Abstract
CD4 T cells activated in vitro by anti-CD3/28–coated beads are resistant to infection by CC chemokine receptor 5 (CCR5)-dependent HIV-1 isolates. In vivo, antigen-presenting cells (APCs) activate CD4 T cells in part by signaling through the T cell receptor and CD28, yet cells stimulated in this manner are susceptible to HIV-1 infection. We show that cytotoxic T lymphocyte antigen 4 (CTLA-4) engagement counteracts the CD28 antiviral effects, and that the ratio of CTLA-4 to CD28 engagement determines the susceptibility of HIV-1 infection. Furthermore, unopposed CTLA-4 signaling provided by CD28 blockade promotes vigorous HIV-1 replication, despite minimal T cell proliferation. Finally, CTLA-4 antibodies decrease the susceptibility of antigen-activated CD4 T cells to HIV, suggesting a potential approach to prevent or limit viral spread in HIV-1–infected individuals.
Keywords: HIV, costimulation, T cells, chemokine receptors, chemokines
Introduction
Cell activation is required for productive HIV-1 infection 1 2. HIV-1 can infect resting CD4 T cells, but the infection is aborted unless the cell reaches the G1b stage of the cell cycle within hours after infection 3. The necessity for T cell activation reflects, at least in part, a requirement for induction of nuclear factor of activated T cells (NFATc) 4. In vivo, optimal T cell activation occurs when an APC engages the TCR–CD3 complex and a costimulatory molecule on the same cell 5. CD28, the most potent costimulator, is highly expressed on resting T cells and upon engaging either B7.1 or B7.2 on the surface of APCs, it activates multiple pathways involved in cell growth and effector functions 6 7.
Cells stimulated in vitro by polystyrene beads coated with anti-CD3 and anti-CD28 antibodies (CD3/28 beads) undergo long-term polyclonal expansion and are resistant to infection by HIV-1 isolates that use the β-chemokine receptor, CC chemokine receptor 5 (CCR5), as a coreceptor (R5 isolates) 8. R5 isolates are implicated in transmission and are found during the early stages of HIV infection 9. CD28 costimulation induces the downregulation of CCR5 mRNA levels as well as CCR5 surface expression, and enhances production of the β-chemokines regulated on activation, normal T cell expressed and secreted protein (RANTES), macrophage inflammatory protein (MIP)-1α, and MIP-1β 10 11 12 13. These β-chemokines are the natural ligands for CCR5, and their secretion can inhibit entry of R5 viruses 14.
While cells stimulated by CD3/28 beads in vitro are resistant to infection with R5 HIV-1 isolates, CD4 T cells stimulated in vivo by APCs are the primary targets of HIV infection. We hypothesized that interplay between other costimulatory molecules may oppose or alter the antiviral effects of CD28 costimulation. CTL antigen 4 (CTLA-4), a costimulatory molecule structurally related to CD28, is able to bind members of the B7 family with a higher affinity than CD28 and unlike CD28, is not detectable on the surface of resting cells 15 16 17. CTLA-4 engagement leads to decreased cell activation and appears to function as an immune attenuator 18. A genetic approach involving germline disruption of CTLA-4 expression revealed a striking inhibitory role of CTLA-4 function on proliferation of mature T cells, particularly cells of the CD4 lineage 19 20 21. Another approach involved stimulating cells in the presence of anti–CTLA-4 antibodies. Anti–CTLA-4 antibodies and Fab fragments augment T cell proliferation under certain conditions, suggesting that blocking CTLA-4–B7 interactions prevents the delivery of a negative signal 22 23 24. In this report, we find that CTLA-4 engagement renders cells highly susceptible to HIV infection. CTLA-4 ligation blocks both the CD28-mediated downregulation of CCR5 expression and upregulation of β-chemokine expression. These results show that dynamic interactions between CD28, CTLA-4, and their ligands govern the outcome infection of CD4 T cells by HIV-1.
Materials and Methods
Cell Separation and Generation of Dendritic Cells.
Peripheral blood lymphocytes were isolated over Percoll (Amersham Pharmacia Biotech) gradient centrifugation from leukopacks obtained by apheresis of healthy donors. CD28+CD4+ T cells were purified by negative selection using magnetic beads (Dynal) as described previously 25 and were routinely >98% CD3+, >98% CD28+, and <3% CD8+ as judged by flow cytometry. To generate blood-derived dendritic cells (DCs), monocytes were isolated by Percoll gradient centrifugation and further enriched by gravity sedimentation. The monocytes differentiated into DCs after culture with IL-4 (a gift of Schering-Plough, Levallois-Perret, France) and GM-CSF (Immunex) 26. If the DCs were to be used for antigen-specific stimulation, tetanus toxoid (TT; Lederle Laboratories) was added before maturation. The DCs were then matured in the presence of TNF-α (R&D Systems) for an additional 4 d before use.
CD4 T Cell Stimulation Using Magnetic Beads.
Cells were stimulated with magnetic beads coated with a constant amount of anti-CD3 (humanized OKT3; a gift of Dr. Jeffrey Bluestone, University of Chicago, Chicago, IL) and various amounts (indicated in the text) of anti-CD28 (clone 9.3) 27, anti-MHC class I (W6/32; American Tissue Type Collection), or anti–CTLA-4 (3D6) 28 as described previously 8 29. To verify the amounts of antibody loaded, the beads were stained with pretitered amounts of goat anti–mouse IgG2a-PE, IgG2b-PE, or IgG1-PE (Southern Biotechnology Associates, Inc.). This approach was not possible with the MHC class I (W6.32)-coated beads, as the isotype of this antibody and CD28 mAbs were the same. Goat anti–human IgG was used to detect the humanized OKT3 mAb. Flow cytometry was then used to quantitate antibody bound to the beads. Alternatively, cells were stimulated with 3–5 μg/ml PHA (Sigma-Aldrich) and 100 U/ml IL-2 (Chiron). Cells were cultured at 1 × 106/ml in complete medium RPMI 1640 (BioWhittaker) supplemented with 10% fetal bovine serum (Hyclone), 2 mM l-glutamine (BioWhittaker), and 20 mM Hepes (BioWhittaker). Cell volume and cell number were monitored on a Coulter counter (model ZM; Beckman Coulter) and Channelyzer (model 256; Beckman Coulter). The culture medium was renewed at 2–3-d intervals, and the T cells were maintained at a concentration of 1–2 × 106 cells/ml.
CD4 T Cell Stimulation by DCs.
Allogeneic MLR was performed by mixing 106 freshly isolated CD4 T cells with allogeneic DCs irradiated at 3,000 rads from a 137Cs source at a 20:1 ratio in AIM V (GIBCO BRL) supplemented with 3% heat-inactivated human serum (NorML Cera-Plus®; North American Biological, Inc.). After 3 d of culture, the medium was supplemented with 100 U/ml of IL-2 and this level was maintained throughout the experiment.
TT-specific CD4 T cell lines were generated by repeated stimulations (three to seven) of CD4 T cells with autologous TT-pulsed DCs. For each restimulation, TT-specific CD4 T cells were cultured at 106 cells per well in 24-well plates in AIM V supplemented with 3% heat-inactivated human serum with autologous irradiated TT-pulsed DCs at a 40:1 ratio for 6 d. 100 U/ml of rIL-2 was added after 3 d of culture and IL-2 was replenished throughout the experiment. The culture medium was renewed at 2–3-d intervals, and the T cells were maintained at a concentration of 1–2 × 106 cells/ml.
Generation and Use of Fab Fragments.
Fab of anti-CD28 (9.3) and F(ab′)2 fragments of anti–CTLA-4 (CT26) were prepared using ImmunoPure IgG1 Fab and F(ab′)2 Preparation kit according to the manufacturer's instructions (Pierce Chemical Co.). In preliminary experiments, we found that Fab fragments of the CTLA-4 mAb did not retain binding activity, whereas full activity was retained with F(ab′)2 fragments of the CTLA-4 mAb. Where indicated, these fragments were added to CD4 T cells 1 h before stimulation and maintained at 25 μg/ml for the first 6 d of culture.
Flow Cytofluorometric Analysis.
106 CD4 T cells were stained with anti-CCR5–PE, anti-CXC chemokine receptor 4 (CXCR4)–PE, or an equivalent amount of an isotype control (BD PharMingen) for 30 min at 4°C. After washing in PBS with 0.01% sodium azide and 0.05% BSA (wash buffer), stained cells were analyzed immediately by flow cytometry on a FACScan™ (Becton Dickinson) after gating on live lymphocytes based on a standard light scatter histogram (integral forward scatter versus log 90°). Data was analyzed using WinMDI software (J. Trotter, The Scripps Research Institute, La Jolla, CA). Markers were set so that the isotype control would be 2% positive.
Acute Infection and PCR/Liquid Hybridization Procedures.
Resting CD4 T cells as well as cells stimulated for 6 d were infected with either HIV-1US-1 30 or HIV-1NL4-3 31 as described previously 10 12. In brief, for each infection, 5 × 106 cells were resuspended in 400 μl of 50% conditioned medium containing 1–3 × 105 half-maximal tissue culture infectious doses (TCID50) of HIV-1. The cells were incubated at 37°C for 2 h, washed three times in complete medium to remove excess virus, and resuspended in 50% conditioned medium at 106/ml. Antibody-coated beads were removed immediately before the start of the infection. In experiments in which resting CD4 T cells were infected, the cells were stimulated immediately after infection. In cases where Fab fragments were added, the cells were infected first, then incubated with the appropriate Fab fragment for 1 h after which they were stimulated. At designated time points, 106 cells were pelleted by centrifugation and frozen at –70°C. The cell pellets were lysed, amplified by PCR using HIV gag-specific primers, and the amplified sequences were detected by hybridization to a radiolabeled internal probe 32 33. The hybridized products were resolved by electrophoresis on 10% polyacrylamide gels, exposed to a PhosphorImager® screen overnight, and developed on PhosphorImager® 445 SI (Molecular Dynamics). To ensure that the reactions were performed within the linear range of the assay, log increments of HIV gag plasmid standards were amplified at the same time (not shown). Human β-globin sequences were PCR amplified to assure that equivalent levels of input DNA were present in each PCR reaction 32 33. Data were analyzed using ImageQUANT™ software (Molecular Dynamics).
Chemokine Measurements.
Levels of MIP-1α, MIP-1β, and RANTES in cell supernatants were measured using ELISA kits from R&D Systems according to the manufacturer's instructions.
Chemokine Receptor Reverse Transcription PCR Assay.
Total RNA was isolated from cells using RNA STAT-60 (Tel-Test, Inc.) and cDNA was synthesized using the StrataScript reverse transcriptase (RT)-PCR kit (Stratagene). cDNA products were diluted in H2O to predetermined optimal concentrations (1:3 for CCR5, 1:3,000 for glyceraldehyde 3-phosphate dehydrogenase gene [GAPDH]) and amplified using the following program: 95°C, 30 s; 55°C, 30 s; and 72°C, 90 s (25 cycles) as described previously 34. For CCR5-specific amplifications, the following primers were used: CCR5-42 (5′-GGG TGG AAC AAG ATG GAT TAT CAA GTG TCA-3′) and CCR5-640 (5′-ATG TCT GGA AAT TCT TCC AGA ATT GAT ACT-3′). For GAPDH-specific amplifications, the following primers were used: GAPDH-61 (5′-ATG GGG AAG GTG AAG GTC GGA GTC AAC GGA-3′) and GAPDH-433 (5′-AGG GGG CAG AGA TGA TGA CCC TTT TGG CTC-3′). A portion of the PCR reaction was hybridized as described 34 with end-labeled oligonucleotide probes specific for CCR5 (5′-GGG CTC CGA TGT ATA ATA ATT GAT GTC ATA-3′) or GAPDH (5′-TCG CTC CTG GAA GAT GGT GAT GGG ATT TCC-3′). The hybridized products were separated on 6% polyacrylamide gels, exposed to PhosphorImager® screens overnight and developed on a PhosphorImager® 445 SI (Molecular Dynamics). Figures were generated using ImageQUANT™ software (Molecular Dynamics).
Results
CTLA-4 Engagement Prevents the CD28-mediated Downregulation of CCR5 Expression.
The interplay between the costimulatory effects of CD28 and CTLA-4 and resulting susceptibility to HIV infection was examined initially by varying the ratio of the costimulatory signals delivered. We prepared immunobeads containing a constant level of anti-CD3 combined with varying ratios of anti-CD28 and anti–CTLA-4 29. Corresponding control sets of immunobeads were prepared containing anti-CD3 combined with varying ratios of anti-CD28 and anti-MHC class I (anti–MHC I). To simplify nomenclature, beads containing anti-CD3 coupled with one part anti-CD28 and nine parts anti–CTLA-4 are referred to as 1:9 CD28/CTLA-4. Similarly, beads that comprised three parts anti-CD28 and seven parts anti–MHC I and a constant amount of anti-CD3, are termed 3:7 CD28/MHC I. Freshly isolated CD4 T cells were stimulated with immunobeads for 3 d, after which the beads were removed and the cells were examined for CCR5 expression, β-chemokine expression, and susceptibility to infection with R5 isolates of HIV-1.
Resting CD4 T cells are characterized by a small cell volume and moderate levels of CCR5 surface expression 13 35. As we demonstrated previously, stimulation with 1:9 CD28/CTLA-4 beads induced little or no cell activation, as judged by the maintenance of small resting volumes and minimal cell proliferation 29. In contrast, cells treated with all other combinations of beads (Fig. 1) were strongly activated, as indicated by marked increases in cell volume and induction of cell proliferation. Since HIV-1 infection is influenced by the activation state of the T cell 36 37, we focused on studying the effects of CTLA-4 ligation on HIV-1 infection by using cells activated with 3:7 CD28/CTLA-4 beads, as their matched controls stimulated with 3:7 CD28/MHC I beads had similar growth kinetics.
Figure 1.
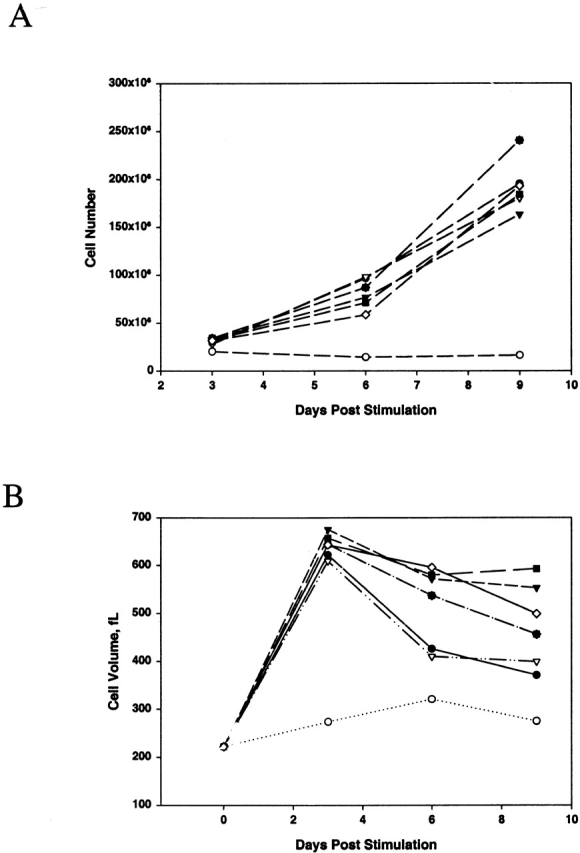
Cell growth (A) and cell volume (B) changes after stimulation with polystyrene beads containing varying ratios of CD28 to CTLA-4. Freshly isolated CD4 T cells were stimulated with immunobeads containing different ratios of anti-CD28 to anti–CTLA-4 or anti–MHC class I at a fixed amount of anti-CD3. Cell volume and growth kinetics were measured every 3 d. Data shown are representative of four experiments. •, 1:9 CD28/MHC I; ○, 1:9 CD28/CTLA-4; ▾, 3:7 CD28/MHC I; ▿, 3:7 CD28/CTLA-4; ▪, 5:5 CD28/MHC I; □, 5:5 CD28/CTLA-4; ♦, 7:3 CD28/MHC I; and ⋄, 7:3 CD28/CTLA-4. fL, femtoliters.
We next examined the effects of CD3, CD28, and CTLA-4 ligation on the induction of CCR5 expression. CCR5 expression in CD4 cells stimulated with 3:7 CD28/MHC I beads was strongly downregulated to undetectable levels, suggesting that this level of CD28 occupancy was sufficient to mediate the CD28 antiviral effect (Fig. 2). In contrast, a substantial fraction (63%) of CD4 cells stimulated with 3:7 CD28/CTLA-4 beads expressed high levels of CCR5. When cells were stimulated with beads containing higher ratios of anti-CD28 to anti–CTLA-4, the ability of CTLA-4 costimulation to maintain surface CCR5 expression diminished (data not shown). Thus, these results suggest that the ratio between signals delivered by CD28 and CTLA-4 in the context of CD3 ligation governs surface CCR5 expression.
Figure 2.
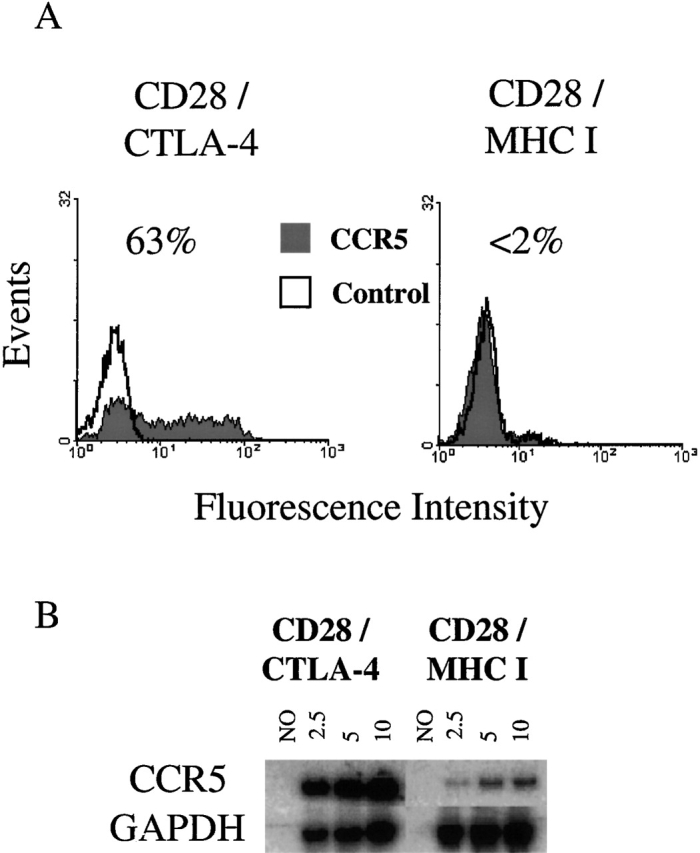
CCR5 levels in CD4 T cells stimulated with anti-CD3/28/CTLA-4 immunobeads. CD4 T cells were isolated and stimulated with beads containing a 3:7 ratio of anti-CD28 to anti–CTLA-4 or anti–MHC class I beads for 6 d. (A) Cytofluorometric analysis of CCR5 (red) or isotype control (open) fluorescence. Values of 2% or less are considered background. (B) RT-PCR analysis of CCR5 mRNA levels. RNA was isolated from CD4 T cells stimulated with 3:7 CD28/CTLA-4 and 3:7 CD28/MHC I beads, and cDNA was synthesized and diluted to the optimal level (1:3 for CCR5 and 1:3,000 for GAPDH). 2.5, 5, or 10 μl of the RT product was used in the subsequent PCR and liquid hybridization reaction to demonstrate that the assay was performed within the linear response range. NO, the amplification of a cDNA reaction in a sample from which the RT was omitted. Data shown are representative of four experiments.
Previously, we have shown that optimal levels of CD28 costimulation leads to downregulation of steady state CCR5 RNA levels 12 13. Furthermore, CD3/28 bead–stimulated cells are unable to transcribe heterologous genes linked to the CCR5 promoter 11. These observations suggested that CTLA-4 ligation may interfere with the CD28-mediated downregulation of CCR5 transcript levels. Therefore, we isolated RNA from both 3:7 CD28/MHC I bead– and 3:7 CD28/CTLA-4 bead–stimulated cells and used a semiquantitative RT-PCR method to examine CCR5 mRNA levels (Fig. 2 B). As expected in cells stimulated by 3:7 CD28/MHC I beads, only trace levels of CCR5 could be detected. In contrast, cells stimulated by 3:7 CD28/CTLA-4 had high levels of CCR5 RNA, suggesting that CTLA-4 ligation blocks the CD3/CD28-mediated downregulation of CCR5 transcript levels.
CTLA-4 Costimulation Exerts Only Modest Effects on β-Chemokine Production.
The CD28 antiviral effect comprises at least two components: downregulation of CCR5 expression and enhancement of β-chemokine production 10 12 13. Table shows the levels of β-chemokine secretion by CD4 cells after 3 d of stimulation with the CD28/CTLA-4 or CD28/MHC I beads. In all three donors, CD4 T cell stimulation with either CD28/MHC I or CD28/CTLA-4 beads resulted in high levels of β-chemokine production. In most cases, less than a twofold difference was observed between the CD28/MHC I– and CD28/CTLA-4–stimulated cells, suggesting that CD28/CTLA-4 did not markedly alter β-chemokine production. The notable exception is CD28/MHC I stimulated cells, which produced ∼10-fold more MIP-1α than the CTLA-4–stimulated cells in two of the three donors. At this point, it is unclear whether or not CTLA-4 has a direct role in MIP-1α production. Thus, in contrast to CCR5 downregulation, β-chemokine production appeared to be relatively insensitive to CTLA-4 engagement, suggesting that different costimulatory pathways or different thresholds of CD28 signaling are required to regulate CCR5 expression and β-chemokine production.
Table 1.
CD4 T Cell β-Chemokine Secretion after Stimulation with Anti-CD3/28/CTLA-4 Beads
| RANTES | MIP-1α | MIP-1β | ||
|---|---|---|---|---|
| ng/ml | ng/ml | ng/ml | ||
| Donor 1 | CD28/CTLA-4 | 1 | 23 | 107 |
| CD28/MHC I | 3 | 195 | 145 | |
| Donor 2 | CD28/CTLA-4 | 8 | 54 | 39 |
| CD28/MHC I | 11 | 93 | 66 | |
| Donor 3 | CD28/CTLA-4 | 6 | 15 | 43 |
| CD28/MHC I | 7 | 160 | 97 |
CD4 T cells were purified and stimulated with immunobeads coated with a constant level of anti-CD3 and three parts anti-CD28 with either seven parts anti–CTLA-4 or anti–MHC I for 3 d as described in Materials and Methods. Supernatants were collected and β-chemokine levels were measured by ELISA.
CTLA-4 Ligation Permits R5 Infection.
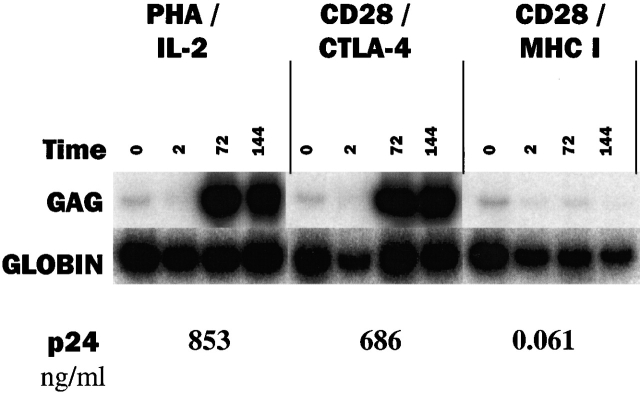
Our data suggest that CCR5 expression, and hence the balance of CD28 to CTLA-4 signaling, governs susceptibility to R5 infection. We tested this hypothesis by first stimulating CD4 T cells with 3:7 CD28/CTLA-4 or 3:7 CD28/MHC I beads for 3 d followed by infection with an R5 virus. Fig. 3 shows the level of HIV-1 gag DNA present in the infected cells and the amount of p24Gag antigen produced by the cells after infection. We found that 3:7 CD28/CTLA-4–stimulated cells were highly susceptible to R5 infection, as high levels of HIV gag DNA and p24 antigen were observed. As a reference, CD4 T cells stimulated with PHA/IL-2 were also infected and similar values were obtained demonstrating the high susceptibility of CD4 cells stimulated with 3:7 CD28/CTLA-4 to R5 infection. In contrast, 3:7 CD28/MHC I–stimulated cells remained resistant to R5 infection, suggesting that without CTLA-4 engagement this level of CD28 costimulation is able to prevent R5 infection 8 11 12.
Figure 3.
CTLA-4 ligation permits HIV-1 infection of CD3/28 bead–stimulated cells. CD4 T cells were stimulated as described in the legend to Fig. 1 for 3 d and infected with the R5 isolates as described in Materials and Methods. Samples were taken at t = 0, 2, 72, and 144 h after infection and cell pellets were analyzed for gag DNA by a quantitative PCR assay using liquid hybridization. p24 (in ng/ml) was measured in culture supernatants at 144 h after infection. Data shown are representative of three experiments.
Costimulation Modifies Susceptibility of PHA-activated T Cells to HIV-1 Infection.
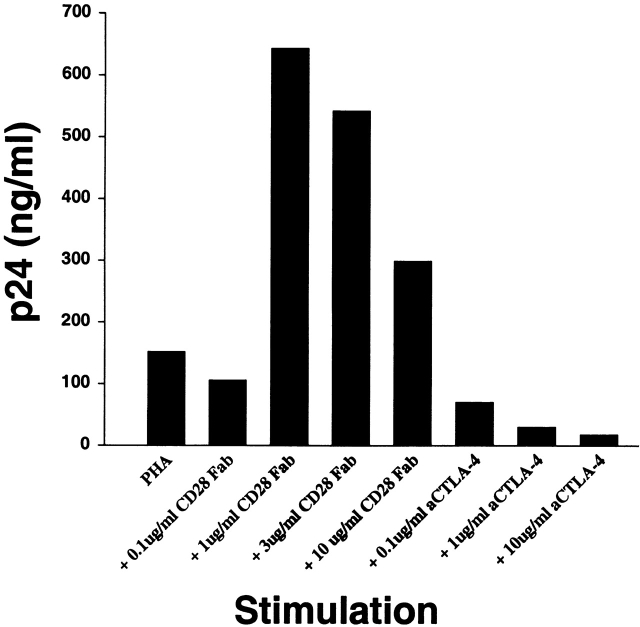
To generalize the previous results obtained with a bead-based system of T cell activation, we carried out experiments with PHA-activated T cells to determine if CTLA-4 has a similar role in a more widely studied model of HIV infection. CD8 cell–depleted PBMCs were infected with R5 HIV-1, and then activated with PHA in the presence of soluble CD28 and CTLA-4 antibodies (Fig. 4). We have previously shown that the addition of CD28 Fab fragments does not activate T cells as assessed by IL-2 production and proliferation 38. A surprising augmentation of HIV replication was observed after PHA activation in the presence of CD28 blockade despite decreased T cell proliferation (data not shown). Dose-dependent increases in HIV infection were not observed in cells incubated in higher concentrations (>1 μg/ml) of CD28 Fabs. This may be due to the overriding deleterious effects of minimal cell activation. In contrast, a striking dose-dependent inhibition of HIV replication was induced when CTLA-4 antibodies were included in the culture of PHA-activated T cells (Fig. 4) in spite of increased cell proliferation (data not shown). Thus, HIV R5 replication was increased under conditions that promote CTLA-4 ligation and inhibited under conditions with enhanced CD28 signaling.
Figure 4.
CD28 or CTLA-4 antibody fragments can modulate the susceptibility of PHA-stimulated T cells to R5 infection. Freshly isolated CD8-depleted PBMCs were infected with HIVUS-1 (R5) and stimulated with PHA alone or PHA in the presence of 0.1, 1, 3, and 10 μg/ml of anti-CD28 Fab, or PHA in the presence of 0.1, 1, and 10 μg/ml of anti–CTLA-4 F(ab′)2 fragments. p24 (in ng/ml) was measured in culture supernatants collected at 6 d after infection. Data are representative of three experiments using two different donors.
Costimulation Modifies Susceptibility of Antigen-specific Cell Lines to HIV-1 Infection.
In vivo, T cell activation is regulated through interactions with APCs. The CD80 (B7.1) and CD86 (B7.2) ligands on the surface of the APCs are able to bind and compete for binding to CD28 and CTLA-4 on the surface of T cells. In the next series of experiments, we examined whether antigen-specific CD4 T cells activated by autologous, antigen-loaded DCs were susceptible to HIV-1 infection. First, we generated several TT-specific CD4 T cell lines. In preliminary studies, we found that the TT lines were dependent on costimulation, as blockade with anti-B7 antibodies or CTLA4Ig prevented the stimulatory effects of antigen-pulsed DCs, consistent with previous data with tetanus-specific lines 39. These cell lines had stimulation indices ranging from 50 to 100, indicating a high degree of TT specificity (data not shown). The antigen-specific cells were stimulated for 6 d with either TT-loaded DCs or with CD3/28 beads. After stimulation, the cells were infected with either an R5 or X4 isolate of HIV-1. gag DNA and p24Gag antigen were measured (Fig. 5). Consistent with our previous reports, which used polyclonal, freshly isolated CD4 T cells 8 12 13, antigen-specific cells stimulated by CD3/28 beads remained susceptible to infection with X4 isolates while they were refractory to infection with R5 HIV isolates (Fig. 5). In contrast, when antigen-specific cells were stimulated by TT-loaded DCs, they were susceptible to both R5 and X4 strains of HIV-1, in agreement with previous studies 40 41. X4 isolates use the coreceptor CXCR4 42, and typically are not detected until the later stages of HIV-1 disease 9.
Figure 5.
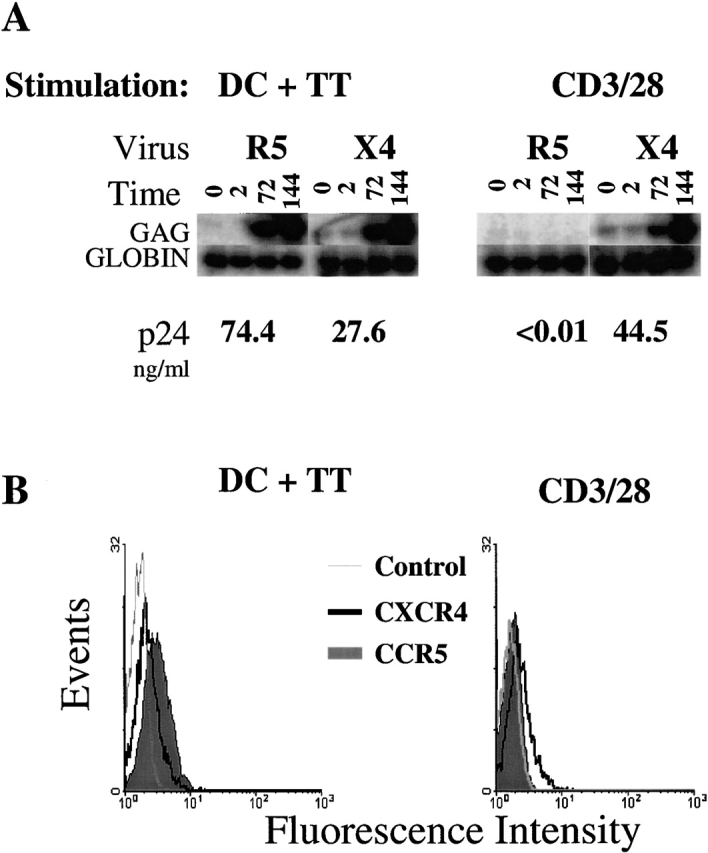
Antigen-specific cell lines are susceptible to R5 infection when stimulated with autologous DCs. (A) TT-specific CD4 T cells were stimulated with TT-loaded autologous DCs for 6 d and infected with either HIVUS-1(R5) or HIVNL4-3 (X4) as described in Materials and Methods. Samples were taken at t = 0, 2, 72, and 144 h after infection and cell pellets were analyzed for gag DNA by a quantitative PCR assay using liquid hybridization. p24 (in ng/ml) was measured in culture supernatants at 144 h after infection. (B) Cytofluorometric analysis of CCR5 (green histogram), CXCR4 (dark open histogram), or isotype control (light open histogram) of the cells described above before infection. Data are representative of three independent experiments.
We next used flow cytometric analysis to determine the status of CD4 T cell HIV-1 coreceptor expression after stimulation with antigen or anti-CD3/28. The coreceptor surface expression correlated with the infection data: antigen-mediated stimulation resulted in increases in CCR5 expression, whereas cells given CD3/28 bead stimulation had no detectable CCR5 expression (Fig. 5 B). In contrast, both antigen and CD3/28 stimulation increased CXCR4 expression. This latter finding is consistent with our previous reports, which showed increases in CXCR4 expression in T cells after CD3/CD28 stimulation 12. In the experiments with tetanus-specific CD4 cell lines, we noted a major difference (>1,000-fold) in the level of β-chemokines secreted after antigen and CD3/28 bead stimulation, with nearly undetectable levels found after antigen stimulation (data not shown). Taken together, high CCR5 levels and low β-chemokine production explain the high levels of virus replication seen in the antigen-specific cells. Thus, despite B7/CD28 interactions, the antigen-specific cells are highly susceptible to R5 isolates, consistent with the notion that other costimulatory molecules such as CTLA-4 may be interfering with the CD28-mediated antiviral effects.
Modulation of HIV Infection After Antigen Stimulation with CTLA-4 and CD28 Antibody Fragments.
To ascertain whether or not the susceptibility of antigen-activated cells could be modulated by altering the levels of CD28 and CTLA-4 costimulation, we generated Fab fragments to CD28 and F(ab′)2 fragments to CTLA-4. As with the PHA experiment shown in Fig. 4, we reasoned that these fragments, by virtue of their ability to bind but not cross-link their target molecules by Fc receptors, could promote the use of one costimulatory pathway at the expense of the other. Fab fragments of anti-CD28 mAb 9.3 have been shown to block allogeneic MLR reactions 43 and intact and Fab fragments of anti–CTLA-4 mAbs have been shown to enhance MLRs and T cell clonal expansion 23 44. Fab fragments to CD28 would be predicted to sterically prevent B7–CD28 interaction, favoring B7–CTLA-4 interactions and thus making antigen-activated cells more susceptible to R5 infection.
The ability of Fab fragments to alter the susceptibility of CD4 T cells to HIV infection was first tested by infecting resting TT-specific clones with both R5 and X4 strains of HIV-1. The infection was allowed to proceed for 2 h, after which unbound virus was washed out. The CD4 T cells were then stimulated immediately with autologous, TT-pulsed DCs, either in the presence or absence of anti-CD28 or anti–CTLA-4 fragments. As a control, infected cells were also stimulated with CD3/28 beads. Supernatants were removed from the cultures 6 d after infection and analyzed for the presence of HIV p24Gag antigen (Fig. 6). In CD4 T cells first infected with an R5 strain and then stimulated with CD3/28 beads, p24Gag antigen was undetectable (Fig. 6 A). It is likely that the CD28-mediated downregulation of CCR5 and upregulation of β-chemokine production prevented viral spread, similar to observations made when CD4 T cells from HIV-infected individuals were stimulated in vitro with CD3/28 beads 8. In contrast, a robust infection ensued in antigen-stimulated CD4 T cells stimulated with DCs. Stimulation with DCs in the presence of anti–CTLA-4 fragments rendered these cells much less permissive to a spreading HIV-1 infection (sevenfold reduction in p24), whereas stimulation of CD4 T cells by DCs in the presence of anti-CD28 Fab fragments markedly increased the amount of p24 produced (Fig. 6 A). These observations suggested that the anti–CTLA-4 fragments impeded with B7–CTLA-4 interactions, promoting stronger B7–CD28 signaling, whereas anti-CD28 fragments interfered with B7–CD28 interactions, thus strengthening B7–CTLA-4 interactions.
Figure 6.
Anti-CD28 Fab and anti–CTLA-4 F(ab′)2 fragments can modulate the susceptibility of antigen-stimulated CD4 T cells to HIV-1 infection. (A) Before restimulation, TT-specific CD4 T cells were infected with an R5 strain (HIVUS-1) and stimulated with antigen-loaded DCs in the presence or absence of 25 μg/ml of anti-CD28 or anti–CTLA-4 fragments. Alternately infected cells were stimulated with CD3/28 beads. Supernatant p24 (in ng/ml) was measured 6 d after infection. (B) Cells were stimulated as in A and infected with X4 isolate HIV-1NL4-3. Data shown are representative of two experiments.
Further support for the role of CTLA-4 in regulating CD4 T cell susceptibility to R5 infection was gathered by examining CCR5 expression in the treated cells. As predicted, treatment with anti–CTLA-4 fragments resulted in a decrease in CCR5-expressing cells. 30% of the CD4 T cells stimulated with DCs plus TT expressed CCR5 compared with 21% when the anti–CTLA-4 F(ab′)2 were added (data not shown). In contrast, treatment with anti-CD28 Fab fragments resulted in an increase in the number of cells expressing CCR5 (52%), suggesting that CCR5 levels are predictive of the level of HIV-1 production in this model system.
Manipulations of costimulatory molecule interactions in cells infected with an X4 virus isolate produced strikingly different results (Fig. 6 B). Unlike CCR5, CXCR4 expression (30 ± 5%) remained unchanged by the different methods of activation. Nonetheless, a marked decrease in p24Gag production in antigen-specific stimulated cells in the presence of anti-CD28 Fabs was observed, suggesting that the reduced level of cell activation caused by the anti-CD28 Fabs is inhibiting viral replication. This result underscores the differences seen in Fig. 6 A and implies that even larger differences might be observed if similar levels of cell activation were achieved. Likewise, treatment with anti–CTLA-4 F(ab′)2 slightly increased X4 replication rather than diminishing it as seen with an R5 virus.
Modulation of HIV Infection After Allogeneic Stimulation with CTLA-4 and CD28 Antibody Fragments.
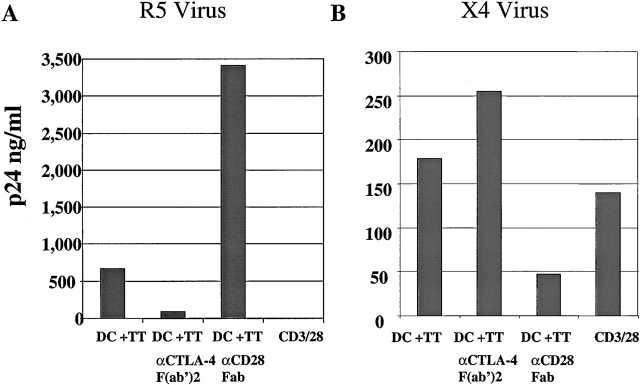
A potential limitation of data obtained from antigen-specific cell lines is that these cells have been cultured ex vivo. They have undergone multiple cycles of stimulation and cell division and thus are reasonable models for recall responses, but may not adequately reflect T cell behavior in vivo for primary responses to antigen. To address this concern, we examined CD28/CTLA-4 modulation and susceptibility to HIV infection in an MLR involving allogeneic (allo) DCs mixed with fresh primary CD4 T cells. Previous studies found that allostimulation has dichotomous effects on replication of R5 strains of HIV-1 in that it activates HIV expression in previously infected cells but inhibits HIV entry by the production of β-chemokines 45. Purified resting CD4 T cells were infected with an R5 HIV-1 isolate and then stimulated with CD3/28 beads, allo DCs, or allo DCs plus anti–CTLA-4 or anti-CD28 antibody fragments. p24Gag analysis was performed on day 9 after infection supernatant. Consistent with the data obtained from antigen-specific cells, CD4 T cells stimulated with DCs alone were susceptible to infection and those stimulated with CD3/28 beads were resistant to infection. Moreover, addition of anti–CTLA-4 fragments decreased viral production 5-fold, whereas the addition of anti–CD28 Fab fragments increased the amount of virus produced 18-fold (Fig. 7 A). Thus, addition of anti-CTLA-4 and anti-CD28 fragments to cells stimulated by allo DCs generated outcomes similar to those observed in the antigen-specific lines.
Figure 7.
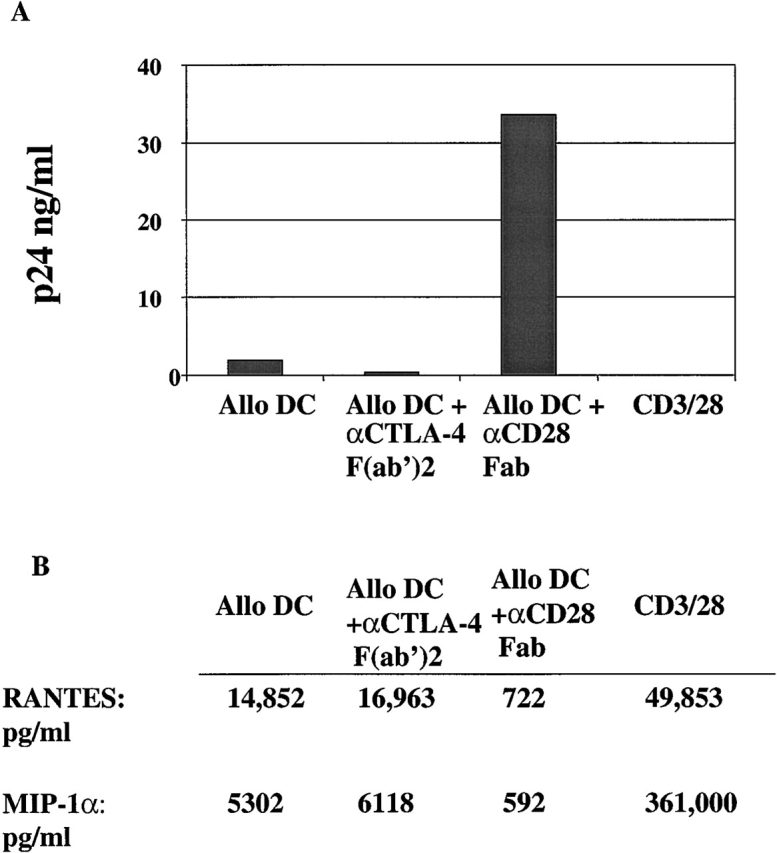
CD28 or CTLA-4 antibody fragments can modulate the susceptibility of MLR-stimulated CD4 T cells to R5 infection. Freshly isolated CD4 T cells were infected with HIVUS-1 (R5) and stimulated with allo DCs, allo DCs in the presence of either 25 μg/ml of anti–CD28 Fab or 25 μg/ml anti-CTLA-4 F(ab′)2 fragments, or CD3/28 beads. p24 (in ng/ml) was measured in culture supernatants collected 9 d after infection. Data are representative of three independent experiments in which the mean fold difference for the cultures treated with anti–CTLA-4 was −7.1 ± 2.2, and the mean fold difference for culture treated with anti-CD28 fragments was 14.6 ± 4.3. (B) RANTES and MIP-1α levels were measured 9 d after the stimulations described in A.
Overall, the level of CCR5 expression in cells stimulated by allo DCs was low (4%), and this probably contributes to the decreased level of viral replication that occurs in cells stimulated in this manner. Nonetheless, CCR5 expression was consistently increased by the addition of anti-CD28 fragments (6%) and decreased by anti–CTLA-4 fragments (2%). We also examined the levels of β-chemokines present in the supernatant at the time of infection. Overall, allo DC–stimulated cells produced lower levels of β-chemokines than CD3/28-stimulated cells. The addition of anti–CTLA-4 Fabs to the MLR led to modest yet reproducible increases in β-chemokine production, whereas the addition of anti-CD28 Fabs dramatically lowered β-chemokines levels (Fig. 7 B). These reduced levels of β-chemokine production coupled with the increased levels of CCR5 may explain the high levels of R5 replication seen in these cells. As with the antigen-specific cell model, infection of the MLR in the presence of a CD28 Fab fragments using an X4 virus led to much lower levels of virus replication (data not shown), supporting the role of cell activation in HIV infection and accentuating the differences seen when an R5 virus is used.
Discussion
CD28 Antiviral Effect.
These results refine a role for CTLA-4 in HIV-1 infection. The resistance of CD3/CD28-stimulated CD4 T cells to HIV infection contrasts sharply with the susceptibility of CD4 T cells to HIV infection in vivo, where they are activated by APCs. A key difference between CD3/CD28 bead costimulation and antigen-driven costimulation is that the APCs can also engage CTLA-4. We have confirmed that CD4 cells activated by DCs are highly susceptible to infection by R5 HIV-1 isolates 40 46. Furthermore, by specifically blocking B7–CD28 interactions, we have enhanced HIV replication in the target cells. Conversely, blocking B7–CTLA-4 interactions rendered the cells resistant to HIV infection. The results were robust, as similar effects were also observed in cells stimulated with lectins and soluble CD28 and CTLA-4 antibodies as with antigen-specific T cell activation–mediated peptide–MHC complexes on DCs. Thus, our results suggest that in vivo, the function of CTLA-4 is critical for inducing HIV susceptibility.
Previous studies have demonstrated the importance of costimulatory signal transduction for HIV infection of CD4 cells. Several investigators have shown that CTLA4Ig, a soluble form of CTLA-4, can decrease or prevent HIV infection of CD4 T cells. DC-mediated transmission of X4 strains to CD4 cells was increased by anti-CD28 and blocked by CTLA4Ig 47. Haffar et al. found that infected T cells could present alloantigen to fresh, uninfected CD4 T cells, leading to increased proliferation and virus spread to the activated cells, and that both of these events were blocked by CTLA4Ig 48. Mature DCs in peripheral blood were shown to bind HIV and induce infection when added to autologous CD4 T cells in the absence of added stimuli, and this infection was inhibited by CTLA4Ig 40. However, these studies did not reveal the discrete roles of CD28 and CTLA-4, nor have they distinguished between requirements for cellular activation and the subsequent inability to sustain productive HIV infection.
The mechanism by which CD3/28 bead costimulation regulates CCR5 and β-chemokine expression is unknown. Moriuchi et al. used a heterologous gene reporter system to show that CD28 costimulation inhibits transcription from the CCR5 promoter 11. As CD28 costimulation affects both the transcription rate and the mRNA stability of many genes 6, it remains to be seen whether other factors are involved in CCR5 regulation. How CTLA-4 blocks CD28's actions represents an even more complex question. It is not yet known whether signaling through CTLA-4 is essential for this effect or whether simple competition for CD28 through limiting amounts of B7 availability is sufficient for this function of CTLA-4. New insights into how CTLA-4 may inhibit cell growth show that upon cell activation CTLA-4 engagement inhibits tyrosine phosphorylation of TCR-ζ 49. Currently, it is unknown whether CTLA-4 exerts all of its mechanisms through TCR-ζ or whether other targets are involved in direct signal transduction by CTLA-4. For example, it has been shown that CTLA-4 can induce TGF-β secretion 50, and TGF-β can increase HIV transcription 51. The present results suggest that partial inhibition of CTLA-4 signal transduction has the potential to prevent or limit HIV-1 infection.
CD28/CTLA-4 Competition, Cell Activation, and Susceptibility to HIV Infection.
Our results argue that the strength of the costimulatory signals delivered by anti-CD28 and anti–CTLA-4 play an essential role in many cellular events, from early activation events to susceptibility to HIV infection. By constructing artificial APCs that have anti-CD3 coupled with various ratios of anti-CD28 to anti–CTLA-4, we have shown that different quantities of anti–CTLA-4 are required to block distinct subsets of CD28-mediated events. These results suggest that there is a hierarchy of events elicited by different ratios of CD28 and CTLA-4 ligation. For instance, a bead prepared at a 1:9 ratio of anti-CD28/anti–CTLA-4 is required to block IL-2 production, β-chemokine production, and cell growth. However, these same beads are unable to prevent Bcl-XL induction in naive T cells 29. Moreover, 1:9 anti-CD28/anti–MHC I (i.e., anti–CTLA-4 deficient) beads, while promoting vigorous cell proliferation, are unable to downregulate CCR5 levels (data not shown). However, 3:7 anti-CD28/anti–MHC I beads efficiently downregulate CCR5 expression, suggesting that stronger CD28 signaling is required for CCR5 downregulation than for cell growth initiation. In contrast, 3:7 anti-CD28/anti–CTLA-4 beads induced full activation of T cell growth, high level CCR5 expression, and supported vigorous HIV-1 infection. Thus, our results suggest a hierarchy of events that is elicited by different amounts or ratios of CD28 and CTLA-4 ligation.
In vivo, the existence of different signaling thresholds for CD28-mediated effects may serve to limit nonspecific activation, and through Bcl-XL induction, to prevent apoptosis. Recently, several groups have shown that MHC class II molecules are required for long-term CD4 cell survival 52 53. Presumably, tonic interactions between MHC class II molecules on APCs and the TCR complex deliver cell survival signals. These tonic interactions between MHC class II and TCR may also include B7–CD28–CTLA-4 complexes. It is likely that CTLA-4–B7 interactions would be favored, due to the higher affinity of CTLA-4 for B7 17. Thus, CD28-mediated signaling would be diminished to levels insufficient to induce proliferation but sufficient to induce Bcl-XL expression, thereby blocking the apoptosis cascade initiated by CD3 cross-linking.
Tonic interactions between costimulatory molecules may have important implications for HIV-1 infection. As APCs and T cells are in frequent contact with each other, partial CD4 T cell activation may be common, whereas full activation (mediated by CD28 signaling) would be a more rare event. We have examined two instances of partially activated CD4 cells: T cells stimulated with either PHA or APCs in the presence of CD28 Fab fragments. In both cases, despite low levels of cellular proliferation, these cells were highly susceptible to HIV-1 infection presumably due to the high levels of CCR5 expression and low levels of β-chemokine secretion. These results were surprising and challenge the absolute link between cellular activation, and HIV-1 infection; however, it must be noted that limited cellular activation is required for a productive infection. Excess anti-CD28 Fab fragments in these models severely inhibited cellular proliferation, and this resulted in little to no enhancement of HIV-1 replication (Fig. 4 and data not shown). Thus, a careful titration of anti-CD28 Fab fragments was required in order to prevent CD28 from downregulating CCR5 levels while at the same time permitting enough cellular activation to permit a productive infection. These models suggest that during the short time span in which cells are partially activated, they are highly susceptible to HIV-1 infection and this may permit HIV-1 replication to occur in the absence of full activation induced by an immune response against a pathogen. Recently, Haase and colleagues have demonstrated that shortly after infection, cells with a “resting” phenotype contain a significant fraction of the viral load 54. Perhaps, these highly infectable cells are the consequence of partial activation caused by CTLA-4 engagement.
Could Targeting CTLA-4 Limit the Spread of HIV-1 Infection?
The results presented here argue that B7–CTLA-4 interactions induce an HIV-sensitive state in CD4 T cells. Blocking this interaction may limit initial HIV-1 spread and thus lower long-term viral load. Targeting B7–CTLA-4 interactions may also have beneficial effects beyond the initial infection stage. Using an alloantigen stimulation model, Haffar et al. showed that CD28 expression decreases over time in infected individuals, and that viral production increases concurrently with the CD28 decrease 48. Furthermore, CTLA-4 expression on CD4 T cells in patients increases over the course of HIV-1 infection 55. Taken together with the data presented here, these studies suggest that over time the CD4 T cells in an HIV-1–infected individual become more susceptible to HIV-1 after B7 stimulation. Thus, inhibition of B7–CTLA-4 interactions, and the concomitant promotion of B7–CD28 interactions, may permit enhanced immunological control of HIV-1 and contribute to the maintenance of low viral loads. Oligonucleotide-based strategies to prevent or diminish CD28 expression have demonstrated some efficacy in rodents 56. It is possible that similar reagents could be developed to regulate CTLA-4 expression. However, since CTLA-4 has a role in downregulating immune responses, care must be used to prevent potentially harmful side effects resulting from immune hyperactivation 57.
In conclusion, we have demonstrated that susceptibility to HIV infection is controlled by interactions between APCs and costimulatory molecules on the CD4 T cell surface. CD28- and CTLA-4–mediated signaling results in a diametrically opposed phenotype susceptibility to R5 HIV strains. Blocking B7-mediated ligation of CTLA-4 permits the establishment of an HIV-resistant state, and approaches to limit CTLA-4 function may represent a therapeutic modality for HIV infection.
Acknowledgments
We would like to thank Tara Francomano and Jennifer Tench for technical assistance and Drs. Chris Broeren and Bruce Levine for insightful discussions.
This work was supported by United States Army contract DAMD17-93-V-3004, the Henry M. Jackson Foundation, the Buddy Taub Foundation, and the Leonard and Madlyn Family Cancer Research Institute. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Navy, Department of Defense, or the United States Government.
Footnotes
Abbreviations used in this paper: allo, allogeneic; CCR5, CC chemokine receptor 5; CTLA-4, CTL antigen 4; CXCR4, CXC chemokine receptor 4; DC, dendritic cell; RANTES, regulated on activation, normal T cell expressed and secreted; MIP, macrophage inflammatory protein; RT, reverse transcriptase; TCID50, half-maximal tissue culture infectious dose; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TT, tetanus toxoid.
References
- Zagury D., Bernard J., Leonard R., Cheynier R., Feldman M., Sarin P.S., Gallo R.C. Long-term cultures of HTLV-III-infected T cellsa model of cytopathology of T-cell depletion in AIDS. Science. 1986;231:850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]
- Zack J.A., Cann A.J., Lugo J.P., Chen I.S. HIV-1 production from infected peripheral blood T cells after HTLV-I induced mitogenic stimulation. Science. 1988;240:1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]
- Korin Y.D., Zack J.A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita S., Chen B.K., Kaneshima H., Nolan G.P. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Allison J.P. Co-stimulation in T cell responses. Curr. Opin. Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- June C.H., Bluestone J.A., Nadler L.M., Thompson C.B. The B7 and CD28 receptor families. Immunol. Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Lenschow D.J., Walunas T.L., Bluestone J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Levine B.L., Mosca J.D., Riley J.L., Carroll R.G., Vahey M.T., Jagodzinski L.L., Wagner K.F., Mayers D.L., Burke D.S., Weislow O.S. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272:1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- Connor R.I., Sheridan K.E., Ceradini D., Choe S., Landau N.R. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J. Exp. Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J.L., Carroll R.G., Levine B.L., Bernstein W., St. Louis D.C., Weislow O.S., June C.H. Intrinsic resistance to T cell infection with HIV type 1 induced by CD28 costimulation. J. Immunol. 1997;158:5545–5553. [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Fauci A.S. Cloning and analysis of the promoter region of CCR5, a coreceptor for HIV-1 entry. J. Immunol. 1997;159:5441–5449. [PubMed] [Google Scholar]
- Carroll R.G., Riley J.L., Levine B.L., Feng Y., Kaushal S., Ritchey D.W., Bernstein W., Weislow O.S., Brown C.R., Berger E.A. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- Riley J.L., Levine B.L., Craighead N., Francomano T., Kim D., Carroll R.G., June C.H. Naive and memory CD4 T cells differ in their susceptibilities to human immunodeficiency virus type 1 infection following CD28 costimulationimplications for transmission and pathogenesis. J. Virol. 1998;72:8273–8280. doi: 10.1128/jvi.72.10.8273-8280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Linsley P.S., Greene J.L., Tan P., Bradshaw J., Ledbetter J.A., Anasetti C., Damle N.K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D., Wang Z., Donovan C., He H., Mark D., Guan G., Wang Y., Walunas T., Bluestone J., Listman J. Regulation of CTLA-4 expression during T cell activation. J. Immunol. 1996;156:4154–4159. [PubMed] [Google Scholar]
- van der Merwe P.A., Bodian D.L., Daenke S., Linsley P., Davis S.J. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Sullivan T.J., Allison J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Bakker C.Y., Bluestone J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney E.R., Walunas T.L., Karr R.W., Morton P.A., Loh D.Y., Bluestone J.A., Jenkins M.K. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C.H., Ledbetter J.A., Gillespie M.M., Lindsten T., Thompson C.B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol. Cell. Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J.A., Martin P.J., Nowinski R.C. Monoclonal antibodies identifying a novel T-cell antigen and Ia antigens of human lymphocytes. Immunogenetics. 1980;10:247–260. [Google Scholar]
- Gribben J.G., Freeman G.J., Boussiotis V.A., Rennert P., Jellis C.L., Greenfield E., Barber M., Restivo V.A.J., Ke X., Gray G.S. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc. Natl. Acad. Sci. USA. 1995;92:811–815. doi: 10.1073/pnas.92.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair P.J., Riley J.L., Levine B.L., Lee K.P., Craighead N., Francomano T., Perfetto S.J., Gray G.S., Carreno B.M., June C.H. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-XL induction. J. Immunol. 1998;160:12–15. [PubMed] [Google Scholar]
- Mascola J.R., Louwagie J., McCutchan F.E., Fischer C.L., Hegerich P.A., Wagner K.F., Fowler A.K., McNeil J.G., Burke D.S. Two antigenically distinct subtypes of human immunodeficiency virus type 1viral genotype predicts neutralization serotype. J. Infect. Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- Adachi A., Gendelman H.E., Koenig S., Folks T., Willey R., Rabson A., Martin M.A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey M.T., Wong M.T. Quantative liquid hybridization employing phosphor technology. In: Dieffenbach C.W., Dveksler G.S., editors. PCR PrimerA Laboratory Manual. Cold Spring Harbor Laboratory Press; Plainview, NY: 1995. p. 313. [Google Scholar]
- Vahey M.T., Wong M.T., Michael N.L. A standard PCR protocolrapid isolation of DNA and PCR assay for β-globin. In: Dieffenbach C.W., Dveksler G.S., editors. PCR PrimerA Laboratory Manual. Cold Spring Harbor Laboratory Press; Plainview, NY: 1995. p. 17. [Google Scholar]
- Riley J.L., Carroll R.G. Quantitation of HIV-1 cofactor expression. In: Michael N.L., Kim J.H., editors. HIV Protocols. Humana Press; Totowa, NJ: 1998. pp. 219–226. [Google Scholar]
- Bleul C.C., Wu L., Hoxie J.A., Springer T.A., Mackay C.R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T.L., Dempsey M.P., Lamonica C.A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda S.D., Stein B.S., Mohagheghpour N., Benike C.J., Engleman E.G. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J. Immunol. 1989;142:773–780. [PubMed] [Google Scholar]
- Ledbetter J.A., Imboden J.B., Schieven G.L., Grosmaire L.S., Rabinovitch P.S., Lindsten T., Thompson C.B., June C.H. CD28 ligation in T-cell activationevidence for two signal transduction pathways. Blood. 1990;75:1531–1539. [PubMed] [Google Scholar]
- Gimmi C.D., Freeman G.J., Gribben J.G., Gray G., Nadler L.M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc. Natl. Acad. Sci. USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D., Barker T.D., Fauci A.S. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J. Exp. Med. 1996;183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P.A., Roderiquez G.L., Peden K.W., Norcross M.A. Human immunodeficiency virus type 1 infection of antigen-specific CD4 cytotoxic T lymphocytes. AIDS Res. Hum. Retroviruses. 1998;14:1397–1406. doi: 10.1089/aid.1998.14.1397. [DOI] [PubMed] [Google Scholar]
- Feng Y., Broder C.C., Kennedy P.E., Berger E.A. HIV-1 entry cofactorfunctional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Tan P., Anasetti C., Hansen J.A., Melrose J., Brunvand M., Bradshaw J., Ledbetter J.A., Linsley P.S. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J. Exp. Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Fauci A.S. Induction of HIV-1 replication by allogeneic stimulation. J. Immunol. 1999;162:7543–7548. [PubMed] [Google Scholar]
- Pope M., Betjes M.G., Romani N., Hirmand H., Cameron P.U., Hoffman L., Gezelter S., Schuler G., Steinman R.M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Pinchuk L.M., Polacino P.S., Agy M.B., Klaus S.J., Clark E.A. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity. 1994;1:317–325. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Haffar O.K., Smithgall M.D., Wong J.G., Bradshaw J., Linsley P.S. Human immunodeficiency virus type 1 infection of CD4+ T cells down-regulates the expression of CD28effect on T cell activation and cytokine production. Clin. Immunol. Immunopathol. 1995;77:262–270. doi: 10.1006/clin.1995.1152. [DOI] [PubMed] [Google Scholar]
- Lee K.M., Chuang E., Griffin M., Khattri R., Hong D.K., Zhang W., Straus D., Samelson L.E., Thompson C.B., Bluestone J.A. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Wahl S.M. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4+ T cells. J. Exp. Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.M., Shen X., Hu P.P., Wang X.F. Transforming growth factor beta stimulates the human immunodeficiency virus 1 enhancer and requires NF-kappaB activity. Mol. Cell. Biol. 1998;18:110–121. doi: 10.1128/mcb.18.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II–expressing dendritic cells. J. Exp. Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirberg J., Berns A., von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex–encoded molecules. J. Exp. Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Schuler T., Zupancic M., Wietgrefe S., Staskus K.A., Reimann K.A., Reinhart T.A., Rogan M., Cavert W., Miller C.J. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Steiner K., Waase I., Rau T., Dietrich M., Fleischer B., Broker B.M. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin. Exp. Immunol. 1999;115:451–457. doi: 10.1046/j.1365-2249.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam R.C., Phan U.T., Milovanovic T., Pai B., Lim C., Bard J., He L. Oligonucleotide-mediated inhibition of CD28 expression induces human T cell hyporesponsiveness and manifests impaired contact hypersensitivity in mice. J. Immunol. 1997;158:200–208. [PubMed] [Google Scholar]
- Daikh D., Wofsy D., Imboden J.B. The CD28-B7 costimulatory pathway and its role in autoimmune disease. J. Leukoc. Biol. 1997;62:156–162. doi: 10.1002/jlb.62.2.156. [DOI] [PubMed] [Google Scholar]