Abstract
There is no published information on the causes of bacteremia in the Lao PDR (Laos). Between 2000 and 2004, 4512 blood culture pairs were taken from patients admitted to Mahosot Hospital, Vientiane, Laos, with suspected community-acquired bacteremia; 483 (10.7%) cultures grew a clinically significant community-acquired organism, most commonly Salmonella enterica serovar typhi (50.9%), Staphylococcus aureus (19.0%), and Escherichia coli (12.4%). S. aureus bacteremia was common among infants (69.2%), while children 1–5 years had a high frequency of typhoid (44%). Multi–drug-resistant S. Typhi was rare (6%). On multiple logistic regression analysis, typhoid was associated with younger age, longer illness, diarrhea, higher admission temperature, and lower peripheral white blood cell count than non-typhoidal bacteremia. Empirical parenteral ampicillin and gentamicin would have some activity against ∼ 88% of clinically significant isolates at a cost of US $1.4/day, an important exception being B. pseudomallei. Bacteremic infants in this setting require an anti-staphylococcal antibiotic.
INTRODUCTION
Despite being a major cause of hospital admission and mortality, there is relatively little information available on community-acquired bacteremia in the tropics.1-10 The Lao PDR (Laos) is mostly situated between the Mekong River and the Annamite Mountains, the majority of the population of 5.2 million people are rural rice farmers, the per capita income is U.S. $304/year and life expectancy is 54 years.11 In comparison to wealthier countries in Asia, there is minimal information on the epidemiology of bacterial disease in Laos.12-16 There has been no accessible blood culture service and hence no information on the causes of blood stream bacterial infections, nor information on the drug sensitivity patterns of pathogenic organisms or evidence-based antibiotic treatment guidelines.
In 2000 we began the provision of the first routine, freely available inpatient blood culture service in Laos and describe the microbiological and clinical features of community-acquired bacteremia among patients admitted to Mahosot Hospital, the main internal medicine primary-tertiary hospital in Vientiane, the capital city.
PATIENTS AND METHODS
Study site
The study was conducted between February 2000 and 2004 among inpatients on all wards at Mahosot Hospital, Vientiane, a 365-bed hospital with approximately 1200 admissions/month.15 Peritoneal and hemodialysis, cancer and antiretroviral therapy, and central venous catheterization were not available. Ethical clearance was granted by the Ethical Review Committee of the Faculty of Medical Sciences, National University of Laos.
Clinical procedures and sampling
We recruited all patients admitted with suspected community-acquired bacteremia if they gave informed verbal consent. No formal definition was used and recruitment was at the discretion of the responsible physician. History and clinical examination were recorded on standard forms. A venous blood sample was drawn aseptically for 2 blood cultures (based on tryptic hydrolysate of casein and soy peptone) (Pharmaceutical Factory No 2, Vientiane). The volume of blood taken was 5 mL/bottle for adults (≥ 15 years), 2 ml/bottle for children > 1 year, and 1 mL/bottle for infants. The ratio of blood to media was 1:10.
Microbiological procedures and definitions
Vented blood culture bottles were incubated in air at 37°C for 7 days. All bottles were examined daily and if turbid were subcultured onto blood and chocolate agar. ‘Blind’ subculture was performed on day 7 post-inoculation. Bacteria and fungi isolated were identified using standard biochemical tests.17,18 Goat blood was used for blood agars.19 Antibiotic susceptibilities were determined by the Kirby-Bauer disk diffusion method using antibiotic impregnated disks (Oxoid, Basingstoke, UK),20 and minimum inhibitory concentrations (MICs) using standard broth dilution methods or Etests™ (AB Biodisk, Solna, Sweden). Methicillin susceptibility testing of S. aureus was performed only during the last 6 months of the study.
True bacteremia, hospital-acquired bacteremia, and blood culture contaminants were defined using conventional criteria.6,21 Community-acquired bacteremia was defined as clinically relevant positive blood cultures taken within 48 hours of hospital admission, or if the blood culture was drawn after 48 hours and the clinical presentation and identified pathogen were consistent with community-acquired disease. A single episode was recorded when the same organism was isolated from multiple blood culture sets during the same hospital admission.
Statistical analysis
Analysis was performed using STATA v8 (Stata Corporation, College Station, TX). Categorical variables were compared with χ2 and Fisher's exact test and continuous variables by the Student's t test, ANOVA, Mann-Whitney U, and Kruskal-Wallis tests as appropriate. Multiple logistic regression analysis (backward) was used to examine relationships between culture results and clinical details and the predictive value of the model was measured using the area under the receiver operating characteristic curve (AUROCC). Continuous variables entered in multiple logistic regressions were categorized into clinically meaningful categories and their relationship with outcome was examined using both continuous and categorical representations. None of the variables showed evidence for non-linear relationships with the outcome, so they were modeled as continuous variables.
RESULTS
Of the ∼57,000 admissions during the 4 years, a total of 4512 blood culture pairs from 4460 patients were taken; 754 (16.7%) yielded an organism, of which 483 (10.7%) were considered clinically significant community-acquired organisms, 4 (0.1%) were significant hospital-acquired organisms, and 267 (5.9%) yielded an organism that was classified as a contaminant (Table 1). The mean (95%CI) monthly percentage of patients admitted who had a blood culture drawn was 7.6% (6.9–8.3), of which 0.9% (0.7–1.1) had a clinically significant positive culture; 2772 (61%) blood cultures were taken from adults, and 1740 (39%) were from children (age < 15 years) including 341 (7.6%) from infants (< 1 year). In an analysis performed after organisms were classified according to clinical significance, an organism was cultured in both bottles for 343 of 449 patients with clinically significant bacteremia (76%) and for 157 of 246 patients with an organism regarded as a contaminant (64%) (P < 0.001). Of 782 blood cultures taken more than 2 days after admission, 40 (5.1%) grew a clinically significant organism, in comparison to 3515 taken within 2 days of admission of which 425 (12.1%) grew a clinically significant organism. The estimated minimum incidence of community-acquired bacteremia in the catchment area was 4.5 of 100,000 people/year and 8.4 of 1000 admissions/year. This assumes a catchment population of 900,000 and that Mahosot Hospital contains 30% of hospital beds in Vientiane. The mean (95%CI; range) number of clinically significant pathogenic species isolated per month was 3.8 (3.3–4.3; 1–8).
Table 1.
Organisms isolated from blood cultures, interpreted as community acquired and as contaminants, from 4,512 blood culture pairs at Mahosot Hospital, Vientiane, Laos, 2000–2004
| Clinically significant |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All |
Adults |
Children 1–15 |
Infants |
Probable contaminants |
||||||
| Organism | N | % | N | % | N | % | N | % | N | % |
| All organisms | 483 | 10.7 | 311 | 11.2 | 146 | 10.0 | 26 | 9.1 | 267 | 5.9 |
| Gram-negative organisms | 373 | 77.2 | 253 | 81.4 | 114 | 78.1 | 6 | 23.1 | 102 | 38.2 |
| Salmonella enterica serovar typhi | 246 | 50.9 | 159 | 51.1 | 87 | 59.6 | 0 | 0 | 0 | 0 |
| Escherichia coli | 60 | 12.4 | 48 | 15.4 | 10 | 6.8 | 2 | 7.7 | 0 | 0 |
| Klebsiella pneumoniae* | 20 | 4.1 | 15 | 4.8 | 3 | 2.1 | 2 | 7.7 | 12 | 4.5 |
| Burkholderia pseudomallei | 14 | 2.9 | 12 | 3.9 | 2 | 1.4 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 13 | 2.7 | 4 | 1.3 | 8 | 5.5 | 1 | 3.8 | 0 | 0 |
| Salmonella species | 9 | 1.9 | 6 | 1.9 | 3 | 2.1 | 0 | 0 | 0 | 0 |
| Salmonella cholerasuis | 3 | 0.6 | 3 | 1.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aeromonas hydrophilia | 3 | 0.6 | 2 | 0.6 | 1 | 0.7 | 0 | 0 | 0 | 0 |
| Edwardsiella tarda | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Proteus vulgaris | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Citrobacter freundii | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shigella flexneri | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Haemophilus influenzae | 1 | 0.2 | 0 | 0 | 0 | 0 | 1 | 3.8 | 0 | 0 |
| Enterobacter species | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 9.7 |
| Pseudomonas species† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 6.7 |
| Acinetobacter species | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 3.7 |
| Burkholderia cepacia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 2.9 |
| Klebsiella species | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2.2 |
| Ochrobacterium anthropi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.4 |
| Others‡ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 7.9 |
| Gram-positive organisms | 109 | 22.6 | 57 | 18.3 | 32 | 21.9 | 20 | 76.9 | 163 | 61.0 |
| Staphylococcus aureus | 92 | 19.0 | 49 | 15.8 | 25 | 17.1 | 18 | 69.2 | 0 | 0 |
| Streptococcus pneumoniae | 6 | 1.2 | 3 | 1.0 | 3 | 2.1 | 0 | 0 | 0 | 0 |
| Streptococcus Group C | 3 | 0.6 | 0 | 0 | 2 | 1.4 | 1 | 3.8 | 0 | 0 |
| Unspeciated viridans streptococci | 2 | 0.4 | 1 | 0.3 | 1 | 0.7 | 0 | 0 | 0 | 0 |
| Streptococcus species | 2 | 0.4 | 1 | 0.3 | 1 | 0.7 | 0 | 0 | 5 | 1.9 |
| Streptococcus Group B | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Streptococcus Group G | 1 | 0.2 | 0 | 0 | 0 | 0 | 1 | 3.8 | 0 | 0 |
| Streptococcus milleri | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Streptococcus bovis | 1 | 0.2 | 1 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coagulase negative Staphylococcus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95 | 35.6 |
| Enterococcus species | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 3.4 |
| Aerococcus viridans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.1 |
| Others§ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 51 | 19.1 |
| Fungi | 1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.8 |
| Candida species | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.8 |
| Cryptococcus neoformans | 1 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
An additional 4 Klebsiella pneumoniae isolates were thought to have been hospital-acquired.
Unidentified Pseudomonas spp. (6), Ps. fluorescens (7), Ps. putida (1), Ps. stutzeri (3), Ps. [Chryseomonas] luteola (1).
Unidentified Gram-negative organisms (8), Pantoea spp. (6), Serratia spp. (3), Morganella sp. (1), Flavimonas sp. (1), Agrobacterium sp. (1), Alcaligenes sp. (1).
Unidentified Gram-positive organisms (10), Micrococcus sp. (1), Staphylococcus species of uncertain identity (36), Corynebacterium spp. (4).
Blood culture isolates
Clinically significant isolates were obtained from 11.2% of adults, 9.9% of children, and 8.2% of infants (see Table 1). Of the clinically significant organisms grown from patients with community-acquired bacteremia, 373 (77%) were Gram-negative, 109 (23%) were Gram-positive, and 1 patient had cryptococcemia. Of these, 23 were isolated more than 2 days after admission (Salmonella enterica serovar typhi (S. Typhi) (N = 16) and B. pseudomallei (N = 7)). Overall, 3 pathogens made up 80% of all isolates, with S. Typhi being the most frequent isolate (50.9%) followed by S. aureus (19.0%) and E. coli (12.4%). Four Klebsiella pneumoniae infections were thought to be hospital acquired. There were no significant differences in the spectrum of bacteria isolated from children (< 15 years) and adults (P > 0.02). The organisms most commonly isolated from children aged 1–5 years were S.Typhi (44%), S. aureus (17.7%), and E.coli (17.7%). For children aged < 1 year, the common species isolated were S. aureus (69.2%), E. coli (7.7%), and K. pneumoniae (7.7%) with no S.Typhi isolated. The frequency of S.Typhi was lower (P < 0.0001) and the frequency of S. aureus higher (P < 0.0001) in those < 1 year old in comparison to those 1–5 years old.
None of the 7 patients with Ps. fluorescens cultured had received a blood transfusion,22 and none of 10 patients with Acinetobacter species cultured had pneumonia.23 Ten patients < 1 week old had blood cultures positive for coagulase-negative staphylococci, but none of these infants were premature and these were considered to be contaminants.
Clinical features
Comparison of patients with Gram-positive, non-typhoidal Gram-negative and S.Typhi bacteremia suggested that the 3 groups differed significantly (P < 0.02) for 23 variables (Table 2). Subsequent paired analysis suggested that patients with S.Typhi bacteremia differed significantly from those with Gram-positive and non-typhoidal Gram-negative bacteremia for 16 and 18 variables, respectively (see Table 2).
Table 2.
Clinical features of patients with and without clinically significant blood culture isolates, Gram-negative non-typhoidal bacteremia, Gram-positive bacteremia and with S. Typhi bacteremia
| Blood cultures judged clinically |
Community-acquired bacteremia |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable* | Negative or not significant |
Significant† | P | Non-typhoidal Gram-negative bacteria |
P‡ | Gram-positive bacteria |
P‡ | S. Typhi† | P§ |
| N | 4029 | 483 | 127 | 109 | 246 | ||||
| Age/years∥ | 20 (0–100)3981 | 22 (0–95)480 | 0.1 | 37 (0–95)127 | < 0.0001 | 15 (0–90)108 | 0.4 | 20 (1–74)244 | 0.0001 |
| No (%) < 15 years | 1568/4029 (39) | 172/483 (35) | 0.2 | 33/127 (26) | 0.07 | 52/109 (48) | 0.03 | 87/246 (35) | 0.002 |
| No (%) male | 2331/3995 (58) | 265/483 (55) | 0.1 | 60/127 (47) | – | 66/109 (61) | – | 138/246 (56) | 0.1 |
| No days ill∥ | – | 6 (1–180)357 | – | 4 (1–60)78 | < 0.0001 | 4 (1–120)79 | < 0.0001 | 7 (1–180)200 | 0.0001 |
| Headache | 2667/3843 (70) | 306/461 (66) | 0.2 | 79/119 (66) | – | 56/101 (56) | – | 170/240 (71) | 0.02 |
| Abdominal pain | 1320/3812 (35) | 202/460 (45) | < 0.001 | 50/119 (42) | 0.1 | 31/101 (31) | 0.001 | 121/239 (51) | 0.003 |
| Diarrhea | 1060/3801 (28) | 171/463 (37) | < 0.001 | 28/119 (24) | < 0.001 | 27/103 (27) | < 0.001 | 116/240 (48) | < 0.001 |
| Vomiting | 1198/3810 (31) | 118/343 (34) | 0.01 | 29/119 (25) | 0.6 | 23/101 (23) | 0.4 | 66/242 (27) | 0.8 |
| Dyspnea | 839/3769 (22) | 107/463 (23) | 0.7 | 39/120 (33) | < 0.001 | 33/102 (33) | < 0.001 | 34/240 (14) | < 0.001 |
| Diabetes mellitus | 134/3746 (4) | 48/452 (10) | < 0.001 | 25/115 (22) | < 0.001 | 12/101 (12) | 0.02 | 11/235 (5) | < 0.001 |
| Chronic renal failure | 54/3736 (1) | 11/448 (3) | 0.1 | 7/114 (6) | 0.001 | 3/98 (3) | 0.04 | 1/235 (0.4) | 0.005 |
| Systemic lupus | 80/3688 (2) | 5/440 (1) | 0.2 | 4/111 (4) | 0.004 | 1/196 (0.5) | 0.1 | 0/232 | 0.01 |
| Renal calculi | 77/3752 (2) | 11/451 (3) | 0.6 | 6/112 (6) | 0.002 | 4/100 (4) | 0.01 | 1/238 (0.4) | 0.01 |
| Steroid use | 106/3747 (3) | 20/454 (4) | 0.06 | 10/115 (9) | 0.001 | 7/100 (7) | 0.004 | 3/238 (1) | 0.002 |
| Smoker | 539/3713 (15) | 73/454 (16) | 0.4 | 30/116 (26) | < 0.001 | 17/97 (18) | 0.07 | 25/240 (10) | 0.001 |
| Temperature/°C¶ | 38.7 (38.6–38.7)3831 | 39.0 (38.9–39.0)455 | < 0.0001 | 38.9 (38.8–39.1)122 | 0.3 | 38.7 (38.4–38.9)102 | < 0.0001 | 39.1 (39.0–39.2)230 | 0.0002 |
| Pulse/minute¶ | 101.7 (101.1–102.3)3842 | 102.2 (100.4–104.1)457 | 0.6 | 103.7 (100.2–107.1)118 | 0.1 | 109.8 (104.5–115.0)103 | < 0.0001 | 98.2 (96.3–100.2)235 | < 0.0001 |
| BP systolic mm Hg¶ | 104.9 (104.4–105.5)3304 | 104.1 (102.4–105.8)402 | 0.3 | 108.4 (104.0–112.9)104 | 0.004 | 105.7 (100.6–110.7)75 | 0.2 | 101.6 (100.0–103.1)223 | 0.003 |
| BP diastolic mm Hg¶ | 67.2 (66.8–67.6)3294 | 66.1 (65.0–67.1)399 | 0.06 | 68.4 (65.5–70.6)103 | – | 66.0 (63.5–68.4)74 | – | 65.2 (64.0–66.4)222 | 0.07 |
| Respiratory rate/min∥ | 25 (10–140)3403 | 25 (10–100)376 | 0.5 | 24 (10–100)108 | 0.6 | 28 (18–90)81 | 0.001 | 25 (18–90)186 | 0.006 |
| Jaundiced | 728/3695 (20) | 91/439 (21) | 0.6 | 34/116 (29) | 0.001 | 24/93 (26) | 0.02 | 33/229 (14) | 0.002 |
| Hepatomegaly | 599/3684 (16) | 80/442 (18) | 0.3 | 28/115 (24) | – | 15/95 (16) | – | 37/231 (16) | 0.1 |
| Splenomegaly | 190/3651 (5) | 30/441 (7) | 0.2 | 11/115 (10) | – | 8/94 (9) | – | 11/231 (5) | 0.2 |
| Rash | 355/3642 (10) | 37/436 (9) | 0.4 | 6/115 (5) | 0.6 | 21/94 (22) | < 0.001 | 9/226 (4) | < 0.001 |
| Meningism | 263/3646 (7) | 21/440 (5) | 0.06 | 8/114 (7) | 0.01 | 9/97 (9) | 0.002 | 4/228 (2) | 0.006 |
| Septic arthritis | 139/3638 (4) | 19/432 (4) | 0.6 | 8/113 (7) | 0.004 | 8/89 (9) | < 0.001 | 3/229 (1) | 0.003 |
| Abscess | 89/3597 (3) | 20/431 (5) | 0.009 | 7/113 (6) | < 0.01 | 10/93 (11) | < 0.001 | 3/224 (1) | 0.001 |
| White cell count 109/L∥ | – | 10.8 (1.2–61.8)333 | – | 12.7 (2.2–39.3)74 | < 0.0001 | 13.0 (1.3–44.2)72 | < 0.0001 | 9.2 (2.3–38.6)187 | 0.0001 |
| Neutrophils%∥ | – | 69 (2–94)332 | – | 78 (2–94)73 | < 0.0001 | 75 (10–93)72 | 0.004 | 67 (35–94)187 | 0.0001 |
| Mortality | 306/3489 (9) | 46/420 (11) | 0.1 | 24/103 (23) | < 0.001 | 18/98 (18) | < 0.001 | 3/218 (1) | < 0.001 |
NOTE:
P value for ANOVA, Krusksall-Wallis, test or χ2 test—if P < 0.02
P value for comparison with patients with S. Typhi bacteremia given (Sceffe test if ANOVA performed). Superscript numbers refer to the sample size for that category.
There were no significant differences (P > 0.02) for all comparisons for the following variables that are not shown in the table: nausea, vomiting, cough, sputum, dysuria, arthralgia, sore throat, alcohol excess, illicit IV drug use, level of consciousness, hematocrit and platelet count.
Comparison of those with non-typhoidal bacteremia and S.Typhi bacteremia suggested that S.Typhi patients were younger (P = 0.003), ill for longer (P < 0.0001), had a higher frequency of abdominal pain (P = 0.003) and diarrhea (P < 0.001), lower frequency of dyspnea (P < 0.001), diabetes (P < 0.001), chronic renal failure (P = 0.004), renal calculi (P = 0.003), steroid use (P = 0.001), smoking (P = 0.001), jaundice (P = 0.001), rash (P < 0.001), meningism (P = 0.002), septic arthritis (P = 0.001) and abscess(es) (P = 0.001), higher admission temperature (P = 0.003), lower admission pulse (P < 0.0001), systolic blood pressure (P = 0.001), peripheral white count (P < 0.001) and neutrophil percentage (P < 0.0001).
Median (range)
Mean (95% CI).
In comparison to patients with typhoid, patients with Gram-positive bacteremia were ill for fewer days, were less likely to have abdominal pain or diarrhea and more likely to have used steroids or have had dyspnea, rash, meningism, septic arthritis, abscess or renal calculi, had a higher pulse rate, respiratory rate, peripheral white count and percentage of neutrophils, and lower admission temperature (see Table 2). In comparison to patients with typhoid, those with non-S.Typhi Gram-negative bacteremia were older, ill for fewer days, and less commonly had diarrhea, but more commonly had dyspnea, jaundice, meningism, septic arthritis, or abscesses and a history of diabetes, chronic renal failure, systemic lupus, renal calculi, to have used steroids or to be a smoker, and to have a higher systolic blood pressure and percentage of neutrophils in the peripheral white count.
Of 18 infants with S. aureus bacteremia, 13 (72%) were neonates, 9 were dyspneic, 7 were jaundiced, 4 had diarrhea, and 1 had a cutaneous abscess. None had scabies. Blood cultures from a 31-year-old fisherman admitted with fever and jaundice, with a history of cholecystectomy and postoperative biliary tract obstruction, grew Edwardsiella tarda. A 67-year-old housewife, with diabetes and using steroids, was admitted with 5 days of diarrhea and fever. Shigella flexneri was grown from her blood cultures and she deteriorated and was taken home moribund. B.pseudomallei was isolated from the blood of 14 (3%) patients; 6 had pneumonia, 2 had septic arthritis, and 5 had diabetes.
Antimicrobial susceptibility patterns
Antibiotic disk diffusion susceptibility testing of 226 S.Typhi isolates demonstrated that 12% were resistant to ampicillin, 11% resistant to co-trimoxazole, and 12% resistant to chloramphenicol, while none were resistant to nalidixic acid; 6% were multi-drug resistant (resistant to ampicillin, co-trimoxazole, and chloramphenicol; MDR) (Table 3). No methicillin-resistant S. aureus were identified. Two of four S. pneumoniae tested were resistant to oxacillin by disc diffusion (penicillin MICs were determined for 2 isolates to be 0.032 (sensitive) and 1.0 μg/mL (intermediately resistant)). The mean (95%CI) co-trimoxazole E-test MIC for B. pseudomallei was 0.20 (0.12–0.28) μg/mL; all were sensitive.24
Table 3.
Percentage of isolates susceptible to selected antibiotics for organisms isolated from blood cultures
| Organism | Ampicillin | Co-trimoxazole | Chloramphenicol | Ceftriaxone | Nalidixic acid | Gentamicin | Penicillin | Erythromycin |
|---|---|---|---|---|---|---|---|---|
| Salmonella enterica serovar typhi* | 198/226 (88%) |
197/221 (89%) |
199/225 (88%) |
50/50† | 168/168 | – | – | – |
| Salmonella cholerasuis | 2/2 | 2/2 | 2/2 | – | 1/1 | – | – | – |
| Salmonella species | 7/8 (88%) |
7/8 (88%) |
6/7 (86%) |
3/3 | 7/7 | – | – | – |
| Edwardsiella tarda | 0/1 | 1/1 | 0/1 | 1/1 | 1/1 | – | – | – |
| Escherichia coli | 13/51 (25%) |
17/47 (36%) |
34/55 (62%) |
23/25 (92%) |
39/45 (87%) |
11/12 (92%) |
– | – |
| Klebsiella pneumoniae | 1/18 (6%) |
12/17 (71%) |
12/18 (67%) |
6/6 | 15/18 (83%) |
9/9 | – | – |
| Aeromonas hydrophilia | 0/1 | 3/3 | 3/3 | 1/1 | 2/2 | – | – | – |
| Burkholderia pseudomallei | – | 10/10† | 11/12 (92%) |
– | – | – | – | – |
| Staphylococcus aureus | – | 58/74 (78%) |
63/80 (79%) |
– | – | 66/67 (99%) |
9/72 (13%) |
32/78 (41%) |
| Streptococcus pneumoniae | – | 1/5 (20%) |
4/4 | – | – | – | 2/4‡ (50%) |
2/5 (40%) |
MIC = minimum inhibitory concentrations.
MICs were determined for 163 S.Typhi isolates; 50 by broth dilution and 113 by E test. 19/163 (11.7%) and 2/163 (1.5%) were resistant or had intermediate resistance to co-trimoxazole, 19/163 (11.7%) and 1/163 (0.6%) were resistant or had intermediate resistance to chloramphenicol, 19/161 (11.8%) and 1/161 (0.6%) were resistant or had intermediate resistance to ampicillin. 18/161 (11.2%) were MDR. None were resistant to nalidixic acid (N = 163), ofloxacin (N = 135), and ceftriaxone (N = 50). The median (range) MICs (μg/mL) were co-trimoxazole 0.03 (0.016– > 512), chloramphenicol 4 (0.15– > 256), ampicillin 0.5 (0.05– > 512), ceftriaxone 0.03 (0.015–0.125), nalidixic acid 1.5 (1–8) and ofloxacin 0.06 (0.015–1.0).
As determined from oxacillin disc diffusion testing.
Disk diffusion technique, except† by E test. No S. aureus isolates were methicillin resistant.
Predictors of all-cause bacteremia and S.Typhi bacteremia
Univariate analysis indicated that abdominal pain (P < 0.001), vomiting (P = 0.01), diarrhea (P < 0.001), a history of diabetes mellitus (P < 0.001), an axillary temperature (P < 0.0001), and the presence of an abscess (P = 0.009) were associated with a positive clinically significant community-acquired bacteremia among those who had a blood culture drawn (see Table 2). Multiple logistic regression, with these variables as a starting set, identified all variables as independent predictors of a positive blood culture (Table 4).
Table 4.
Multiple Logistic Regression of variables predictive of S.Typhi bacteremia, community-acquired bacteremia and mortality
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Predictive of blood cultures growing S. Typhi versus a non-S. Typhi clinically significant cause of community-acquired bacteremia AUROCC (95%CI) = 0.809 (0.760–0.857) | |||
| Age/years | 0.95 | 0.93–0.97 | < 0.001 |
| Days ill | 1.04 | 1.00–1.08 | < 0.001 |
| Diarrhea | 3.56 | 1.63–7.83 | 0.001 |
| Axillary temperature °C | 1.94 | 1.20–3.12 | 0.007 |
| Peripheral white count 109/L | 0.88 | 0.83–0.94 | < 0.001 |
|
Predictive of blood cultures growing or not growing a clinically significant cause of community-acquired bacteremia AUROCC (95%CI) = 0.645 (0.615–0.674) | |||
| Abdominal pain | 1.40 | 1.09–1.79 | 0.007 |
| Vomiting | 0.74 | 0.58–0.95 | 0.018 |
| Diarrhea | 1.44 | 1.12–1.86 | 0.005 |
| Diabetes mellitus | 3.16 | 2.16–4.62 | <0.001 |
| Abscess | 2.29 | 1.33–3.94 | 0.003 |
| Axillary temperature °C | 1.45 | 1.28–1.65 | < 0.001 |
|
Predictive of death among patients with community-acquired bacteremia AUROCC (95%CI) = 0.738 (0.643–0.833) | |||
| Axillary temperature °C | 0.47 | 0.30–0.76 | 0.002 |
| Neutrophils% | 1.06 | 1.02–1.10 | 0.005 |
| Pulse/minute | 1.02 | 1.00–1.04 | 0.041 |
AUROCC (95%CI) = area under the receiver operating characteristic curve with 95% confidence interval.
The mean (95%CI) ratio of admission pulse to temperature was lower in those with S.Typhi (2.53 (2.48–2.58) min−1/°C) than those with non-typhoidal bacteremia (2.76 (2.68–2.84) min−1/°C) (P < 0.0001). AUROCC (95%CI) for the pulse: temperature ratio as predictive of S.Typhi bacteremia was 0.386 (0.333–0.439).
Multiple logistic regression was performed to identify independent clinical and laboratory features predicting the diagnosis of S.Typhi bacteremia versus non-S.Typhi bacteremia with the 20 variables significant (P < 0.02) on univariate analysis (see Table 2) included. Typhoid was associated with a lower peripheral white count, higher admission temperature, a longer duration of illness, the presence of diarrhea, and a younger age (see Table 4).
Outcome
Data on outcome were available for 3909 patients (87%), of whom 16 were transferred, 189 are known to have died, and 163 were taken from the hospital to die at home, giving an overall mortality of 9.0%. Patients taken home to die were moribund, received no further known effective medical care, and the overwhelming majority are expected to have died. Of 483 patients whose blood grew a clinically significant community-acquired organism, outcome is known for 420 of whom 31 (7.4%) are known to have died, 3 were transferred, and 15 were taken home to die. The overall mortality for patients with a clinically significant blood culture was therefore 11.0%. The mortalities in those with Gram-positive and Gram-negative sepsis were 18.4 and 8.4%, respectively (P = 0.006). The mortality of non-typhoidal Gram-negative sepsis was 23%. The organism specific mortalities (including patients taken home to die) were 1.4% for S.Typhi, 12% for E.coli, 17% for S.aureus, 18% for K. pneumoniae, and 60% for B. pseudomallei. Twenty-one patients who had an axillary temperature < 37.5°C on admission and discharge information grew an organism thought to represent community-acquired bacteremia and 6 of 21 (29%) died. In contrast, only 37 of 373 (9.9%) of those admitted with a temperature ≥ 37.5°C died (P = 0.018). Four of 16 infants (25%) with S. aureus bacteremia and outcome data died or were taken home moribund.
Univariate comparison of the variables in Table 2 between those with community-acquired bacteremia who lived and died gave significant (P < 0.02) differences for 5 variables. The median (range) number of days of illness for those who died was 7 (1–120) days and for those who lived 3.5 (1–60) days (P = 0.005). Dyspnea was recorded for 73 of 356 (21%) of survivors and 17 of 44 (39%) for those who died (P = 0.007). Mean (95%CI) admission temperature was lower in those who died (38.6 (38.2–38.9)°C) than in those who survived (39.0 (38.9–39.1)°C) (P = 0.008). Mean (95%CI) admission pulse was higher in those who died (111.2 (103.2–119.2)/min) than in those who survived (101.3 (99.3–103.3)/min) (P = 0.002). The median (range) percentage of neutrophils in the peripheral blood of those who lived and died were 69% (2–94) and 79% (35–93), respectively (P = 0.01). Multiple logistic regression, using the above 5 variables, identified admission temperature, pulse, and neutrophil % as independently associated with death (see Table 4).
DISCUSSION
S.Typhi was the predominant organism isolated from blood cultures among patients presenting with community-acquired bacteremia to a hospital in Vientiane. This is similar to the situation in southern Vietnam, where 67% of clinically significant bacteria cultured from patients admitted with community-acquired bacteremia were S.Typhi.8 Typhoid is considered rare in southern Laos, an impression supported by the paucity of reports of intestinal perforation from hospitals in this area. In adjoining northeast Thailand typhoid is extremely rare,4,5 emphasizing the heterogeneity of typhoid epidemiology in southeast Asia. Study limitations include the lack of information on antibiotic treatment before and after discharge and detailed information on co-morbidity. The decision as to whether a blood culture should be drawn was the responsibility of a diversity of doctors and some septic patients may not have had cultures taken. Only 11% of the Lao population lives in the relatively urbanized areas of Vientiane City, and the results of this study may not be generalizable to the rest of the country. In Vientiane a considerable percentage (25–50%) of patients admitted with fever had taken prior antibiotics, usually ampicillin or co-trimoxazole, and this will have reduced our probability of growing clinically significant organisms, especially S. pneumoniae (Khennavong M and others, unpublished data).
The admission pulse:temperature ratio was significantly lower in those with S.Typhi in comparison to those with other causes of community-acquired bacteremia, consistent with the finding of relative bradycardia in typhoid. However, the difference is insufficient to be clinically useful and will be confounded by relative bradycardia associated with other clinically similar diseases such as scrub typhus,25 which is also common in Vientiane.15
The S.Typhi isolated from patients in Vientiane had a relatively low MDR frequency. This contrasts with findings from the Indian subcontinent, Burma, and Vietnam, where multi-drug resistance has spread widely. In Rangoon 86–93% of S.Typhi isolates were MDR.10 As first-line drugs have become ineffective, there has been an increase in community use of fluoroquinolones, to which Salmonellae were originally exquisitely sensitive.26 Resistance has followed rapidly. In this series all isolates were sensitive to nalidixic acid. This contrasts with India and southern Vietnam, where the majority of isolates are both MDR and moderately resistant to fluoroquinolones.26 The relative susceptibility of S.Typhi in Laos probably relates to limited population movement and extreme poverty limiting the use of anti-infective medicines. However, in May 2004 a 20-year-old rice farmer from the Plain of Jars (Xieng Khuang Province) was admitted with blood cultures growing MDR S.Typhi also resistant to nalidixic acid. She is not known to have visited Vietnam.
H. influenzae, S. pneumoniae, and N. meningitidis were either very rarely isolated or not found in this series, in contrast to the situation in Europe and North America, but similar to previous reports from Asia,5,7,8 although the relative unimportance of H. influenzae in southeast Asia has been questioned.28 E. tarda bacteremia has been described in 4 Thai patients,29,30 and is a Mekong River fish pathogen. Fishing is a very important source of food in rural Laos and that organisms derived from fish and freshwater may be common is also suggested by the isolation of A. hydrophilia from 3 patients. Thirteen patients with P. aeruginosa bacteremia were identified in this series. Community-acquired P. aeruginosa bacteremia has been described among otherwise healthy children in Taiwan.31 In our series, 9 were children and the median (range) number of days between admission before blood culture was 0 (0–4), 3 had diarrhea, and 3 died. Whether the patients in our series had genuine community-acquired P. aeruginosa bacteremia was difficult to determine with certainty, although no other cause was found to account for their illness.
S.Typhi was the commonest pathogen cultured from children as well as from adults. In children aged 1–5 years typhoid was responsible for 44% of community-acquired bacteremias. In contrast, among 19,339 Kenyan children (< 13 years) only 1 child had S.Typhi bacteremia; the organisms isolated most commonly were S. pneumoniae and non-typhoidal Salmonella.3 The high proportion of Lao infants with S. aureus bacteremia (69%) is higher than in most previous reports.3,32,33 As 72% of these infants were neonates, at least some of these infections may have been hospital acquired. Of 43 Kenyan infants with S. aureus bacteremia, 25 (58%) had no identifiable focus and presented with pneumonia and gastroenteritis.33 Absence of a focus was associated with high mortality perhaps because they tended not to be treated with a specific anti-staphylococcal antibiotic. With a mortality of 25% in this Lao series, anti-staphylococcal antibiotic therapy should be considered in infants, especially neonates, irrespective as to whether they have a potential infected focus.33,34
No S. Paratyphi were cultured in the 4 years of this study, in striking contrast to the situation in Nepal and India.35 Non-typhoidal salmonellae were rare causes of bacteremia in Vientiane. The prevalence of HIV infection in Laos is lower than in adjoining countries, with a 1.1% prevalence of HIV antibodies among ‘women working in bars, night clubs, and guesthouses’ in Vientiane in 2001.36 As it is likely that the HIV prevalence in Laos will rise, the incidence of non-typhoidal Salmonella and Cryptococcus bacteremia may also increase.5
To estimate the necessary duration of a study to determine the main causes of community-acquired bacteremia, the cumulative number of clinically significant taxa isolated is plotted against study duration in Figure 1. The number of taxa did not reach an asymptote after 4 years and the first year of the study would have identified only 12 (52%) of the total taxa that were identified in 4 years. However, in one year the main causes of bacteremia, responsible for 97% of the cases during the 4 years, would have been identified.
Figure 1.
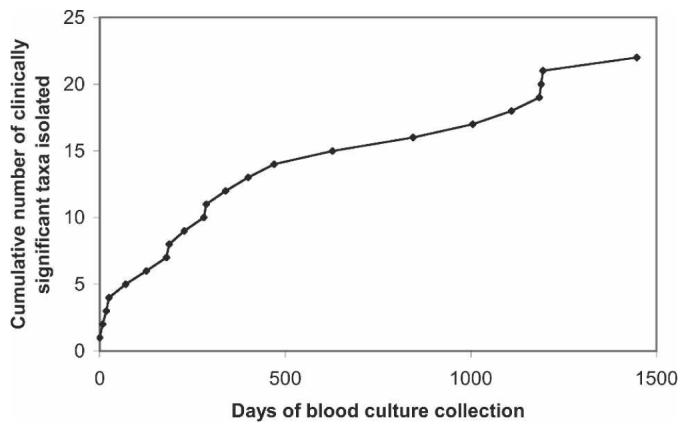
Cumulative number of bacterial and fungal taxa cultured during the 4 years of the bacteremia study.
Public health measures, such as improved sanitation and vaccination, to reduce the incidence of typhoid, may have a significant impact on morbidity and mortality in northern Laos, as it appears to have done in Thailand.37 However, vaccination is relatively expensive in Laos costing ∼US $8/dose. With improved trans-border road communications the prevalence of MDR and nalidixic acid resistant typhoid may increase in Laos.
Antibiotic guidelines have recently been introduced for typhoid and melioidosis. For community-acquired bacteremia, empirical therapy with parenteral ampicillin and gentamicin would have some activity against ∼88% of clinically significant organisms identified at a local cost of ∼ US $1.4/day in adults. This regimen would not benefit patients with melioidosis, which is difficult to diagnose clinically and has a very high mortality. Ceftriaxone would have activity against a broader range of organisms and is likely to have some benefit in patients with melioidosis (although it is inferior to ceftazidime),38 but the local daily drug cost would be higher at ∼US $3.0/day. Intravenous cloxacillin is not widely available and is also relatively expensive at ∼US $3.6/day.
Acknowledgments
We are very grateful to all the patients who participated in this study, the hospital staff, especially Ammala Xayyavong, Viengmone Davong, Olay Lattana, Manivanh Vongsouvath, Anisone Changthongthip, Viengmala Siharath, Oday Phimmasone, Soulignasack Thongpaseuth, Sengmani Symanivong, Kaiamporn Keopaseuth, and Aratsany Chandara. We are very grateful for the help and advice of Christopher Parry, Kasia Stepniewska, Jay Berkley, Miriam Laufer, Chirapha Darasavath, Oudayvone Rattanavong, Siho Sisouphone, Ko Chang, Vannaporn Wuthiekanun, Andrew Simpson, Jenny Short, Nicholas Day, and the physicians of Health Frontiers. Syseng Khounsy and the Department of Livestock and Fisheries kindly provided goat blood. SP is supported by a Wellcome Trust Career Development Award in clinical tropical medicine. We are very grateful to the Minister of Health, His Excellency Dr Ponmek Dalaloy, and the Director of the Curative Department, Ministry of Health, Professor Sommone Phounsavath for their support for this study, which was part of the Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration.
Financial support: This work was supported by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Alausa KO, Montefiore D, Sogbetun AO, Ashiru JO, Onile BA, Sobayo E. Septicaemia in the tropics. A prospective epidemiological study of 146 patients with a high case fatality rate. Scand J Infect Dis. 1977;9:181–185. doi: 10.3109/inf.1977.9.issue-3.05. [DOI] [PubMed] [Google Scholar]
- 2.Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis. 2001;7:302–305. doi: 10.3201/eid0702.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, Davis TM, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 5.Chierakul W, Rajanuwong A, Wuthiekanun V, Teerawattanasook N, Gasiprong M, Simpson A, Chaowagul W, White NJ. The changing pattern of bloodstream infections associated with the rise of HIV prevalence in northeastern Thailand. Trans R Soc Trop Med Hyg. 2004;98:678–686. doi: 10.1016/j.trstmh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Douglas MW, Lum G, Roy J, Fisher DA, Anstey NM, Currie BJ. Epidemiology of community-acquired and nosocomial blood stream infections in tropical Australia: a 12-month prospective study. Trop Med Int Hlth. 2004;9:795–804. doi: 10.1111/j.1365-3156.2004.01269.x. [DOI] [PubMed] [Google Scholar]
- 7.French GL, Cheng AFB, Duthie R, Cockram CS. Septicaemia in Hong Kong. J Antimicrob Chemother. 1990;25(Suppl. C):115–125. doi: 10.1093/jac/25.suppl_c.115. [DOI] [PubMed] [Google Scholar]
- 8.Hoa NT, Diep TS, Wain J, Parry CM, Hien TT, Smith MD, Walsh AL, White NJ. Community-acquired septicaemia in southern Viet Nam: the importance of multidrug-resistant Salmonella typhi. Trans R Soc Trop Med Hyg. 1998;92:503–508. doi: 10.1016/s0035-9203(98)90891-4. [DOI] [PubMed] [Google Scholar]
- 9.Macfarlane DE, Narla VR. Bacteraemia at the University Hospital of the West Indies-a report of 222 cases. J Infect. 1985;10:126–142. doi: 10.1016/s0163-4453(85)91564-6. [DOI] [PubMed] [Google Scholar]
- 10.Shwe TN, Nyein MM, Yi W, Mon A. Blood culture isolates from children admitted to Medical Unit III, Yangon Children's Hospital, 1998. Southeast Asian J Trop Med Public Hth. 2002;33:764–771. [PubMed] [Google Scholar]
- 11.UNDP Human Development Report. 2004. [Accessed 11th September 2005]. Available at: http://www.undp.org/hdr2003/indicator/cty_f_LAO.html.
- 12.Higa N, Sithivong N, Phantouamath B, Insisienngmay S, Miyazato T, Iwanaga M. Initial stage of hospital contamination with methicillin-resistant Staphylococcus aureus in Lao People's Democratic Republic. J Hosp Inf. 2004;56:125–130. doi: 10.1016/j.jhin.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Phetsouvanh R, Phongmany S, Newton PN, Mayxay M, Ramsay A, Wuthiekanun V, White NJ. Melioidosis and Pandora's box in Lao PDR. Clin Infect Dis. 2001;32:653–654. doi: 10.1086/318713. [DOI] [PubMed] [Google Scholar]
- 14.Phongmany S, Phetsouvanh R, Sisouphone S, Darasavath C, Vongphachane P, Rattanavong O, Phongmany S, Phetsouvanh R, Sisouphone S, Darasavath C, Vongphachane P, Rattanavong O, Mayxay M, Ramsay AC, Blacksell SD, Thammavong C, Syhavong B, White NJ, Newton PN. A randomized comparison of oral chloramphenicol versus ofloxacin in the treatment of uncomplicated typhoid fever in Laos. Trans R Soc Trop Med Hyg. 2005;99:451–458. doi: 10.1016/j.trstmh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B, Phiasakha K, Soukkhaseum S, Frichithavong K, Chu V, Keolouangkhot V, Martinez-Aussel B, Chang K, Darasavath C, Rattanavong O, Sisouphone S, Mayxay M, Vidamaly S, Parola P, Thammavong C, Heuangvongsy M, Syhavong B, Raoult D, White NJ, Newton PN. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–262. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashiro T, Nakasone N, Higa N, Iwanaga M, Insisiengmay S, Phounane T, Munnalath K, Sithivong N, Sisavath L, Phanthauamath B, Chomlasak K, Sisulath P, Vongsanith P. Etiological study of diarrheal patients in Vientiane, Lao People's Democratic Republic. J Clin Microbiol. 1998;36:2195–2199. doi: 10.1128/jcm.36.8.2195-2199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrow GI, Feltham RKA. Cown And Steel's Manual For The Identification Of Medical Bacteria. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 18.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. Manual of Clinical Microbiology. 7th ed. Washington, DC: American Society for Microbiology Press; 1999. [Google Scholar]
- 19.Anand C, Gordon R, Shaw H, Fonseca K, Olsen M. Pig and goat blood as substitutes for sheep blood in blood-supplemented agar media. J Clin Microbiol. 2000;38:591–594. doi: 10.1128/jcm.38.2.591-594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCCLS . Performance Standards for Antimicrobial Susceptibility Testing, 1998. Eighth Informational Supplement (M100-S8) Wayne, PA: NCCLS; [Google Scholar]
- 21.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimmer LG, Parmigiani G, Reller LB. The Clinical Significance of Positive Blood Cultures in the 1990's: a Prospective Comprehensive Evaluation of the Microbiology, Epidemiology, and Outcome of Bacteremia and Fungemia in Adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 22.Murray AE, Bartzokas CA, Shepherd AJ, Roberts FM. Blood transfusion-associated Pseudomonas fluorescens septicaemia: is this an increasing problem? J Hosp Infect. 1987;9:243–248. doi: 10.1016/0195-6701(87)90120-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang J-T, McDonald LC, Chang S-C, Ho M. Community-acquired Acinetobacter baumanii bacteremia in adult patients in Taiwan. J Clin Microbiol. 2002;40:1526–1529. doi: 10.1128/JCM.40.4.1526-1529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piliouras P, Ulett GC, Ashurst-Smith C, Hirst RG, Norton RE. A comparison of antibiotic testing methods for cotrimoxazole with Burkholderia pseudomallei. Int J Antimicrob Agents. 2002;19:427–429. doi: 10.1016/s0924-8579(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 25.Aronoff DM, Watt G. Prevalence of relative bradycardia in Orientia tsutsugamushi infection. Am J Trop Med Hyg. 2003;68:477–479. [PubMed] [Google Scholar]
- 26.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 27.Connerton P, Wain J, Hien TT, Ali T, Parry C, Chinh NT, Vinh H, Ho VA, Diep TS, Day NP, White NJ, Dougan G, Farrar JJ. Epidemic typhoid in Vietnam: molecular typing of multiple-antibiotic-resistant Salmonella enterica serotype typhi from four outbreaks. J Clin Microbiol. 2000;38:895–897. doi: 10.1128/jcm.38.2.895-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gessner BD, Sutanto A, Linehan M, Djelantik IG, Fletcher T, Gerudug IK, Ingerani Mercer D, Moniaga V, Moulton LH, Moulton LH, Mulholland K, Nelson C, Soemohardjo S, Steinhoff M, Widjaya A, Stoeckel P, Maynard J, Arjoso S. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 29.Jaruratanasirikul S, Kalnauwakul S. Edwardsiella tarda: a causative agent in human infections. Southeast Asian J Trop Med Public Hlth. 1991;22:30–34. [PubMed] [Google Scholar]
- 30.Osiri M, Tantawichien T, Deesomchock U. Edwardsiella tarda bacteremia and septic arthritis in a patient with diabetes mellitus. Southeast Asian J Trop Med Public Hlth. 1997;28:669–672. [PubMed] [Google Scholar]
- 31.Huang Y-C, Lin T-Y, Wang C-H. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: analysis of forty-three episodes. Pediatr Infect Dis J. 2002;21:1049–1052. doi: 10.1097/00006454-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Hill PC, Wong CG, Voss LM, Taylor SL, Pottumarthy S, Drinkovic D, Morris AJ. Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand. Pediatr Infect Dis J. 2001;20:868–873. doi: 10.1097/00006454-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Ladhani S, Konnana OS, Mwarumba S, English MC. Bacteremia due to Staphylococcus aureus. Arch Dis Child. 2003;89:568–571. doi: 10.1136/adc.2003.026781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The WHO Young Infants Study Group Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. Ped Infect Dis J. 1999;18(Suppl):S17–S22. doi: 10.1097/00006454-199910001-00004. [DOI] [PubMed] [Google Scholar]
- 35.Maskey AP, Day JN, Phung QT, Thwaites GE, Campbell JI, Zimmerman M, Farrar JJ, Basnyat B. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis. 2006;42:1247–1253. doi: 10.1086/503033. [DOI] [PubMed] [Google Scholar]
- 36.Phimphachanh C, Sayabounthavong K. The HIV/AIDS/STI situation in Lao People's Democratic Republic. AIDS Educ Prev. 2004;16:91–99. doi: 10.1521/aeap.16.3.5.91.35523. [DOI] [PubMed] [Google Scholar]
- 37.Bodhidatta L, Taylor DN, Thisyakorn U, Echeverria P. Control of typhoid fever in Bangkok, Thailand, by annual immunization of schoolchildren with parenteral typhoid vaccine. Rev Infec Dis. 1987;9:841–845. doi: 10.1093/clinids/9.4.841. [DOI] [PubMed] [Google Scholar]
- 38.Chaowagul W, Simpson AJ, Suputtamongkol Y, White NJ. Empirical cephalosporin treatment of melioidosis. Clin Infect Dis. 1999;28:1328. doi: 10.1086/517787. [DOI] [PubMed] [Google Scholar]


