Abstract
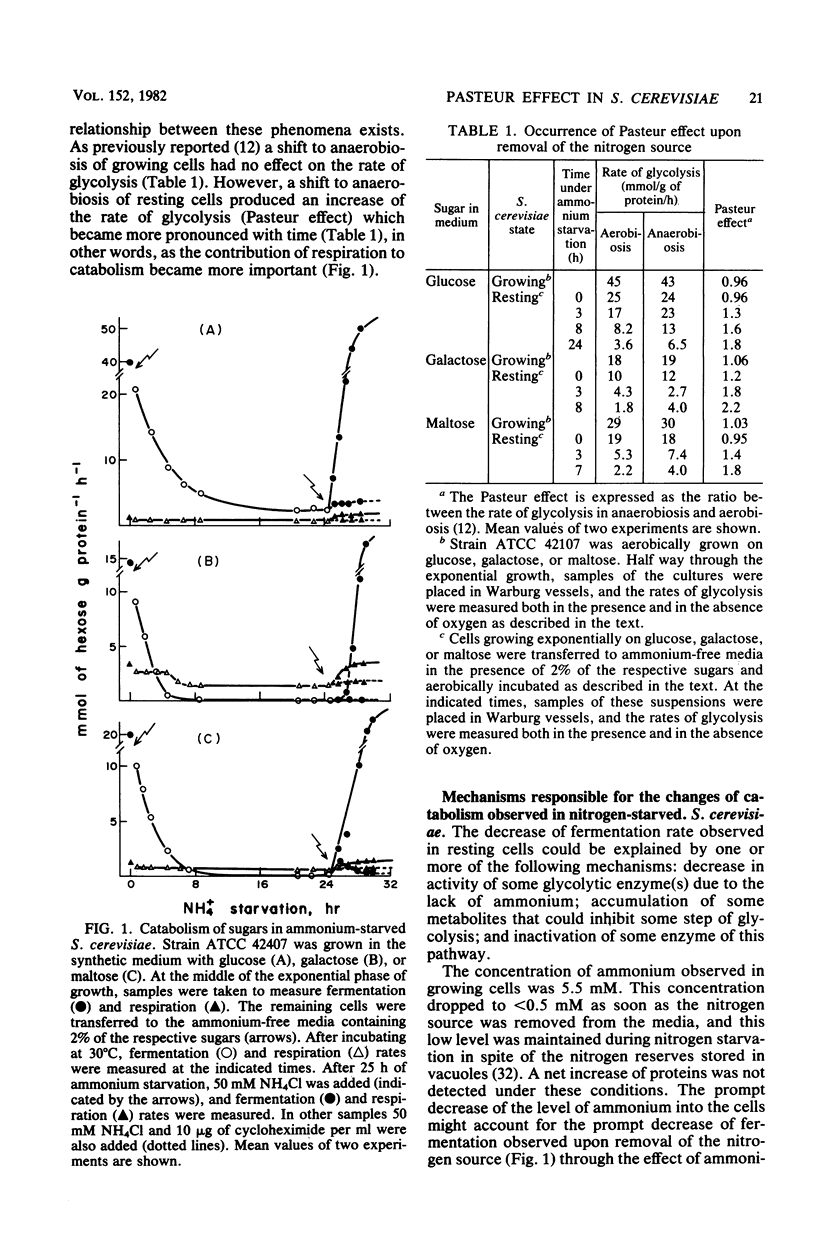
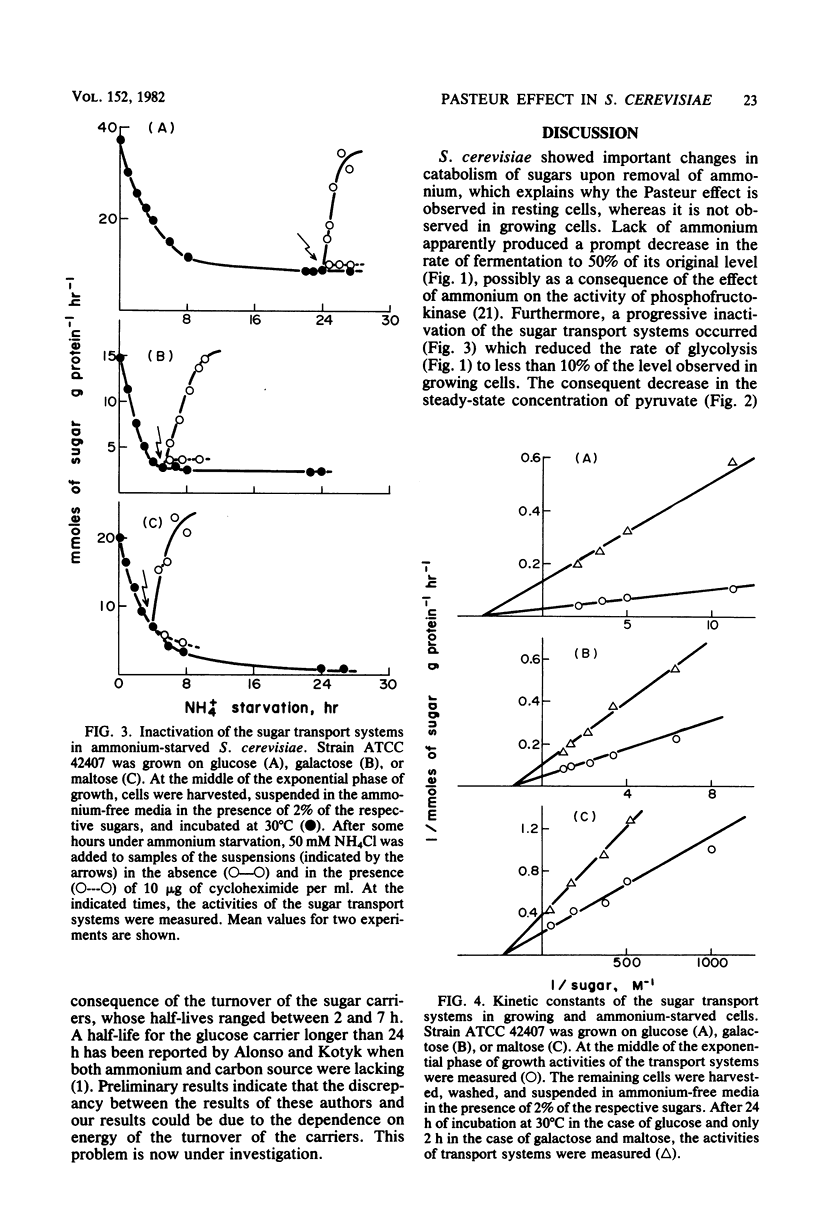
Saccharomyces cerevisiae does not show a noticeable Pasteur effect (activation of sugar catabolism by anaerobiosis) when growing with an excess of sugar and nitrogen source, but it does do so after exhaustion of the nitrogen source in the medium (resting state). We have found that this different behavior of growing and resting S. cerevisiae seems due to differences in the contribution of respiration to catabolism under both states. Growing S. cerevisiae respired only 3 to 20% of the catabolized sugar, depending on the sugar present; the remainder was fermented. In contrast, resting S. cerevisiae respired as much as 25 to 100% of the catabolized sugar. These results suggest that a shift to anaerobiosis would have much greater energetic consequences in resting than in growing S. cerevisiae. In resting S. cerevisiae anaerobiosis would strongly decrease the formation of ATP; as a consequence, various regulatory mechanisms would switch on, producing the observed increase of the rate of glycolysis. The greater significance that respiration reached in resting cells was not due to an increase of the respiratory capacity itself, but to a loss of fermentation which turned respiration into the main catabolic pathway. The main mechanism involved in the loss of fermentation observed during nitrogen starvation was a progressive inactivation of the sugar transport systems that reduced the rate of fermentation to less than 10% of the value observed in growing cells. Inactivation of the sugar transports seems a consequence of the turnover of the sugar carriers whose apparent half-lives were 2 to 7 h.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO F. Substrate specificity of Saccharomyces fragilis galactokinase. Biochim Biophys Acta. 1960 Jul 1;41:233–238. doi: 10.1016/0006-3002(60)90005-6. [DOI] [PubMed] [Google Scholar]
- Alonso A., Kotyk A. Apparent half-lives of sugar transport proteins in Saccharomyces cerevisiae. Folia Microbiol (Praha) 1978;23(2):118–125. doi: 10.1007/BF02915311. [DOI] [PubMed] [Google Scholar]
- Carrasco L., Barbacid M., Vazquez D. The trichodermin group of antibiotics, inhibitors of peptide bond formation by eukaryotic ribosomes. Biochim Biophys Acta. 1973 Jun 23;312(2):368–376. doi: 10.1016/0005-2787(73)90381-x. [DOI] [PubMed] [Google Scholar]
- Görts C. P. Effect of glucose on the activity and the kinetics of the maltose-uptake system and of alpha-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1969 Jul 30;184(2):299–305. doi: 10.1016/0304-4165(69)90032-4. [DOI] [PubMed] [Google Scholar]
- HOLZER H., HOLZER E., SCHULTZ G. Zusammenhang zwischen Wachstum und aerober Gärung. I. Versuche mit Hefezellen. Biochem Z. 1955;326(6):385–404. [PubMed] [Google Scholar]
- HOLZER H. Regulation of carbohydrate metabolism by enzyme competition. Cold Spring Harb Symp Quant Biol. 1961;26:277–288. doi: 10.1101/sqb.1961.026.01.034. [DOI] [PubMed] [Google Scholar]
- Heredia C. F., Sols A., DelaFuente G. Specificity of the constitutive hexose transport in yeast. Eur J Biochem. 1968 Aug;5(3):321–329. doi: 10.1111/j.1432-1033.1968.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Lagunas R. Energetic irrelevance of aerobiosis for S. cerevisiae growing on sugars. Mol Cell Biochem. 1979 Nov 1;27(3):139–146. doi: 10.1007/BF00215362. [DOI] [PubMed] [Google Scholar]
- López S., Gancedo J. M. Effect of metabolic conditions on protein turnover in yeast. Biochem J. 1979 Mar 15;178(3):769–776. doi: 10.1042/bj1780769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H., Holzer H. Catabolite inactivation of the galactose uptake system in yeast. J Biol Chem. 1977 Sep 25;252(18):6399–6402. [PubMed] [Google Scholar]
- Racker E. History of the Pasteur effect and its pathobiology. Mol Cell Biochem. 1974 Nov 15;5(1-2):17–23. doi: 10.1007/BF01874168. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Energetic efficiency and maintenance. Energy characteristics of Saccharomyces cerevisiae (wild type and petite) and Candida parapsilosis grown aerobically and micro-aerobically in continuous culture. Arch Microbiol. 1974;99(1):25–46. doi: 10.1007/BF00696220. [DOI] [PubMed] [Google Scholar]
- SALAS M. L., VINUELA E., SALAS M., SOLS A. CITRATE INHIBITION OF PHOSPHOFRUCTOKINASE AND THE PASTEUR EFFECT. Biochem Biophys Res Commun. 1965 Apr 23;19:371–376. doi: 10.1016/0006-291x(65)90471-7. [DOI] [PubMed] [Google Scholar]
- Serrano R., Delafuente G. Regulatory properties of the constitutive hexose transport in Saccharomyces cerevisiae. Mol Cell Biochem. 1974 Dec 20;5(3):161–171. doi: 10.1007/BF01731379. [DOI] [PubMed] [Google Scholar]
- Serrano R. Energy requirements for maltose transport in yeast. Eur J Biochem. 1977 Oct 17;80(1):97–102. doi: 10.1111/j.1432-1033.1977.tb11861.x. [DOI] [PubMed] [Google Scholar]
- Sáez M. J., Lagunas R. Determination of intermediary metabolites in yeast. Critical examination of the effect of sampling conditions and recommendations for obtaining true levels. Mol Cell Biochem. 1976 Nov 30;13(2):73–78. doi: 10.1007/BF01837056. [DOI] [PubMed] [Google Scholar]
- Zacharski C. A., Cooper T. G. Metabolite compartmentation in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):490–497. doi: 10.1128/jb.135.2.490-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



