Abstract
Sulindac is a nonsteroidal antiinflammatory drug with a chemopreventive effect in patients with familial adenomatous polyposis (FAP). In vivo, the active form of sulindac is sulindac sulfide, which is inactivated by the hepatic microsomal enzyme, flavin monooxygenase 3 (FMO3). In humans, numerous polymorphisms exist in FMO3, which alter enzymatic activity and subsequent substrate metabolism. We recently showed that certain polymorphic forms of FMO3 with reduced activity were associated with a more favorable response to sulindac in preventing the formation of adenomas in patients with FAP without polyps at baseline. Here, we determined whether these FMO3 polymorphisms correlated with the ability of sulindac to regress polyposis in patients with FAP who had polyps prior to treatment. Nineteen patients were treated with 150 mg sulindac twice a day for 6 months. The size and number of polyps in each patient was assessed at baseline (prior to the administration of sulindac), and at 3 and 6 months. Genotyping was done on seven established FMO3 polymorphisms with functional significance—M66I, E158K, P153L, V257M, E305X, E308G, and R492W. Statistical analyses were done with Wilcoxon rank sum test. Of the loci examined, only E158K and E308G showed polymorphic changes. Six patients exhibited polymorphisms in both E158K and E308G loci and were designated as genotype combination 1. The remaining patients were designated as genotype combination 2. Over the course of treatment, patients with genotype combination 1 had a greater reduction in both the size and number of polyps than those with genotype combination 2. These results suggest that combined polymorphic changes in the E158K and E308G alleles may protect against polyposis in patients with FAP treated with sulindac.
Introduction
Colorectal cancer is a major cause of mortality and morbidity from cancer in the U.S. In 2005, an estimated 145,000 new cases and 56,000 deaths from colorectal cancer will occur, making this neoplasm the second leading cause of cancer death in the U.S. (American Cancer Society—Cancer facts and figures, 2005; http://www.cancer.org/downloads/STT/CAFF2005f4PWSecured.pdf). Encouragingly, enormous effort has been spent in the last decade to promote the early detection (screening) of colorectal cancer and its precursor, adenoma. This effort has resulted in a declining trend in the mortality rate from this disease.
An additional strategy to reduce the incidence and complications from colorectal neoplasia is prevention with pharmacologic or nutritional agents. This approach, called chemoprevention, has generated a significant amount of interest and led to the identification of agents that prevent colorectal neoplasia (1, 2). For instance, epidemiologic evidence indicates that nonsteroidal antiinflammatory drugs (NSAID) are effective chemopreventive agents for colorectal neoplasia (3, 4). Further investigation revealed that daily use of aspirin reduced the incidence of colorectal adenomas in patients with previous colorectal adenoma or cancer (5, 6). Other studies support the notion that inhibition of cyclooxygenases, especially cyclooxygenase-2, is in part responsible for the chemopreventive effects of NSAIDs (7, 8).
Familial adenomatous polyposis (FAP) is an autosomal dominant disorder characterized by the development of numerous adenomatous polyps in the colon at an early age in affected individuals (9). If left untreated, patients with FAP inevitably develop colorectal cancer at a median age of 40 years. Because of the predictable and accelerated nature of polyposis in FAP, this disease has served as a model for clinical trials involving chemoprevention. Thus, results of randomized controlled trials showed that NSAIDs, such as sulindac and celecoxib, reduce the burden (number and size) of colorectal adenomas in patients with FAP (10-13). Moreover, in patients treated with sulindac, regression of polyposis correlates with reduction in levels of prostaglandins in the rectal mucosa (14, 15). Because prostaglandins are products of cyclooxygenases, these results support a mechanism by which inhibition of cyclooxygenase activity is responsible in part for the chemopreventive ability of NSAIDs in colorectal neoplasia.
Administered orally, sulindac is a pro-drug containing a racemic sulfoxide moiety, which is reduced by the gut flora to the active sulfide form before absorption (16, 17). In vivo, the sulfide form is reoxidized to the sulfoxide, and then, the sulfone form; the latter two compounds relatively inactive compared with the sulfide form (16). The two oxidation steps leading from sulindac sulfide to sulfone are carried out by flavin monooxygenase 3 (FMO3), a microsomal enzyme enriched in hepatocytes (18, 19). FMO3 is also involved in the metabolism of a host of other nucleophilic heteroatom–containing chemicals and drugs including trimethylamine, clozapine, (S)-nicotine, and ranitidine (20, 21).
Studies indicate substantial interindividual and interethnic differences in FMO3 activity (22). Much of this variation is attributed to genetic polymorphisms, of which, 26 have been identified to date (21, 23). Most of these result in variant enzymes with amino acid substitutions, some of which lead to altered enzymatic activity. Because FMO3 is implicated in the oxidative inactivation of sulindac sulfide (18) and sulindac is effective in chemoprevention against FAP (10-12, 15), we recently examined the effect of FMO3 polymorphisms on the clinical outcome of a sulindac-mediated primary chemoprevention trial in FAP (24). We identified two variant loci in FMO3, E158K, and E308G, both known to reduce FMO3 activity (21), that were associated with an increased efficacy in the prevention of adenoma formation in patients treated with sulindac (25). In the current study, we examined the impact of FMO3 polymorphisms on the ability of sulindac to regress polyposis in patients with FAP who had adenomas before the initiation of sulindac treatment.
Materials and Methods
Study Population
The study population included 19 patients with FAP previously treated with sulindac for polyp regression at the Johns Hopkins University School of Medicine. The inclusion and exclusion criteria have been reported (11). Three additional patients in that report were not included in the present study because sera were not available for genotyping. Each patient was treated with 150 mg sulindac orally twice a day for 6 months.
The study design was previously described (11). Briefly, assessment of the number and size of rectal polyps was done in each patient before the administration of sulindac (time 0 or baseline), and at 3 and 6 months of sulindac treatment. Enrolled patients had a minimum of five rectal polyps at baseline. Polyp size and number were determined during examination of the colorectal region with a flexible sigmoidoscope. At time 0, the rectal mucosa 20 cm from the anal verge were tattooed with sterile Indian ink. The examiner counted the total number of polyps in the entire circumference of the rectum from the tattoo mark to the anal verge. The size of each of the first five polyps just distal to the tattoo was measured with a graduated millimeter scale. The mean size of the five polyps was then recorded for each patient. Patient compliance was assessed by tablet counts and weekly telephone contact. The protocol was approved by the Johns Hopkins Medical Institution Joint Committee on Clinical Investigation.
FMO3 Genotyping
FMO3 genotyping was done as previously described (25). Briefly, genomic DNA was extracted from stored sera of the study patients using the QIAgen Blood Minikit Protocol (QIAgen, Inc., Valencia, CA). Twenty micro-liters of proteinase K was added to 400 μL serum followed by the addition of 200 μL buffer AL. The solution was thoroughly mixed and incubated at 50°C followed by the addition of 200 μL ethanol. The mixture was then loaded onto a QIAmp spin column and centrifuged at 6,000 × g for 1 minute, after which the filtrate was discarded. The same process was repeated with buffer AW1 and AW2. Finally, bound DNA was eluted from the spin column with 50 μL DNase- and RNase-free ultrapure water.
Seven established FMO3 polymorphic loci shown to be associated with altered enzymatic functions were selected for genotyping: M66I, P153L, E158K, V257M, E305X, E308G, and R492W (21). Polymorphisms were determined by PCR-based RFLP using previously published primer sequences (26). Genotypes were classified as homozygous wild-type (WT/WT), heterozygous polymorphic (WT/P), or homozygous polymorphic (P/P).
Statistical Analysis
Due to the small sample size, nonparametric methods were used. Summary statistics were the median (50th percentile) and the first and third quartiles (25th and 75th percentiles, respectively). To test for a difference in location of two independent populations, the Wilcoxon rank sum test was used to assess significance and calculate P values. The Wilcoxon test was used to test for a difference between genotype combinations 1 and 2 in the reduction in polyp size and number from baseline to 3 months and from baseline to 6 months. Analyses and graphs were produced using the statistical computing program, R (http://www.R-project.org).
Results
Of the seven polymorphic loci examined in the 19 patients, only two, E158K and E308G, showed polymorphic changes. The other five were wild-type. Table 1 shows the allelic distribution of the E158K and E308G loci in all 19 patients. The overall frequency of distribution of the two polymorphisms is similar to our previous study in a different patient population (25).
Table 1. Distribution of the E158K and E308G polymorphisms in the study population.
| Polymorphisms | E158K | E308G |
|---|---|---|
| WT/WT | 4 (21) | 13 (68) |
| WT/P | 9 (47) | 6 (32) |
| P/P | 6 (32) | 0 (0) |
NOTE: Values are expressed as number (%).
Recently, we identified a particular genotype combination in FMO3 associated with an increased efficacy of sulindac to prevent polyposis in a primary chemoprevention trial in patients with FAP (25). Patients with this genotype combination, called genotype 1, exhibit either heterozygous (WT/P) or homozygous (P/P) variant alleles at both the E158K and E308G loci. In the present study, six patients (32%) had genotype combination 1. This frequency is similar to that previously reported in the primary chemoprevention trial (22%; ref. 25). The remainder of the patients in the present study was assigned genotype combination 2.
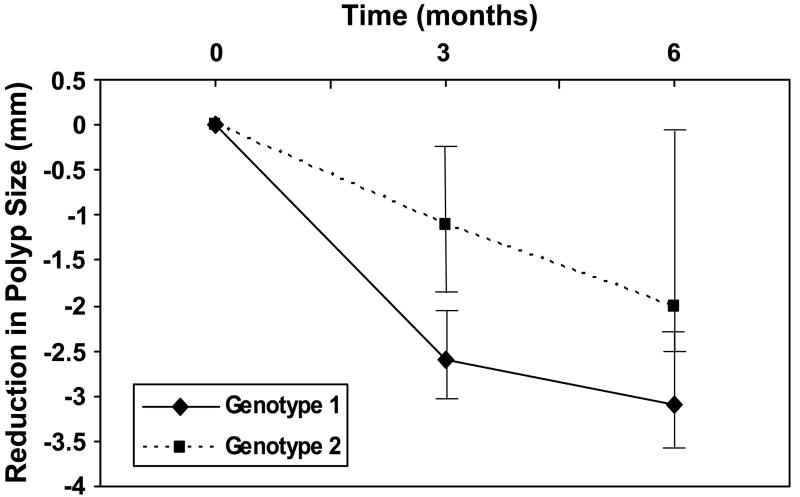
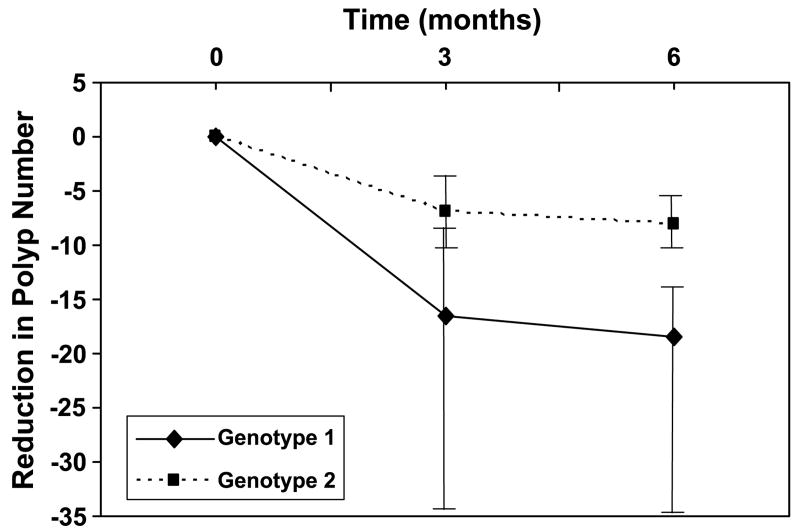
Table 2 shows the median polyp size and number for the two genotype combinations at baseline. The differences between the two genotypes were not statistically significant. Table 3 shows the changes in polyp size and number over the 6-month course of sulindac treatment and the FMO3 genotype combination for all 19 patients. Figure 1 contrasts the reduction in polyp size over the 6 months of sulindac treatment between patients with FMO3 genotype combinations 1 and 2. The median reduction in polyp size from baseline to 3 months for genotype combinations 1 and 2 was 2.6 and 1.1 mm, respectively (P = 0.018). At 6 months, the median reduction was 3.1 and 2.0 mm for genotype combinations 1 and 2, respectively (P = 0.069). Similarly, Fig. 2 shows the result of reduction in polyp number over the 6-month period. The median reduction in polyp number from baseline to 3 months for genotype combinations 1 and 2 was 16.5 and 7.0, respectively (P = 0.24). At 6 months, the median reduction was 18.5 and 8.0 for genotype combinations 1 and 2, respectively (P = 0.057).
Table 2. Comparison of baseline polyp size and number between genotype combinations 1 and 2.
| Genotype 1 | Genotype 2 | |
|---|---|---|
| Polyp size (mm) | 3.5 (2.9, 4.8) | 2.8 (2.1, 3.0) |
| Polyp number | 20.5 (18.5, 40.5) | 14.0 (9.5, 19.5) |
NOTE: Values are expressed as median (25th and 75th percentiles).
Table 3. Changes in polyp size and number over the course of treatment and FMO3 genotypes in the study patients.
| Patient | Polyp size (mm)
|
Polyp number
|
FMO3 genotype
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | E158K | E308G | Class | |
| 1 | 5 | 2.6 | 1.2 | 7 | 4 | 4 | WT/P | WT/P | 1 |
| 2 | 3 | 1.9 | 1 | 21 | 16 | 8 | P/P | WT/P | 1 |
| 3 | 5.5 | 4.5 | 2 | 160 | 160 | 160 | WT/P | WT/WT | 2 |
| 4 | 1.7 | 0.4 | 0.6 | 8 | 1 | 2 | WT/P | WT/WT | 2 |
| 5 | 3 | 2.3 | 2.9 | 34 | 27 | 29 | WT/WT | WT/WT | 2 |
| 6 | 1.5 | 1.3 | 1.8 | 7 | 8 | 14 | WT/WT | WT/WT | 2 |
| 7 | 1.7 | 3.5 | 2.1 | 21 | 11 | 14 | P/P | WT/WT | 2 |
| 8 | 4 | 1.1 | 0.6 | 20 | 5 | 1 | WT/P | WT/P | 1 |
| 9 | 3 | 3 | 0.6 | 10 | 6 | 3 | WT/P | WT/WT | 2 |
| 10 | 2.8 | 1.1 | 0.1 | 12 | 8 | 3 | P/P | WT/WT | 2 |
| 11 | 2.8 | 2.5 | 1.2 | 16 | 16 | 6 | WT/P | WT/WT | 2 |
| 12 | 2.4 | NA | 0.4 | 19 | 3 | 3 | WT/WT | WT/WT | 2 |
| 13 | 2.5 | 0.6 | 1 | 47 | 7 | 6 | P/P | WT/P | 1 |
| 14 | 3.1 | 0 | 0 | 7 | 0 | 0 | WT/WT | WT/WT | 2 |
| 15 | 2.8 | 0 | 0 | 18 | 0 | 0 | P/P | WT/P | 1 |
| 16 | 2.2 | 0 | 0 | 10 | 0 | 0 | P/P | WT/WT | 2 |
| 17 | 2.1 | 0 | 0 | 16 | 0 | 0 | WT/P | WT/WT | 2 |
| 18 | 3.8 | 2.7 | 3.6 | 80 | 30 | 40 | WT/P | WT/WT | 2 |
| 19 | 6.2 | 2.3 | 2.8 | 80 | 22 | 12 | WT/P | WT/P | 1 |
Figure 1.
The reduction in polyp size from baseline in patients with genotype combinations 1 and 2 over the 6-month course of sulindac treatment. Medians of polyp size for patients with genotype combination 1 (solid line) and 2 (dashed line) over the course of treatment with sulindac are shown. The vertical bars span between the first and third quartiles.
Figure 2.
The reduction in polyp number from baseline in patients with genotype combinations 1 and 2 over the 6-month course of sulindac treatment. Medians of polyp number for patients with genotype combination 1 (solid line) and 2 (dashed line) over the course of treatment with sulindac are shown. The vertical bars span between the first and third quartiles.
Discussion
Colorectal cancer is a significant health concern due to high prevalence in Western populations. Approaches designed to reduce the incidence of colorectal neoplasia may, therefore, lead to a reduction in mortality from colorectal cancer. Numerous studies show that NSAIDs have a beneficial effect in reducing the incidence of colorectal neoplasia (1, 4). However, the heterogenous nature of the response to these agents has not been examined.
Our group has used FAP as a model to study NSAID-mediated chemoprevention. In several independent trials, we showed that sulindac is effective in regressing colorectal adenomas in patients with FAP (10-12). These studies also revealed the relatively heterogeneous interindividual response to sulindac in treated patients. One of the studies examined various factors possibly responsible for the different clinical outcome (11). It identified prior subtotal colectomy with ileorectal anastomosis as a predictive factor when compared with patients with no prior surgery (11). In addition, a lower level of mucosal prostanoids was correlated with a better clinical response to sulindac treatment (14). This finding supports the notion that cyclooxygenase inhibition, as reflected by mucosal prostanoid levels, is an important mechanism by which sulindac exerts its chemopreventive effect.
To identify additional predictive factors that distinguish the clinical efficacy of sulindac-mediated chemoprevention, we examined FMO3 polymorphisms in a group of patients with FAP treated with sulindac. FMO3 is the primary enzyme involved in the oxidative inactivation of the active form of sulindac, sulindac sulfide (18). In addition, many polymorphisms in FMO3 result in an alteration of enzymatic activity against various substrates (21). Results of the present study showed that in patients with a particular genotype combination (genotype 1), which includes both variants (heterozygous or homozygous) E158K and E308G alleles, sulindac has a greater efficacy in reducing polyp size (Fig. 1) and number (Fig. 2) compared with patients with other genotypes. Of note is that both E158K and E308G variants are associated with decreased FMO3 activity (21). Therefore, these results support the notion that patients with genotype combination 1 are more likely to benefit from sulindac to regress polyposis, and this increased benefit is probably due to the reduced inactivation of sulindac. The functional correlation between FMO3 polymorphisms and sulindac metabolism is similar to previous reports in which abnormal metabolism of carcinogens by certain drug-metabolizing enzymes, such as glutathione-S-transferase, might contribute to the development of intestinal tumors in patients with FAP or HNPCC (27, 28).
We recently performed a survey of FMO3 polymorphisms in an independent cohort of patients with FAP treated with sulindac. These patients belonged to a randomized, placebo-controlled trial and were polyp-free at trial entry. The study showed that sulindac, when compared with placebo, failed to prevent the development of colorectal adenomas over a 4-year trial period (24). However, among the patients using sulindac, mucosal prostanoid levels were significantly lower in those who remained polyp-free compared with those developing polyps (29). Moreover, sulindac-treated patients with either the E158K or E308G allele were more likely to remain polyp-free (25). Importantly, polymorphisms in the E158K or E308G allele were associated with a greater reduction in mucosal prostanoid levels in treated patients. These results add support to our present study that FMO3 polymorphism is a factor in determining the clinical efficacy of sulindac-mediated chemoprevention of polyposis. However, as our study was limited to a subset of established FMO3 polymorphisms, the results of the study would suggest that the polymorphisms examined in this study is only one such factor. Additional studies are needed to establish the functional relevance of other FMO3 polymorphisms in contributing to sulindac-mediated chemoprevention in FAP.
If inactivating FMO3 polymorphisms is indeed correlated with a therapeutic benefit of sulindac in preventing polyposis, it is also possible that the same polymorphisms are associated with increased toxicities or adverse events due to sulindac. However, sulindac was well-tolerated by patients in both the primary chemoprevention trial (24) and regression trial (11). Very few and relatively minor adverse events were reported by patients in either trial. It is therefore not possible to correlate FMO3 polymorphisms to sulindac-induced toxicities in these studies.
The beneficial effect of certain FMO3 polymorphisms on sulindac to regress or prevent polyposis in FAP is reminiscent of a recent study examining the effect of polymorphisms in the gene encoding UGT1A6 on the association between aspirin use and risk of colorectal adenoma (30). Functional polymorphisms in UGT1A6 are associated with the impaired metabolism of aspirin. Among the study subjects with variant UGT1A6 genotypes, regular use of aspirin was associated with a decreased risk of colorectal adenoma. In contrast, in those with the wild-type UGT1A6 genotype, regular aspirin use was not associated with a reduced risk. Thus, functional polymorphisms in UGT1A6 significantly modify the effect of aspirin on colorectal neoplasia. Combining the results of studies examining FMO3 and UGT1A6 polymorphisms on NSAID metabolism, the introduction of pharmacogenetics in the design of future chemoprevention trials should be considered.
Acknowledgments
Grant support: Supported in part by NIH grants [CA84197, DK64399 (to V.W. Yang); CA53801 (to F.M. Giardiello)], and the John G. Rangos, Sr. Charitable Foundation and the Clayton Fund (to F.M. Giardiello). V.W. Yang is the recipient of a Georgia Cancer Coalition Distinguished Cancer Clinician Scientist Award.
References
- 1.Hawk ET, Umar A, Viner JL. Colorectal cancer chemoprevention—an overview of the science. Gastroenterology. 2004;126:1423–47. doi: 10.1053/j.gastro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Janne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960–8. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- 3.Hawk ET, Viner J, Richmond E, Umar A. Non-steroidal anti-inflammatory drugs (NSAIDs) for colorectal cancer prevention. Cancer Chemother Biol Response Modif. 2003;21:759–89. doi: 10.1016/s0921-4410(03)21036-x. [DOI] [PubMed] [Google Scholar]
- 4.Arber N, DuBois RN. Nonsteroidal anti-inflammatory drugs and prevention of colorectal cancer. Curr Gastroenterol Rep. 1999;1:441–8. doi: 10.1007/s11894-999-0027-1. [DOI] [PubMed] [Google Scholar]
- 5.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 6.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 7.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 8.DuBois RN. Cyclooxygenase-2 and colorectal cancer. Prog Exp Tumor Res. 2003;37:124–37. doi: 10.1159/000071370. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Correa M, Giardiello FM. Familial adenomatous polyposis. Gastrointest Endosc. 2003;58:885–94. doi: 10.1016/s0016-5107(03)02336-8. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–5. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 11.Giardiello FM, Offerhaus JA, Tersmette AC, et al. Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut. 1996;38:578–81. doi: 10.1136/gut.38.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 13.Steinbac G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 14.Yang VW, Geiman DE, Hubbard WC, et al. Tissue prostanoids as biomarkers for chemoprevention of colorectal neoplasia: correlation between prostanoid synthesis and clinical response in familial adenomatous polyposis. Prostaglandins Other Lipid Mediat. 2000;60:83–96. doi: 10.1016/s0090-6980(99)00054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardiello FM, Spannhake EW, DuBois RN, et al. Prostaglandin levels in human colorectal mucosa: effects of sulindac in patients with familial adenomatous polyposis. Dig Dis Sci. 1998;43:311–6. doi: 10.1023/a:1018898120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duggan DE, Hooke KF, Risley EA, Shen TY, Arman CG. Identification of the biologically active form of sulindac. J Pharmacol Exp Ther. 1977;201:8–13. [PubMed] [Google Scholar]
- 17.Etienne F, Resnick L, Sagher D, Brot N, Weissbach H. Reduction of Sulindac to its active metabolite, sulindac sulfide: assay and role of the methionine sulfoxide reductase system. Biochem Biophys Res Commun. 2003;312:1005–10. doi: 10.1016/j.bbrc.2003.10.203. [DOI] [PubMed] [Google Scholar]
- 18.Hamman MA, Haehner-Daniels BD, Wrighton SA, Rettie AE, Hall SD. Stereoselective sulfoxidation of sulindac sulfide by flavin-containing monooxygenases. Comparison of human liver and kidney microsomes and mammalian enzymes. Biochem Pharmacol. 2000;60:7–17. doi: 10.1016/s0006-2952(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 19.Dolphin CT, Cullingford TE, Shephard EA, Smith RL, Phillips IR. Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FMO3 and FM04. Eur J Biochem. 1996;235:683–9. doi: 10.1111/j.1432-1033.1996.00683.x. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler DM. Recent studies on the structure and function of multisubstrate flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 1993;33:179–99. doi: 10.1146/annurev.pa.33.040193.001143. [DOI] [PubMed] [Google Scholar]
- 21.Cashman JR. The implications of polymorphisms in mammalian flavin-containing monooxygenases in drug discovery and development. Drug Discov Today. 2004;9:574–81. doi: 10.1016/S1359-6446(04)03136-8. [DOI] [PubMed] [Google Scholar]
- 22.Cashman JR, Zhang J. Interindividual differences of human flavin-containing monooxygenase 3: genetic polymorphisms and functional variation. Drug Metab Dispos. 2002;30:1043–52. doi: 10.1124/dmd.30.10.1043. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez D, Addou S, Lee D, Orengo C, Shephard EA, Phillips IR. Trimethylaminuria and a human FMO3 mutation database. Hum Mutat. 2003;22:209–13. doi: 10.1002/humu.10252. [DOI] [PubMed] [Google Scholar]
- 24.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–9. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hisamuddin IM, Wehbi MA, Chao A, et al. Genetic polymorphisms of human flavin monooxygenase 3 in sulindac-mediated primary chemoprevention of familial adenomatous polyposis. Clin Cancer Res. 2004;10:8357–62. doi: 10.1158/1078-0432.CCR-04-1073. [DOI] [PubMed] [Google Scholar]
- 26.Sachse C, Ruschen S, Dettling M, et al. Flavin monooxygenase 3 (FMO3) polymorphism in a white population: allele frequencies, mutation linkage, and functional effects on clozapine and caffeine metabolism. Clin Pharmacol Ther. 1999;66:431–8. doi: 10.1053/cp.1999.v66.a102203. [DOI] [PubMed] [Google Scholar]
- 27.Moisio AL, Sistonen P, Mecklin JP, Jarvinen H, Peltomaki P. Genetic polymorphisms in carcinogen metabolism and their association to hereditary nonpolyposis colon cancer. Gastroenterology. 1998;115:1387–94. doi: 10.1016/s0016-5085(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 28.Spigelman AD, Nugent KP, Penna C, Foulds S, Phillips RK. Glutathione S-transferase Mu phenotype in patients with familial adenomatous polyposis and in unaffected controls. Cancer Detect Prev. 1994;18:253–8. [PubMed] [Google Scholar]
- 29.Giardiello FM, Casero RA, Jr, Hamilton SR, et al. Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis. Gastroenterology. 2004;126:425–31. doi: 10.1053/j.gastro.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan AT, Tranah GJ, Giovannucci EL, Hunter DJ, Fuchs CS. Genetic variants in the UGT1A6 enzyme, aspirin use, and the risk of colorectal adenoma. J Natl Cancer Inst. 2005;97:457–60. doi: 10.1093/jnci/dji066. [DOI] [PubMed] [Google Scholar]