PubMed] [Google Scholar]
- Skeen LC. Odor-induced patterns of deoxyglucose consumption in the olfactory bulb of the tree shrew, Tupaia glis. Brain Res. 1977;124:147–153. doi: 10.1016/0006-8993(77)90871-x. [DOI] [PubMed] [Google Scholar]
- Skiri HT, Galizia CG, Mustaparta H. Representation of primary plant odorants in the antennal lobe of the moth Heliothis virescens using calcium imaging. Chem Senses. 2004;29:253–267. doi: 10.1093/chemse/bjh026. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Bisulco S. Detection and discrimination of carvone enantiomers in rats with olfactory bulb lesions. Neuroscience. 2003;121:451–457. doi: 10.1016/s0306-4522(03)00402-0. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Graham S, Laing DG, Bell GA. Detection of propionic acid vapor by rats with lesions of olfactory bulb areas associated with high 2-DG uptake. Brain Res. 1987;417:343–346. doi: 10.1016/0006-8993(87)90460-4. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Panhuber H, Bell GA, Laing DG. Odor-induced metabolic activity in the olfactory bulb of rats trained to detect propionic acid vapor. Brain Res. 1989;500:161–168. doi: 10.1016/0006-8993(89)90310-7. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Bell GA, Panhuber H, Laing DG. Detection and discrimination of propionic acid after removal of its 2-DG identified major focus in the olfactory bulb: A psychophysical analysis. Brain Res. 1997;762:89–96. doi: 10.1016/s0006-8993(97)00357-0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WB, Kauer JS, Shepherd GM. Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol. 1979;185:715–734. doi: 10.1002/cne.901850407. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Storan MJ, Key B. Septal organ of Gruneberg is part of the olfactory system. J Comp Neurol. 2006;494:834–844. doi: 10.1002/cne.20858. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Wanner I, Krieger J, Raming K, Breer H. Expression of odorant receptors in spatially restricted subsets of chemosensory neurones. Neuroreport. 1992;3:1053–6. doi: 10.1097/00001756-199212000-00005. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Hoppe R, Conzelmann S, Feinstein P, Mombaerts P, Breer H. Small subfamily of olfactory receptor genes: structural features, expression pattern and genomic organization. Gene. 1999;236:281–291. doi: 10.1016/s0378-1119(99)00275-9. [DOI] [PubMed] [Google Scholar]
- Strotmann J, Conzelmann S, Beck A, Feinstein P, Breer H, Mombaerts P. Local permutations in the glomerular array of the mouse olfactory bulb. J Neurosci. 2000;20:6927–38. doi: 10.1523/JNEUROSCI.20-18-06927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Szyszka P, Ditzen M, Galkin A, Galizia CG, Menzel R. Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J Neurophysiol. 2005;94:3303–3313. doi: 10.1152/jn.00397.2005. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol. 2004a;92:2413–2427. doi: 10.1152/jn.00236.2004. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Nagayama S, Mori K. Detection and masking of spoiled food smells by odor maps in the olfactory bulb. J Neurosci. 2004b;24:8690–8694. doi: 10.1523/JNEUROSCI.2510-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Stewart WB, Kauer JS, Shepherd GM. Suckling pheromone stimulation of a modified glomerular region in the developing rat olfactory bulb revealed by the 2-deoxyglucose method. Brain Res. 1980;194:530–535. doi: 10.1016/0006-8993(80)91237-8. [DOI] [PubMed] [Google Scholar]
- Teyke T, Gelperin A. Olfactory oscillations augment odor discrimination not odor identification by Limax CNS. Neuroreport. 1999;10:1061–1068. doi: 10.1097/00001756-199904060-00030. [DOI] [PubMed] [Google Scholar]
- Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24:8383–8390. doi: 10.1523/JNEUROSCI.2222-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar HB, Feinstein P, Mombaerts P, Greer CA. Specificity of glomerular targeting by olfactory sensory axons. J Neurosci. 2002;22:2469–2477. doi: 10.1523/JNEUROSCI.22-07-02469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A, Yoshihara S, Yamazaki N, Kasai H, Asai-Tsuboi H, Komatsu M, Serizawa S, Ishii T, Matsuda Y, Nagawa F, Sakano H. Olfactory neurons expressing closely linked and homologous odorant receptor genes tend to project their axons to neighboring glomeruli on the olfactory bulb. J Neurosci. 1999;19:8409–8418. doi: 10.1523/JNEUROSCI.19-19-08409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A, Miyazaki T, Imai T, Sakano H. Olfactory sensory neurons expressing class I odorant receptors converge their axons on an antero-dorsal domain of the olfactory bulb in the mouse. Eur J Neurosci. 2006;23:1436–1444. doi: 10.1111/j.1460-9568.2006.04675.x. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nuñez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vedin V, Slotnick B, Berghard A. Zonal ablation of the olfactory sensory neuroepithelium of the mouse: effects on odorant detection. Eur J Neurosci. 2004;20:1858–1864. doi: 10.1111/j.1460-9568.2004.03634.x. [DOI] [PubMed] [Google Scholar]
- Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odour-plume dynamics influence the brain's olfactory code. Nature. 2001;410:466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Correspondence between odorant-evoked patterns of receptor neuron input and intrinsic optical signals in the mouse olfactory bulb. J Neurophysiol. 2003;89:1623–1639. doi: 10.1152/jn.00747.2002. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Denk W, Friedrich RW. Functional organization of sensory input to the olfactory bulb glomerulus analyzed by two-photon calcium imaging. Proc Natl Acad Sci U S A. 2004;101:9097–9102. doi: 10.1073/pnas.0400438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- Wes PD, Bargmann CI. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature. 2001;410:698–701. doi: 10.1038/35070581. [DOI] [PubMed] [Google Scholar]
- Willhite DC, Nguyen KT, Masurkar AV, Greer CA, Shepherd GM, Chen WR. Viral tracing identifies distributed columnar organization in the olfactory bulb. Proc Natl Acad Sci U S A. 2006;103:12592–12597. doi: 10.1073/pnas.0602032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. J Neurophysiol. 1988;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci Biobehav Rev. 2003;27:307–328. doi: 10.1016/s0149-7634(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- Wise PM, Cain WS. Latency and accuracy of discriminations of odor quality between binary mixtures and their components. Chem Senses. 2000;25:247–265. doi: 10.1093/chemse/25.3.247. [DOI] [PubMed] [Google Scholar]
- Woo CC, Hingco EE, Johnson BA, Leon M. 2004 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. Consistent odorant-evoked patterns of glomerular activity following short or long exposure to an odorant using the 2-deoxyglucose technique. (Program No. 531.3). Online. [Google Scholar]
- Woo CC, Hingco EE, Johnson BA, Leon M. Broad activation of the glomerular layer enhances subsequent olfactory responses. Chem Senses. 2007;32:51–55. doi: 10.1093/chemse/bjl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Kida I, Hyder F, Shulman RG. Assessment and discrimination of odor stimuli in rat olfactory bulb by dynamic functional MRI. Proc Natl Acad Sci U S A. 2000;97:10601–10606. doi: 10.1073/pnas.180321397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2003;100:11029–11034. doi: 10.1073/pnas.1832864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Gutierrez-Osuna R. Estimating the number of modules in rat olfactory bulb by prediction of odorant descriptors. Chem Senses. 2006;31:A76. [Google Scholar]
- Yang X, Renken R, Hyder F, Siddeek M, Greer CA, Shepherd GM, Shulman RG. Dynamic mapping at the laminar level of odor-elicited responses in rat olfactory bulb by functional MRI. Proc Natl Acad Sci U S A. 1998;95:7715–7720. doi: 10.1073/pnas.95.13.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Schwob JE. Odorant identification and quality perception following methyl bromide-induced lesions of the olfactory epithelium. Behav Neurosci. 2006;120:1346–1355. doi: 10.1037/0735-7044.120.6.1346. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Markert LM, Mozell MM, Hornung DE. A method for establishing a five-odorant identification confusion matrix task in rats. Physiol Behav. 1990;47:1053–1059. doi: 10.1016/0031-9384(90)90352-5. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL, Youngentob LM. OMP gene deletion results in an alteration in odorant quality perception. Behav Neurosci. 2001;115:626–631. doi: 10.1037//0735-7044.115.3.626. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C, Sobel N. Humans as an animal model for systems-level organization of olfaction. Neuron. 2005;48:431–454. doi: 10.1016/j.neuron.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- Zhao K, Dalton P, Yang GC, Scherer PW. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chem Senses. 2006;31:107–118. doi: 10.1093/chemse/bjj008. [DOI] [PubMed] [Google Scholar]
- Zou Z, Horowitz LF, Montmayeur JP, Snapper S, Buck LB. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001;414:173–179. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]
- Zou Z, Li F, Buck LB. Odor maps in the olfactory cortex. Proc Natl Acad Sci USA. 2005;102:7724–7729. doi: 10.1073/pnas.0503027102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]