Abstract
Hepatic stem cells (oval cells) proliferate within the liver after exposure to a variety of hepatic carcinogens and can generate both hepatocytes and bile duct cells. Oval cell proliferation is commonly seen in the preneoplastic stages of liver carcinogenesis, often accompanied by an inflammatory response. Tumor necrosis factor (TNF), an inflammatory cytokine, is also important in liver regeneration and hepatocellular growth. The experiments reported here explore the relationship among the TNF inflammatory pathway, liver stem cell activation, and tumorigenesis. We demonstrate that TNF is upregulated during oval cell proliferation induced by a choline-deficient, ethionine-supplemented diet and that it is expressed by oval cells. In TNF receptor type 1 knockout mice, oval cell proliferation is substantially impaired and tumorigenesis is reduced. Oval cell proliferation is impaired to a lesser extent in interleukin 6 knockout mice and is unchanged in TNF receptor type 2 knockout mice. These findings demonstrate that TNF signaling participates in the proliferation of oval cells during the preneoplastic phase of liver carcinogenesis and that loss of signaling through the TNF receptor type 1 reduces the incidence of tumor formation. The TNF inflammatory pathway may be a target for therapeutic intervention during the early stages of liver carcinogenesis.
Keywords: cytokines; carcinoma, hepatocellular; interleukin 6; stem cells; liver regeneration
Introduction
Hepatocytes, the main cellular component of the liver, are highly differentiated cells that retain the capacity to proliferate. In the normal liver, hepatocytes are metabolically active but rarely divide. However, when cell or tissue deficits are imposed on the liver by tissue resection (partial hepatectomy) or acute chemical injury (carbon tetrachloride poisoning being the most common example), almost all hepatocytes proliferate to correct the deficit. Recent experiments involving the repopulation of damaged mouse livers by hepatocyte transplantation as well as serial hepatocyte transplantation have demonstrated that these cells have a remarkable proliferative capacity 1 2.
Despite these observations, a large number of studies make it clear that the adult liver also contains a progenitor or stem cell compartment (for review see references 3 and 4). These cells, thought to be located at the canals of Hering (structures that connect hepatocyte biliary canaliculi with the bile duct system) have epithelial morphology but differ from small hepatocytes or bile duct cells 5 6. Although the definitions may not be entirely accurate, we will use the term “stem cell” to refer to canals of Hering cells capable of generating lineage 6.
Stem cells are activated under conditions of liver injury in which hepatocytes cannot perform their regenerative functions. Under these circumstances, they give rise to the oval cells, a heterogeneous, proliferative cell population capable of differentiating into hepatocytes and cells of the biliary system 7 8 9 10. Recent data suggest that oval cells as well as hepatocytes can be generated from bone marrow cells 11 12. Oval cells express α-fetoprotein (AFP), a marker of fetal hepatocytes, and fetal and adult isozymes of aldolase and pyruvate kinase as well as phenotypic features of bile duct cells, such as expression of cytokeratins 7 and 19 (CK7 and CK19) and surface antigens including A6 and G7 (for review see references 13 and 14). Proliferation of oval cells in rodents occurs after induction of liver injury with a variety of agents. In these situations, oval cells may generate hepatocytes that repopulate damaged liver 8 15 16 17 18 19 20. However, as oval cells may be targets for carcinogens, oval cells may also function as tumor progenitors through the generation of abnormal hepatocytes. Particularly well studied are oval cell proliferation and differentiation models induced by 2-acetylamino fluorene and partial hepatectomy 7 21, dipin and partial hepatectomy 9, administration of chemicals such as galactosamine and retrorsine 17 22, azo dye carcinogens 15 23, and feeding rats a choline-deficient, ethionine-supplemented (CDE) diet 24 25. Oval cells have also been identified in human livers in a variety of conditions, including chronic liver diseases that may be associated with acute liver failure 6 26.
Understanding the mechanisms by which liver progenitor cells can repopulate the liver or give rise to tumors is of fundamental importance. Several potentially important oval cell growth factors have been identified, including hepatocyte growth factor, transforming growth factor α, epidermal growth factor, stem cell factor, fibroblast growth factor, leukemia inhibitory factor, and IFN-γ 27 28 29 30 31 32 33. Whether these factors are required for proliferation, migration, and/or differentiation of the oval cell compartment is not known. Nagy et al. 34 reported that dexamethasone inhibits oval cell proliferation in the 2-acetylamino fluorene and partial hepatectomy model, an effect that is probably mediated through glucocorticoid inhibition of nuclear factor (NF)-κB activation 35, an important downstream component of the TNF receptor type 1 (TNF R1) signaling pathway. In this context, it is of interest that although oval cell proliferation commonly occurs when hepatocytes are not capable of responding to a growth stimulus, oval cells are not detected in livers of mice lacking TNF R1 in which hepatocyte replication is deficient after partial hepatectomy or carbon tetrachloride injury 36 37 38. These observations suggest that signaling through TNF R1 might be important for oval cell proliferation.
Kirillova et al. 39 have recently shown that TNF stimulates the proliferation of oval cells in culture (LE-6 cell line) and acts through the activation of NF-κB. The studies presented in this paper are designed to determine whether oval cell proliferation in the liver in vivo requires TNF signaling. For these experiments, the rat CDE diet model of oval cell proliferation and carcinogenesis was adapted for mice to permit the use of animals with the appropriate targeted mutations. The results show that deficiency of TNF R1, but not TNF R2, inhibits the oval cell response as well as tumor development in the livers of mice receiving a carcinogenic diet.
Materials and Methods
Animals
Mice of two strains were used in this study. For the evaluation of TNF-α levels, wild-type (WT) BDF-1 strain mice (purchased from Animal Resources Centre) were used. Knockout (KO) mice were of the C57BL/6 strain. TNF R2 KO mice were bred into the C57BL/6 lineage by backcrossing 37. WT C57BL/6 and IL-6 KO mice were purchased from The Jackson Laboratory. TNF R1 KO mice bred in the laboratory were also used 36 37 38 40 41. Animals were housed in temperature-controlled rooms with alternating 12-h light and dark cycles. Animal experiments were performed in accordance with the guidelines of the University of Washington School of Medicine or the National Health and Medical Research Council of Australia.
Dietary Conditions.
4-wk-old male mice were administered a diet consisting of normal laboratory chow and drinking water (control), 100% choline-deficient chow (ICN Biomedicals) and drinking water supplemented with 0.165% (wt/vol) ethionine (ICN Biomedicals) (100% CDE diet), or a 1:1 mixture of choline-deficient and normal chow and drinking water supplemented with 0.15% ethionine (50% CDE diet).
Liver Damage Assessment.
For assessment of liver damage, blood samples from duplicate WT, TNF R1 KO, TNF R2 KO, and IL-6 KO mice after 2, 3, or 4 wk of 50% CDE feeding were analyzed for alanine aminotransferases by the University of Washington Veterinary Service. Averaged levels for duplicate KO mice were expressed as a percentage of WT mice at each time point.
Cell Isolations
Livers were dissociated by a three-step collagenase perfusion via the hepatic portal vein modified from the method of Seglen et al. 42. In brief, livers were perfused at a rate of 2–3 ml/min, first with perfusion buffer (KCl [0.4 g/liter]/NaCl [6.8 g/liter]/HEPES [4.8 g/liter]/ NaHCO3 [2.1 g/liter]/glucose [1 g/liter]) containing 0.63 mM EGTA (50 ml), then with perfusion buffer alone (25 ml), and finally with perfusion buffer containing 5.1 mM CaCl2 and 6 U type H collagenase (75 ml; Roche). For hepatocyte isolation, dissociated liver was dispersed in Leibowitz (L15) medium (GIBCO BRL), and hepatocytes were purified by filtration and centrifugation as described by Seglen et al. 42. For oval and inflammatory cell isolation, dissociated livers were incubated (25 min, 37°C) with shaking in Joklik medium (GIBCO BRL) containing 10.25 U type VIII collagenase (Sigma-Aldrich) and 0.002% DNase I (Sigma-Aldrich). Cells were filtered through gauze (45 μm) and centrifuged (270 g) between and after treatments to remove debris. Cell pellets were then washed and resuspended in Joklik medium containing 10% FCS (GIBCO BRL) and 0.0045% DNase I. Cells were then fractionated by centrifugal elutriation using a Beckman JE6B rotor (Beckman Coulter) at 1,206 g as described by Yaswen et al. 25. Inflammatory cells were eliminated from the oval cell fraction (collected at 40 ml/min) using a MiniMACS™ MS+ separation system (Miltenyi Biotec) with an anti-CD45 bead conjugate (Miltenyi Biotec) according to the manufacturer's instructions. CD45+ inflammatory cells were also collected once oval cells were purified. Cell cytospins were stained for inflammatory cells using a cocktail of antibodies (directed against CD4, CD8, CD3, B220, J11d, Mac-1, and F4-80), and positive cells were counted in a fixed area. Phase contrast illumination was used to assess the total number of cells in the same area. The percentage of inflammatory cells in the oval cell fraction was estimated based on the average of eight areas of cells.
Analysis of TNF-α mRNA Expression
Total RNA was isolated from whole liver (100 mg) or purified cells (5 × 106) using TRIZOL RNA isolation reagent (Life Technologies) according to the manufacturer's instructions and quantitated using spectrophotometry. Expression of TNF-α mRNA levels was determined from 5–20 μg of total RNA using RiboQuant™ RNase Protection assay with the mCK-3 template set (BD PharMingen) according to the manufacturer's instructions. Bands were visualized using a BAS-1000 PhosphorImager (Fuji) and quantified by densitometry. TNF-α levels were normalized to glyceraldehyde 3 phosphate dehydrogenase (GAPDH) to account for loading differences between lanes.
Histology and Immunohistochemistry
Portions of liver tissue (5 mm3) were immerse fixed in Carnoy's fixative for 2 h and then processed and paraffin embedded. Portions (10 × 10 × 2 mm) were also snap frozen for cryostat sectioning. 5-μm paraffin or 10-μm cryostat sections (post-fixed at 4°C in 1:1 methanol/acetone) were immunohistochemically stained for M2 pyruvate kinase (M2PK; diluted 1:500; Schebo Technologies), CK19 (diluted 1:10; Amersham Pharmacia Biotech), A6 (reference 43; diluted 1:10; a gift from Dr. V. Factor, National Cancer Institute, Bethesda, MD), AFP (diluted 1:500; ICN Biochemicals), or proliferating cell nuclear antigen (PCNA; diluted 1:50; Leinco Technologies) using a two- (A6, CK19, PCNA, AFP) or three-step (M2PK) indirect method. Peroxidase activity was detected using Liquid DAB (Dako).
Cell Scores
Oval cells were identified as small, oval-shaped cells with scant cytoplasm that stained positively for M2PK or CK19. Proliferating oval cells were scored as cells bearing the morphology of oval cells (as determined by hematoxylin counterstain) that expressed PCNA in their nuclei. Care was taken to exclude round inflammatory cells, elongated endothelial cells, and larger hepatocytes. Inflammatory cells were identified using two-step immunohistochemistry with a cocktail of antibodies directed against CD4, CD8, and J11d.
For each section for a given mouse, 10 areas were scored using a 40× objective, and the number of oval cells was normalized to that of hepatocytes. Averages for the 10 areas were determined and then pooled for 5 experiments, and the overall average and standard error of the mean calculated. Oval cell numbers between mouse types were compared using Student's t test.
Pathological Assessment of Carcinogenesis
Liver sections from long-term CDE mice were examined independently without knowledge of genotype by two groups of scientists (G. Yeoh and B. Knight; N. Fausto and J. Rhim) and scored for evidence of fibrosis, dysplasia, and neoplasia. Multiple (four to five) samples of liver from each animal were used for evaluations. Data on tumor incidence between genotypes was analysed using a one-tailed analysis of variance test.
Results
TNF-α Expression Is Associated with Oval Cell Proliferation.
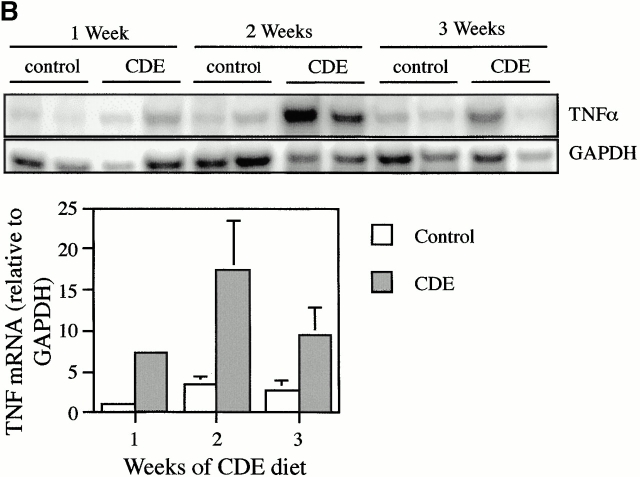
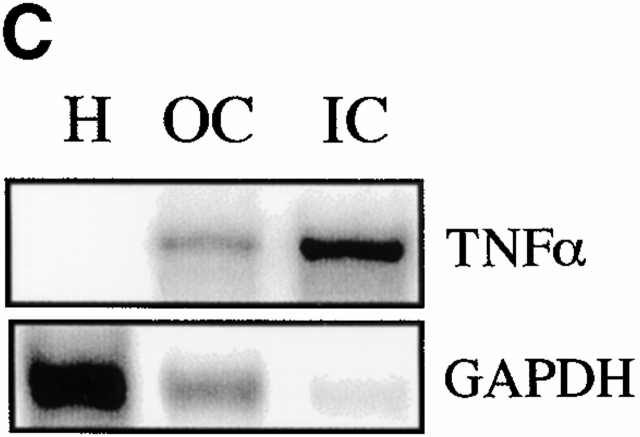
TNF-α expression was evaluated in mice fed a 100% CDE diet. A short-term study showed that TNF mRNA is rapidly induced 1 d after administration of the diet and continues to increase during the next 2 d (Fig. 1 A). Longer term feeding of the CDE diet caused increases in TNF mRNA of four- to sevenfold above those of mice maintained on the normal diet. A representative autoradiograph for this experiment is shown, along with quantitation data pooled from six experimental animals. (Fig. 1 B). To determine which cells express TNF-α mRNA, ribonuclease protection analysis was performed on RNA extracted from hepatocytes and oval and inflammatory cells isolated from mice that received the CDE diet for 3 wk. Inflammatory cell contamination in the oval cell fraction was determined to be 0.96 ± 0.25%. Strong expression of TNF-α mRNA was detected in oval and inflammatory cells, but not hepatocytes (Fig. 1 C). These results demonstrate that the feeding of the carcinogenic diet leads to a rapid and persistent increase in TNF in the liver, mostly due to oval cells and inflammatory cells that invade the liver.
Figure 1.
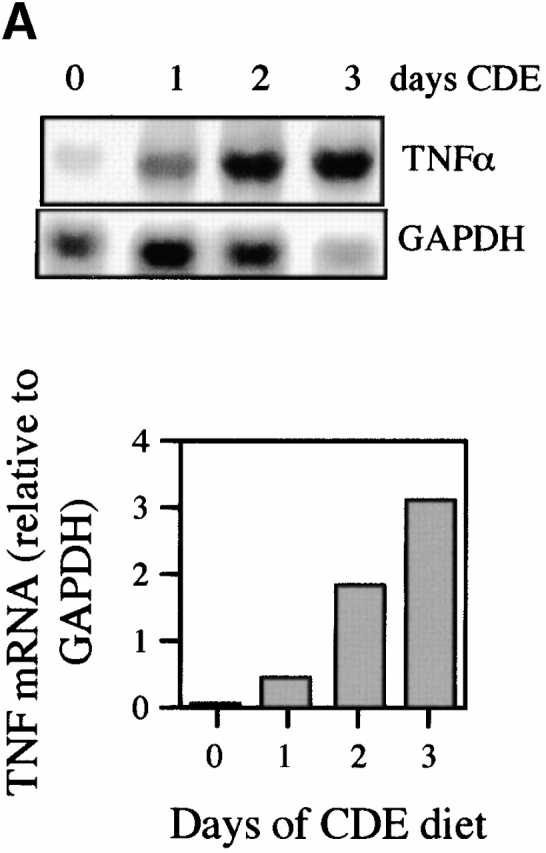
Increased expression of TNF-α during CDE diet feeding. TNF-α levels were detected by ribonuclease protection assay and quantified by densitometry relative to GAPDH. TNF levels increased during the first few days of the CDE diet (A). A peak in TNF-α expression in CDE samples (gray bars) compared with WT (white bars) was seen at 2 wk (B). Repeat experiments show the result illustrated in the representative autoradiograph to be reproducible (bars represent mean ± SEM, n = 6). Analysis of TNF-α mRNA in purified cell types showed expression in oval (OC) and inflammatory (IC) cells but not in hepatocytes (H) (C).
Oval Cell Response Is Deficient in Mice Lacking TNF R1.
A comparison between WT and TNF R1 KO mice fed a 50% CDE diet revealed that the oval cell response was diminished in TNF R1 KO mice (Fig. 2). This is evident from histological examination of liver sections from WT (Fig. 2 A) and TNF R1 KO (Fig. 2 B) mice. Immunohistochemistry showed that the attenuated oval cell response in TNF R1 KO mice could be demonstrated regardless of the oval cell marker used. Staining for CK19 (Fig. 2C and Fig. D) and A6 (Fig. 2E and Fig. F) both show fewer oval cells in TNF R1 KO (Fig. 2D and Fig. F) than WT (Fig. 2C and Fig. E) mice.
Figure 2.
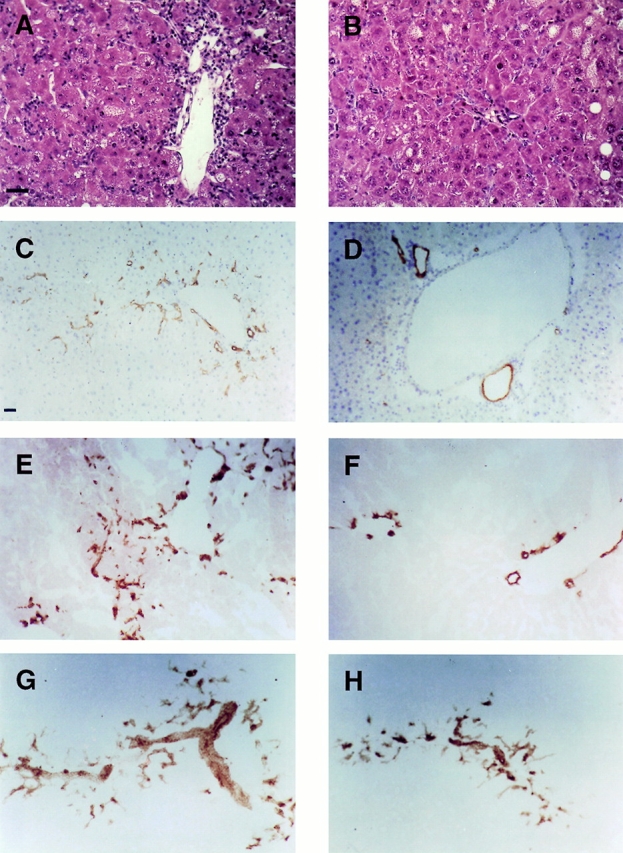
Diminished oval cell response in TNFR1 KO mice. Hematoxylin and eosin staining of livers after 2 wk of CDE diet shows a decreased oval cell response in TNF R1 KO compared with WT mice (A and B). Immunohistochemistry for CK19 (C and D) and A6 (E and F) after 2 wk of CDE diet in WT (C and E) and TNF R1 KO (D and F) highlights the reduced oval cell numbers in TNF R1 KO mice. By contrast, A6 staining in TNF R2 KO (G) shows no difference from WT. A6-positive oval cells in IL-6 KO mice (H) are more abundant than in TNF R1 KO (compare with F). Bar = 60 μM.
We used two markers to estimate the number of oval cells present in the livers of mice fed the carcinogenic diet: CK19 and M2PK, the fetal liver isozyme of pyruvate kinase. The absolute numbers of oval cells scored based on CK19 or M2PK staining differed (cell counts based on M2PK were ∼30% lower than those estimated by CK19 staining). This is not surprising, as CK19 stains mature bile duct cells as well as oval cells. Nevertheless, the relative comparison of oval cell numbers between WT and TNF R1 KO mice using each of these markers was not significantly different (Chi-squared analysis, P > 0.01). In these studies, M2PK was used to quantitate oval cell numbers. This marker was considered superior, as it can be used on paraffin sections, allowing cell counts to be performed based on both staining and morphology. Furthermore, unlike CK19, it discriminates between oval and biliary cells and, unlike A6, has commercially available antibodies. Results show that TNF R1 KO mice have, on average, 50% fewer oval cells than WT after the same dietary treatment (Fig. 3 A). This difference was found to be of statistical significance at all time points (P < 0.01). Thus, the oval cell response after the feeding of a carcinogenic diet is deficient in mice that lack TNF R1.
Figure 3.
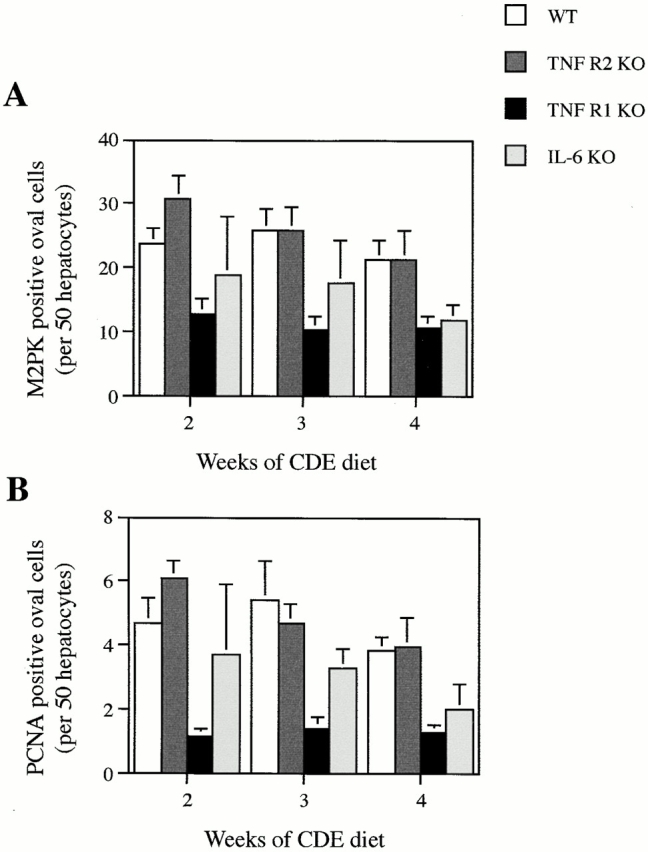
Quantitation of oval cell numbers in WT and KO mice. Livers were stained using either M2PK (for specific detection of oval cells) or PCNA (to detect proliferating cells), and positive oval cells were scored and normalized to hepatocyte number in the same area. Results shown are WT (white bars), TNF R2 KO (dark gray bars), TNF R1 KO (black bars), and IL-6 KO (light gray bars). Results are expressed as mean ± SEM, n = 5 (WT, TNF R1 KO, TNF R2 KO) or mean + range, n = 2 (IL-6 KO). Both M2PK- (A) and PCNA-based (B) oval cell counts reflect the pattern of oval cell response documented in Fig. 2.
To determine the extent of oval cell proliferation in WT and KO mice fed the CDE diet, we counted the number of proliferating oval cells using PCNA as a marker. A deficiency in oval cell proliferation in TNF R1 KO mice was evident. Numbers of PCNA-positive oval cells in TNF R1 KO mice were, on average, only 28% of WT (Fig. 3 B), a significant difference at all time points (P < 0.01).
Analysis of Oval Cell Response in TNF R2 and IL-6 KO Mice.
Although TNF R2 is not essential for initiation of liver regeneration and hepatocyte proliferation after partial hepatectomy, it participates in the transduction of TNF signals in the liver 37. To examine the importance of TNF R2 signaling in the oval cell response, we determined whether the response to a 50% CDE diet is altered in TNF R2 KO mice. Immunohistochemistry for A6, an oval cell surface antigen, shows no difference between TNF R2 KO and WT mice after the same dietary treatment (Fig. 2 G). This is reflected in the number of oval cells quantitated by M2PK staining (Fig. 3 A). Oval cell proliferation, determined by PCNA staining, was also not impaired in TNF R2 KO mice (Fig. 3 B). All comparisons between WT and TNF R2 KO revealed no significant difference between genotypes (P > 0.1).
The TNF R1 signaling pathway activated after partial hepatectomy involves the downstream activation of IL-6 36 37. KO mice deficient in IL-6 have impaired liver regeneration 44. In addition, IL-6 has been shown to be a mitogen for bile duct cells 45 46. Based on these observations, we evaluated whether the oval cell response is altered in IL-6 KO mice. A small but consistent reduction in oval cell numbers in IL-6 KO mice fed the CDE diet was apparent from sections stained for A6 (Fig. 2 H). This was confirmed by quantitation of oval cell numbers; IL-6 KO mice had, on average, a 30% reduction in M2PK-positive and a 50% reduction in PCNA-positive oval cells (Fig. 3A and Fig. B). Statistical evaluation of the data revealed no significant difference between WT and IL-6 KO for M2PK-positive cells at all time points (P > 0.05) and a significant difference in PCNA-positive oval cell counts only at 3 wk of dietary treatment (P < 0.05). Cell counts differed with some significance between IL-6 KO and TNF R1 KO at 3 (P < 0.05) and 4 wk (P < 0.07), but not 2 wk (P > 0.1).
Assessment of Liver Damage.
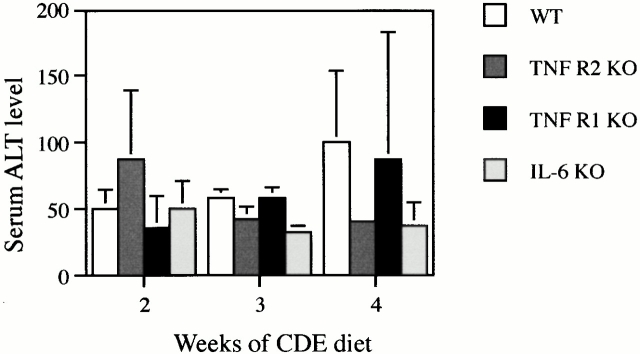
It could be argued that TNF R1 KO mice had a diminished oval cell response because the lack of signaling through TNF R1 might protect them from liver damage that stimulates oval cell production. To examine this possibility, we measured serum alanine aminotransferase (ALT) levels in WT, TNF R1, TNF R2, and IL-6 KO mice maintained on a 50% CDE diet for 2–4 wk (Fig. 4). ALT activity was, on average, 87, 96, and 65% of WT levels for TNF R1, TNF R2, and IL-6 KO mice (not significant; P > 0.05). Fat accumulation after 3 or 4 wk of CDE feeding was present in some of the livers. The extent of steatosis varied from animal to animal, but no difference between genotypes was observed in fat accumulation. Inflammatory cell infiltration, which often accompanies oval cell proliferation 47, was observed in all livers. The distribution of inflammatory cells (detected by CD8, CD4, and J11D antibodies) did not differ between animal types, indicating that the inflammatory response is not diminished in the KO mice (data not shown).
Figure 4.
Assessment of liver damage in KO mice. Serum from duplicate mice were analyzed for ALT activity and the results expressed as mean + range. Results shown are WT (white bars), TNF R2 KO (dark gray bars), TNF R1 KO (black bars), or IL-6 KO (light gray bars). There is no difference between genotypes at any time point in the experiment.
Liver Tumor Incidence Is Reduced in TNF R1 KO Mice.
Prolonged feeding of the CDE diet to rats causes the development of liver tumors after 10–12 mo 48 49. There has been much debate over the relationship between oval cell proliferation and liver tumor development in models of hepatocarcinogenesis in which oval cell proliferation is prominent. It has been assumed that oval cells, either directly or indirectly through the generation of hepatocytes, function as tumor progenitors. Because the oval cell response is impaired in TNF R1 KO mice, it was of interest to determine whether carcinogenesis in these animals would also be inhibited. To test this hypothesis, WT and TNF R1 KO mice were fed a 50% CDE diet for ∼9 mo. Of eight WT mice killed at 36 wk, five had well developed tumors, one had hepatic dysplasia and only neoplastic foci, and two had histologically normal livers. In contrast, only one in seven TNF R1 KO mice fed the CDE diet had well developed liver tumors, while four had normal livers and two had evidence of dysplasia and early neoplastic foci. Tumor incidence in WT mice was found to be significantly greater than in TNF R1 KO by analysis of variance (P < 0.05). Tumors ranged from 2 to 8 μm in diameter. Multiple nodules were seen in four animals (four to seven nodules per animal); nodules were distributed over all liver lobes.
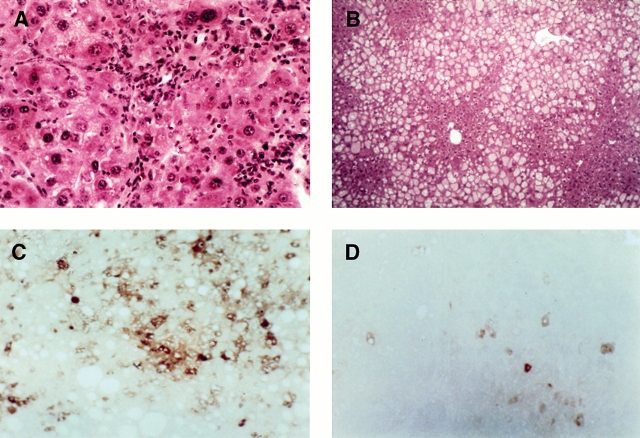
All livers examined showed some disruption, and steatosis was evident in most. The extent of fat accumulation varied from animal to animal but did not correlate with genotype. Additionally, evaluation of liver to body weight ratios revealed a small but significant (P = 0.05) elevation in liver weight in TNF R1 KO animals after long-term diet feeding (data not shown). Hematoxylin and eosin staining shows steatosis in both mice, with tumor nodules evident in WT but not TNF R1 KO (Fig. 5A and Fig. B). Staining of long-term–exposed CDE livers for AFP, a liver tumor cell marker, showed strong expression in nodular areas of hepatocytes in WT (Fig. 5 C) and scattered positive cells in TNF R1 KO mice (Fig. 5 D). The staining pattern seen in KO liver is typical of early-stage carcinogenesis induced by a short-term CDE diet 50; it does not imply tumor formation but does indicate that some preneoplastic changes have occurred.
Figure 5.
Tumor formation after long-term CDE diet. Liver tissue from WT (A and C) and TNF R1 KO (B and D) mice after 36 wk of CDE diet. Hematoxylin and eosin stain shows oval cells within a tumor in the liver of a WT mouse (A) and absence of tumor and oval cell proliferation but extensive fat accumulation in a TNF R1 KO mouse (B). The tumor contains AFP-positive cells (C), but only a few cells stain for AFP in the liver of the KO animal (D). Original magnifications: A, ×200, B–D, ×100.
Discussion
Oval cell proliferation after chronic liver injury has been well documented in a variety of models. The association of oval cells with liver repopulation and carcinogenesis and their ability to differentiate bipotentially suggest that oval cells function as a liver progenitor cell compartment. Previous work has suggested roles for several cytokines in mediating the growth of oval cells 27 28 29 30 31 32 33. However, a precise understanding of signaling pathways involved in oval cell proliferation is lacking. TNF-α has been shown to be a growth factor for an oval cell line in vitro 39 and has also been indirectly implicated in mediating oval cell proliferation in vivo 34. In this study, we sought to determine whether TNF signaling is involved in the oval cell response in mice.
Using a modification of the CDE diet protocol for inducing oval cells in rats, we were able to induce oval cell proliferation in mice within 1 wk. Analysis of TNF-α mRNA levels showed that liver TNF rapidly increases after commencement of the diet and is further elevated during the initial stages of oval cell proliferation. Peak TNF levels were detected after 2 wk of CDE feeding. Expression of TNF in CDE liver was localized to oval and inflammatory cells; expression was not detected in hepatocytes. The early, sustained elevations in expression indicate that TNF may play a role in both induction and maintenance of oval cell proliferation in this model. In cultured oval cells, TNF reinitiates growth in arrested cells and induces proliferation through NF-κB activation 39. It remains to be established whether NF-κB is a mediator of oval cell growth in vivo.
To directly evaluate the importance of TNF signaling in the oval cell response in vivo, we used KO mice lacking TNF R1 or TNF R2 and showed that signaling through the type 1 but not the type 2 receptor is essential for optimal oval cell response to the CDE diet. Compared with WT and TNF R2 KO animals, TNF R1 KO mice had fewer oval cells and a lower level of oval cell proliferation. The impairment of the oval cell response in TNF R1 KO mice was not a result of reduced damage caused by lack of TNF R1 signaling, because similar levels of fat accumulation, inflammatory cell infiltration, and serum ALT levels were observed in TNF R1 KO and WT mice. In addition, there was no difference between mortality of WT and TNF R1 KO mice on the diet in either the short or long term (data not shown), suggesting that the deficiency in oval cell proliferation did not impair liver regeneration. It is presumed that residual oval cell proliferation is sufficient to maintain parenchyma in the TNF R1 KO animals. Furthermore, although substantially impaired, liver regeneration is detected in the KO mice 36. The CDE diet compromises the replicative capacity of hepatocytes. However, some hepatocyte growth may still occur, and even in the absence of TNF R1, hepatocyte regeneration may contribute to maintenance of liver function. Interestingly, an increase in the ratio of liver/body weight was observed in long-term TNF R1 KO mice. Untreated KO mice have been reported to have normal liver weight 37, indicating that administration of the CDE diet, in the long term, has resulted in liver enlargement. The mechanisms underlying this finding have not been addressed but may involve proapoptotic signaling downstream of TNF R1. TNF R1 KO mice have impaired apoptosis in several models, including acute hepatic injury 51 52.
Current research suggests that TNF R1 is the dominant TNF receptor 53 54, and after partial hepatectomy, it is this receptor, not TNF R2, that is appears to be active in promoting hepatocyte growth 37. The signaling pathway employed by regenerating hepatocytes proceeds via activation of NF-κB and IL-6 36 44. A similar mechanism is suggested for oval cell–associated liver regeneration, because dexamethasone, which inhibits oval cell proliferation 34, inhibits NF-κB nuclear translocation 35. However, divergence of the pathways mediating oval cell and hepatocyte regeneration must exist, as oval cell proliferation does not occur after partial hepatectomy. Evaluation of the oval cell response in IL-6 KO mice suggests that IL-6 is involved in oval cell proliferation induced by the CDE diet. However, while oval cell proliferation is impaired in IL-6 KO mice, the extent of impairment is lesser in these mice than in TNF R1 KO. This suggests that IL-6 is not the sole downstream factor responsible for the TNF R1–mediated effect. By contrast, after partial hepatectomy, IL-6 and TNF R1 KO mice both have profound deficiencies in hepatocyte replication 36 44. This indicates that the factors mediating oval cell proliferation induced by the CDE diet and hepatocyte replication after partial hepatectomy may differ with respect to involvement of IL-6, although both may require TNF R1 signaling. It is likely that the optimal oval cell response involves several other cytokines, which may include other members of the IL-6 family.
Oval cell proliferation is often seen in the early stages of disease states that lead to cancer in humans 55 56 57 58 and rodents 48 59 60 61 62. This implicates the oval cell compartment in the carcinogenic process. Based on this, it could be hypothesized that the number of oval cells would positively correlate with the likelihood of tumor formation. This could explain the inhibition of tumor development in TNF R1 KO mice. However, the possibility that TNF is itself mediating tumor progression cannot be excluded. TNF has been shown to direct differentiation of several cell types 63 64 65. Absence of TNF has also been shown to impair skin tumor formation 66. TNF enhances growth factor mitogenicity 67 and may, therefore, affect transformation of these cells. Thus, while the experiments showed that absence of TNF R1 reduces tumor formation, the exact role of TNF signaling in the carcinogenic process is still unclear.
These studies, although they demonstrate a role for TNF signaling in oval cell proliferation, also highlight the complexity of the system mediating oval cell growth and differentiation. Absence of TNF R1 is sufficient to reduce oval cell numbers to approximately half of WT levels, but it does not completely abolish the oval cell response to CDE feeding. This indicates that additional growth factors or signaling pathways may supplement that of TNF R1 in oval cell signaling. Other cytokines may be induced for the different phases of the oval cell response: infiltration, growth, and differentiation. In this study, we have focused on the growth phase and show that TNF R1 is important for oval cell proliferation in this stage. By contrast, Matsusaka et al. 30 show that stem cell factor, while promoting liver oval cell infiltration, does not mediate the growth of these cells within the liver parenchyma. The association of a proinflammatory response with oval cell proliferation indicates that many cytokine systems may be activated during this process and that multiple factors may be required for the full oval cell response to liver damage. However, regardless of additional factors, this study demonstrates the involvement of TNF signaling in oval cell proliferation and carcinogenesis.
Acknowledgments
This work was supported by funding from the National Health and Medical Research Council of Australia and grant CA 74131 from the National Cancer Institute.
Footnotes
Abbreviations used in this paper: AFP, α-fetoprotein; CK, cytokeratin; KO, knockout; M2PK, M2 pyruvate kinase; NF, nuclear factor; PCNA, proliferating cell nuclear antigen; WT, wild-type.
References
- Rhim J.A., Sandgren E.P., Degen J.L., Palmiter R.D., Brinster R.L. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- Overturf K., al-Dhalimy M., Ou C.N., Finegold M., Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am. J. Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Fausto N. Liver stem cells. In: Arias I.M., Boyer J.L., Fausto N., Jakoby W.B., Schachter D.A., Shafritz D.A., editors. The Liver. Biology and Pathobiology. 3rd ed. Raven Press; New York: 1994. pp. 1501–1518. [Google Scholar]
- Thorgeirsson S.S. Hepatic stem cells in liver regeneration. FASEB J. 1996;10:1249–1256. [PubMed] [Google Scholar]
- Saxena R., Theise N.D., Crawford J.M. Microanatomy of the human liver—exploring the hidden interfaces. Hepatology. 1999;30:1339–1346. doi: 10.1002/hep.510300607. [DOI] [PubMed] [Google Scholar]
- Theise N.D., Saxena R., Portmann B.C., Thung S.N., Yee H., Chirboga L., Kumar A., Crawford J.M. The canals of hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- Evarts R.P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S.S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R.P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S.S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- Factor V.M., Radaeva S.A., Thoreirsson S.S. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am. J. Pathol. 1994;145:409–422. [PMC free article] [PubMed] [Google Scholar]
- Lazaro C.A., Rhim J.A., Yamada Y., Fausto N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998;58:5514–5522. [PubMed] [Google Scholar]
- Petersen B.E., Bowen W.C., Patrene K.D., Mars W.M., Sullivan A.K., Murase N., Boggs S.S., Greenberger J.S., Goff J.P. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Theise N.D., Badve S., Saxena R., Henegariu O., Sell S., Crawford J.M., Krause D.S. Derivation of hepatocytes from bone marrow cells in mice after radiation induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Sell S., Ilic Z. Liver stem cells and hepatocarcinogenesisexperimental models. In: Sell S., Ilic Z., editors. Liver Stem Cells. R.G. Landes Co; Austin, TX: 1997. pp. 117–165. [Google Scholar]
- Fausto, N., J.M. Lemire, and N. Shiojiri. 1992. Oval cells in liver carcinogenesis: cell lineages in hepatic development and the identification of facultative cells in normal liver. In The Role of Cell Types in Hepatocarcinogenesis. A.E. Sirica, editor. CRC Press, Inc., Boca Raton, FL. 89–108.
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylaminofluorene and 3-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- Lemire J.M., Shiojiri N., Fausto N. Oval cell proliferation an the origin of small hepatocytes in liver injury induced by d-galactosamine. Am. J. Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- Dabeva M.D., Shafritz D.A. Activation, proliferation and differentiation of progenitor cells into hepatocytes in the d-galactosamine model of liver regeneration. Am. J. Pathol. 1993;143:1606–1620. [PMC free article] [PubMed] [Google Scholar]
- Yavorkovsky L., Lai E., Ilic Z., Sell S. Participation of small intraportal stem cells in the restitutive response of the liver to periportal necrosis induced by allyl alcohol. Hepatology. 1995;21:1702–1712. [PubMed] [Google Scholar]
- Alison M., Golding M., Lalani E.N., Nagy P., Thorgeirsson S., Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J. Hepatol. 1997;26:343–352. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- Coleman W.B., McCullough K.D., Esch G.L., Faris R.A., Hixson D.C., Smith G.J., Grisham J.W. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am. J. Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H.C., Nagy P., Santonirugiu E., Thorgeirsson S.S. Modulation of keratin 14 and alpha-fetoprotein expression during hepatic oval cell proliferation and liver regeneration. J. Cell Physiol. 1994;159:475–484. doi: 10.1002/jcp.1041590312. [DOI] [PubMed] [Google Scholar]
- Dabeva M.D., Laconi E., Oren R., Petkov P.M., Hurston E., Shafritz D.A. Liver regeneration and alpha-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998;58:5825–5834. [PubMed] [Google Scholar]
- He X.Y., Smith G.J., Enno A., Nicholson R.C. Short-term diethylnitrosamine-induced oval cell responses in three strains of mice. Pathology. 1994;26:154–160. doi: 10.1080/00313029400169401. [DOI] [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R.M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978;38:1092–1098. [PubMed] [Google Scholar]
- Yaswen P., Hayner N.T., Fausto N. Isolation of oval cells by centrifugal leutriation and comparison with other cell types purified from normal and preneoplastic livers. Cancer Res. 1994;44:324–331. [PubMed] [Google Scholar]
- Gerber M.A., Thung S.N., Shen S., Stromeyer F.W., Ishak K.G. Phenotypic characterization of hepatic proliferation. Antigenic expression by proliferating epithelial cells in fetal liver, massive hepatic necrosis, and nodular transformation of the liver. Am. J. Pathol. 1983;110:70–74. [PMC free article] [PubMed] [Google Scholar]
- Nagy P., Bisgaard H.C., Santoni-Rugiu E., Thorgeirsson S.S. In vivo infusion of growth factors enhances the mitogenic response of rat hepatic ductal (oval) cells after administration of 2-acetylaminofluorene. Hepatology. 1996;23:71–79. doi: 10.1002/hep.510230111. [DOI] [PubMed] [Google Scholar]
- Fujio K., Evarts R.P., Hu Z., Marsden E.R., Thorgeirsson S.S. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab. Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- Fujio K., Hu Z., Evarts R.P., Marsden E.R., Niu C.H., Thorgeirsson S.S. Coexpression of stem cell factor and c-kit in embryonic and adult liver. Exp. Cell Res. 1996;224:243–250. doi: 10.1006/excr.1996.0134. [DOI] [PubMed] [Google Scholar]
- Matsusaka S., Tsujimura T., Toyosaka A., Nakasho K., Sugihara A., Okamoto E., Uematsu K., Terada N. Role of c-kit receptor tyrosine kinase in development of oval cells in the rat 2-acetylaminofluorene partial hepatectomy model. Hepatology. 1999;29:670–676. doi: 10.1002/hep.510290304. [DOI] [PubMed] [Google Scholar]
- Hu Z., Evarts R.P., Fujio K., Marsden E.R., Thorgeirsson S.S. Expression of fibroblast growth factor receptors flg and bek during hepatic ontogenesis and regeneration in the rat. Cell Growth Differ. 1995;6:1019–1025. [PubMed] [Google Scholar]
- Omori N., Evarts R.P., Omori M., Hu Z., Marsden E.R., Thorgeirsson S.S. Expression of leukemia inhibitory factor and its receptor during liver regeneration in the adult rat. Lab. Invest. 1996;75:15–24. [PubMed] [Google Scholar]
- Bisgaard H.C., Muller S., Nagy P., Rasumussen L.J., Thorgeirsson S.S. Modulation of the gene network connected to interferon-gamma in liver regeneration from oval cells. Am. J. Pathol. 1999;155:1075–1085. doi: 10.1016/s0002-9440(10)65210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P., Kiss A., Schnur J., Thorgeirsson S.S. Dexamethasone inhibits the proliferation of hepatocytes and oval cells but not bile duct cells in rat liver. Hepatology. 1998;28:423–429. doi: 10.1002/hep.510280220. [DOI] [PubMed] [Google Scholar]
- Auphan N., DiDonato J.A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoidsinhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kirillova I., Peschon J.J., Fausto N. Initiation of liver growth by tumor necrosis factordeficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Webber E.M., Kirillova I., Peschon J.J., Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptorrequirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–970. doi: 10.1002/hep.510280410. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Fausto N. Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am. J. Pathol. 1998;152:1577–1589. [PMC free article] [PubMed] [Google Scholar]
- Kirillova I., Chaisson M., Fausto N. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor kappaB activation. Cell Growth Differ. 1999;10:819–828. [PubMed] [Google Scholar]
- Zheng L., Fisher G., Miller R.E., Peschon J., Lynch D.J., Lenardo M.J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Mariathasan S., Nahm M.H., Baranyay F., Peschon J.J., Chaplin D.D. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- Seglen P.O. Preparation of rat liver cells. Exp. Cell Res. 1973;82:391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Engelhardt N.V., Factor V.M., Yasova A.K., Poltoranina V.S., Baranov V.N., Lasareva M.N. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation. 1990;45:29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Cressman D.E., Greenbaum L.E., DeAngelis R.A., Ciliberto G., Furth E.E., Poli V., Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sakamoto T., Ezure T., Yokomuro S., Murase N., Michalopoulos G., Demetris A.J. Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in miceinteractions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology. 1998;28:1260–1268. doi: 10.1002/hep.510280514. [DOI] [PubMed] [Google Scholar]
- Sugawara H., Yasoshima M., Katayanagi K., Kono N., Watanabe Y., Harada K., Nakanuma Y. Relationship between interleukin-6 and proliferation and differentiation in cholangiocarcinoma. Histopathology. 1998;33:145–153. doi: 10.1046/j.1365-2559.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- Cassell H.S., Price P., Olver S.D., Yeoh G.C.T. The association between murine cytomegalovirus induced hepatitis and the accumulation of oval cells. Int. J. Exp. Pathol. 1998;79:433–441. doi: 10.1046/j.1365-2613.1998.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H.J., Steinberg P., Toshkov I., Oesch R., Bannasch P. Persistence of the cholangiocellular and hepatocellular lesions observed in rats fed a choline deficient, DL-ethionine supplemented diet. Carcinogenesis. 1992;13:271–276. doi: 10.1093/carcin/13.2.271. [DOI] [PubMed] [Google Scholar]
- Tarsetti F., Lenzi R., Salvi R., Schuler E., Rijhsinghani K., Lenzen R., Tavoloni N. Liver carcinogenesis associated with feeding of ethionine in a choline-free dietevidence against a role of oval cells in the emergence of hepatocellular carcinoma. Hepatology. 1993;18:596–603. [PubMed] [Google Scholar]
- Smith P.G.J., Tee L.B.G., Yeoh G.C.T. Appearance of oval cells in the liver of rats after long-term exposure to ethanol. Hepatology. 1996;23:145–154. doi: 10.1002/hep.510230120. [DOI] [PubMed] [Google Scholar]
- Zhao Y.X., Lajoie G., Zhang H., Chiu B., Payne U., Inman R.D. Tumor necrosis factor receptor p55-deficient mice respond to acute Yersinia enterocolitica infection with less apoptosis and more efficient host resistance. Infect. Immun. 2000;68:1243–1251. doi: 10.1128/iai.68.3.1243-1251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M., Gaines G.C., Rosenberg J., Minter R., Bahjat F.R., Rectenwald J., MacKay S.L.D., Edwards C.K., Moldawer L.L. LPS-induced liver injury in d-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am. J. Physiol. 2000;278:R1202–R1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- Bazzoni F., Beutler B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- Wallach D., Varfolomeev E.E., Malinin N.L., Goltsev Y.V., Kovalenko A.V., Boldin M.P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Hsia C.C., Evarts R.P., Nakatsukasa H., Marsden E.R., Thorgeirsson S.S. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992;16:1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- Lowes K.N., Brennan B.A., Yeoh G.C., Olynyk J.K. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am. J. Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams T., De Vos R., Van Eyken P., Myazaki H., Van Damme B., Desmet V. Hepatic OV-6 expression in human liver disease and rat experimentsevidence for hepatic progenitor cells in man. J. Hepatol. 1998;29:455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- Robrechts C., De Vos R., Van den Heuvel M., Van Cutsem E., Van Damme B., Desmet V., Roskams T. Primary liver tumour of intermediate (hepatocyte-bile duct cell) phenotypea progenitor cell tumour? Liver. 1998;18:288–293. doi: 10.1111/j.1600-0676.1998.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Tee L.B.G., Kirilak Y., Huang W.-H., Smith P.G.J., Morgan R.H., Yeoh G.C.T. Dual phenotypic expression of hepatocytes and bile ductular markers in developing and preneoplastic rat liver. Carcinogenesis. 1996;17:251–259. doi: 10.1093/carcin/17.2.251. [DOI] [PubMed] [Google Scholar]
- Bennoun M., Rissel M., Engelhardt N., Guillouzo A., Briand P., Weber-Benarous A. Oval cell proliferation in early stages of hepatocarcinogenesis in simian virus 40 large T transgenic mice. Am. J. Pathol. 1993;143:1326–1336. [PMC free article] [PubMed] [Google Scholar]
- Kim C.M., Koike K., Saito I., Miyamura T., Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- Richards W.G., Yoder B.K., Isfort R.J., Detilleux P.G., Foster C., Neilsen N., Woychik R.P., Wilkinson J.E. Oval cell proliferation associated with the murine insertional mutation TgN737Rpw. Am. J. Pathol. 1996;149:1919–1930. [PMC free article] [PubMed] [Google Scholar]
- Sawamura M., Murakami H., Tsuchiya J. Tumor necrosis factor alpha and interleukin 4 in myeloma cell precursor differentiation. Leuk. Lymphoma. 1996;21:31–36. doi: 10.3109/10428199609067576. [DOI] [PubMed] [Google Scholar]
- Xu H., Sethi J.K., Hotamisligil G.S. Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 1999;274:26287–26295. doi: 10.1074/jbc.274.37.26287. [DOI] [PubMed] [Google Scholar]
- Piemonti L., Monti P., Allavena P., Sironi M., Soldini L., Leone B.E., Socci C., Di Carlo V. Glucocorticoids affect human dendritic cell differentiation and maturation. J. Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- Suganuma M., Okabe S., Marino M.W., Sakai A., Sueoka E., Fujiki H. Essential role of tumor necrosis factor a (TNF-α) in tumor promotion revealed by TNF-α deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- Webber E.M., Bruix J., Pierce R.H., Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]