Abstract
Defects in cilia cause a broad spectrum of human diseases known collectively as the ciliopathies. Although all ciliopathies arise from defective cilia, the range of symptoms can vary significantly, and only a small subset of the possible ciliary disease symptoms may be present in any given syndrome. This complexity is puzzling until one realizes that the cilia are themselves exceedingly complex machines that perform multiple functions simultaneously, such that breaking one piece of the machine can leave some functions intact while destroying others. The clinical complexity of the ciliopathies can therefore only be understood in light of the basic cell biology of the cilia themselves, which I will discuss from the viewpoint of cell biological studies in model organisms.
The ciliopathies: one organelle, many diseases
Cilia are organelles that extend out from the cell surface and are composed of nine parallel microtubule doublets surrounded by an extension of the plasma membrane (Fig. 1). Cilia act as antennas to sense developmental signaling molecules (Eggenschwiler and Anderson, 2007) and physiological ligands (Christensen et al., 2007). Cilia also generate flows of mucus and cerebrospinal fluid and can act as mechanosensors and flow meters (Praetorius and Spring, 2003). The different functions of cilia are reflected in the structural diversity of cilia even within a single organism. For example, the dynein arms that power motile cilia are missing in purely sensory cilia. Sensory cilia themselves can attain a wide variety of structures; for example, in Caenorhabditis elegans, some sensory cilia have unusual fanlike shapes, whereas other sensory cilia in the same animal have more canonical structures (Perkins et al., 1986).
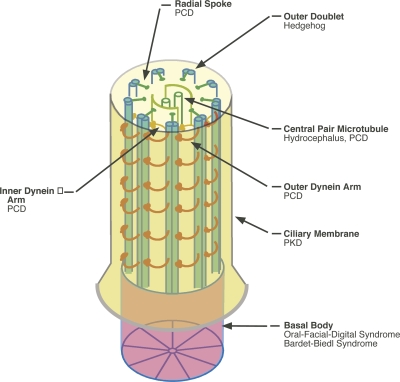
Figure 1.
The anatomy of the cilium. Cilia are arrays of nine microtubule doublets that extend from the basal body (a modified centriole) and push out an extension of the plasma membrane (called the ciliary membrane). Some cilia also contain a pair of singlet microtubules in the middle called the central pair that is involved in regulating motility. Motile power is provided by two sets of dynein arms: the inner and outer arms. Motility is regulated by radial spokes that interact with the central pair. Defects in any of these structures can lead to ciliary diseases, although the precise symptoms that arise appear to depend on which structure is altered.
Given the multiple roles of cilia in development and physiology, it is not surprising that defects in cilia cause multiple human diseases (Table I; for reviews see Afzelius, 2004; Badano et al., 2006; Fliegauf et al., 2007). Perhaps the most puzzling aspect of ciliopathies is that different ciliary diseases involve different, often partially overlapping sets of symptoms. For instance, Bardet-Biedl patients suffer from obesity, retinal degeneration, and cystic kidneys, whereas Oral-Facial-Digital syndrome patients suffer from polydactyly and cystic kidneys. However, both types of diseases result from defects in genes whose protein products localize to the ciliary basal body. Why don't all ciliary defects produce the same set of symptoms? The key to this question is to realize that despite the growing wealth of genomic and proteomic data on cilia composition (Inglis et al., 2006), cilia are not just lists of genes but complex organelles with variable ultrastructures that must assemble and function in different tissue contexts.
Table I. Human ciliary disease genes and their cell biological functions.
| Disease | Gene | Cellular function | Protein localization |
|---|---|---|---|
| PKD | PKD1 | Mechanosensing | Cilia |
| PKHD1 | Unknown | Cilia, basal body | |
| Immotile cilia syndrome | DNAH5 | Ciliary motility | Outer dynein arms |
| Bronchiectasis | DNAI1 | Ciliary motility | Outer dynein arms |
| Chronic sinusitis | |||
| Situs inversus | |||
| Bardet-Biedl syndrome | BBS1-12 | Ciliogenesis | Basal body and IFT complexes |
| Obesity | |||
| Retinal degeneration | |||
| Cystic kidneys | |||
| Meckel-Gruber syndrome | Cep290 | Unknown | Basal body |
| Brain malformation | MKS1 | Ciliogenesis | Basal body |
| Polydactyly | MKS3 | Ciliogenesis | Ciliary membrane |
| Cystic kidneys | |||
| Oral-Facial-Digital syndrome | OFD1 | Ciliogenesis | Basal body |
| Craniofacial abnormality | |||
| Polydactyly | |||
| Cystic kidneys | |||
| Nephronophthisis | NPHP1-5 | Uncertain | Basal body and cilia |
| Retinitis pigmentosa | RPGR | Retinal transport | Basal body |
| Situs inversus | DNAH11 | Ciliary motility | Dynein arms |
For reviews of human ciliopathies, see Afzelius, 2004; Badano et al., 2006; Fliegauf, 2007.
Why might defects in two cilia-related genes lead to distinct diseases? There are several ways this can happen: (1) some ciliary genes may have additional cilia-unrelated gene functions; (2) some mutations may affect the ciliogenesis of only a subset of all cilia in the body; and (3) genetic defects may affect different ultrastructural modules of cilia and, thus, only influence a subset of ciliary functions.
Genes with dual functions
Some genes involved in ciliary assembly or function may have additional functions unrelated to cilia so that when mutated, they cause a combination of cilia-related and -unrelated symptoms. One example is the huntingtin-interacting protein Hippi/IFT57, which appears to play roles in both ciliogenesis and apoptosis (Houde et al., 2006). Diseases or mutations altering Hippi function can thus affect both body symmetry (cilia related) as well as apoptotic stress response in the brain (presumably cilia unrelated). Similarly, many cell polarity proteins are required for ciliogenesis (Schermer et al., 2006; Fan et al., 2007), so mutations in such genes might cause ciliary defects in combination with other polarity-related phenotypes. Even proteins that play very specific roles in ciliary function may have additional functions. For example, the intraflagellar transport (IFT) motor KIF3 also functions in axonal transport (Kondo et al., 1994). Similarly, GAS11 is a component of the dynein regulatory complex, a protein complex attached to the outer doublets that regulates dynein arm activity, but it also localizes to the Golgi, where it may mediate Golgi–microtubule interactions (Colantonio et al., 2006). These results should sound a cautionary note every time one sees the phrase cilia specific.
Differences in mechanisms of ciliogenesis
Studies in model organisms such as C. elegans have shown that not all cilia are the same in terms of the machinery needed to build and maintain them. It is known that in some cell types, cilia microtubules undergo ongoing turnover (Stephens, 1997), whereas in others, the axonemal microtubules appear to be much less dynamic. Therefore, the degree to which the machinery of ciliogenesis, including IFT (see Scholey on p. 23 of this issue), is required in these different types of cilia will clearly differ, with more dynamic cilia requiring a higher efficacy of continual assembly. Such cilia would be the first to go in a disease mutation that partially reduced IFT. Therefore, hypomorphic alleles of ciliogenesis genes might cause defects in only a subset of cilia, leading to an overall phenotype that differs from that of a null mutant.
There are also clearly different requirements for the assembly of cilia in different cell types within a single organism. In C. elegans, the different sensory cilia have dramatically different morphologies, with some having elaborate branched or fanlike structures and others having more canonical cylindrical cilia shapes. In addition to these structural differences, the different types of cilia also differ in their requirement for different parts of the IFT machinery. Two different kinesins, kinesin-II and OSM-3, normally cooperate to build sensory cilia, with OSM-3 specifically required to build the distal half of the cilium, which contains only singlet microtubules and is presumably specialized for sensory functions (Ou et al., 2005). Interestingly, in the cilia of the AWB neurons, the two motors are no longer coupled, and, unlike in the other ciliary types previously analyzed, OSM-3 is no longer required to build the distal segment (Mukhopadhyay et al., 2007). In an even more extreme case, some ciliary structures such as sperm flagella in Drosophila melanogaster do not require IFT at all for their assembly (Han et al., 2003). In diatoms, the lack of retrograde IFT motor along with IFT complex A and Bardet-Biedl syndrome proteins (Scholey, 2008) from the genome suggests that these parts of the IFT systems are dispensable in some cases (Merchant et al., 2007). If a similar variability in the requirement for IFT is seen between different cell and tissue types in humans, one could imagine that genetic defects in different components of the IFT machinery might have more severe ciliary defects in some cell types than in others.
A dramatic difference in the mechanism of ciliogenesis is seen in multiciliated epithelia. In contrast to primary cilia in most cells, cilia in the airway and ependymal cells are nucleated by basal bodies that form de novo in large spherical arrays called deuterosomes (Dirksen, 1991). Defects in deuterosome-specific genes might result in cilia defects specifically in multiciliated epithelia without having any effect on sensory primary cilia.
Another important consideration is the relative timing of gene loss in different tissues. Adult-onset ciliopathy can result from the spontaneous loss of heterozygosity of a ciliary gene in patients carrying one mutant allele. This second hit would occur long after embryogenesis; thus, developmental defects such as polydactyly would not be seen. Timing of ciliopathy onset can be studied using inducible Cre-mediated knockout alleles (Garcia-Gonzalez et al., 2007).
Defects affecting different ultrastructural modules of cilia
Cilia have a modular organization at the ultrastructural level (Fig. 1), and the individual structural modules (for example, central pair, dynein arms, and radial spokes) are involved in different functions. For instance, the dynein arms or radial spokes are needed for motile but not for sensory functions, whereas ciliary membrane channels may be required for sensing but not for motion. Thus, a particular disease mutation can affect some particular subset of ciliary structural or functional features while leaving others intact.
This effect is clearly seen in primary ciliary dyskinesia (PCD), which is also known as immotile cilia syndrome. PCD generally involves defects in dynein arms, radial spokes, or the central pair (which is to say, components of the motile machinery). Defects in such structures would not be expected to affect signaling; for example, one does not typically observe polydactyly or other hedgehog signaling defects during development in PCD patients. Similarly, PCD patients do not suffer from cystic kidneys, obesity, or retinal degeneration because these symptoms arise from defects in ciliary structures that are not involved in motility.
One can further subdivide PCD cases into those that affect different motile structures. The main symptoms of PCD, namely defects in mucus clearance, are seen with any defect in motile structures. Another symptom commonly associated with immotile cilia is hydrocephalus, reflecting a role of ependymal cell cilia in moving cerebrospinal fluid. Hydrocephalus appears to be specifically associated with defects in the central pair microtubules as typified by the mouse hydin mutant, which has hydrocephalus and lacks the central pair (Davy and Robinson, 2003; Lechtreck and Witman, 2007). A third symptom of immotile cilia is situs inversus, an inversion of the normal left-right asymmetry of internal organs. Situs inversus occurs because the motion of primary cilia in the mammalian node generates a leftward fluid flow that breaks the left/right symmetry during development. However, the node cilia appear to lack the central pair microtubules, at least early in development, and, consistent with an absence of the central pair at this stage, mutations that lead to loss of the central pair microtubules (e.g., hydin mutants) do not generally lead to situs inversus.
Distinct ciliary substructures may also play distinct and separable roles in cilia-mediated signaling. A mouse mutation, hennin, causes an alteration in the spatial extent of hedgehog signaling during development (Caspary et al., 2007). Interestingly, the effect on hedgehog may occur via alteration in the structure of the cilium itself (Caspary et al., 2007), causing the B tubule of the outer doublets to be incomplete, with a gap at the point where it meets the A tubule toward the interior of the cilium. The protofilament of the B tubule that interacts with the A tubule in this region has an unusual appearance in electron micrographs and may not even be composed of tubulin (for review see Linck and Stephens, 2007), and so it is indeed interesting to speculate on the functional consequences of losing this very unusual structural element. The zebrafish fleer mutant also has a defect in B-tubule joining to the A tubule, apparently as the result of a loss of fleer-specific tubulin polyglutamylation. However, unlike the hennin phenotype, in fleer mutants, the gap between the A and B tubules is at the other A–B junction facing the ciliary membrane (Pathak et al., 2007). These mutants show defects in ciliary motility but no dramatic hedgehog phenotypes. It is difficult to compare mouse and fish mutants directly because the large maternal stores in the fish embryo can mask early developmental phenotypes. At the very least, these results raise the possibility that specific protofilaments of the B tubule might play specific roles in signaling.
If structures involved in motility also play roles in signaling during development, one would expect human patients with defects in these structures to be less likely to survive past birth. This might explain why mutations in the outer dynein arms are so frequently seen as the cause of PCD in patients (Hornef et al., 2006) because mutations in the outer doublets or the central pair may end up leading to more severe symptoms.
The ciliary membrane is another component of the cilium in which functional molecules reside. A wide range of receptors and channels are located in this specialized membrane, which are crucial for ciliary function. For example, transient receptor potential channels have been observed to actively move along the ciliary membrane (Qin et al., 2005), although the purpose of this motion for signal transduction remains unknown. One of the best-defined ciliary membrane channels is the mechanical stress sensor consisting of the polycystins polycystic kidney disease 1 (PKD1) and PKD2, mutations in which cause autosomal dominant PKD. These proteins localize within the cilium (Barr et al., 2001; Yoder et al., 2002) and are thought to confer on cilia the ability to sense flow in the kidney. PKD patients with defects in these proteins do not show other ciliary defects, suggesting the PKD proteins are not required for ciliogenesis or other ciliary functions. Ciliary signaling ultimately transmits information to the cytoplasm and nucleus. Defects in particular downstream signaling pathways could mimic the effect of ciliary loss in cell types that rely on these pathways while leaving ciliary function in other cell types unaffected.
Defects in the trafficking and biogenesis of the ciliary membrane may also lead to disease. The Bardet-Biedl syndrome protein complex interacts with a guanine nucleotide exchange factor for Rab8, which itself appears to regulate the recruitment of membrane to the cilium (Nachury et al., 2007). Defects in ciliary membrane trafficking might produce subtle changes in ciliary function.
Finally, we consider what happens if the cilia are completely normal in terms of structure and composition but are simply in the wrong place at the wrong time. Motile cilia must be properly oriented to drive flows in the right direction. Cilia must also be on the right surface of the cell (for instance, facing the lumen of a duct) to sense the right environment. One of the most dramatic types of ciliary mislocalization that has been reported in patients is the targeting of cilia to intracellular vacuoles rather than the cell surface (Hagiwara et al., 2000). In these cases, one can imagine that sensory cilia function would continue to operate except that instead of sensing the environment that the cell thinks it is sensing, for instance a duct lumen, the cilia are instead sensing the environment within the vacuole. This could differ in the extreme from the normal extracellular environment and might inappropriately activate cilia-based signaling pathways with drastic negative consequences for the cell's function.
Future directions
The bottom line is that cilia are complex, and details of the complexity vary from one cell type to another. To understand the full spectrum of ciliary diseases, therefore, we must learn much more about cell type–specific differences in cilia. A key first step would be to use proteomics to identify differences in the composition of different types of cilia. Another important step would be the development of simple assays for ciliary function and structure that can be applied in a range of tissues, both in animal models and in samples from human patients, to understand how different mutations affect different types of cilia.
Abbreviations used in this paper: IFT, intraflagellar transport; PCD, primary ciliary dyskinesia; PKD, polycystic kidney disease.
References
- Afzelius, B.A. 2004. Cilia-related diseases. J. Pathol. 204:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7:125–148. [DOI] [PubMed] [Google Scholar]
- Barr, M.M., J. DeModena, D. Braun, C.Q. Nguyen, D.H. Hall, and P.W. Sternberg. 2001. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11:1341–1346. [DOI] [PubMed] [Google Scholar]
- Caspary, T., C.E. Larkins, and K.V. Anderson. 2007. The graded response to sonic hedgehog depends on cilia architecture. Dev. Cell. 12:767–778. [DOI] [PubMed] [Google Scholar]
- Christensen, S.T., L.B. Pedersen, L. Schneider, and P. Satir. 2007. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 8:97–100. [DOI] [PubMed] [Google Scholar]
- Colantonio, J.R., J.M. Bekker, S.J. Kim, K.M. Morrissey, R.H. Crosbie, and K.L. Hill. 2006. Expanding the role of the dynein regulatory complex to non-axonemal functions: association of GAS11 with the Golgi apparatus. Traffic. 7:538–548. [DOI] [PubMed] [Google Scholar]
- Davy, B.E., and M.L. Robinson. 2003. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum. Mol. Genet. 12:1163–1170. [DOI] [PubMed] [Google Scholar]
- Dirksen, E.R. 1991. Centriole and basal body formation during ciliogenesis revisited. Biol. Cell. 72:31–38. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler, J.T., and K.V. Anderson. 2007. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23:345–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, S., V. Fogg, Q. Wang, X.W. Chen, C.J. Liu, and B. Margolis. 2007. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin β interactions. J. Cell Biol. 178:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf, M., T. Benzig, and H. Omran. 2007. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8:880–893. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez, M.A., L.F. Menezes, K.B. Piontek, J. Kaimori, D.L. Huso, T. Watnick, L.F. Onuchic, L.M. Guay-Woodford, and G.G. Germino. 2007. Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum. Mol. Genet. 16:1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, H., N. Ohwada, T. Aoki, and K. Takata. 2000. Ciliogenesis and ciliary abnormalities. Med. Electron Microsc. 33:109–114. [DOI] [PubMed] [Google Scholar]
- Han, Y.G., B.H. Kwok, and M.J. Kernan. 2003. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 13:1679–1686. [DOI] [PubMed] [Google Scholar]
- Hornef, N., H. Olbrich, J. Horvath, M.A. Zariwala, M. Fliegauf, N.T. Loges, J. Wildhaber, P.G. Noone, M. Kennedy, S.E. Antonarakis, et al. 2006. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am. J. Respir. Crit. Care Med. 174:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde, C., R.J. Dickinson, V.M. Houtzager, R. Cullum, R. Montpetit, M. Metzler, E.M. Simpson, S. Roy, M.R. Hayden, P.A. Hoodless, and D.W. Nicholson. 2006. Hippi is essential for node cilia assembly and sonic hedgehog signaling. Dev. Biol. 300:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis, P.N., K.A. Boroevich, and M.R. Leroux. 2006. Piecing together a ciliome. Trends Genet. 22:491–500. [DOI] [PubMed] [Google Scholar]
- Kondo, S., R. Sato-Yoshitake, Y. Noda, H. Aizawa, T. Nakata, Y. Matsuura, and N. Hirokawa. 1994. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J. Cell Biol. 125:1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck, K.F., and G.B. Witman. 2007. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 176:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck, R.W., and R.W. Stephens. 2007. Functional protofilament numbering of ciliary, flagellar, and centriolar microtubules. Cell Motil. Cytoskeleton. 64:489–495. [DOI] [PubMed] [Google Scholar]
- Merchant, S.S., S.E. Prochnik, O. Vallon, E.H. Harris, S.J. Karpowicz, G.B. Witman, A. Terry, A. Salamov, L.K. Fritz-Laylin, L. Maréchal-Drouard, et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 318:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, S., Y. Lu, H. Qin, A. Lanjuin, S. Shaham, and P. Sengupta. 2007. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 26:2966–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury, M.V., A.V. Loktev, Q. Zhang, C.J. Westlake, J. Peranen, A. Merdes, D.C. Slusarski, R.H. Scheller, J.F. Bazan, V.C. Sheffield, and P.K. Jackson. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 129:1201–1213. [DOI] [PubMed] [Google Scholar]
- Ou, G., O.E. Blacque, J.J. Snow, M.R. Leroux, and J.M. Scholey. 2005. Functional coordination of intraflagellar transport motors. Nature. 436:583–587. [DOI] [PubMed] [Google Scholar]
- Pathak, N., T. Obara, S. Mango, Y. Liu, and I.A. Drummond. 2007. The Zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol. Biol. Cell. 18:4353–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, L.A., E.M. Hedgecock, J.H. Thomson, and J.G. Culotti. 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117:456–487. [DOI] [PubMed] [Google Scholar]
- Praetorius, H.A., and K.R. Spring. 2003. The renal cell primary cilium functions as a flow sensor. Curr. Opin. Nephrol. Hypertens. 12:517–520. [DOI] [PubMed] [Google Scholar]
- Qin, H., D.T. Burnette, Y.K. Bae, P. Forscher, M.M. Barr, and J.L. Rosenbuam. 2005. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15:1695–1699. [DOI] [PubMed] [Google Scholar]
- Schermer, B., C. Ghenious, M. Bartram, R.U. Mueller, F. Kotsis, M. Hoehne, W. Kuehn, M. Rapka, R. Nitschke, H. Zentgraf, et al. 2006. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 175:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey, J.M. 2008. Intraflagellar transport motors in cilia: moving along the cell's antenna. J. Cell. Biol. 180:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, R.E. 1997. Synthesis and turnover of embryonic sea urchin ciliary proteins during selective inhibition of tubulin synthesis and assembly. Mol. Biol. Cell. 8:2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder, B.K., X. Hou, and L.M. Guay-Woodford. 2002. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13:2508–2516. [DOI] [PubMed] [Google Scholar]