Abstract
Intraflagellar transport (IFT), the motor-dependent movement of IFT particles along the axoneme, is critical for the assembly, maintenance, and function of motile and sensory cilia, and, consequently, this process underlies ciliary motility, cilium-based signaling, and ciliopathies. Here, I present my perspective on IFT as a model system for studying motor-driven cargo transport. I review evidence that kinesin-2 motors physically transport IFT particles as cargo and hypothesize that several accessory kinesins confer cilia-specific functions by augmenting the action of the two core IFT motors, kinesin-2 and dynein 1b, which assemble the cilium foundation.
The intraflagellar transport machinery and cilium biogenesis
The recognition that cilia are ubiquitous structures that have critical cellular and developmental functions has generated renewed interest in these organelles and in the mechanism of their assembly by intraflagellar transport (IFT). The discovery of IFT was the culmination of a long, careful series of studies that exploited the Chlamydomonas flagellum (herein called a cilium) as a model system for studying the biogenesis of subcellular organelles (for review see Rosenbaum et al., 1999). The assembly of the cilium was found to depend on the addition of precursors onto the distal tip of the growing axoneme, suggesting that these building blocks must be moved in the anterograde direction from their site of synthesis in the cell body to the microtubule (MT) plus ends at the cilium tip (Johnson and Rosenbaum, 1992). Subsequent work suggested that the precursors are transported in association with electron-dense multimeric protein complexes called IFT particles that move bidirectionally along the axoneme at rates of the order of a micrometer per second (Kozminski et al., 1993; Cole et al., 1998). The IFT particles consist of two subcomplexes, IFTA (linked to retrograde transport) and IFTB (linked to anterograde transport), which furnish multiple sites for binding the large number of axoneme, matrix, and membrane proteins that must be delivered to the distal tip of the cilium, and they may be evolutionally related to the protein coats of intracellular transport vesicles (Cole et al., 1998; Piperno et al., 1998;Iomini et al., 2001; Lucker et al., 2005; Jekely and Arendt, 2006). Exciting recent advances have linked IFT not only with the delivery of ciliary components required for the assembly, maintenance, and length control of motile and sensory cilia but also for carrying cilium-based signals that control cell function, gene expression, cell division, animal development, and the onset of some human diseases (Bisgrove and Yost, 2006; Scholey and Anderson, 2006; Pan and Snell, 2007; Wemmer and Marshall, 2007). Given the important biological functions of IFT, we need to develop a precise understanding of how IFT particles and their associated proteins are moved as cargo along the cilium.
Motors required for IFT and ciliogenesis: the basic mechanism of bidirectional IFT
Two types of MT-based motors, kinesin-2 and dynein 1b, are proposed to drive the anterograde and retrograde movement of IFT particles along the axoneme, respectively (Fig. 1 a). Heterotrimeric kinesin-2 was first purified from echinoderm egg/embryo cytosol as a plus end–directed MT motor consisting of two heterodimerized kinesin-related motor subunits and an accessory subunit, termed kinesin-2–associated protein (KAP) (Cole et al., 1993; Wedaman et al., 1996). This motor emerged as a candidate anterograde IFT motor based on three parallel lines of research: in sea urchin embryos, disrupting kinesin-2 function inhibited the assembly of motile cilia at the blastula stage; in vertebrate embryos, it inhibited the formation of nodal cilia that promote nodal flow and left-right asymmetry; and in Chlamydomonas, it disrupted the transport of IFT particles in both directions along cilia (Kozminski et al., 1995; Morris and Scholey, 1997; Cole et al., 1998; Nonaka et al., 1998). On the other hand, cytoplasmic dynein 1b was identified as a dynein heavy chain that is up-regulated before ciliary assembly in sea urchin embryos (Gibbons et al., 1994). Its inhibition led to the formation of swollen, stunted cilia containing large quantities of accumulated IFT particles in Chlamydomonas and Caenorhabditis elegans, which is consistent with a role for dynein 1b in the retrograde transport of IFT particles from the cilium tip back to the cell body (Pazour et al., 1999; Porter et al., 1999; Signor et al., 1999). Several accessory subunits associate with the dhc1b heavy chain, including light chains (LC8), light intermediate chains (D1bLIC), and a novel putative intermediate chain, FAP133, of unknown function that may participate in IFT along motile but not sensory cilia (Perrone et al., 2003; Schafer et al., 2003; Pfister et al., 2006; Rompolas et al., 2007). Although it is reasonable to assume that dynein 1b is a minus end–directed MT motor, its in vitro motility properties have not yet been characterized. Collectively, the data strongly support the hypothesis that kinesin-2 and dynein 1b are required for the bidirectional transport of IFT particles along cilia.
Figure 1.
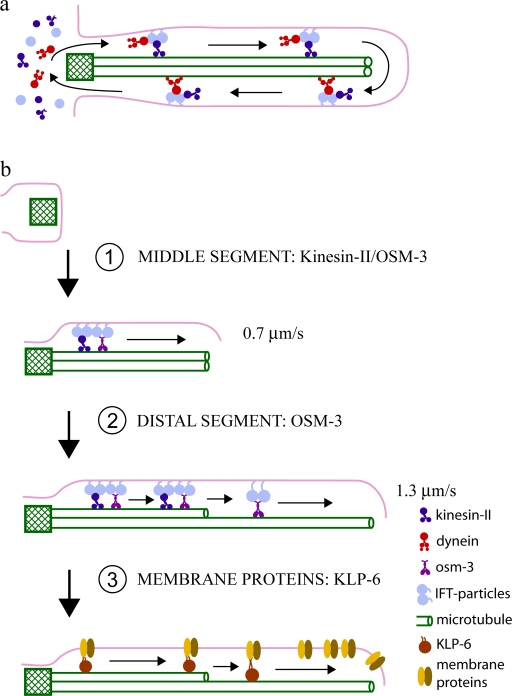
Pathways of IFT. (a) The canonical pathway of IFT. Heterotrimeric kinesin-2 moves IFT particles, ciliary precursors, and dynein 1b from the basal body to the distal tip of the cilium, where cargo unloading, motor switching, and turnaround occurs. Then, dynein 1b moves kinesin-2, turnover products, and IFT particles back to the cell body. This basic pathway is thought to build the core of the cilium in organisms such as Chlamydomonas and in C. elegans osm-3 mutants (Table I). (b) Anterograde transport pathways can be augmented by accessory kinesins. For example, in C. elegans sensory cilia, this produces sequential pathways of IFT. First, the concerted action of two members of the kinesin-2 family, kinesin-II and OSM-3, transports IFT particles along the middle segment of the axoneme in a process required to build the middle segment (corresponding to the cilium core). Second, OSM-3 alone then moves along the distal singlets, building and maintaining the distal segment as it goes. Finally, the kinesin-3 KLP-6 is required to target membrane proteins to the ciliary membrane, where they may be moved by KLP-6 itself and/or by other motors. Other accessory kinesins may target other ciliary components to the cilium, control ciliary length, or control ciliary motility, and the functional modulation of these motors may contribute to ciliary diversity.
Kinesin-2 as an anterograde IFT particle transporter in Chlamydomonas: Fla'd evidence?
Heterotrimeric kinesin-2 is an attractive candidate for moving IFT particles as cargo from the base to the tip of the axoneme based on the anterograde MT motility driven by the purified motor (Cole et al., 1993) combined with its loss of function phenotype (Kozminski et al., 1995), but it is difficult to prove that it does so. For example, in Chlamydomonas, fla10 and fla8 (also known as fla1) genes encode the motor subunits of kinesin-2, whereas fla3 encodes the KAP subunit, and mutations in these genes are associated with changes in the rate, frequency, and processivity of anterograde IFT, ultimately leading to its cessation (Kozminski et al., 1995; Iomini et al., 2001; Miller et al., 2005; Mueller et al., 2005). These observations demonstrate that kinesin-2 is required for IFT and ciliogenesis, and they are consistent with the widely accepted hypothesis that kinesin-2 drives the anterograde transport of IFT particles (Fig. 1 a; for review see Rosenbaum et al., 1999), but they certainly do not prove it. For example, heterotrimeric kinesin-2 purified from phylogenetically diverse organisms drives anterograde motility at only 0.3–0.6 μm/s, whereas IFT particles move significantly faster from the base to the tip of the Chlamydomonas cilium at 2 μm/s (Cole et al., 1993; Kozminski et al., 1993). If Chlamydomonas kinesin-2 on its own drives the anterograde transport of IFT particles, it must be atypically fast, or perhaps its velocity is enhanced by factors present in cilia.
Although this idea is plausible, in the absence of data on the motility properties of purified Chlamydomonas kinesin-2 or on the rate at which it moves along the axoneme in vivo, I think that the published data from Chlamydomonas could be interpreted differently. For example, careful analysis of KAP mutants reveals severe defects in the localization of kinesin-2 around the basal body where the IFT machinery normally enters the cilium (Mueller et al., 2005). In addition, its cilia contain abnormally large anterograde IFT particles that move along the axoneme at reduced frequencies (0.5 s−1 compared with 1.3 s−1 in controls), move at modestly reduced speeds (1.6 μm/s compared with 1.8 μm/s in controls), and pause more often than in control cilia (Mueller et al., 2005). An alternative interpretation of these results is that kinesin-2 serves to load IFT particles into the base of the cilium, where they, together with kinesin-2 motors, are moved as cargo along the axoneme by a faster kinesin so that defects in the kinesin-2–dependent loading process in KAP mutants give rise to the lower frequency of transport events observed. Moreover, the abnormally large IFT particles present in KAP mutants could be subject to increased drag forces or steric hindrance caused by obstacles they encounter as they move from the base to the tip of the axoneme, causing them to pause and move more slowly than smaller, wild-type IFT particles. Thus, more information is needed to determine the precise role of kinesin-2 motors in anterograde IFT, and such information has now emerged from studies done mainly in C. elegans (discussed in the following sections). This information provides compelling evidence that kinesin-2 does indeed transport IFT particles anterogradely along the axoneme and also suggests that accessory kinesins can sometimes be deployed to play important roles in modulating IFT and ciliogenesis.
Ciliary kinesins
Cilia contain multiple kinesins in addition to heterotrimeric kinesin-2 (Fox et al., 1994), some of which contribute to cilium biogenesis. A homodimeric form of kinesin-2 (OSM-3) contributes to axoneme assembly in C. elegans (see next paragraph), and its close relative, KIF17, serves to target cyclic nucleotide–gated channels to mammalian primary cilia (Jenkins et al., 2006), but it is not yet known whether KIF17, like OSM-3 (Snow et al., 2004), also participates directly in IFT. A member of the kinesin-3 family, KLP-6 in C. elegans, was found to be required for the ciliary targeting of the polycystins, which form mechanosensory ion channels in the membranes of cilia on male-specific sensory neurons involved in mating (Peden and Barr, 2005). Such membrane proteins are proposed to be moved in the plane of the ciliary membrane by the IFT machinery, but the motor involved and the function of this transport remain unclear (Qin et al., 2005). In Giardia and Leishmania, the MT depolymerase kinesin-13 is proposed to localize to the distal tips of ciliary axonemes, where it may depolymerize axonemal MTs and cooperate with the IFT machinery to control the length of the cilium (Blaineau et al., 2007; Dawson et al., 2007). In Chlamydomonas, a member of the kinesin-9 family, whose in vitro motility properties remain poorly characterized, localizes to the C2 central pair MT specific to motile cilia, and its depletion by RNA inhibition leads to slow swimming as a result of a decrease in the ciliary beat frequency (from 60 to 36 Hz) or to a complete loss of swimming motility as a result of ciliary paralysis (Yokoyama et al., 2004). Thus, this kinesin-9 motor may interact with the radial spokes located between the central pair and outer doublets to control dynein-dependent axoneme beating (Yokoyama et al., 2004). It will be interesting to determine whether these kinesin-13 and -9 motors are delivered to the ciliary tips and central pair MTs by the IFT machinery. Interestingly, a calmodulin-binding kinesin-14 concentrates around the Chlamydomonas basal body and also localizes to cytoplasmic MTs and ciliary membrane matrix fractions (Dymek et al., 2006). Because kinesin-14 motors are minus end directed, this protein could function in retrograde IFT or some other form of ciliary membrane–associated motility, or it could transport components of the IFT machinery along cytoplasmic MTs to the base of the cilium, where it concentrates before entering the cilium. Functional data are currently lacking, however. Finally, phylogenetic analyses have implicated two new families of kinesin motors in ciliary functions, namely kinesin-16 and -17, but their biological roles have so far not been reported (Wickstead and Gull, 2006).
Among these kinesins, the function of C. elegans OSM-3 has been studied using fluorescence microscopy of living animals expressing ciliary markers combined with serial section EM and 3D reconstructions of cilia. The results suggest that this homodimeric kinesin-2 cooperates with a heterotrimeric kinesin-2, named kinesin-II, to assemble cilia on the dendritic endings of chemosensory neurons (Perkins et al., 1986; Snow et al., 2004; Evans et al., 2006). In wild-type animals, these cilia project from a modified basal body called a transition zone and contain axonemes organized into two domains: the middle segment consisting of nine doublet MTs forming the cilium core, from which extend the distal segments consisting of nine singlet extensions of the middle segment A tubules (note that such distal singlets are also found elsewhere [for example, in frog olfactory cilia and Chlamydomonas cilia during mating] and may provide a domain that accumulates membrane-bound molecules required for cilium-based signaling; Reese, 1965; Flannery et al., 2006; Scholey and Anderson, 2006). Significantly, klp-11 or kap-1 mutants lacking the function of kinesin-II have full-length cilia, and osm-3 mutants lacking the function of OSM-3 have intact middle segments but no distal segments. However, in klp-11; osm-3 or kap-1; osm-3 double mutants lacking the function of both motors, the entire ciliary axoneme is missing. Therefore, OSM-3 and kinesin-II function redundantly to assemble the middle segment of these axonemes, with either motor being able to do the job in the absence of the other motor, and OSM-3 alone specifically extends the distal segments (Snow et al., 2004; Evans et al., 2006). As discussed in the following section, to accomplish this step-wise assembly mechanism, kinesin-II and OSM-3 cooperate in a surprising way to form two sequential pathways of IFT (Fig. 1 b).
Kinesin-2 motors physically move IFT particles as cargo along the cilium
The finding that two forms of kinesin-2 build sensory cilia on C. elegans neurons was fortuitous in the sense that it allowed a decisive test of the hypothesis that kinesin-2 motors drive the anterograde transport of IFT particles (for review see Rosenbaum et al., 1999). This exploited a combination of in vitro motility assays of purified motors together with in vivo transport assays of IFT in mutants defective in either kinesin-II or OSM-3 function (Table I; Orozco et al., 1999; Snow et al., 2004; Pan et al., 2006). Under appropriate conditions, purified kinesin-II drives plus end–directed MT motility at a rate of ∼0.4 μm/s in vitro. In living animals, both GFP-tagged kinesin-II and IFT particles move at a similar velocity along the intact middle segments of cilia in mutants lacking OSM-3 function, but transport is blocked after the additional loss of kinesin-II function (in osm-3; kap-1 or osm-3; klp-11 double mutants). Conversely, purified OSM-3 drives MT motility at ∼1.1 μm/s, a similar speed to that of tagged OSM-3 and IFT particles along the full length of the cilium of animals lacking kinesin-II function, and this in vivo transport stops after the loss of OSM-3 function in the double mutants. Finally, in competitive MT gliding assays performed in the presence of varying molar ratios of kinesin-II/OSM-3 at a mole fraction of 0.6–0.8 OSM-3, MTs were moved at an intermediate speed of 0.7 μm/s. This rate is similar to the rate of movement of kinesin-II, OSM-3, and IFT particles along the middle segments of wild-type cilia, where both motors apparently act together in a concerted fashion to move the same IFT particles along the axoneme (Fig. 1 b). The data support the hypothesis that kinesin-2 motors do indeed move IFT particles as cargo from the base to the tip of the cilium (Table I; Snow et al., 2004; Pan et al., 2006) and provide among the best available evidence documenting the intracellular transport of a specific cargo by a specific motor. This underscores the usefulness of IFT as a model system for studying motor-cargo transport.
Table I. Kinesin-2 motors transport IFT particles as cargo anterogradely along the cilium.
| IFT motor activity | Pure motor | Motor in cilium | IFT particles | Assembly of: |
|---|---|---|---|---|
| μm/s | μm/s | μm/s | ||
| Kinesin-II (osm-3) | 0.4 | 0.5 | 0.5 | Middle segment |
| OSM-3 (klp-11 or kap-1) | 1.1 | 1.3 | 1.2 | Full-length cilium/distal segment |
| Kinesin-II and OSM-3 | 0.7 | 0.7 | 0.7 | Middle segment (redundant) |
Results of in vivo transport assays performed in C. elegans sensory cilia combined with in vitro motility assays of purified kinesin-II and OSM-3. In the first column, the italicized text in parentheses refers to the mutant background for the in vivo assay of individual motor activity.
The logic of the system: core IFT motors and accessory kinesins?
The results raise the obvious question “why use more than one motor?” We hypothesize that heterotrimeric kinesin-2 and cytoplasmic dynein 1b are core IFT motors that assemble and maintain the cilium foundation, whereas the other ciliary kinesins (e.g., OSM-3) are accessory motors that confer cilia-specific functions. The accessory motors may function to modulate IFT itself, they may target specific proteins to cilia, or they may function as stable components of the cilium itself. For example, in wild-type C. elegans, sensory cilia are assembled by two members of the kinesin-2 family, the core motor kinesin-II and the accessory kinesin OSM-3 (Fig. 1 b), whereas osm-3 mutants resemble Chlamydomonas, Tetrahymena, Drosophila, sea urchin, and mouse, in which disrupting the function of heterotrimeric kinesin-2 alone leads to the loss of cilia (Morris and Scholey, 1997; Cole et al., 1998; Nonaka et al., 1998; Brown et al., 1999; Sarpal et al., 2003). The peculiar feature of C. elegans is that in klp-11 and kap-1 single mutants lacking kinesin-II function, OSM-3 alone appears capable of building the full-length cilium (Snow et al., 2004; Evans et al., 2006). Thus, in wild types, both motors function redundantly to carry out the important task of building the cilium foundation, which can form after the loss of either kinesin-II or OSM-3. Subsequently, the accessory motor OSM-3 alone specifically extends the distal singlets to confer cilia-specific chemosensory functions (i.e., osmotic avoidance).
Why are two motors used to carry the same IFT particles at speeds intermediate between the unloaded velocity of either motor alone rather than using each motor to move its own cargo independently (Snow et al., 2004; Pan et al., 2006)? One possibility is that the actual speed of transport is somehow important (e.g., for controlling ciliary length). IFT particles are moved along wild-type cilia at a mean speed of 0.93 μm/s, yet these cilia have the same length as cilia in mutants lacking kinesin-II function, in which IFT particles are moved at the faster speed of 1.3 μm/s by OSM-3 alone. This supports the hypothesis that other factors (e.g., the frequency of IFT or the transport of specific length-regulating cargoes) may be more important than the actual rate of IFT (Wemmer and Marshall, 2007). Another possibility is that this mechanism for building the middle segment is an accident of natural selection and that early on in its evolution, OSM-3 was moved passively to the tip of the middle segment by kinesin-II–driven transport, where it takes over the transport of IFT particles required to build the distal tips. This is pure speculation though, and it remains puzzling why kinesin-II is used at all if OSM-3 on its own can build a full-length cilium. However, one possible advantage of using two motors has emerged because the functional modulation of kinesin-II and OSM-3 appears to be capable of contributing to the diversity of structurally and functionally distinct cilia that sense chemicals, mechanical stimuli, and temperature in the C. elegans nervous system (Bae et al., 2006; Evans et al., 2006; Mukhopadhyay et al., 2007). For example, the work described above on IFT motors in C. elegans cilia applies specifically to the bundles of linear, amphid channel cilia that detect hydrophilic molecules, but, in the adjacent fan-shaped amphid wing cilia that detect volatile odorants, kinesin-II and OSM-3 redundantly build the cilium core consisting of MT doublets, but no distal singlets are assembled. Thus, OSM-3 extends distal singlets on some cilia but not others.
Accessory motors such as the kinesins-2, -3, -9, -13, -14, -16, and -17 listed above (see Ciliary kinesins section) may also cooperate with heterotrimeric kinesin-2 to confer distinct properties to functionally distinct cilia; for example, by controlling axoneme assembly, dynamics, and length, by inserting and moving membrane proteins along sensory cilia, or by modulating the beating of motile cilia (Yokoyama et al., 2004; Peden and Barr, 2005; Qin et al., 2005; Bae et al., 2006; Dymek et al., 2006; Jenkins et al., 2006; Blaineau et al., 2007; Dawson et al., 2007). In C. elegans male-specific sensory cilia, for example, the functional cooperation of kinesin-II, OSM-3, and KLP-6 could produce a sequence of transport events that build the middle segment, the distal segments, and finally insert membrane-bound channels for axial movement along the cilium (Fig. 1 b; Snow et al., 2004; Peden and Barr, 2005; Qin et al., 2005).
Regulators of IFT motors
Although progress has been made in elucidating the roles of kinesin-2, dynein 1b, and accessory kinesins in IFT and ciliogenesis, much needs to be learned about how the activity of these motors are regulated so as to generate the coherent pathways of bidirectional IFT that assemble and maintain functional cilia (Pedersen et al., 2006). Looking at the pathways presented in Fig. 1, it seems plausible that the functions of IFT motors are controlled at several points, including (1) the transport of IFT motors and cargo from the site of synthesis within the cell body to the base of the cilium, where the IFT machinery accumulates; (2) the entry of IFT motors and cargo into the cilium, the docking of IFT motors onto IFT particles, and their movement along the axoneme; (3) motor switching and turnaround at the tips of the axoneme (including both the middle and distal segments in C. elegans); (4) the loading and unloading of ciliary precursors and molecules involved in cilium-based signaling at the base and tips of the cilium; and (5) the modulation of IFT motor activity in different types of cilia to generate cilia with diverse structures and functions.
Research addressing the problem of IFT motor regulation has been initiated, but this remains a poorly understood aspect of IFT. For example, there exists evidence that both heterotrimeric and homodimeric kinesin-2 can exist in compact and extended conformations (Wedaman et al., 1996; Imanishi et al., 2006), which, based on in vitro ATPase and motility assays, are proposed to correspond to autoinhibited and active species, respectively (Imanishi et al., 2006). This suggests that the motors can be activated by factors that relieve autoinhibition, possibly IFT particle binding or posttranslational modifications. In the case of heterotrimeric kinesin-2, it is plausible to think that the KAP subunit (Wedaman et al., 1996), whose functions are poorly understood, plays a role in regulating motor activity, for example by targeting the motor to cilia, docking it onto IFT particles, or controlling its motor activity. In support of this idea, work in Chlamydomonas shows that KAP is required for the localization of kinesin-2 to the basal body and for processive transport along the axoneme (Mueller et al., 2005). Work done in C. elegans suggested that a complex of Bardet-Biedl syndrome proteins (the BBSome; Nachury et al., 2007) may contribute to the coordinate action of kinesin-II and OSM-3 by maintaining the association of IFT subcomplexes A and B, whereas a novel tetratrico repeat protein, DYF-1, is required to activate OSM-3, possibly by docking this motor onto IFT particles and relieving its autoinhibition (Ou et al., 2005). However, subsequent work suggests that a DYF-1 homologue in zebrafish, namely Fleer, serves to enhance tubulin polyglutamylation and MT doublet integrity, indicating that it may influence OSM-3 motility by modifying the motor's tracks (Pathak et al., 2007). Therefore, it might be informative to perform in vitro assays of rates of OSM-3 motility along MTs labeled with varying levels of polyE. Furthermore, in vertebrates, the BBSome cooperates with the GTPase Rab8 (which is one of several ciliogenesis-related G proteins and G-protein cofactors; Yoshimura et al., 2007) to promote ciliary membrane biogenesis (Nachury et al., 2007). Therefore, although these putative IFT motor cofactors move as components of the IFT machinery in C. elegans (Ou et al., 2005), further work is required to determine how DYF-1 and the BBSome contribute to IFT motor activity in different systems. Finally, the MT plus tip tracker, EB1, localizes to the ciliary tips, where it is proposed to control motor switching and turnaround of the IFT machinery (Pedersen et al., 2003; Sloboda and Howard, 2007). The MAPK DYF-5 may also contribute to this aspect of IFT by facilitating the dissociation of kinesin-2 motors from IFT particles (Burghoorn et al., 2007), but, again, further work is needed to determine the precise roles of EB1 and DYF-5.
Conclusions
The requirement of kinesin-2 and dynein 1b for IFT particle transport along the axoneme led to the proposal that these motors physically move IFT particles in the anterograde and retrograde direction, respectively (Fig. 1 a; for review see Rosenbaum et al., 1999). The use of in vivo transport assays of tagged IFT proteins combined with in vitro assays with purified kinesin-2 motors has now provided strong support for this hypothesis and further suggests that anterograde transport can be augmented by the activity of accessory kinesins to confer cilia-specific properties (Fig. 1 b; Pan et al., 2006). As a result of research on this topic by multiple laboratories, the anterograde transport of IFT particles by kinesin-2 motors along the axoneme now represents one of the best systems available for analyzing motor-driven cargo transport. Work performed so far should provide a useful foundation for future studies addressing the poorly understood yet fascinating problem of how the regulation of IFT motors generates orderly pathways of IFT to assemble cilia and, thus, to contribute to cell motility, sensory reception, signal transduction, the control of gene expression, cell behavior, animal development, and disease.
Acknowledgments
I thank Drs. Scott Dawson, Mel Thein, Guangshuo Ou, Gul Civelekoglu-Scholey, and the four anonymous reviewers for useful comments.
Work in my laboratory on IFT motors is supported by National Institutes of Health grant GM50718.
Abbreviations used in this paper: IFT, intraflagellar transport; KAP, kinesin-2–associated protein; MT, microtubule.
References
- Bae, Y.K., H. Qin, K.M. Knobel, J. Hu, J.L. Rosenbaum, and M.M. Barr. 2006. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 133:3859–3870. [DOI] [PubMed] [Google Scholar]
- Bisgrove, B.W., and H.J. Yost. 2006. The roles of cilia in developmental disorders and disease. Development. 133:4131–4143. [DOI] [PubMed] [Google Scholar]
- Blaineau, C., M. Tessier, P. Dubessay, L. Tasse, L. Crobu, M. Pages, and P. Bastien. 2007. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr. Biol. 17:778–782. [DOI] [PubMed] [Google Scholar]
- Brown, J.M., C. Marsala, R. Kosoy, and J. Gaertig. 1999. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol. Biol. Cell. 10:3081–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghoorn, J., M.P. Dekkers, S. Rademakers, T. de Jong, R. Willemsen, and G. Jansen. 2007. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 104:7157–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, D.G., S.W. Chinn, K.P. Wedaman, K. Hall, T. Vuong, and J.M. Scholey. 1993. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 366:268–270. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., D.R. Diener, A.L. Himelblau, P.L. Beech, J.C. Fuster, and J.L. Rosenbaum. 1998. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141:993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, S.C., M.S. Sagolla, J.J. Mancuso, D.J. Woessner, S.A. House, L. Fritz-Laylin, and W.Z. Cande. 2007. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis.Eukaryot. Cell. 6:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek, E.E., D. Goduti, T. Kramer, and E.F. Smith. 2006. A kinesin-like calmodulin-binding protein in Chlamydomonas: evidence for a role in cell division and flagellar functions. J. Cell Sci. 119:3107–3116. [DOI] [PubMed] [Google Scholar]
- Evans, J.E., J.J. Snow, A.L. Gunnarson, G. Ou, H. Stahlberg, K.L. McDonald, and J.M. Scholey. 2006. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J. Cell Biol. 172:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery, R.J., D.A. French, and S.J. Kleene. 2006. Clustering of cyclic-nucleotide-gated channels in olfactory cilia. Biophys. J. 91:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, L.A., K.E. Sawin, and W.S. Sale. 1994. Kinesin-related proteins in eukaryotic flagella. J. Cell Sci. 107:1545–1550. [DOI] [PubMed] [Google Scholar]
- Gibbons, B.H., D.J. Asai, W.J. Tang, T.S. Hays, and I.R. Gibbons. 1994. Phylogeny and expression of axonemal and cytoplasmic dynein genes in sea urchins. Mol. Biol. Cell. 5:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi, M., N.F. Endres, A. Gennerich, and R.D. Vale. 2006. Auto-inhibition regulates the motility of the C. elegans intraflagellar transport motor OSM-3. J Cell Biol. 174:931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini, C., V. Babaev-Khaimov, M. Sassaroli, and G. Piperno. 2001. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 153:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely, G., and D. Arendt. 2006. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 28:191–198. [DOI] [PubMed] [Google Scholar]
- Jenkins, P.M., T.W. Hurd, L. Zhang, D.P. McEwen, R.L. Brown, B. Margolis, K.J. Verhey, and J.R. Martens. 2006. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16:1211–1216. [DOI] [PubMed] [Google Scholar]
- Johnson, K.A., and J.L. Rosenbaum. 1992. Polarity of flagellar assembly in Chlamydomonas. J. Cell Biol. 119:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K.G., K.A. Johnson, P. Forscher, and J.L. Rosenbaum. 1993. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA. 90:5519–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K.G., P.L. Beech, and J.L. Rosenbaum. 1995. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131:1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker, B.F., R.H. Behal, H. Qin, L.C. Siron, W.D. Taggart, J.L. Rosenbaum, and D.G. Cole. 2005. Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J. Biol. Chem. 280:27688–27696. [DOI] [PubMed] [Google Scholar]
- Miller, M.S., J.M. Esparza, A.M. Lippa, F.G. Lux III, D.G. Cole, and S.K. Dutcher. 2005. Mutant kinesin-2 motor subunits increase chromosome loss. Mol. Biol. Cell. 16:3810–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, R.L., and J.M. Scholey. 1997. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J. Cell Biol. 138:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, J., C.A. Perrone, R. Bower, D.G. Cole, and M.E. Porter. 2005. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol. Biol. Cell. 16:1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, S., Y. Lu, H. Qin, A. Lanjuin, S. Shaham, and P. Sengupta. 2007. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 26:2966–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury, M.V., A.V. Loktev, Q. Zhang, C.J. Westlake, J. Peranen, A. Merdes, D.C. Slusarski, R.H. Scheller, J.F. Bazan, V.C. Sheffield, and P.K. Jackson. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 129:1201–1213. [DOI] [PubMed] [Google Scholar]
- Nonaka, S., Y. Tanaka, Y. Okada, S. Takeda, A. Harada, Y. Kanai, M. Kido, and N. Hirokawa. 1998. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 95:829–837. [DOI] [PubMed] [Google Scholar]
- Orozco, J.T., K.P. Wedaman, D. Signor, H. Brown, L. Rose, and J.M. Scholey. 1999. Movement of motor and cargo along cilia. Nature. 398:674. [DOI] [PubMed] [Google Scholar]
- Ou, G., O.E. Blacque, J.J. Snow, M.R. Leroux, and J.M. Scholey. 2005. Functional coordination of intraflagellar transport motors. Nature. 436:583–587. [DOI] [PubMed] [Google Scholar]
- Pan, J., and W. Snell. 2007. The primary cilium: keeper of the key to cell division. Cell. 129:1255–1257. [DOI] [PubMed] [Google Scholar]
- Pan, X., G. Ou, G. Civelekoglu-Scholey, O.E. Blacque, N.F. Endres, L. Tao, A. Mogilner, M.R. Leroux, R.D. Vale, and J.M. Scholey. 2006. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J. Cell Biol. 174:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak, N., T. Obara, S. Mangos, Y. Liu, and I.A. Drummond. 2007. The Zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol. Biol. Cell. 18:4353–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G.J., B.L. Dickert, and G.B. Witman. 1999. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden, E.M., and M.M. Barr. 2005. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr. Biol. 15:394–404. [DOI] [PubMed] [Google Scholar]
- Pedersen, L.B., S. Geimer, R.D. Sloboda, and J.L. Rosenbaum. 2003. The microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr. Biol. 13:1969–1974. [DOI] [PubMed] [Google Scholar]
- Pedersen, L.B., S. Geimer, and J.L. Rosenbaum. 2006. Dissecting the molecular mechanisms of intraflagellar transport in Chlamydomonas. Curr. Biol. 16:450–459. [DOI] [PubMed] [Google Scholar]
- Perkins, L.A., E.M. Hedgecock, J.N. Thomson, and J.G. Culotti. 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117:456–487. [DOI] [PubMed] [Google Scholar]
- Perrone, C.A., D. Tritschler, P. Taulman, R. Bower, B.K. Yoder, and M.E. Porter. 2003. A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in Chlamydomonas and mammalian cells. Mol. Biol. Cell. 14:2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, K.K., P.R. Shah, H. Hummerich, A. Russ, J. Cotton, A.A. Annuar, S.M. King, and E.M. Fisher. 2006. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno, G., E. Siuda, S. Henderson, M. Segil, H. Vaananen, and M. Sassaroli. 1998. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J. Cell Biol. 143:1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, M.E., R. Bower, J.A. Knott, P. Byrd, and W. Dentler. 1999. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol. Biol. Cell. 10:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, H., D.T. Burnette, Y.K. Bae, P. Forscher, M.M. Barr, and J.L. Rosenbaum. 2005. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15:1695–1699. [DOI] [PubMed] [Google Scholar]
- Reese, T.S. 1965. Olfactory cilia in the frog. J. Cell Biol. 25:209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas, P., L.B. Pedersen, R.S. Patel-King, and S.M. King. 2007. Chlamydomonas FAP133 is a dynein intermediate chain associated with the retrograde intraflagellar transport motor. J. Cell Sci. 120:3653–3665. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, J.L., D.G. Cole, and D.R. Diener. 1999. Intraflagellar transport: the eyes have it. J. Cell Biol. 144:385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal, R., S.V. Todi, E. Sivan-Loukianova, S. Shirolikar, N. Subramanian, E.C. Raff, J.W. Erickson, K. Ray, and D.F. Eberl. 2003. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr. Biol. 13:1687–1696. [DOI] [PubMed] [Google Scholar]
- Schafer, J.C., C.J. Haycraft, J.H. Thomas, B.K. Yoder, and P. Swoboda. 2003. XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans. Mol. Biol. Cell. 14:2057–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey, J.M., and K.V. Anderson. 2006. Intraflagellar transport and cilium-based signaling. Cell. 125:439–442. [DOI] [PubMed] [Google Scholar]
- Signor, D., K.P. Wedaman, J.T. Orozco, N.D. Dwyer, C.I. Bargmann, L.S. Rose, and J.M. Scholey. 1999. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda, R.D., and L. Howard. 2007. Localization of EB1, IFT polypeptides, and kinesin-2 in Chlamydomonas flagellar axonemes via immunogold scanning electron microscopy. Cell Motil. Cytoskeleton. 64:446–460. [DOI] [PubMed] [Google Scholar]
- Snow, J.J., G. Ou, A.L. Gunnarson, M.R. Walker, H.M. Zhou, I. Brust-Mascher, and J.M. Scholey. 2004. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6:1109–1113. [DOI] [PubMed] [Google Scholar]
- Wedaman, K.P., D.W. Meyer, D.J. Rashid, D.G. Cole, and J.M. Scholey. 1996. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP85/95) complex. J. Cell Biol. 132:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer, K.A., and W.F. Marshall. 2007. Flagellar length control in Chlamydomonas–paradigm for organelle size regulation. Int. Rev. Cytol. 260:175–212. [DOI] [PubMed] [Google Scholar]
- Wickstead, B., and K. Gull. 2006. A “holistic” kinesin phylogeny reveals new kinesin families and predicts protein functions. Mol. Biol. Cell. 17:1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, R., E. O'Toole, S. Ghosh, and D.R. Mitchell. 2004. Regulation of flagellar dynein activity by a central pair kinesin. Proc. Natl. Acad. Sci. USA. 101:17398–17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, S., J. Egerer, E. Fuchs, A.K. Haas, and F.A. Barr. 2007. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]