Abstract
The skin epidermis and its appendages provide a protective barrier that is impermeable to harmful microbes and also prevents dehydration. To perform their functions while being confronted with the physicochemical traumas of the environment, these tissues undergo continual rejuvenation through homeostasis, and, in addition, they must be primed to undergo wound repair in response to injury. The skin's elixir for maintaining tissue homeostasis, regenerating hair, and repairing the epidermis after injury is its stem cells, which reside in the adult hair follicle, sebaceous gland, and epidermis. Stem cells have the remarkable capacity to both self-perpetuate and also give rise to the differentiating cells that constitute one or more tissues. In recent years, scientists have begun to uncover the properties of skin stem cells and unravel the mysteries underlying their remarkable capacity to perform these feats. In this paper, I outline the basic lineages of the skin epithelia and review some of the major findings about mammalian skin epithelial stem cells that have emerged in the past five years.
The epidermis
I refer the reader to several fine reviews on avian skin epithelial stem cells (Chuong et al., 2006) and other stem cells of the mammalian skin (Nishikawa and Osawa, 2006; Tiede et al., 2007; Waters et al., 2007; Fernandes et al., 2008). The mammalian skin epidermis has been the paradigm for exploring homeostasis and injury repair in a stratified epithelium. The epidermis maintains a single inner (basal) layer of proliferative cells that adhere to an underlying basement membrane rich in ECM and growth factors (Fig. 1). Basal cells express several characteristic markers, including keratins and transcription factors. Periodically, these cells withdraw from the cell cycle, commit to differentiate terminally, move outward, and are eventually shed from the skin surface. This magnificent architecture allows the epidermis to generate a self-perpetuating barrier that keeps harmful microbes out and essential body fluids in.
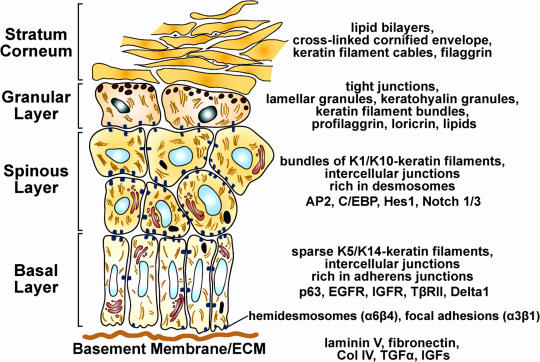
Figure 1.
Epidermal differentiation. The program of epidermal differentiation is shown in this schematic, illustrating the basement membrane at the base, the proliferative basal layer, and the three differentiation stages: spinous layer, granular layer, and outermost stratum corneum. At the right, key molecular markers are shown, which are described in the first section of this paper.
Upon commitment to terminally differentiate, an epidermal keratinocyte progresses through three distinct differentiation stages: spinous, granular, and stratum corneum (Fig. 1). Major changes in transcription, morphology, and function occur at the basal/spinous layer transition and again at the granular/stratum corneum transition such that differentiated cells reaching the skin surface are enucleated cellular skeletons that are packed with cables of keratin filaments encased by a γ-glutamyl-ε-lysine cross-linked cornified envelope of proteins. An additional final step in the differentiation process is the extrusion of a lipid bilayer that creates a saran wrap seal to the body surface (de Guzman Strong et al., 2006; Elias, 2007). The process is in a dynamic flux so that surface cells are continually sloughed and replaced by inner cells differentiating and moving outward. In human epidermis, the self-renewing capacity of epidermal stem cells is enormous, and, within 4 wk, a basal cell has terminally differentiated and exited at the skin surface. In mice, the postnatal trunk epidermis becomes thinner, and proliferation slows substantially as the hair coat develops and becomes the primary line of protection.
Although researchers have known for years that stem cells exist within the basal layer of adult epidermis, it is still not clear whether all cells within the basal layer are stem cells or whether only a small number of stem cells exist within this layer. The epidermal proliferative unit (EPU) has been architecturally defined as a bed of 10 tightly packed basal cells yielding a stack of increasingly larger and flatter cells that culminate with a single hexagonal surface cell (Potten, 1974). This has led to the hypothesis that there is one self-renewing stem cell per EPU and that the other basal cells are so-called transit-amplifying (TA) cells (i.e., committed cells that divide several times and then exit the basal layer and terminally differentiate; Potten, 1974; Mackenzie, 1997). In support of this notion are in vitro studies that show that human epidermal cells with the highest level of surface β1 integrins give rise to the largest colonies (holoclones) that can be passaged long term, whereas cells with lower levels of β1 integrins produce smaller meroclones that do not survive passaging (Barrandon and Green, 1987; Jones et al., 1995). αβ1 Integrins are the transmembrane core components of focal adhesions (FAs), which are required for basement membrane assembly, cell substratum adhesion, and cell survival/proliferation.
Based largely on in vitro observations, it has long been surmised that basal epidermal cells divide symmetrically and give rise to equal daughter cells. In this scenario, a basal cell would progressively reduce its adhesiveness to the underlying basement membrane, delaminate, and commit to terminally differentiate (Fig. 2; Watt et al., 1984; Watt and Hogan, 2000). In vivo, regional variations within the basement membrane and microenvironment have been described to suggest how distinct populations of integrin-rich stem cells and TA cells might arise within the basal layer (Lavker and Sun, 1982; Jensen et al., 1999). Recent studies suggest that basal epidermal cells can divide asymmetrically, affording a different view of how one basal stem cell and one committed cell might arise (Lechler and Fuchs, 2005; Clayton et al., 2007).
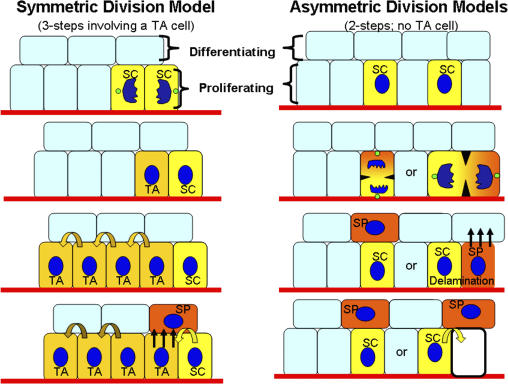
Figure 2.
Models for the generation of a single innermost (basal) layer of cells with proliferative potential and multiple layers of suprabasal cells. Self-renewing stem cells (SCs) exist in the basal layer of the epidermis. Symmetrical divisions produce two stem cells, a process which can serve to replenish vacancies in the basal layer. In the three-step model, a transit-amplifying (TA) intermediate arises, which has been postulated to divide four to five times before delaminating (straight arrows) and entering into a terminal differentiation program. In the two-step model, a stem cell divides asymmetrically to preferentially partition proliferation-associated factors into the stem cell daughter while providing differentiation-inducing components to the other daughter, which is fated to become a spinous cell (SP). Depending on the orientation of the spindle, the divisions could either result in detachment of the SP daughter from the basement membrane or, if lateral, would then necessitate subsequent delamination of the committed SP daughter. The spinous cells enter a program of terminal differentiation as they move outward and are eventually sloughed from the skin surface (see Fig. 1). Differentiating cells are continually replaced by a flux of inner cells committing to terminally differentiate and moving outward.
In the first study reporting asymmetrical divisions, at the onset of stratification, mouse embryonic basal cells were shown to shift their spindle orientation from a lateral to a more perpendicular orientation to undergo asymmetrical divisions (Lechler and Fuchs, 2005). Asymmetrical divisions soon accounted for ∼70% of all basal cell mitoses, whereas ∼30% remained symmetric (Lechler and Fuchs, 2005). Although the marked reduction in postnatal basal cell divisions posed technical difficulties in measuring asymmetrical divisions in most regions of adult mouse skin, ear skin as well as tongue displayed >60% asymmetrical divisions. The asymmetrical divisions observed provide a natural means of maintaining one proliferative basal daughter cell rich in growth-promoting receptor tyrosine kinases (RTKs) and integrins and one suprabasal daughter with reduced RTKs and integrin levels (Fig. 2). Moreover, loss of function experiments demonstrated a role for αβ1 integrins and also α-catenin (a core member of cell–cell adherens junctions) in setting up the cell polarity required for proper spindle pole orientation and maintenance of homeostasis (Lechler and Fuchs, 2005).
In a more recent study, lineage tracing experiments on adult mouse tail skin (another area diminished in hair follicles) also provided evidence that divisions in the basal layer are both asymmetric and symmetric, but, in this case, only ∼30% occurred at an angle of >20° relative to the basement membrane (Clayton et al., 2007). In this case, it was proposed that asymmetrical divisions might leave both daughters physically adhering to their substratum but that the committed daughter cell asymmetrically inherits a stronger Notch signal (Clayton et al., 2007), a key transcriptional determinant of the spinous cell fate (discussed in more detail later in this section; Rangarajan et al., 2001; Pan et al., 2004; Blanpain et al., 2006; Nguyen et al., 2006a; Lee et al., 2007). In this model, the daughter inheriting the Notch signal would differentiate, detach from the underlying basement membrane, and enter the spinous layer, whereas the daughter spared of the Notch signal would remain a stem cell (Clayton et al., 2007).
Although additional studies will be needed to resolve these different models for basal cell divisions, they lead to distinct implications for the numbers of epidermal stem cells and the location of the epidermal stem cell niche. The EPU model implies the existence of only a small number of basal stem cells, suggesting that within the basal layer, there should be spatially organized microenvironments that constitute a mini-niche for a single stem cell. In contrast, the asymmetrical division models suggest that a single progenitor population within the basal layer can give rise to committed cells by differentially partitioning proteins to the daughters. The lateral asymmetrical division model implies that asymmetrical divisions are intrinsic to the epider-mal stem cell, alleviating the need for a microenvironmental change to trigger commitment. The perpendicular asymmetrical division model suggests that the basement membrane itself may constitute the epidermal stem cell niche, naturally placing the committed cell into the spinous layer. The lateral symmetric divisions, which would yield two stem cells, might then provide a mechanism to replenish old or damaged basal stem cells (in the case of perpendicular asymmetrical divisions) or spinous cells (after delamination in the case of lateral asymmetrical divisions). This perpendicular asymmetrical division model is analogous to that of Drosophila melanogaster germ cell development, in which preservation of contact with the niche maintains stemness, whereas perpendicular asymmetrical divisions drive fate determination of the daughter cells that depart the niche (Fuller and Spradling, 2007).
Irrespective of the orchestrator of epidermal cell fate, the basement membrane demarcating the epidermis and dermis plays a critical role in governing the proliferative capacity of the epidermis. Although its mechano-physical properties alone are likely to impact the proliferative properties of basal cells (Dobereiner et al., 2005), the basement membrane is also composed of a rich array of ECM polymers and growth factors that provide a complex repertoire of stimuli for basal cells. Among them is laminin 5, which promotes anchorage and signaling/migration through its respective abilities to act as ligand for α6β4-rich hemidesmosomes and α3β1-rich FAs (Owens and Watt, 2003; Raghavan et al., 2003; Manohar et al., 2004). Through downstream α3β1 integrin activation of FAK, integrin-linked kinase, and the RTK Src, integrin signaling not only stimulates the Ras–MAPK pathway but also induces FA adhesion turnover and epidermal migration (Lorenz et al., 2007; Schober et al., 2007). Notably, transgenic suprabasal expression of integrins promotes tumorigenesis (Carroll et al., 1995). Conversely, conditional loss of FAK in the skin of mice leads to an increased resistance to chemically induced skin tumorigenesis (McLean et al., 2004), and, in vitro, FAK-deficient keratinocytes exhibit defects in cell migration and FA turnover under conditions in which apoptosis and proliferation are unaffected (Schober et al., 2007). Such studies underscore the importance of integrin/FAK signaling in balancing homeostasis, wound repair, and tumorigenesis in the skin.
The basement membrane is also rich in proteoglycans and other proteins that act as molecular sinks for growth factors, such as TGFβs, which restrict epidermal proliferation, and TGFα/EGFs and insulin growth factors, which promote it (for review see Fuchs, 2007). A recent study has shown that when TGFβ signaling is compromised through loss of the TβRII receptor, epidermal homeostasis is maintained, but the basal layer displays increased proliferation counterbalanced by increased apoptosis (Guasch et al., 2007). Although homeostasis can be maintained, it is precarious: wounds heal faster, and with just one additional oncogenic mutation, the tissue transforms quickly to squamous cell carcinoma of the skin. Interestingly, enhanced FAK/integrin signaling and accelerated migration are also seen when TGFβ signaling is compromised in the epidermis (Guasch et al., 2007). Conversely, elevated integrin signaling has been shown to suppress TGFβ signaling (Owens and Watt, 2003), suggesting that this molecular route may be a two-way street.
Although TGFβ signaling places the brake on epidermal proliferation and migration, EGF receptor (EGFR) signaling enhances proliferation and migration in the epidermis (Sibilia et al., 2000). A recent study shows that Lrig1, an inhibitory ligand for EGFR signaling, is expressed within a less proliferative, β1-enriched subset of cells within the basal layer of human epidermis (Jensen and Watt, 2006). Gene targeting has revealed that the epidermis of mice lacking Lrig1 hyperproliferates (Suzuki et al., 2002), and human keratinocytes treated with Lrig1 short hairpin RNA produce larger colonies than normal. These observations have led to the hypothesis that Lrig1 plays a role in basal stem cell quiescence (Jensen and Watt, 2006). Mitogen-inducible gene 6 is also a suppressor of EGFR signaling, and mice deficient in mitogen-inducible gene 6 also display epidermal hyperproliferation and increased tumor susceptibility (Ferby et al., 2006). Intriguingly, EGFR signaling promotes not just proliferation but also cell migration. One possible mechanism is through the ability of activated EGFR to phosphorylate β4 integrin and promote hemidesmosome disassembly (Wilhelmsen et al., 2007).
Thus, through regulation of two opposing tyrosine kinase receptor pathways and two integrin structures, the basement membrane plays an integral role in controlling the ability of basal epidermal stem cells to maintain homeostasis under normal and injury conditions. When this regulation goes awry, there is an increased propensity to developing squamous cell carcinoma. As the roles for additional basement membrane constituents and their associates are uncovered, our understanding of the special features of the epidermal basement membrane and its ability to regulate epidermal stem cell biology should continue to unfold.
The extrinsic signals received by the microenvironment and translated through transmembrane receptors are likely to couple with the intrinsic properties of the basal epidermal cells to define their ability to self-renew and undergo homeostasis and wound repair. One transcription factor that is likely to play a key role in regulating the self-renewal and long-term proliferative capacity of the stem cell is p63, a member of the p53 family of protooncogenes. The ΔN isoform of p63 is preferentially expressed in basal epidermal cells (Laurikkala et al., 2006). Its importance in epidermal biology surfaced when two groups made the fortuitous discovery that mice lacking p63 are severely impaired in their ability to generate the epidermis (Mills et al., 1999; Yang et al., 1999). The groups differed in whether the thin epidermis was caused by a defect in stem cell renewal (Yang et al., 1999), an absence of lineage commitment, and/or an early block in epidermal differentiation (Mills et al., 1999), and recent studies still leave this issue unresolved.
Supporting a role for ΔNp63 in stem cell self-renewal are clonal analyses on cultured thymic epithelial and epidermal cells, which suggest that when p63 mRNA is knocked down through small hairpin RNAs, the cells form smaller colonies with reduced proliferation rates (Senoo et al., 2007). Experiments on human epidermal raft cultures suggest that p63's function in basal cells is mediated by its ability to inhibit its relative p53, thereby promoting cell survival and longevity, whereas its effects on differentiation are p53 independent (Truong and Khavari, 2007). Consistent with this notion, the p63 knockdown experiments of Senoo et al. (2007) revealed the increased expression of terminal differentiation and apoptosis markers. In contrast, the studies of Koster et al. (2007) propose a role for p63 in triggering basal cells to switch from proliferation to terminal differentiation through the induction of IκB kinase-α, a putative regulator of differentiation (Hu et al., 2001; Gareus et al., 2007). Future studies will be needed to resolve this controversy.
Once cells become suprabasal, p63 is down-regulated by an underlying mechanism that is not yet clear. It has been reported that suprabasal signaling through Notch transmembrane receptors leads to suprabasal repression of p63 and inhibition of proliferation (Nguyen et al., 2006a). Similarly, loss of function experiments on the basally expressed Notch ligand, Delta1, results in hyperproliferation as well as the repression of differentiation, suggesting that Delta1 functions by repressing epidermal proliferation as well as promoting differentiation (Estrach et al., 2007). That said, conditional ablation of all canonical Notch signaling in the embryonic epidermis results in basal cell hypoproliferation and repression of spinous layer differentiation, with no obvious suprabasal expression of p63 (Blanpain et al., 2006). Recent studies by Lee et al. (2007) suggest that the epidermal hyperproliferation seen when skin is partially compromised in Notch signaling may arise from noncell autonomous changes in the underlying dermis. Although the effects of Notch on proliferation appear to be complex, the collective view emerging from the present studies is that Notch signaling plays a key role in the transition between basal and suprabasal cells in the epidermis, where it functions in activating the basal to suprabasal fate switch (Fig. 3).
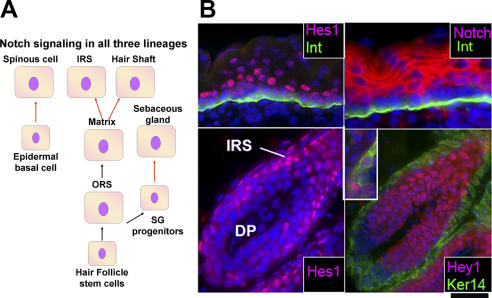
Figure 3.
Predicted roles of Notch signaling in the three different lineages of the epidermis. (A) Model. In the skin, Notch ligands are often basal, whereas Notch receptors are typically suprabasal (Pan et al., 2004; Blanpain et al., 2006; Estrach et al., 2006, 2007; Nguyen et al., 2006a). Notch signaling results in the release of Notch intracellular domain, which acts as a transcription cofactor for the DNA-binding protein RBPj (Pan et al., 2004). Downstream targets of Notch intracellular domain/RBPj include Hes1 and Hey1, which bind DNA and often function as transcriptional repressors (Hurlbut et al., 2007). In the skin lineages, Notch signaling, as indicated by either the expression of Notch intracellular domain (Pan et al., 2004), Notch target genes Hes1 and Hey1 (Blanpain et al., 2006), or loss of function studies (Blanpain et al., 2006; Estrach et al., 2006, 2007), is particularly prominent at the transition of fates from proliferative to differentiating. The arrows denote molecular steps along the pathways for each of the three major epithelial lineages of the skin. Those marked in red indicate the steps at which Notch signaling acts. (B) Immunofluorescence. Immunofluorescence microscopy was used on frozen mouse skin sections of E18.5 (epidermis) and postnatal day 28 (hair follicle bulb) to illustrate the localization of Notch3, Hes1, and Hey1. All samples were counterstained with DAPI (blue) to mark the nuclei. Where indicated, antibodies against β4 integrin (Int) were used to mark the dermo-epidermal boundary, and keratin 14 (Ker14) was used to denote the basal layer of the ORS. The top two panels illustrate the coexpression of Notch3 and Hes1, which is particularly strong at the basal to suprabasal transition of the epidermis. The bottom two panels show that Hes1 is prominent in the differentiating cells of the IRS (left), whereas Hey1 is more broadly expressed in both the precortex and IRS (right). The inset in the image in the bottom right frame shows a close-up view of hair shaft precursor cells that have been labeled with antibodies against hair keratins in green and Hey1 in red to show that nuclear Hey1 colocalizes with cells that generate the hair shaft. Note that the highly proliferative matrix cells at the base of the hair bulb are negative for signs of Notch signaling. For further information and similar images (courtesy of C. Blanpain and W.E. Lowry), see Blanpain et al. (2006). Bar, 45 mm.
Overall, the basal expression of Notch ligands such as Delta1 and the suprabasal expression of Notch receptors and Notch downstream target genes such as Hes1 are consistent with a role for Notch signaling in controlling this switch. A role for Notch as the gatekeeper of the commitment of basal epidermal cells to terminally differentiate is particularly intriguing in light of a Notch inhibitor, Numb, which functions as an intrinsic cell fate determinant that is segregated into only one of the two daughter cells during neural precursor division in Drosophila neuroblasts (for review see Yu et al., 2006). In flies, Numb localization is determined by opposing signals from the Par3–atypical PKC complex, and this machinery is also present in basal epidermal cells and is present at the right time and place to control asymmetrical divisions (Lechler and Fuchs, 2005; Clayton et al., 2007). Although much still remains to be learned about how basal epidermal cells become activated to withdraw from the cell cycle and commit to terminally differentiate, the action appears to converge at the base of the epidermis between the basal and first suprabasal layers.
The hair follicle
One of the most remarkable features of the vertebrate epidermis is its extraordinary ability to generate elaborate appendages. The scales of a fish, the horn of a rhinocerous, the claws of an eagle, the mane of a lion, and the sweat (eccrine) and oil (sebaceous) glands of our skin are all epidermal appendages. Knowledge of how epidermal appendages develop has been gained by studying the embryonic skin at a stage when it exists as a single-layered epithelium.
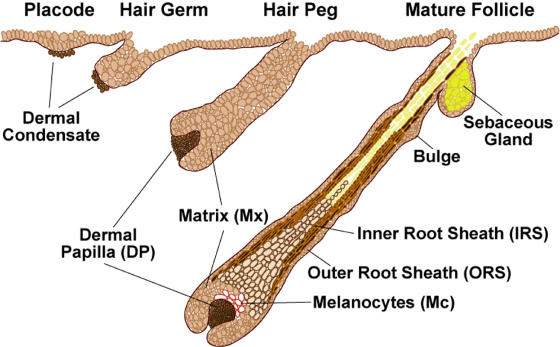
Hair follicle morphogenesis begins early in embryonic development as dermal cells populate the skin, and signals from the epithelium induce the formation of dermal condensates. Dermal condensates are the precursors to the dermal papillae (DP), which are the permanent mesenchymal component of the hair follicle. At approximately embryonic day (E) 14.5 of mouse development, mesenchymal–epithelial interactions result in the formation of the first wave of hair placodes, which appear as small epidermal invaginations into the dermis (Fig. 4). Once initiated, these placodes undergo marked proliferation and downgrowth to produce first the hair germs (∼E15.5) and then hair pegs (∼E16.5–17.5). The follicle cells at the leading front (matrix) remain highly proliferative, and, through contact with the DP, they engage in a program of gene expression that is distinct from the root. The outer root sheath (ORS) maintains contact with the basement membrane, whereas the inner root sheath (IRS) provides the channel for the emerging hair (∼E18.5). At birth, the most mature hairs begin to break the skin surface, and the sebaceous gland (SG) precursor cells become established in the upper segment of the root. Maturation continues through the first postnatal week (Fig. 4; for review see Schmidt-Ullrich and Paus, 2005). There are four waves of follicle morphogenesis, with the large primary guard hairs forming first (E14.5) and the bulk of the hair coat follicles initiating within a few days after this.
Figure 4.
Embryonic stages of hair follicle morphogenesis. The process of follicle morphogenesis occurs relatively late in embryonic development. In the mouse, discrete waves of placodes are seen beginning at approximately E14.5 and ending near birth. Once initiated, newly formed follicles continue to mature postnatally until approximately day 9, at which time matrix (TA) cells at the base of the follicle continue to rapidly proliferate and differentiate, generating hair growth. For a comprehensive analysis of the regional, temporal, and strain-specific variations in the timing of hair follicle morphogenesis, see the reviews Muller-Rover et al., 2001 andSchmidt-Ullrich and Paus, 2005.
One dermal cue that appears to be critical is the bone morphogenetic protein (BMP) inhibitory protein Noggin, whose absence severely impairs hair follicle morphogenesis (Botchkarev et al., 1999). Ectodermal Wnt signals, which include Wnt10b, are thought to act in concert with dermal signals to instruct the epidermal cells to grow downward (Botchkarev et al., 1999; DasGupta and Fuchs, 1999; Reddy et al., 2001; Andl et al., 2002). At the crux of this signaling cross talk is the bipartite transcription factor complex composed of LEF1 and stabilized β-catenin, which is critical for hair follicle morphogenesis (van Genderen et al., 1994; Zhou et al., 1995; Gat et al., 1998) and whose activity can be monitored by the Wnt reporter gene TOPGAL (DasGupta and Fuchs, 1999; Millar, 2002). Inhibition of BMP signaling is permissive for LEF1 expression and also promotes the stabilization of β-catenin (Jamora et al., 2003; Kobielak et al., 2003; Andl et al., 2004; Zhang et al., 2006), whereas Wnts are well-established instigators of β-catenin stabilization (for review see Clevers, 2006). Interestingly, although this paradigm serves the bulk of follicle morphogenesis, the first wave of follicle morphogenesis, resulting in formation of the large primary guard hairs, diverges somewhat, particularly in the external cues guiding these internal regulatory processes. This first wave is uniquely dependent on a TNF-like ligand–receptor complex composed of ectodysplasin (EDA) and EDA receptor, which, instead of inducing Noggin, induces two different BMP inhibitors. EDA is a target of Wnt signaling (Laurikkala et al., 2001), and signs of Wnt reporter gene activity and LEF1/β-catenin are present by E14.5, when the first wave of follicle morphogenesis occurs (DasGupta and Fuchs, 1999; Pummila et al., 2007; for review see Schmidt-Ullrich and Paus, 2005).
Just how powerful is the stabilization of β-catenin to promoting hair follicle morphogenesis? Studies over the past decade indicate that it is indeed central to the decision-making process. Thus, when excess β-catenin is stabilized in the epidermis of transgenic mice, ectopic hair follicles appear within the interfollicular epidermis, generating super-furry mice (Gat et al., 1998), and, when the Wnt inhibitor Dickoff 1 (Dkk1) is expressed ectopically or when β-catenin is conditionally targeted for ablation, hair follicle morphogenesis is blocked altogether (Huelsken and Birchmeier, 2001; Andl et al., 2002). Recently, Ito et al. (2007) showed that when the skin of mice is severely wounded, endogenous Wnt signaling is elevated in the skin, and this leads to the induction of hair follicle formation from epidermal stem cells. Collectively, these findings underscore the role for stabilized β-catenin in governing the choice of whether to become epidermis or hair follicle.
Once the placode forms, signaling events downstream of Wnts/BMPs drive the downgrowth and maturation of hair follicles. Sonic hedgehog (Shh) is an early gene expressed downstream of Wnt/BMP receptor (BMPR) signaling (and EDA/EDA receptors in the case of guard hairs) and placode formation (Dahmane et al., 1997; Oro et al., 1997; Gat et al., 1998; Morgan et al., 1998; St-Jacques et al., 1998). Shh plays a critical role in organizing the dermal cells into a condensate, or DP, which thereafter becomes a permanent component of the hair follicle (Hardy, 1992; St-Jacques et al., 1998; Oro and Higgins, 2003; Levy et al., 2007). Overall, the formation of the hair bud involves many changes in gene expression as exemplified by the differences between the transcriptional profile of placode cells and their epidermal counterparts (Rhee et al., 2006). Among the early steps is the activation of additional key transcription factors (e.g., Lhx2 and Sox9) whose absence results in a failure to maintain follicle stem cell behavior in mice (Vidal et al., 2005; Rhee et al., 2006). Additional changes involve down-regulation of some adhesion proteins (e.g., epithelial cadherin [E-cadherin]) and up-regulation of others (Jamora et al., 2003). The field is still in the midst of elucidating how multiple signaling pathways converge on the ectoderm to elicit changes in transcription factors that then subsequently change the expression and dynamics of the extracellular matrix, cytoskeleton, and cell matrix and cell–cell junctions that remodel the epithelium from its single layer to a hair bud. Once understood, this process is likely to be relevant to other morphogenetic processes ranging from development of the mammary gland to that of the tooth.
The hair cycle and hair follicle stem cells
The hair follicle undergoes cycles of degeneration and regeneration throughout life. Matrix cells have been referred to as TA cells because they proliferate rapidly during the growth (anagen) phase of the cycle but then suddenly undergo apoptosis by a mechanism that is still poorly understood. During this 3–4-d destructive phase (catagen), the hair bulb and root shrivel to an epithelial strand, which pulls the DP upward to rest at the base of the permanent, noncycling portion of the follicle. In mice, backskin follicles remain dormant for 1–2 d in the first resting phase (telogen) but then rest for >3 wk in the second telogen. Old hairs can remain in their socket through several bouts of hair cycling, rendering the hair coat impervious to this cyclic behavior.
At the onset of each new anagen, the cycling portion of the follicle regenerates, a process that necessitates a reservoir of follicle stem cells. These stem cells reside in the lowest permanent portion of the follicle, within the ORS, which bulges around the existing old hair pocket to enable the new hair to emerge through the same orifice at the skin surface. The cells within the bulge of the ORS cycle less frequently than other epithelial cells within the skin (Cotsarelis et al., 1990). At the start of each new hair cycle, a cluster of stem cells at the base of the bulge become activated to proliferate. Grafting and lineage tracing experiments have shown that some bulge cells are stem cells and that offspring of a single bulge stem cell can form SGs and epidermis as well as new hair follicles (Blanpain et al., 2004; Morris et al., 2004; Claudinot et al., 2005). That said, although follicle stem cells are multipotent, unless the skin is wounded, follicle stem cells only function in hair follicle homeostasis and do not contribute to interfollicular epidermis (Ito et al., 2005; Levy et al., 2005, 2007).
To understand the special features of the bulge, researchers have conducted microarray profiling. Approximately 150 genes are preferentially expressed in the bulge relative to the proliferating basal cells of the epidermis (Blanpain et al., 2004; Morris et al., 2004; Tumbar et al., 2004). The purification of bulge stem cells has been accomplished by FACS based on either (1) bulge cell surface markers α6 and CD34 coupled with K14-GFP expression (Blanpain et al., 2004), (2) K15-GFP expression (Morris et al., 2004), or (3) a fluorescent histone pulse-chase experiment in mice expressing a tetracycline-inducible transcriptional regulatory repressor (Tetoff) under the control of a K5 promoter and histone H2B-GFP under the control of a tetracycline regulatory element (Tumbar et al., 2004). Although each of these different procedures purifies slightly different cell populations, the array data are in quite good agreement, enabling researchers to exploit this information to learn more about follicle stem cells.
Like the embryonic epidermis, bulge cells are Wnt responsive, as judged by their expression of two LEF1-related DNA-binding proteins, TCF3 and TCF4, and several Wnt receptor proteins (Fzds). However, at least in telogen, most bulge cells appear to be in a state of Wnt inhibition, as judged by the array data and by the lack of detectable nuclear β-catenin or TOPGAL activity (DasGupta and Fuchs, 1999). These findings are consistent with a recent study showing that TCF3 can function as a repressor when β-catenin is absent or underrepresented and that TCF3 on its own functions to repress the differentiation of skin stem cells (Nguyen et al., 2006b).
Although Wnt inhibition seems to be a feature of the dormant stem cell niche in the skin, stabilized β-catenin is a feature of activated stem cells. By transgenically elevating the levels of stabilized β-catenin, hair follicles precociously enter anagen (Van Mater et al., 2003; Lo Celso et al., 2004). Conditional loss of BMPR1a in telogen follicles also results in the precocious entry of follicles into anagen and the stabilization of β-catenin, and, at least in part, this appears to be caused by the activation of PI3 kinase signaling, which, in turn, phosphorylates and inactivates GSK3β, the kinase that otherwise targets β-catenin for phosphorylation and ubiquitin-mediated degradation (Zhang et al., 2006; Kobielak et al., 2007). These studies further clarify how the inhibition of BMP signaling and activation of Wnt signaling converge to regulate stem cell activation.
Once follicle stem cells are activated, Wnt3 signaling appears to be required for hair shaft differentiation, whereas BMP signaling is required for IRS and directly or indirectly is also required for hair shaft differentiation (Millar, 2002; Kobielak et al., 2003; Andl et al., 2004; Yuhki et al., 2004). Thus, when β-catenin is conditionally targeted for depletion in adult skin when follicles are in telogen phase, the bulge stem cell compartment cannot be maintained, and the new hair cycle does not reinitiate (Lowry et al., 2005); when stabilized β-catenin is constitutively activated, tumors develop that are composed of a center of hair shaft cells surrounded by matrix-like cells expressing nuclear LEF1/β-catenin (Gat et al., 1998).When the gene encoding BMPR1a is conditionally targeted for depletion in adult follicles in telogen phase, bulge stem cells become activated, as judged by their loss of BrdU label retention and their entry into S phase (Andl et al., 2004; Kobielak et al., 2007). However, the cells cannot terminally differentiate, and, within weeks, tumors develop that display markers such as Lhx2, Sox4, Sox9, and Shh, which are features of activated stem cells. Despite the fact that they are no longer slow cycling and do not express appreciable CD34, the follicle cells lacking BMPR1a are still able to repair epidermis in a wound response, and they generate what appear to be long-lived tumors (Zhang et al., 2006; Kobielak et al., 2007). These studies are tantalizing in that they suggest that quiescence may not be an essential feature of follicle stem cells.
Overall, these data suggest that BMP signaling plays an important role in follicle stem cell quiescence. Consistent with this view are immunofluorescence studies that detect phosphorylated Smad1, which is reflective of active BMP signaling, and the behavior of cultured bulge cells exposed to BMP6, which results in their transient withdrawal from the cell cycle (Blanpain et al., 2004; Kobielak et al., 2007). TGFβ signaling also appears to be up-regulated in bulge cells (Tumbar et al., 2004) and is known to dampen the cell cycle (Massague, 2007). However, conditional ablation of the TGFβ receptor did not appear to compromise the slow-cycling capability of the follicle stem cells in the bulge (Guasch et al., 2007).
Although studies in recent years have led to major advances in our knowledge of bulge stem cells and the signaling pathways involved in their activation at the start of the hair cycle (for review see Fuchs, 2007), the precise nature of the signals and where they come from are still poorly understood. Thus, despite the established role for β-catenin stabilization in follicle stem cell activation, documentation for the involvement of a specific Wnt is still lacking. Similarly, although the condensate of DP cells typically rests at the base of the bulge in telogen follicles and expresses several BMP inhibitory factors and Wnts that are candidates for follicle stem cell activation, it is not clear precisely what this inductive signal is. Further complicating the issue is that in the whisker cycle of rodents, the DP never reaches the bulge before the start of a new anagen. It has been proposed that stem cells from the bulge therefore migrate along the ORS to the base of the follicle, where they become activated (Oshima et al., 2001). Although further studies are required, most studies to date are consistent with the notion that this may also happen in backskin follicles. If so, it would imply that Sox9, Tcf3, and Lhx2 may be markers of follicle stem cells irrespective of whether they are quiescent or proliferative and that there are likely to be factors beyond DP signals that are required to activate the hair cycle.
SGs
Appendages of the hair follicle, SGs, are located above the bulge and just below the hair shaft orifice at the skin surface. The major role of the gland is to generate terminally differentiated sebocytes, which degenerate to release lipids and sebum and lubricate the skin surface. SG homeostasis necessitates a population of progenitor cells that gives rise to a continual flux of proliferating and differentiating cells that are sloughed through the hair canal. Lineage tracing by retroviral-mediated gene transfer suggests that a small population of cells near or at the base of the SG might be stem cells (Ghazizadeh and Taichman, 2001).
Recently, the transcriptional repressor protein Blimp1 was identified in a genetic screen for hair follicle transcription factors, and it was shown to mark a small population of cells at the SG base (Horsley et al., 2006). These Blimp1-positive cells appeared to be in close association with the basement membrane surrounding the gland and are contiguous with the BM that demarcates the dermal–epidermal border and surrounds the hair follicle. The Blimp1-positive SG cells also express K5/K14. Genetic lineage tracing experiments reveal that the Blimp1-positive SG cells can regenerate the entire gland, including the sebocytes. When it was conditionally targeted for ablation, SGs got larger. The likely explanation stems from Blimp1's role in B lymphocytes, in which it transcriptionally represses c-myc (Chang et al., 2000), a gene known to induce SG hyperplasia and sebocyte differentiation at the expense of hair follicle differentiation (Arnold and Watt, 2001; Waikel et al., 2001). However, if Blimp1 truly marks the progenitor/stem cell population of the SG, why don't the enlarged glands eventually recede in Blimp1's absence even if hyperplasia is the initial outcome?
Although further studies are needed, the underlying explanation could be rooted in the ability of the bulge to become mobilized when the SG progenitor cell population is depleted. As judged by BrdU pulse and pulse-chase experiments, Blimp1-negative bulge cells show signs of active cycling and reduced label retention in the absence of Blimp1 (Horsley et al., 2006). In this regard, the behavior of bulge stem cells in response to a loss of Blimp1 in SG progenitors resembles their response to the epidermis lost upon injury in normal mice. Such precursor-product relation between the bulge and other skin stem cell populations has also been documented by engraftment experiments with isolated bulge stem cells (Blanpain et al., 2004; Claudinot et al., 2005).
Common themes in skin stem cells
An emerging view of skin stem cells is that there appears to be at least three distinct niches for skin stem cells: the follicle bulge, the base of the SG, and the basal layer of the epidermis. It is not yet resolved whether the basal layer contains a subset of stem cells as originally posited by Potten (1974) or whether the basal compartment is composed of a single progenitor population, as more recently proposed by Clayton et al. (2007).
Are there unifying features of keratinocyte stem cells and their activation mechanisms that guide them along specific lineages? Although the answer to this question remains unknown, it seems reasonable to predict that certain features of keratinocyte stem cells and stem cell activation must be similar given the similarities in stem cell niches.
All three progenitor populations express K5, K14, and p63. Although p63 has also been implicated in differentiation, p63 remains one of the best candidates to date for a gatekeeper of proliferation in epithelial stem cells (Truong et al., 2006; Senoo et al., 2007). Cells within all three progenitor pools also express E-cadherin and display elevated levels of adherens junctions and reduced levels of desmosomes. A reduction in E-cadherin appears to be an essential feature in mobilizing embryonic epidermal cells to invaginate to form a hair follicle (Jamora et al., 2003), and reductions in α-catenin and p120-catenin have also been linked to epithelial cancers, a less organized form of invagination (Scott and Yap, 2006; Reynolds, 2007). Such studies make it tempting to speculate that the mobilization of stem cells to exit their niches and either form a hair follicle or repair wounds may entail the remodeling of intercellular junctions.
Additionally, the three progenitor compartments are typified by their proximity to an underlying basement membrane, and all three types of progenitors express integrins, including α6β4 and α3β1, as well as their associated proteins. The higher the integrin level, the greater the proliferative potential seems to be, as judged by the early studies of Jones et al. (1995) on cultured epidermal stem cells. One intriguing aspect is the up-regulation of β6 integrin and the presence of the αvβ6 integrin ligand, tenascin C, in the bulge stem cells as they transit from telogen to anagen (Tumbar et al., 2004). Similar changes are seen in the epidermal basal layer in response to injury (Fassler et al., 1996), leading to the speculation that these changes might be important in understanding how stem cells become activated to migrate from their niche and embark upon their specific mission.
Thus, although none of these markers is exclusive to the three progenitor populations, they do appear to be general features of keratinocytes that maintain their proliferative potential, whether sustained or transient. It is still formally possible that expression of these markers empowers keratinocytes to retain or maintain their proliferative potential and their stem-ness, in which case down-regulation of these markers might define a point of no return along a stem cell lineage. As future studies are conducted, the extent to which this model is correct should become apparent.
The similarities in keratinocyte stem cell behavior may extend to shared principles and pathways, even if the precise players may differ. One example is Blimp1, which appears to mark SG progenitors and repress c-myc expression (Horsley et al., 2006), whereas Miz1 seems to play a similar c-myc–repressive function in the epidermal basal layer (Gebhardt et al., 2006). The outcome of c-myc expression is keratinocyte proliferation in both the SG and epidermis, leading to the postulate that c-myc might control the conversion of stem cells to so-called TA (i.e., committed) cells (Waikel et al., 2001; Frye et al., 2003). In this regard, it is intriguing that later in the epidermal lineage, Ovol1, yet another transcriptional repressor of c-myc, is expressed suprabasally, where it may act to prevent proliferation after TA cells commit to terminally differentiate (Nair et al., 2006).
Another signaling pathway likely to impact on more than one keratinocyte stem cell niche is Notch, which controls selective cell fate determination through close-range interactions not only in the epidermis but also in the hair follicle and the SG (Yamamoto et al., 2003; Pan et al., 2004; Vauclair et al., 2005; Blanpain et al., 2006; Estrach et al., 2006; Nguyen et al., 2006a). The studies to date support a role for Notch in activating the keratinocyte switch from the undifferentiated to differentiated fate (Fig. 3).
Although Notch signaling appears to affect the transition from proliferation to differentiation throughout keratinocyte populations in the skin, other signaling pathways seem to have more potent effects on one lineage over another. Thus, in the follicle bulge, stem cells enter the new hair cycle when threshold levels of stabilized β-catenin are reached (Gat et al., 1998; Huelsken et al., 2001; Van Mater et al., 2003; Lo Celso et al., 2004; Lowry et al., 2005; Ito et al., 2007). Conversely, a negative role for Wnt signaling has been proposed for SG fate determination because SG hyperplasia occurs when a truncated version of LEF1 is expressed that is unable to associate with β-catenin (Merrill et al., 2001; Niemann et al., 2002). Although epidermal stem cells are seemingly unaffected by the loss of β-catenin (Huelsken et al., 2001), they adopt a hair follicle cell fate when β-catenin is stabilized through either genetic manipulation or wounding (Gat et al., 1998; Ito et al., 2007). As outlined in this paper, growth factor receptor signaling also appears to affect different keratinocyte stem cell populations in distinct ways.
When taken with the fact that cell fate signaling pathways are often interdependent on one another, such differences can have a potent impact on lineage determination, as recently demonstrated by Estrach et al. (2006). In the precortex of the hair follicle, where Wnt signaling is particularly high (DasGupta and Fuchs, 1999), the Wnt target gene Jag1 is expressed, leading to active Notch signaling in these cells, promoting proliferation and differentiation to produce hair (Estrach et al., 2006). Conversely, in the absence of Jag1, hair follicles fail to form, but the epidermis is spared. Thus, by activating the expression of other signaling genes, Wnt signaling can accentuate its effects on a particular lineage within the skin.
In closing, the existence of different skin stem cell compartments and the ability of these niches to respond differentially to environmental cues has been an exciting new development in the field. Increasing knowledge about these stem cell populations over the past 5 yr has begun to shed light on the similarities and differences among these niches. Important questions for the future are now raised. Are these different stem cell populations multipotent, as recent experiments by Ito et al. (2007) suggest? Is the multipotency of stem cells in response to wounding a reflection of mechanical disruption of the niche or a response to growth/differentiation factors released in a wound response? To what extent do the gene expression programs of other progenitor populations overlap with that of follicle stem cells, and do these similarities reflect their self-renewing, undifferentiated features? When do these different proliferative epithelial compartments form during skin development, and to what extent are their differences in gene expression patterns dependent on their distinct in vivo microenvironments? What factors control the differences in the slow-cycling ability of different stem cell populations within the skin? The answers to these questions will bring us ever closer in the strife to understanding how stem cells are maintained in their undifferentiated, growth-restricted state and what prompts them to become activated, exit their niches, and embark upon distinct lineages.
Acknowledgments
I am grateful to the Fuchs' laboratory, past and present, for their enthusiasm, dedication, and contributions to the science and research that I have covered here along with those of many colleagues in the field. A special thank you goes to Jonathan Nowak, Dr. Valerie Horsley, and Dr. Michael Rendl for their helpful comments and constructive criticisms. I also thank Joe Alexander (Information Technology, The Rockefeller University, New York NY) for his assistance in the artwork.
E. Fuchs is an Investigator of the Howard Hughes Medical Institute and receives support for this research from the National Institutes of Health, in particular grant R01-AR050452.
Abbreviations used in this paper: BMP, bone morphogenetic protein; BMPR, BMP receptor; DP, dermal papillae; E-cadherin, epithelial cadherin; EDA, ectodysplasin; EGFR, EGF receptor; EPU, epidermal proliferative unit; FA, focal adhesion; IRS, inner root sheath; ORS, outer root sheath; RTK, receptor tyrosine kinase; SG, sebaceous gland; Shh, Sonic hedgehog; TA, transit amplifying.
References
- Andl, T., S.T. Reddy, T. Gaddapara, and S.E. Millar. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2:643–653. [DOI] [PubMed] [Google Scholar]
- Andl, T., K. Ahn, A. Kairo, E.Y. Chu, L. Wine-Lee, S.T. Reddy, N.J. Croft, J.A. Cebra-Thomas, D. Metzger, P. Chambon, et al. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 131:2257–2268. [DOI] [PubMed] [Google Scholar]
- Arnold, I., and F.M. Watt. 2001. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11:558–568. [DOI] [PubMed] [Google Scholar]
- Barrandon, Y., and H. Green. 1987. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA. 84:2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain, C., W.E. Lowry, A. Geoghegan, L. Polak, and E. Fuchs. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 118:635–648. [DOI] [PubMed] [Google Scholar]
- Blanpain, C., W.E. Lowry, H.A. Pasolli, and E. Fuchs. 2006. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 20:3022–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev, V.A., N.V. Botchkareva, W. Roth, M. Nakamura, L.H. Chen, W. Herzog, G. Lindner, J.A. McMahon, C. Peters, R. Lauster, A.P. McMahon, and R. Paus. 1999. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol. 1:158–164. [DOI] [PubMed] [Google Scholar]
- Carroll, J.M., M.R. Romero, and F.M. Watt. 1995. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 83:957–968. [DOI] [PubMed] [Google Scholar]
- Chang, D.H., C. Angelin-Duclos, and K. Calame. 2000. BLIMP-1: trigger for differentiation of myeloid lineage. Nat. Immunol. 1:169–176. [DOI] [PubMed] [Google Scholar]
- Chuong, C.M., P. Wu, M. Plikus, T.X. Jiang, and R. Bruce Widelitz. 2006. Engineering stem cells into organs: topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr. Top. Dev. Biol. 72:237–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot, S., M. Nicolas, H. Oshima, A. Rochat, and Y. Barrandon. 2005. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. USA. 102:14677–14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton, E., D.P. Doupe, A.M. Klein, D.J. Winton, B.D. Simons, and P.H. Jones. 2007. A single type of progenitor cell maintains normal epidermis. Nature. 446:185–189. [DOI] [PubMed] [Google Scholar]
- Clevers, H. 2006. Wnt/beta-catenin signaling in development and disease. Cell. 127:469–480. [DOI] [PubMed] [Google Scholar]
- Cotsarelis, G., T.T. Sun, and R.M. Lavker. 1990. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 61:1329–1337. [DOI] [PubMed] [Google Scholar]
- Dahmane, N., J. Lee, P. Robins, P. Heller, and A. Ruiz i Altaba. 1997. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 389:876–881. [DOI] [PubMed] [Google Scholar]
- DasGupta, R., and E. Fuchs. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 126:4557–4568. [DOI] [PubMed] [Google Scholar]
- de Guzman Strong, C., P.W. Wertz, C. Wang, F. Yang, P.S. Meltzer, T. Andl, S.E. Millar, I.C. Ho, S.Y. Pai, and J.A. Segre. 2006. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J. Cell Biol. 175:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobereiner, H.G., B.J. Dubin-Thaler, G. Giannone, and M.P. Sheetz. 2005. Force sensing and generation in cell phases: analyses of complex functions. J. Appl. Physiol. 98:1542–1546. [DOI] [PubMed] [Google Scholar]
- Elias, P.M. 2007. The skin barrier as an innate immune element. Semin. Immunopathol. 29:3–14. [DOI] [PubMed] [Google Scholar]
- Estrach, S., C.A. Ambler, C. Lo Celso, K. Hozumi, and F.M. Watt. 2006. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 133:4427–4438. [DOI] [PubMed] [Google Scholar]
- Estrach, S., R. Cordes, K. Hozumi, A. Gossler, and F.M. Watt. 2007. Role of the notch ligand delta1 in embryonic and adult mouse epidermis. J. Invest. Dermatol. 10.1038/sj.jid.5701113. [DOI] [PubMed]
- Fassler, R., T. Sasaki, R. Timpl, M.L. Chu, and S. Werner. 1996. Differential regulation of fibulin, tenascin-C, and nidogen expression during wound healing of normal and glucocorticoid-treated mice. Exp. Cell Res. 222:111–116. [DOI] [PubMed] [Google Scholar]
- Ferby, I., M. Reschke, O. Kudlacek, P. Knyazev, G. Pante, K. Amann, W. Sommergruber, N. Kraut, A. Ullrich, R. Fassler, and R. Klein. 2006. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat. Med. 12:568–573. [DOI] [PubMed] [Google Scholar]
- Fernandes, K.J., J.G. Toma, and F.D. Miller. 2008. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, M., C. Gardner, E.R. Li, I. Arnold, and F.M. Watt. 2003. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 130:2793–2808. [DOI] [PubMed] [Google Scholar]
- Fuchs, E. 2007. Scratching the surface of skin development. Nature. 445:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, M.T., and A.C. Spradling. 2007. Male and female Drosophila germline stem cells: two versions of immortality. Science. 316:402–404. [DOI] [PubMed] [Google Scholar]
- Gareus, R., M. Huth, B. Breiden, A. Nenci, N. Rosch, I. Haase, W. Bloch, K. Sandhoff, and M. Pasparakis. 2007. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat. Cell Biol. 9:461–469. [DOI] [PubMed] [Google Scholar]
- Gat, U., R. DasGupta, L. Degenstein, and E. Fuchs. 1998. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 95:605–614. [DOI] [PubMed] [Google Scholar]
- Gebhardt, A., M. Frye, S. Herold, S.A. Benitah, K. Braun, B. Samans, F.M. Watt, H.P. Elsasser, and M. Eilers. 2006. Myc regulates keratinocyte adhesion and differentiation via complex formation with Miz1. J. Cell Biol. 172:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh, S., and L.B. Taichman. 2001. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch, G., M. Schober, H.A. Pasolli, L. Polak, E.B. Conn, and E. Fuchs. 2007. Loss of TGFb signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelium. Cancer Cell. 12:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, M.H. 1992. The secret life of the hair follicle. Trends Genet. 8:55–61. [DOI] [PubMed] [Google Scholar]
- Horsley, V., D. O'Carroll, R. Tooze, Y. Ohinata, M. Saitou, T. Obukhanych, M. Nussenzweig, A. Tarakhovsky, and E. Fuchs. 2006. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 126:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., V. Baud, T. Oga, K.I. Kim, K. Yoshida, and M. Karin. 2001. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 410:710–714. [DOI] [PubMed] [Google Scholar]
- Huelsken, J., and W. Birchmeier. 2001. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11:547–553. [DOI] [PubMed] [Google Scholar]
- Huelsken, J., R. Vogel, B. Erdmann, G. Cotsarelis, and W. Birchmeier. 2001. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 105:533–545. [DOI] [PubMed] [Google Scholar]
- Hurlbut, G.D., M.W. Kankel, R.J. Lake, and S. Artavanis-Tsakonas. 2007. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 19:166–175. [DOI] [PubMed] [Google Scholar]
- Ito, M., Y. Liu, Z. Yang, J. Nguyen, F. Liang, R.J. Morris, and G. Cotsarelis. 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11:1351–1354. [DOI] [PubMed] [Google Scholar]
- Ito, M., Z. Yang, T. Andl, C. Cui, N. Kim, S.E. Millar, and G. Cotsarelis. 2007. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 447:316–320. [DOI] [PubMed] [Google Scholar]
- Jamora, C., R. DasGupta, P. Kocieniewski, and E. Fuchs. 2003. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 422:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, K.B., and F.M. Watt. 2006. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc. Natl. Acad. Sci. USA. 103:11958–11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, U.B., S. Lowell, and F.M. Watt. 1999. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 126:2409–2418. [DOI] [PubMed] [Google Scholar]
- Jones, P.H., S. Harper, and F.M. Watt. 1995. Stem cell patterning and fate in human epidermis. Cell. 80:83–93. [DOI] [PubMed] [Google Scholar]
- Kobielak, K., H.A. Pasolli, L. Alonso, L. Polak, and E. Fuchs. 2003. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell Biol. 163:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak, K., N. Stokes, J. de la Cruz, L. Polak, and E. Fuchs. 2007. Loss of a quiescent niche but not follicle stem cells in the absence of BMP signaling. Proc. Natl. Acad. Sci. USA. 104:10063–10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, M.I., D. Dai, B. Marinari, Y. Sano, A. Costanzo, M. Karin, and D.R. Roop. 2007. p63 induces key target genes required for epidermal morphogenesis. Proc. Natl. Acad. Sci. USA. 104:3255–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala, J., M. Mikkola, T. Mustonen, T. Aberg, P. Koppinen, J. Pispa, P. Nieminen, J. Galceran, R. Grosschedl, and I. Thesleff. 2001. TNF signaling via the ligand-receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Dev. Biol. 229:443–455. [DOI] [PubMed] [Google Scholar]
- Laurikkala, J., M.L. Mikkola, M. James, M. Tummers, A.A. Mills, and I. Thesleff. 2006. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 133:1553–1563. [DOI] [PubMed] [Google Scholar]
- Lavker, R.M., and T.T. Sun. 1982. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science. 215:1239–1241. [DOI] [PubMed] [Google Scholar]
- Lechler, T., and E. Fuchs. 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 437:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., J.M. Basak, S. Demehri, and R. Kopan. 2007. Bi-compartmental communication contributes to the opposite proliferative behavior of Notch1-deficient hair follicle and epidermal keratinocytes. Development. 134:2795–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, V., C. Lindon, B.D. Harfe, and B.A. Morgan. 2005. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell. 9:855–861. [DOI] [PubMed] [Google Scholar]
- Levy, V., C. Lindon, Y. Zheng, B.D. Harfe, and B.A. Morgan. 2007. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 21:1358–1366. [DOI] [PubMed] [Google Scholar]
- Lo Celso, C., D.M. Prowse, and F.M. Watt. 2004. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 131:1787–1799. [DOI] [PubMed] [Google Scholar]
- Lorenz, K., C. Grashoff, R. Torka, T. Sakai, L. Langbein, W. Bloch, M. Aumailley, and R. Fassler. 2007. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J. Cell Biol. 177:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry, W.E., C. Blanpain, J.A. Nowak, G. Guasch, L. Lewis, and E. Fuchs. 2005. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19:1596–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie, I.C. 1997. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 109:377–383. [DOI] [PubMed] [Google Scholar]
- Manohar, A., S.G. Shome, J. Lamar, L. Stirling, V. Iyer, K. Pumiglia, and C.M. DiPersio. 2004. Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J. Cell Sci. 117:4043–4054. [DOI] [PubMed] [Google Scholar]
- Massague, J. 2007. Sorting out breast-cancer gene signatures. N. Engl. J. Med. 356:294–297. [DOI] [PubMed] [Google Scholar]
- McLean, G.W., N.H. Komiyama, B. Serrels, H. Asano, L. Reynolds, F. Conti, K. Hodivala-Dilke, D. Metzger, P. Chambon, S.G. Grant, and M.C. Frame. 2004. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18:2998–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, B.J., U. Gat, R. DasGupta, and E. Fuchs. 2001. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15:1688–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, S.E. 2002. Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118:216–225. [DOI] [PubMed] [Google Scholar]
- Mills, A.A., B. Zheng, X.J. Wang, H. Vogel, D.R. Roop, and A. Bradley. 1999. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 398:708–713. [DOI] [PubMed] [Google Scholar]
- Morgan, B.A., R.W. Orkin, S. Noramly, and A. Perez. 1998. Stage-specific effects of sonic hedgehog expression in the epidermis. Dev. Biol. 201:1–12. [DOI] [PubMed] [Google Scholar]
- Morris, R.J., Y. Liu, L. Marles, Z. Yang, C. Trempus, S. Li, J.S. Lin, J.A. Sawicki, and G. Cotsarelis. 2004. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 22:411–417. [DOI] [PubMed] [Google Scholar]
- Muller-Rover, S., B. Handjiski, C. van der Veen, S. Eichmuller, K. Foitzik, I.A. McKay, K.S. Stenn, and R. Paus. 2001. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117:3–15. [DOI] [PubMed] [Google Scholar]
- Nair, M., A. Teng, V. Bilanchone, A. Agrawal, B. Li, and X. Dai. 2006. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J. Cell Biol. 173:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, B.C., K. Lefort, A. Mandinova, D. Antonini, V. Devgan, G. Della Gatta, M.I. Koster, Z. Zhang, J. Wang, A. Tommasi di Vignano, et al. 2006. a. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 20:1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, H., M. Rendl, and E. Fuchs. 2006. b. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 127:171–183. [DOI] [PubMed] [Google Scholar]
- Niemann, C., D.M. Owens, J. Hulsken, W. Birchmeier, and F.M. Watt. 2002. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 129:95–109. [DOI] [PubMed] [Google Scholar]
- Nishikawa, S.I., and M. Osawa. 2006. What is a stem cell niche? Ernst Schering Res. Found. Workshop. 1–14. [DOI] [PubMed]
- Oro, A.E., and K. Higgins. 2003. Hair cycle regulation of Hedgehog signal reception. Dev. Biol. 255:238–248. [DOI] [PubMed] [Google Scholar]
- Oro, A.E., K.M. Higgins, Z. Hu, J.M. Bonifas, E.H. Epstein Jr., and M.P. Scott. 1997. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 276:817–821. [DOI] [PubMed] [Google Scholar]
- Oshima, H., A. Rochat, C. Kedzia, K. Kobayashi, and Y. Barrandon. 2001. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 104:233–245. [DOI] [PubMed] [Google Scholar]
- Owens, D.M., and F.M. Watt. 2003. Contribution of stem cells and differentiated cells to epidermal tumours. Nat. Rev. Cancer. 3:444–451. [DOI] [PubMed] [Google Scholar]
- Pan, Y., M.H. Lin, X. Tian, H.T. Cheng, T. Gridley, J. Shen, and R. Kopan. 2004. gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev. Cell. 7:731–743. [DOI] [PubMed] [Google Scholar]
- Potten, C.S. 1974. The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet. 7:77–88. [DOI] [PubMed] [Google Scholar]
- Pummila, M., I. Fliniaux, R. Jaatinen, M.J. James, J. Laurikkala, P. Schneider, I. Thesleff, and M.L. Mikkola. 2007. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 134:117–125. [DOI] [PubMed] [Google Scholar]
- Raghavan, S., A. Vaezi, and E. Fuchs. 2003. A role for alphabeta1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev. Cell. 5:415–427. [DOI] [PubMed] [Google Scholar]
- Rangarajan, A., C. Talora, R. Okuyama, M. Nicolas, C. Mammucari, H. Oh, J.C. Aster, S. Krishna, D. Metzger, P. Chambon, et al. 2001. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20:3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, S., T. Andl, A. Bagasra, M.M. Lu, D.J. Epstein, E.E. Morrisey, and S.E. Millar. 2001. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107:69–82. [DOI] [PubMed] [Google Scholar]
- Reynolds, A.B. 2007. p120-catenin: past and present. Biochim. Biophys. Acta. 1773:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, H., L. Polak, and E. Fuchs. 2006. Lhx2 maintains stem cells character in hair follicles. Science. 312:1946–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich, R., and R. Paus. 2005. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 27:247–261. [DOI] [PubMed] [Google Scholar]
- Schober, M., S. Raghavan, M. Nikolova, L. Polak, H.A. Pasolli, H.E. Beggs, L.F. Reichardt, and E. Fuchs. 2007. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176:667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J.A., and A.S. Yap. 2006. Cinderella no longer: alpha-catenin steps out of cadherin's shadow. J. Cell Sci. 119:4599–4605. [DOI] [PubMed] [Google Scholar]
- Senoo, M., F. Pinto, C.P. Crum, and F. McKeon. 2007. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 129:523–536. [DOI] [PubMed] [Google Scholar]
- Sibilia, M., A. Fleischmann, A. Behrens, L. Stingl, J. Carroll, F.M. Watt, J. Schlessinger, and E.F. Wagner. 2000. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 102:211–220. [DOI] [PubMed] [Google Scholar]
- St-Jacques, B., H.R. Dassule, I. Karavanova, V.A. Botchkarev, J. Li, P.S. Danielian, J.A. McMahon, P.M. Lewis, R. Paus, and A.P. McMahon. 1998. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 8:1058–1068. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y., H. Miura, A. Tanemura, K. Kobayashi, G. Kondoh, S. Sano, K. Ozawa, S. Inui, A. Nakata, T. Takagi, et al. 2002. Targeted disruption of LIG-1 gene results in psoriasiform epidermal hyperplasia. FEBS Lett. 521:67–71. [DOI] [PubMed] [Google Scholar]
- Tiede, S., J.E. Kloepper, E. Bodò, S. Tiwari, C. Kruse, and R. Paus. 2007. Hair follicle stem cells: walking the maze. Eur. J. Cell Biol. 86:355–376. [DOI] [PubMed] [Google Scholar]
- Truong, A.B., and P.A. Khavari. 2007. Control of keratinocyte proliferation and differentiation by p63. Cell Cycle. 6:295–299. [DOI] [PubMed] [Google Scholar]
- Truong, A.B., M. Kretz, T.W. Ridky, R. Kimmel, and P.A. Khavari. 2006. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 20:3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar, T., G. Guasch, V. Greco, C. Blanpain, W.E. Lowry, M. Rendl, and E. Fuchs. 2004. Defining the epithelial stem cell niche in skin. Science. 303:359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen, C., R.M. Okamura, I. Farinas, R.G. Quo, T.G. Parslow, L. Bruhn, and R. Grosschedl. 1994. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8:2691–2703. [DOI] [PubMed] [Google Scholar]
- Van Mater, D., F.T. Kolligs, A.A. Dlugosz, and E.R. Fearon. 2003. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 17:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauclair, S., M. Nicolas, Y. Barrandon, and F. Radtke. 2005. Notch1 is essential for postnatal hair follicle development and homeostasis. Dev. Biol. 284:184–193. [DOI] [PubMed] [Google Scholar]
- Vidal, V.P., M.C. Chaboissier, S. Lutzkendorf, G. Cotsarelis, P. Mill, C.C. Hui, N. Ortonne, J.P. Ortonne, and A. Schedl. 2005. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15:1340–1351. [DOI] [PubMed] [Google Scholar]
- Waikel, R.L., Y. Kawachi, P.A. Waikel, X.J. Wang, and D.R. Roop. 2001. Deregulated expression of c-Myc depletes epidermal stem cells. Nat. Genet. 28:165–168. [DOI] [PubMed] [Google Scholar]
- Waters, J.M., G.D. Richardson, and C.A. Jahoda. 2007. Hair follicle stem cells. Semin. Cell Dev. Biol. 18:245–254. [DOI] [PubMed] [Google Scholar]
- Watt, F.M., and B.L. Hogan. 2000. Out of Eden: stem cells and their niches. Science. 287:1427–1430. [DOI] [PubMed] [Google Scholar]
- Watt, F.M., D.L. Mattey, and D.R. Garrod. 1984. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J. Cell Biol. 99:2211–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen, K., S.H. Litjens, I. Kuikman, C. Margadant, J. van Rheenen, and A. Sonnenberg. 2007. Serine phosphorylation of the integrin beta4 subunit is necessary for epidermal growth factor receptor-induced hemidesmosome disruption. Mol. Biol. Cell. 18:3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M., M. Shirai, K. Sugita, N. Nagai, Y. Miura, R. Mogi, K. Yamamoto, A. Tamura, and K. Arishima. 2003. Effects of maternal exposure to diethylstilbestrol on the development of the reproductive system and thyroid function in male and female rat offspring. J. Toxicol. Sci. 28:385–394. [DOI] [PubMed] [Google Scholar]
- Yang, A., R. Schweitzer, D. Sun, M. Kaghad, N. Walker, R.T. Bronson, C. Tabin, A. Sharpe, D. Caput, C. Crum, and F. McKeon. 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 398:714–718. [DOI] [PubMed] [Google Scholar]
- Yu, F., C.T. Kuo, and Y.N. Jan. 2006. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 51:13–20. [DOI] [PubMed] [Google Scholar]
- Yuhki, M., M. Yamada, M. Kawano, T. Iwasato, S. Itohara, H. Yoshida, M. Ogawa, and Y. Mishina. 2004. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 131:1825–1833. [DOI] [PubMed] [Google Scholar]
- Zhang, J., X.C. He, W.G. Tong, T. Johnson, L.M. Wiedemann, Y. Mishina, J.Q. Feng, and L. Li. 2006. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 24:2826–2839. [DOI] [PubMed] [Google Scholar]
- Zhou, P., C. Byrne, J. Jacobs, and E. Fuchs. 1995. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9:700–713. [DOI] [PubMed] [Google Scholar]