Abstract
Drosophila neuroblasts are similar to mammalian neural stem cells in their ability to self-renew and to produce many different types of neurons and glial cells. In the past two decades, great advances have been made in understanding the molecular mechanisms underlying embryonic neuroblast formation, the establishment of cell polarity and the temporal regulation of cell fate. It is now a challenge to connect, at the molecular level, the different cell biological events underlying the transition from neural stem cell maintenance to differentiation. Progress has also been made in understanding the later stages of development, when neuroblasts become mitotically inactive, or quiescent, and are then reactivated postembryonically to generate the neurons that make up the adult nervous system. The ability to manipulate the steps leading from quiescence to proliferation and from proliferation to differentiation will have a major impact on the treatment of neurological injury and neurodegenerative disease.
Keywords: neural stem cells, neuroblast, asymmetric cell division, temporal identity, quiescence, Drosophila
1. Introduction
Neural stem cells are the precursors that generate the astonishing cell diversity found in the central nervous system (CNS). They are multipotent and can exhibit a high mitotic index. Neural stem cells undergo multiple self-renewing divisions. Self-renewal can take the form of either a symmetric cell division, whereby two equal neural stem cells are born, or an asymmetric cell division, which generates one neural stem cell and one daughter cell with a more restricted developmental potential. In the past few years, neural stem cells have been isolated from many different species, including humans (reviewed by Alvarez-Buylla et al. 2001; Temple 2001).
In the Drosophila CNS, embryonic neuroblasts undergo multiple asymmetric divisions whereby they self-renew and produce intermediate progenitor cells, called ganglion mother cells (GMCs). GMCs divide only once giving rise to two post-mitotic cells that differentiate into neurons or glial cells. In the embryo, 30 neuroblasts are born in each hemisegment in regular columns and rows. Each neuroblast can be identified by its unique gene expression profile, its time and place of birth and its neuronal and glial progeny. Each neuroblast has been assigned a name based on a Cartesian coordinate-like system (e.g. neuroblast NB6-4 is located in row 6 and column 4) and produces a near invariant number of neuronal and glial cells (reviewed by Skeath & Thor 2003). The Drosophila nervous system is an excellent model system in which the underlying molecular mechanisms directing cell diversity can be analysed at single cell resolution.
In the past two decades, great insight has been gained into neural development by studying the specification of neural tissue, neuroblast formation and neuroblast lineage commitment (Skeath & Thor 2003). More recently, the key proteins that play vital roles in setting up neuroblast polarity have been identified. The establishment of polarity is an essential first step in asymmetric cell division and the generation of different daughter cells (reviewed by Wodarz & Huttner 2003; Bardin et al. 2004; Betschinger & Knoblich 2004; Wang & Chia 2005). It has also been shown that the fate of embryonic neuroblasts changes over time, triggered by the sequential expression of a defined set of transcription factors (reviewed by Brody & Odenwald 2002; Pearson & Doe 2004). At the end of embryogenesis, many neuroblasts stop dividing and then resume mitotic activity during larval stages to generate the adult nervous system (reviewed by Maurange & Gould 2005). Therefore, postembryonic neuroblasts are an attractive model to study the transition from quiescence to proliferation. It is an open question as to which factors promote the quiescent phase and which factors are involved in the reactivation of postembryonic neuroblasts. Here, we review each of these developmental steps in a neuroblast's ‘life’ and highlight recent findings that may contribute to the understanding of neural stem cell development. While we will concentrate primarily on Drosophila neuroblasts, we also introduce some intriguing findings from vertebrate research.
2. Neuroectoderm formation
The Drosophila CNS develops from a bilateral sheet of neuroectodermal cells, on the ventral surface of the embryo. The brain develops from the anterior region, known as the procephalic neuroectoderm, whereas the ventral nerve cord (VNC) forms from the more posterior ventral neuroectoderm (Campos-Ortega & Hartenstein 1997). The study of embryonic CNS development in Drosophila has focused on the VNC, due to its relatively simple structure. As a result, this review focuses mostly on the VNC. However, recent advances have been made in our understanding of early brain patterning and neuroblast formation (Urbach & Technau 2003a,b; Urbach et al. 2003).
The dorsoventral borders of the neuroectoderm are determined in the early embryo by a ventral to dorsal nuclear gradient of the Rel/NF κB transcription factor, Dorsal (Dl; reviewed by Stathopoulos & Levine 2002). The Dl gradient functions as a repressor that restricts the expression of decapentaplegic (dpp), a member of the bone morphogenic protein family. Low levels of nuclear Dl induce expression of short gastrulation (sog) in broad bilateral stripes outlining the presumptive neuroectoderm. Sog is a secreted protein with similarities to vertebrate Chordin. The role of Sog is to antagonize the dorsalizing effect of Dpp, preventing the neuroectoderm from becoming dorsal epidermis (Ferguson & Anderson 1992; Francois et al. 1994; Holley et al. 1995; Biehs et al. 1996; Ferguson 1996).
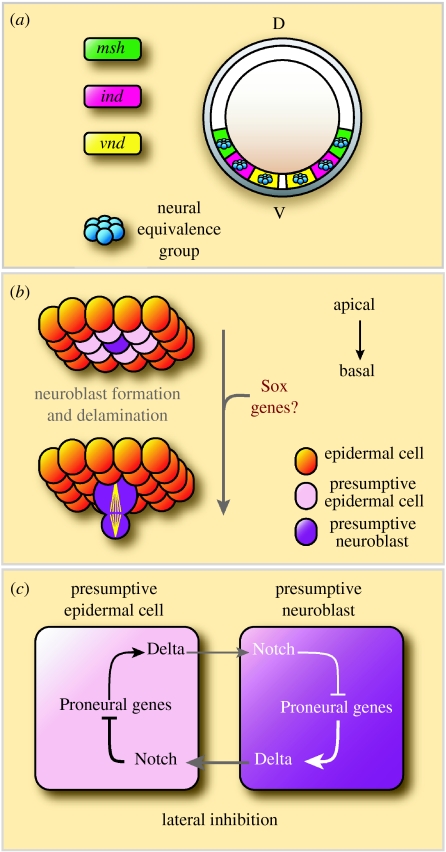
The Dpp and Sog morphogen gradients act together with EGFR (epidermal growth factor receptor) signalling to further subdivide the neuroectoderm into three longitudinal domains (figure 1a; Udolph et al. 1998; Von Ohlen & Doe 2000), each defined by expression of one of the ‘columnar’ genes, ventral nervous system defective (vnd), intermediate neuroblast defective (ind) or muscle segment homeobox (msh; reviewed by Cornell & Von Ohlen 2000). The segment polarity genes, which determine segments along the anteroposterior axis of the embryo, act in combination with the columnar genes to effectively subdivide the embryo into a chequerboard of unique ‘neural equivalence groups’. A neural equivalence group comprises five to six equally competent neuroectodermal cells, of which only one will become a neuroblast (reviewed by Skeath & Thor 2003).
Figure 1.
Neuroectoderm specification and neuroblast formation. (a) A schematic cross-section through a Drosophila embryo. The genes ventral nervous system defective (vnd), intermediate neuroblast defective (ind) and muscle segment homeobox (msh) pattern the neuroectoderm in three columnar domains in response to antagonistic morphogenetic gradients of Dpp and Sog. (b) Single cells are selected to acquire a neuroblast fate from a proneural equivalence group of five to six cells. This is achieved by the process of lateral inhibition and is based on a molecular regulatory loop between the adjacent cells. The selected neuroblast enlarges and delaminates basally into the embryo. The remaining cells of each proneural cluster adopt an alternative epidermal fate. After delamination, each neuroblast begins to divide asymmetrically in a stem cell-like manner along the apico-basal axis. (c) A simplified scheme of lateral inhibition involving Notch, Delta and the proneural genes. Activation of the Notch signalling cascade by the Delta ligand leads to repression of proneural gene expression in the presumptive non-neural cell. Since Delta expression is regulated by proneural transcription factors, downregulation of proneural genes leads to a reduction in Notch activation in the neighbouring cell. As a result, proneural gene activity is maintained in the presumptive neuroblast and repressed in its neighbours.
3. Neurogenesis in Drosophila
(a) Neuroblast formation: Notch signalling and proneural genes
Neurogenesis begins with the delamination of a single neuroblast from each neural equivalence group (figure 1b). All of the cells in an equivalence group initially express proneural genes of the achaete–scute (ac/sc) complex (reviewed by Ghysen & Dambly-Chaudiere 1989; Campuzano & Modolell 1992; Bertrand et al. 2002). These transcription factors drive ectodermal cells towards the neuroblast fate. The cell within the equivalence group that expresses the proneural genes to the highest level will adopt the neuroblast fate, while the other cells adopt an epidermal fate (Cubas et al. 1991; Skeath & Carroll 1992; Skeath & Carroll 1994).
The evolutionarily conserved Notch signalling pathway restricts proneural gene expression to a single neural progenitor cell through a process of lateral inhibition, a molecular regulatory loop between the neighbouring cells (figure 1c; reviewed by Artavanis-Tsakonas & Simpson 1991; Bray 1998). Loss of Notch function in the CNS leads to a severe neurogenic phenotype, with the majority of cells in a neural equivalence group adopting the neuroblast, rather than the epidermal, fate. Transcription factors encoded by the proneural genes positively regulate the expression of the transmembrane ligand Delta. Delta binds to the Notch receptor on adjacent cells, causing Notch to be cleaved and resulting in the nuclear localization of the Notch intracellular domain (NICD). In the nucleus, the NICD interacts with Suppressor of hairless, leading to the expression of the Enhancer of split genes. These genes encode transcriptional repressors that act on the proneural genes. Thus, lower levels of expression of the proneural genes lead to lower levels of Delta expression and a reduced capacity to activate Notch in the surrounding cells. In this way, the cell that initially has higher levels of proneural gene or Delta expression (or perhaps lower levels of Notch) will acquire the neuroblast fate, while extinguishing proneural gene expression in the surrounding cells. This mechanism of lateral inhibition allows the rapid amplification of what might initially be quite small differences in proneural gene expression or Notch pathway activity. The cell that adopts the neuroblast fate goes on to delaminate from the neuroectoderm and divides in a stem cell-like manner.
(b) Sox genes may link neuroectoderm formation and primary neurogenesis
Little is known about the mechanism linking neural tissue formation to primary neurogenesis (reviewed by Sasai 1998). In Drosophila, members of the Sox transcription factor family may play a vital role in transducing the early neurogenic signals and initiating the formation of neuroblasts. Drosophila SoxNeuro (SoxN), encoding a member of the Sox B group transcription factors, is proposed to act upstream and in parallel to the proneural genes to control neuroblast formation in the intermediate and lateral columns of the developing CNS. The results obtained from loss-of-function studies strongly argue for SoxN involvement in establishing proneural clusters and in ‘singling out’ neuroblasts. However, the role of SoxN in neuroblast formation is not mediated through the Notch pathway (Buescher et al. 2002; Overton et al. 2002). Another Drosophila group B Sox gene, Dichaete, is expressed in the medial and intermediate columns of the CNS, where it is similarly involved in this process of neuroblast formation (Buescher et al. 2002; Overton et al. 2002; Zhao & Skeath 2002). Intriguingly, the three vertebrate group B Sox proteins, Sox1, Sox2 and Sox3, have also been shown to play a role in neural development, suggesting that they perform a role that is conserved in evolution. Vertebrate Sox2 was shown to be involved in maintaining rather than establishing the progenitor cell fate in the developing cortex of chicken embryos (Graham et al. 2003). Therefore, Sox genes might have several functions during neurogenesis and further understanding awaits a stepwise analysis of Sox gene function during neural progenitor formation, maintenance and differentiation.
4. Drosophila neuroblasts: maintenance and differentiation
What are the properties that distinguish neural stem cells from intermediate progenitor cells? In the following sections, we address some of these properties in Drosophila neuroblasts and GMCs.
(a) Symmetric versus asymmetric divisions
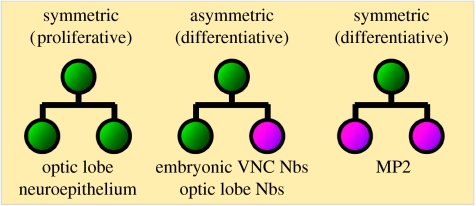
Neural stem cells divide either symmetrically, producing two identical daughter cells, or asymmetrically, producing two different daughter cells. Neural stem cells may also undergo symmetric differentiative divisions, depleting the stem cell pool and producing two developmentally restricted precursors or post-mitotic progeny (figure 2). Proliferative symmetric divisions serve to rapidly increase a progenitor pool, whereas asymmetric divisions are important for differentiation, as they generate daughter cells that can differ in size, mitotic potential and lineage commitment. Vertebrate neuroepithelial cells, which are considered to be neural stem cells, initially undergo several rounds of proliferative symmetric divisions to increase their number. Later, during neurogenesis, asymmetric divisions are observed in the ventricular zone of the developing cortex, when neuroepithelial cells directly generate neurons and self-renew. Recently, differentiative symmetric divisions were also observed in the subventricular zone of the developing cortex, where intermediate progenitors can give rise to two post-mitotic cells (Haubensak et al. 2004; Miyata et al. 2004; Noctor et al. 2004; reviewed by Gotz & Huttner 2005).
Figure 2.
Neural stem cell divisions. Neural stem cells undergo symmetric proliferative or asymmetric differentiative self-renewing divisions. Neural stem cells may also undergo symmetric differentiative divisions, thereby depleting the stem cell pool and producing two developmentally restricted precursors or post-mitotic progeny. Given are some examples for each division type in Drosophila. Nbs, neuroblasts.
In Drosophila, proliferative symmetric divisions are found in the developing optic lobes (White & Kankel 1978; Hofbauer & Campos-Ortega 1990; Ceron et al. 2001; Egger et al. 2007). However, most Drosophila neuroblasts divide asymmetrically by self-renewing and budding-off smaller GMCs. Differentiative divisions occur in specialized progenitor cells (MP2 precursor). This neuroblast-like progenitor divides to generate two post-mitotic neurons (Doe 1992; Spana et al. 1995). Little is known about the molecular mechanisms that maintain the proliferative symmetric division mode in either the vertebrate cortex or the Drosophila optic lobe. How neural progenitors are driven into asymmetric differentiative divisions is also of great interest. The fine tuning of the ratio between symmetric and asymmetric cell divisions clearly affects the neuron number and therefore the outcome of a functional nervous system.
(b) Polarity cues direct the asymmetric segregation of cell-fate determinants
One key mechanism by which a cell can produce two different daughter cells is by partitioning cell-fate determinants unequally between the daughter cells. Experiments performed on neuroblasts in culture suggest that intrinsic factors are the major players directing a neuroblast's ability to divide asymmetrically and to self-renew (Broadus & Doe 1997). In the past decade, a number of polarity cues have been identified that establish the apico-basal polarity of the neuroblast and enable the asymmetric segregation of basally localized cell-fate determinants to the daughter cell (GMC). There are many excellent reviews on this topic (Wodarz & Huttner 2003; Bardin et al. 2004; Betschinger & Knoblich 2004; Wang & Chia 2005), and we will therefore summarize only the key findings and recent new insights in this area.
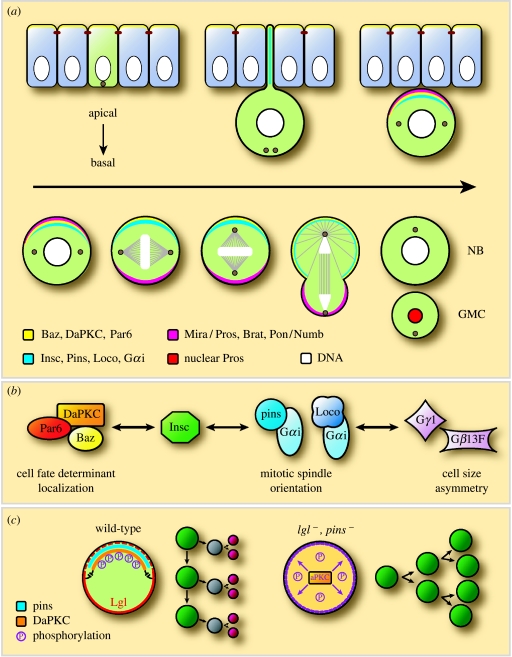
Most of the proteins that direct neuroblast polarity are expressed and localized in epithelial cells, where they act to establish apico-basal polarity. The neuroblast inherits a complement of these proteins prior to delamination (figure 3a). Neuroectodermal cells undergo horizontally oriented divisions, within the epithelial plane, resulting in the symmetric distribution of basally localized cell-fate determinants. After neuroblasts delaminate, their mitotic spindle rotates 90°, so that subsequent divisions are oriented along the apico-basal axis of the embryo (Kaltschmidt et al. 2000). Spindle rotation and the subsequent asymmetric segregation of cell-fate determinants to the basal GMC are dependent on a molecular complex at the apical cortex of the neuroblast. The apical complex (figure 3b) consists of the evolutionarily conserved Par proteins, Bazooka (Baz, Par3) and DmPar6, and the Drosophila homologue of the atypical protein kinase C, DaPKC (Kuchinke et al. 1998; Schober et al. 1999; Wodarz et al. 1999; Petronczki & Knoblich 2001). In neuroblasts, the Baz/DmPar6/DaPKC complex binds through Baz to Inscuteable (Insc), and Insc, in turn, recruits Partner of Inscuteable (Pins) and Locomotion defective (Loco), which are two GDP-dissociation inhibitors (GDIs) that interact with the heterotrimeric G protein subunit, Gαi (Kraut & Campos-Ortega 1996; Parmentier et al. 2000; Yu et al. 2000, 2003, 2005; Schaefer et al. 2001).
Figure 3.
Asymmetric neuroblast division. (a) The subcellular localization of several polarity proteins, cell-fate determinants and their adaptor proteins is indicated in different colours (see the figure). In the neuroectodermal cells, Baz, DmPar6 and DaPKC localize apically (yellow). As the neuroblast delaminates from the neuroectoderm, Insc is expressed and recruits Pins and Gαi to the apical cortex. The adaptor protein Mira becomes localized to the basal cortex, where it anchors the GMC determinants Pros and Brat. After segregating to the GMC, Mira is degraded and releases Pros, which then enters the GMC nucleus (adapted from Wodarz & Huttner 2003). (b) The main functions proposed for the different apical pathways. The Insc/Par pathway is crucial for the localization of basal cell determinants, whereas the Insc/Pins/Gαi pathway is necessary for mitotic spindle orientation. Loco together with the heterotrimeric G proteins are mainly involved in spindle asymmetry (adapted from Bellaiche & Gotta 2005). (c) DaPKC promotes self-renewing neuroblast divisions. In wild-type neuroblasts, Pins is necessary to localize DaPKC to the apical cortex. Basally active Lgl functions to inhibit basal localization of DaPKC and, thus, restrict its function to the apical side for neuroblast self-renewal. In pins lgl double mutants, DaPKC is delocalized and inherited by both daughter cells, and thus neuroblasts undergo continuous symmetric self-renewing divisions (adapted from Lee et al. 2006a).
The apical complex directs cell-fate determinants to the basal cortex. The homeodomain protein Prospero (Pros; Doe et al. 1991; Vaessin et al. 1991; Matsuzaki et al. 1992) and the PTB domain protein Numb (Uemura et al. 1989), as well as pros mRNA, are localized as basal crescents and segregate to the basal GMC (Rhyu et al. 1994; Hirata et al. 1995; Knoblich et al. 1995; Spana & Doe 1995; Li et al. 1997; Broadus et al. 1998). Only Pros appears to act as a cell-fate determinant in the GMC, whereas Numb plays a role later in development, when the GMC divides, to discriminate between the sibling neurons (Spana et al. 1995; Spana & Doe 1996; Buescher et al. 1998). Asymmetric segregation of Pros protein and pros RNA is mediated by the adaptor protein Miranda (Mira). Once segregated to the GMC, Mira is degraded, thereby releasing Pros from the cortex (Ikeshima-Kataoka et al. 1997; Shen et al. 1997; Matsuzaki et al. 1998). Pros can then enter the nucleus, where it has been thought to specify GMC identity by promoting the expression of GMC-specific genes and repressing the expression of neuroblast-specific genes (but see Choksi et al. 2006).
In addition to these apico-basally localized components, two tumour suppressor proteins, Discs large (Dlg) and Lethal (2) giant larvae (Lgl), have been identified as vital factors in the machinery for basal protein targeting (Ohshiro et al. 2000; Peng et al. 2000). Dlg is required to maintain the cortical localization of the Lgl protein and is itself cortically localized. In mutants that interfere with Dlg and Lgl functions, Pros and Mira fail to form a basal crescent and are localized primarily on the mitotic spindle.
A current model for basal protein targeting in embryonic neuroblasts proposes that at the apical cortex, DaPKC phosphorylates and inactivates the tumour suppressor protein Lgl. This in turn allows non-muscle myosin II to associate with cortical actin and to exclude Mira from the apical cortex. Conversely, at the basal side, there is no cortical DaPKC. Lgl is therefore active and inhibits myosin II, enabling Mira to bind to the basal cortex. During cytokinesis, myosin II moves along the cortex towards the cleavage furrow and seems to ‘push’ Mira protein into the GMC (Barros et al. 2003).
(c) Molecular mechanisms of establishing and coordinating polarity
As described earlier, in the Drosophila embryo, neuroblasts occupy the most apical layer of cells within the CNS, just basal to the neuroectoderm. Most of the early VNC neuroblasts produce GMCs towards the interior of the embryo. Therefore, the CNS is a polarized tissue with neuroblasts at the apical side and the neuronal and glial progeny at the basal side. An intriguing question is how neuroblasts are able to repeatedly orient their mitotic spindle and thus their division along the apico-basal axis.
The delaminating neuroblast inherits polarity cues, such as the Par proteins from the overlying neuroectoderm from which it delaminates. The initial position of the ‘Insc/Par’ (Insc/Baz/DmPar6/DaPKC) complex determines the precise and reproducible alignment of the mitotic spindle relative to the surrounding tissues (reviewed by Wodarz & Huttner 2003).
However, what are the cues required to position the Par/Insc complex at the apical neuroblast cortex and for orienting the division axis to the apico-basal plane from division to division? A recent study by Siegrist & Doe (2006) reveals that extrinsic cues play a major role in orienting cell polarity and axis of division. They show that during early neurogenesis, when neuroblasts are still in direct contact with the overlying epithelium, neuroblast divisions are always oriented perpendicular (apico-basal) to the neuroectoderm. Interestingly, when the CNS later loses contact with the overlying ectoderm layer, the initially fixed apico-basal division axis becomes more random. In experiments with cultured neuroblasts, they find that neuroblasts grown in contact with epithelial cells are able to maintain the orientation of the division axis for multiple rounds of divisions. In contrast, single cultured neuroblasts are unable to maintain their orientation of division and produce GMCs in apparently random directions.
Interestingly, in insc mutant embryos, components of the Insc/Par complex fail to localize apically (Wodarz et al. 1999, 2000; Petronczki & Knoblich 2001), and these mutants have misoriented spindles. Therefore, one hypothesis is that components of the Insc/Par complex (Insc/Baz/DmPar6/DaPKC) interact with extracellular signals and form a link with the overlying epithelium to determine the apico-basal neuroblast division axis (Siegrist & Doe 2006). Insc itself is the only molecule in the apical complex that is not expressed in the neuroectoderm, but first appears upon neuroblast delamination. insc transcription in neuroblasts is regulated by the transcription factors of the Snail protein family (Ashraf & Ip 2001; Cai et al. 2001).
A related study by Siegrist & Doe (2005) gives insight into microtubule-based mechanisms of how spindle orientation is coordinated with Insc/Par polarity. They demonstrate that astral microtubules are able to induce an Insc/Par-independent cortical polarity that results in a Pins/Gαi crescent (Pins/Gαi cortical polarity). Their experiments show that Dlg interacts with the Kinesin heavy chain 73 (Khc-73), which is localized at the astral microtubule plus ends. The Dlg/Khc-73 complex then binds to Pins to induce Pins/Gαi cortical polarity in alignment with the mitotic spindle at metaphase. The final step in their model is the alignment of cortical polarity with the apical Insc/Par crescent. In this way, the spindle is aligned perpendicular to the overlying neuroectoderm, which leads to apico-basal-oriented divisions (Siegrist & Doe 2005). Together, these two studies from the Doe group provide an attractive model of how spindle orientation and cortical polarity could be coordinated with the neuroblast's environment in the Drosophila embryo.
Very recently, the mushroom body defect (Mud) has been identified as another linking molecule between the spindle and the neuroblast cortex. Mud shares sequence similarities with vertebrate NuMA and Caenorhabditis elegans Lin-5. The three proteins are considered to be orthologues, which stimulate microtubule polymerization and bind to Pins proteins (AGS3 in mammalians and GPR proteins in C. elegans). In Drosophila mud mutant, neuroblasts fail to align their mitotic spindle with Pins/Gαi cortical polarity (Bowman et al. 2006; Izumi et al. 2006; Siller et al. 2006).
Interestingly, postembryonic neuroblasts in the larval brain completely lose contact with the ectoderm, but, nevertheless, produce ‘pockets’ of neuronal progeny in an apical to basal direction. Considering the lack of an overlying epithelium, what other environmental factors would define the apical–basal orientation? One possibility is that the enwrapping glial sheet serves as a niche (see §4d), which provides the neuroblast clone with positional information.
(d) Mechanisms of self-renewing divisions: from proliferation to differentiation
During CNS development, cell proliferation and differentiation must be precisely regulated to ensure the proper formation of different cell lineages. Premature exit from the cell cycle or overproliferation can lead to the incorrect numbers of particular cell types and thus to CNS malformation. A recent report reveals that cortical DaPKC function is crucial for self-renewing divisions and that the tumour suppressor protein, Lgl, and the apical polarity cue, Pins, restrict DaPKC function to the apical neuroblast upon division (Lee et al. 2006a).
Larval neuroblasts, similar to embryonic neuroblasts, repeatedly divide asymmetrically to self-renew a neuroblast and to produce a smaller GMC. Lee and colleagues find that in pins mutant larval brains, the number of neuroblasts is significantly decreased, and that DaPKC is delocalized from the apical cortex. In lgl mutant brains, however, the number of neuroblasts is notably increased and DaPKC shows weak ectopic cortical localization at metaphase. Surprisingly, in pins lgl double mutants, a massive increase in neuroblast number is observed and DaPKC shows ectopic cortical localization at metaphase. Similarly, overexpression of membrane-tethered DaPKC results in an increase in neuroblast number. All these neuroblasts divide in a symmetric, self-renewing, mode and fail to produce differentiating GMCs. Further genetic experiments led the authors to conclude a simple model whereby Pins anchors DaPKC apically and Lgl inhibits DaPKC localization basally, thereby restricting DaPKC to the apical cortex where it promotes neuroblast self-renewal (figure 3c). The targets of DaPKC involved in self-renewal are currently unknown, but it is possible that DaPKC positively regulates the activity of neuroblast-specific self-renewal proteins and/or negatively regulates the activity of GMC determinants (Lee et al. 2006a).
One of the key factors that regulates aspects of GMC behaviour in the Drosophila CNS is the transcription factor Prospero (Pros). At cell division, Pros is localized, via its adaptor Mira, exclusively to the basal GMC. After cell division, Pros translocates from the cortex to the GMC nucleus. It has been suggested that this translocation coincides with the decision to exit from mitosis and undergo terminal differentiation (Spana & Doe 1995). The current model proposes that Pros regulates GMC identity by repressing neuroblast genes and activating GMC-specific genes. As an example, Pros is required to negatively regulate the expression of cell cycle genes, such as cyclin A, cyclin E and the Drosophila cdc25 homologue, string, and to positively regulate the expression of dacapo, a cyclin-dependent kinase inhibitor (Li & Vaessin 2000; Liu et al. 2002). Thus, the asymmetric distribution and release of Pros to the GMC nucleus results in the repression of multiple cell cycle regulators. It has been suggested that the GMC has just enough previously transcribed and/or translated gene products to complete a single terminal division (Li & Vaessin 2000).
Recently, three research groups reported novel findings about the evolutionarily conserved NHL (NCL-1, HT2A and LIN-41) domain family member, brain tumour (Brat; Bello et al. 2006; Betschinger et al. 2006; Lee et al. 2006b). It was known from earlier studies that mutations in the brat gene cause massive overproliferation in larval brain tissue (Kurzik-Dumke et al. 1992; Woodhouse et al. 1998; Arama et al. 2000). The new findings demonstrate that Brat is also a cargo of Mira and, together with Pros, segregates asymmetrically to GMCs upon neuroblast division. Brat mutant clones reveal an increased number of progenitor cells, which seem to proliferate continuously and lack differentiation markers. Instead, mutant clones express several neuroblast-specific genes and also cyclin E. It is not entirely clear whether the brat mutant ‘GMCs’ are transformed into neuroblasts or whether brat mutant neuroblasts start to proliferate symmetrically. Two of the studies (Bello et al. 2006; Lee et al. 2006b) report the loss of Pros expression in brat mutant clones. However, one of the three studies shows that in brat mutant brains, neuroblasts still bud off smaller GMCs, and they therefore suggest that a number of these brat mutant GMCs may eventually enlarge to become proliferative neuroblasts (Lee et al. 2006b). Interestingly, it has been demonstrated that brat negatively regulates cell growth and ribosomal RNA synthesis (Frank et al. 2002). Betschinger and colleagues show that brat has an inhibitory role for the transcription factor dMyc, which is an important regulator of growth (Betschinger et al. 2006).
The fact that Pros expression is abolished in brat mutant clones suggests that the two proteins may play a role in the same molecular pathway. Indeed, Pros mutant clones in the larval brain also show an increased number of progenitor cells with a high mitotic index and an expression pattern similar to larval neuroblasts. This suggests that Pros acts downstream of Brat and, in support of this, Bello et al. (2006) demonstrate that expression of Prospero in brat mutant clones rescues the brat phenotype (Bello et al. 2006). Recently it has been shown, by whole genome mapping of the genes regulated by Prospero, that Prospero acts as a binary switch between stem cell self-renewal and differentiation, and that GMCs are transformed into neuroblasts in prospero mutant embryos (Choksi et al. 2006). Brat is involved in the control of cell growth, possibly to maintain the size of neuroblasts as they undergo self-renewal.
The challenge now is to determine whether a common molecular pathway underlies the overproliferation phenotypes observed in the case of ectopic aPKC activation and in brat/pros mutant brains. It has been speculated that in some human brain tumours, only a small number of progenitor cells, or cancer stem cells, have the ability to proliferate and self-renew (Singh et al. 2004; reviewed by Read et al. 2006). The studies presented here demonstrate that Drosophila neuroblasts are an excellent model system to investigate the molecular mechanisms that normally regulate neural stem cell differentiation and which, when misregulated, can lead to tumour formation.
(e) Neuroblasts and ganglion mother cells differ in cell size
It is striking that in the embryo the delaminated neuroblast is about twice as big as the GMC (10–12 μm in diameter versus 4–6 μm in diameter; Hartenstein et al. 1987). This simple fact raises the question of how this difference in cell size is achieved.
Studies in worms and flies reveal how cell size differences can be achieved during normal cell division (reviewed by Kaltschmidt & Brand 2002). During Drosophila neuroblast division, the mitotic spindle itself becomes asymmetric, i.e. the neuroblast aster enlarges whereas the GMC aster gets smaller and the midbody moves towards the GMC (Kaltschmidt et al. 2000). Early studies in grasshopper demonstrated that the position of the mitotic spindle is crucial for asymmetric cell division. By using neuroblast culture, it was shown that the spindle can be mechanically shifted to the middle of the dividing cell, which eventually leads to equal-sized daughter cells (Yamashiki & Kawamura 1986).
The Baz/DmPar6/DaPKC and the Pins/Gαi complexes act in a redundant manner for ‘spindle displacement’ and ‘spindle asymmetry’ (reviewed by Wodarz & Huttner 2003; Betschinger & Knoblich 2004; Wang & Chia 2005). In single mutants for baz or pins, spindle asymmetry is impaired but not lost. Only when components of both complexes are mutated (e.g. baz and pins) are symmetric neuroblast divisions observed with 100% penetrance. This suggests that the Par and the Pins/Gαi complexes function through different pathways (Parmentier et al. 2000; Cai et al. 2003). The heterotrimeric G protein subunits Gβ13F and Gγ1, both of which are distributed uniformly at the cortex, have also been shown to participate in the generation of unequal-sized neuroblast daughters (Fuse et al. 2003; Izumi et al. 2004). The current model suggests that Pins and Loco act upstream of Gβγ, but that Gβγ signalling is also necessary to maintain Pins/Gαi and Loco/Gαi complexes at the apical cortex of neuroblasts, which would indicate that there is a feedback loop (reviewed by Bellaiche & Gotta 2005).
As mentioned earlier, Loco is a component of the apical complex that exhibits both GDI activity for Gβγ and GTPase-activating protein (GAP) activity for Gαi. Loco function is required for the apical localization of Gαi and acts in parallel to the Par pathway to mediate unequal daughter cell size (Yu et al. 2005). Yu et al. combine these new data with previous knowledge to propose an interesting model whereby activated Gβγ, maintained through the GDI activity of Pins and Loco, is required for the generation of unequal daughter cells; the equilibrium between GDP–Gαi and GTP–Gαi, at least partially regulated by the GAP activity of Loco, is required for the localization of Insc/Pins/Loco at the apical cortex in neuroblasts (Yu et al. 2005).
How do genetically manipulated, equal-sized, daughter cells behave in subsequent divisions? Do they behave like neuroblasts and undergo asymmetric divisions or can they proceed with additional symmetric divisions? In the spindle shifting experiments described earlier, the resulting daughter cells are sometimes larger than normal GMCs or smaller than neuroblasts. Interestingly, only cells of a certain critical size show neuroblast properties: shorter cell cycle times; high mitotic potential; and unequal division. Cells smaller than this size show GMC properties: longer cell cycles; low mitotic potential; and equal division, or else they stop dividing. Indeed, it was also shown in vivo that the average cell cycle of GMCs is twice as long as that of neuroblasts (Hartenstein et al. 1987). Little is known about what molecular mechanisms govern the intriguing link between cell size and cell cycle length, or cell size and cell fate.
(f) Multipotency: neuroblasts, glioblasts and neuroglioblasts
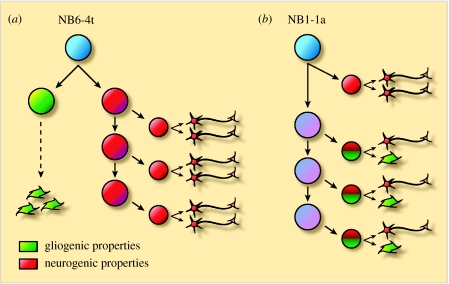
Neurons can be generated in pure neuronal lineages by neuroblasts or in mixed neuronal/glial lineages by neuroglioblasts (figure 4). Glioblasts, on the other hand, produce pure glial lineages (Bossing et al. 1996; Schmid et al. 1997; Schmid et al. 1999). In some neuroglioblast lineages, the bifurcation between the neuronal and the glial lineages occurs in the primary division to produce a neuronal and a glial precursor (figure 4a; Akiyama-Oda et al. 1999; Bernardoni et al. 1999). In other lineages, the neuroglioblast first buds off a GMC before further daughter cells are generated that give rise to neuron/glia sibling pairs (figure 4b; Udolph et al. 2001). In several lineages, neuronal fate precedes glial fate and tightly regulated timing mechanisms are probably responsible for this sequential generation.
Figure 4.
Two division patterns in neuroglial development. (a) One type of neuroglioblast (e.g. NB6-4t) first divides to give a glioblast and a neuroblast, thereby generating precursors with restricted developmental potential that give rise to either glial cells or neurons. (b) Another type of neuroglioblast (e.g. NB1-1a) generates intermediate precursors that have the potential to generate neurons as well as glia via asymmetric cell division. Notch is used to specify the glial part of the lineage (adapted from Udolph et al. 2001).
A novel type of transcription factor, glial cells missing/glial cells deficient (gcm/glide), is necessary and sufficient to induce gliogenesis in all neuroectodermally derived cells. In gcm mutants, presumptive glial cells are transformed into neurons and, conversely, when gcm is ectopically expressed, presumptive neurons enter into a glial differentiation pathway (Hosoya et al. 1995; Jones et al. 1995; Vincent et al. 1996). Thus, gcm is a key regulator in the binary decision between glial and neuronal development. In the current model, activation of gcm-responsive genes promotes glial differentiation and concomitantly represses neuronal differentiation (Giesen et al. 1997).
How is glial fate specified in different glial lineages? The first division of neuroglioblast NB6-4t (t, thoracic) generates a neuronal and a glial precursor. Pros is only transferred into the glial precursor, where it is needed to maintain and enhance the expression of gcm, thereby promoting glial cell fate (Freeman & Doe 2001). In contrast, in glioblast NB6-4a (a, abdominal), Pros is distributed symmetrically, resulting in both daughter cells expressing gcm. A report by Berger et al. (2005) has demonstrated that Cyclin E plays a vital role in this early bifurcation. Cyclin E is expressed just prior to the first division of NB6-4t. Subsequently, cyclin E mRNA is only detected in the neuronal precursor. This suggests that Cyclin E might function in neuronal specification. Indeed, in Cyclin E mutant embryos, neuroglioblast NB6-4t transforms into a glioblast producing only glial cells. Conversely, when Cyclin E is ectopically expressed in glioblast NB6-4a, asymmetric distribution of Pros to one of the two daughter cells is observed just after the first division. Since no other cell cycle regulator could induce a similar cell-fate transformation, the authors concluded that Cyclin E might have a function beyond its classical role as a cell cycle regulator.
In another neuroglioblast lineage (NB1-1a), the Notch pathway acts upstream of gcm to promote glial fate. Notch signalling is required for glial cell-fate specification when the GMC divides asymmetrically to generate a neuronal/glial sibling pair. One of the daughter cells inherits Numb, which acts to inhibit Notch signalling, and this leads to a neuronal cell fate. The clonal analysis of a defined neuroglioblast lineage demonstrated that constitutively activated Notch leads to supernumerary glial cells, while in Notch mutants the GMCs produce two neurons instead of a neuron/glial sibling pair (Udolph et al. 2001). Notch promotes glial cell fate through the activation of gcm, while Numb acts as its antagonist by inhibiting Notch-mediated gcm activation. However, the action of Notch is variable and cell context dependent. For example, in the peripheral nervous system, Notch activation represses glial cell development and gcm activation (Van De Bor & Giangrande 2001). It seems that Notch signalling can result in different outcomes depending on the type of neural progenitor cells in which it is expressed.
5. Specification of temporal identity
During CNS development, many neural progenitors generate distinct cell types over time, contributing to the vast cellular diversity of the CNS. Populations of such multipotent progenitors are found in the vertebrate cortex, retina and spinal cord (reviewed by Pearson & Doe 2004). If we are ever to manipulate the life histories of neural progenitors, we must first elucidate how their behaviour is temporally regulated. What combination of intrinsic and extrinsic factors leads to different potencies and progeny over time?
In Drosophila, DiI lineage tracing studies have shown that embryonic neuroblasts produce near invariant lineages, usually containing distinct cell types (Bossing et al. 1996; Schmid et al. 1997, 1999). Furthermore, progeny are generated in an invariant birth order (Akiyama-Oda et al. 1999; Novotny et al. 2002; Pearson & Doe 2003). Thus, Drosophila embryonic neuroblasts provide a model system for studying how the temporal identity of progenitors is regulated to generate neuronal diversity.
(a) The Hb⇒Kr⇒Pdm⇒Cas transcription factor cascade regulates temporal identity
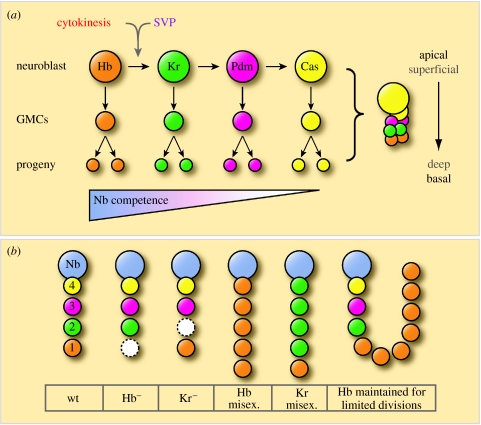
Isolated neuroblasts appear to faithfully recapitulate their in vivo division patterns in vitro (Huff et al. 1989; Brody & Odenwald 2000) and, to date, only intrinsic factors have been implicated in the regulation of their temporal identity. The majority of embryonic neuroblasts sequentially express the transcription factors Hunchback (Hb), Kruppel (Kr), Pdm and Castor (Cas; Brody & Odenwald 2000; Isshiki et al. 2001). GMCs and their neuronal progeny maintain expression of the transcription factor that is present in the neuroblast at the time of the GMC's birth. Thus, Hb is detected in deep layer neurons, with Kr, Pdm and Cas found in progressively more superficial layers (figure 5a; Kambadur et al. 1998; Brody & Odenwald 2000; Isshiki et al. 2001). The expression of these transcription factors correlates with birth order and not with a particular cell type. Hence, for instance, Hb-positive progeny cells are all early born but, depending on the lineage, can give rise to either a motor neuron or a glial cell (Isshiki et al. 2001).
Figure 5.
Temporal neuroblast progression. (a) Neuroblasts sequentially express the transcription factors Hb, Kr, Pdm and Cas. GMCs and their neuronal/glial progeny express the transcription factor present at the time of the GMC's birth. Cytokinesis and Seven-up (Svp) are required for the Hb to Kr transition. The competence of neuroblasts to generate early-born cell fates in response to Hb expression is progressively restricted (adapted from Pearson & Doe 2003). (b) A schematic showing the effects of Hb and Kr loss (Hb− and Kr−) and misexpression on progeny cells. The GMCs are labelled with their birth order, and transcription factor expression is colour-coded as in (a). Dashed white circles represent abnormal GMC development resulting from cell death, cell-fate skipping or transformation (adapted from Isshiki et al. 2001).
It has been shown that Hb and Kr are necessary and sufficient for specifying their respective early-born cell fates (Isshiki et al. 2001). In a number of lineages, the absence of Hb in the embryonic CNS leads to a loss of first-born GMCs (and their corresponding progeny) due to cell death, cell-fate skipping or transformation leading to a duplication of the next-born identity (figure 5b). In contrast, when neuroblasts are forced to continuously express Hb, additional neurons are present in the lineage and all exhibit first-born fates (Isshiki et al. 2001). Similarly, in the absence of Kruppel, second-born cell types are missing, due to cell death or cell-fate skipping. The forced overexpression of Kr in neuroblasts can again lead to extra cells in a lineage, all of which adopt the Kr (second-born) fate (Isshiki et al. 2001). It remains to be seen whether Pdm and Cas regulate temporal identity in the same way as Hb and Kr.
(b) Neuroblast competence to generate early cell fates is progressively restricted
Misexpression of Hb and Kr has been used to start probing the competence of neuroblasts to generate different cell types over time (Pearson & Doe 2003; Cleary & Doe 2006). This is done by misexpressing Hb and Kr in NB7-1 at different times during development and then assaying the effects on the cell fates of its progeny. Each of the first five GMCs (GMC1–5) that NB7-1 produces divides to produce a motor neuron (named U1–U5 with birth order) and a sibling. Each of the U1–U5 motor neurons can be identified by their position and their unique expression of molecular markers (Bossing et al. 1996; Schmid et al. 1999; Pearson & Doe 2003).
Shortly after the normal downregulation of Hb, the neuroblast remains competent to respond to Hb and generates excess (U1) first-born motor neurons. However, when a pulse of Hb expression is induced progressively later, the neuroblast becomes increasingly less competent to respond (Pearson & Doe 2003). Similarly, the competence of neuroblasts to respond to pulses of Kr, and produce excess U3 motor neurons, decreases over time (Cleary & Doe 2006). This is reminiscent of the progressive restriction of mammalian cortical progenitors, which lose their ability to respond to fate-inducing cues in a stepwise fashion (Desai & McConnell 2000).
When Hb expression is maintained in NB7-1 for an extra 10–12 neuroblast cycles, not only are an extra 10–12 U1 motor neurons produced, but the normal complement of later-born U2–U5 neurons are generated afterwards (Grosskortenhaus et al. 2005). This demonstrates that Hb expression can arrest the neuroblast temporal identity timer, keeping the neuroblast in a ‘young’ state.
Kr misexpression is unable to arrest neuroblast temporal identity and competence in the same way as Hb. Once Hb has been downregulated, neuroblast competence begins to be lost. Extended Kr expression, or subsequent pulses of Hb or Kr, can only generate excess motor neurons within this single competence window (Cleary & Doe 2006). The molecular basis of competence, and its progressive restriction, remain unknown. However, the observation that different neuroblasts remain competent to respond to Hb for varying numbers of divisions suggests that a lineage intrinsic mechanism may be more probable than an extrinsic signal (Cleary & Doe 2006).
Pearson & Doe (2003) also asked at what time during neuronal differentiation is the competence to respond to Hb lost? That is to say, at what point is temporal identity fixed? When Hb misexpression is limited to neuroblasts, GMCs and young neurons, there is a large induction of U1 motor neurons, which stably maintain their U1 molecular profile without maintaining Hb expression. Conversely, when Hb misexpression is limited to mature post-mitotic motor neurons, all U1–U5 molecular markers remain wild-type, despite high levels of Hb. Thus, competence to respond to Hb is absent in post-mitotic neurons (Pearson & Doe 2003). Kr misexpression experiments give similar results, with the competence to respond to Kr being lost in post-mitotic neurons (Cleary & Doe 2006).
These results raise the question of how Hb, or indeed any temporal regulator, even when its expression is not maintained, can confer a temporal identity state. Pearson & Doe (2003) have proposed a model whereby Hb interacts with Polycomb complex proteins to confer a lasting temporal identity via chromatin remodelling.
(c) Regulation of temporal identity transitions
Progress is starting to be made in understanding the mechanisms that regulate the transitions between the temporal identity states. Cross-regulatory interactions between Hb, Kr, Pdm and Cas (Kambadur et al. 1998; Brody & Odenwald 2000; Isshiki et al. 2001) led to a model in which each gene can activate the next gene in the pathway and repress the ‘next plus one’ gene (Isshiki et al. 2001). However, these interactions are not found to be necessary for driving this sequential gene expression, indicating the presence of other mechanisms controlling temporal identity transitions (Isshiki et al. 2001).
It has recently been shown that blocking neuroblast cytokinesis prevents the downregulation of Hb transcription, and thus the Hb to Kr transition (Grosskortenhaus et al. 2005). The orphan nuclear receptor Seven-up (Svp) is also necessary for this important transition to occur (Kanai et al. 2005). Svp is expressed transiently within neuroblasts, coinciding with the downregulation of Hb. Loss of Svp function leads to an increase in early-born cell types, as a result of enduring Hb expression. Misexpression of Svp leads to the loss of Hb expression and early-born neuronal types (Kanai et al. 2005). Mettler et al. (2006) have demonstrated that mitosis is necessary for the efficient translation of Svp, which may account for the observed necessity for cytokinesis in the Hb to Kr transition.
After cell division, Svp is inherited by both neuroblast and GMC. How then is Hb expression maintained in the GMC after mitotic division? The answer lies with the asymmetrically segregated transcription factor Prospero (see §4d). In contrast to svp mutants, loss of Pros function leads to a strong reduction in the number of Hb+ cells, due to a lack of Hb maintenance (Mettler et al. 2006). This is the opposite phenotype to Svp loss of function. In Pros/Svp double loss-of-function embryos, an increase in Hb+ cells is observed, similar to Svp loss of function alone. This result suggests that in the GMC, Pros acts to counteract the negative regulation of Hb by Svp (Mettler et al. 2006). Temporal examination of Hb, Svp and svp mRNA in specific neuroblast lineages in wild-type, Pros mutant and Svp mutant embryos suggests that Pros acts on both a transcriptional and a post-transcriptional level to downregulate Svp activity in the GMC (Mettler et al. 2006). In this way, Pros prevents the downregulation of Hb. The precise nature of the Pros/Svp interaction remains to be elucidated, although Pros recently has been shown to regulate directly the expression of both svp and hp (Choksi et al. 2006).
Surprisingly, neither cytokinesis nor cell cycle progression is required for the subsequent Kr⇒Pdm⇒Cas cascade, indicating the presence of an intrinsic, cell cycle-independent, timer (Grosskortenhaus et al. 2005).
It has clearly been shown that this transcription factor cascade, present in most neuroblasts, is a key mechanism by which temporal identity is regulated, but much remains to be elucidated. Are Pdm and Cas true temporal regulators like Hb and Kr? What are the targets of Hb, Kr, Pdm and Cas in the CNS? What are the exact cellular and molecular mechanisms governing competence and the transitions between the temporal states? When Hb is continuously misexpressed in neuroblasts, the lineage expands, but not indefinitely (Isshiki et al. 2001). It is possible that extrinsic signals act to constrain the intrinsic programme of temporal progression. It will be of great interest to see whether analogous intrinsic timing mechanisms, in combination with already established environmental inputs, might govern the temporal identity of vertebrate neural progenitors (reviewed by Temple 2001).
6. The postembryonic phase of neuroblast proliferation
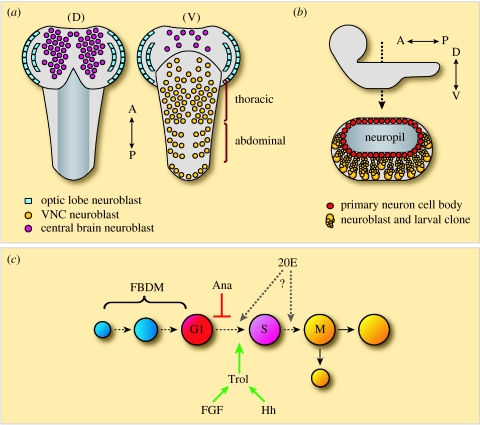
The embryonic period of neurogenesis gives rise to the distinct larval nervous system. Although much of the larval CNS will be remodelled and incorporated into the adult, a postembryonic period of neurogenesis is required to produce the majority of the adult CNS. This postembryonic phase of neurogenesis begins during the first larval instar and extends into pupal stages. It is believed that the neuroblasts responsible for this larval period of neurogenesis are embryonic neuroblasts that have been reactivated postembryonically. Neuroblasts of the central brain and VNC undergo asymmetric self-renewing divisions to produce a ‘pocket’ of neuronal progeny in an apical to basal direction (figure 6a,b). These progeny remain in an immature state until metamorphosis when, in combination with remodelled larval neurons, they form the fully functioning adult CNS (Truman et al. 1993).
Figure 6.
Postembryonic neuroblast development. (a) A schematic of a third instar larval Drosophila brain. Left, dorsal view (D); right, ventral view (V). Anterior is to the top of the page. (b) Lateral view top, with anterior to the left and dorsal to the top of the page. Bottom, cross-section of the thoracic VNC. The cell bodies of the primary neurons produced during embryogenesis surround the neuropil formed by their axons. (c) A schematic of neuroblast reactivation. Enlargement is triggered by the fat body-derived mitogen (FBDM). Anachronism (Ana)-mediated repression keeps the neuroblast in G1 until factors working through terribly reduced optic lobes (Trol) drive the neuroblast into S phase. The role of ecdysone (20E) in this process has yet to be resolved.
(a) Abdominal-A and reaper regulate larval neuroblast number
The patterns of embryonic neuroblast segregation are identical in thoracic and abdominal segments of the VNC (Doe 1992). However, there are large differences in larval neuroblast number between the thoracic and the abdominal segments of the VNC (Truman & Bate 1988). From the initial embryonic complement of 60 neuroblasts per segment, around 47 are present in a larval thoracic segment, versus six in a typical abdominal segment (Truman & Bate 1988; Prokop & Technau 1991). This regional difference is the result of higher levels of neuroblast apoptosis in abdominal segments of the embryo (White et al. 1994), which is regulated by the homeotic genes (Prokop et al. 1998). The removal of proliferative cells via apoptosis as a means to control cell number and sculpt the CNS is also observed in mammals (Blaschke et al. 1998).
In Abdominal-A (Abd-A) loss-of-function mutants, an increased number of neuroblasts persist in abdominal segments and proliferate during the postembryonic phase of neurogenesis (Prokop et al. 1998). Furthermore, misexpression of Abd-A in thoracic neuroblasts shortly after segregation leads to between only four and eight persisting neuroblasts per thoracic segment at the end of embryogenesis (Prokop et al. 1998). Abd-A expression is only detected in neuroblasts during early embryogenesis (Prokop et al. 1998), supporting the idea that Abd-A expression (along with the expression of other Hox genes) bestows enduring fates on neuroblasts, as opposed to directly interacting with the apoptotic machinery.
The apoptosis of embryonic neuroblasts is effected through the proapoptotic gene, reaper (rpr; Peterson et al. 2002). In rpr loss-of-function mutants, both the thoracic and the abdominal regions of the VNC are enlarged, with the most extensive hyperplasia found in the abdominal region (Peterson et al. 2002). This phenotype is caused by the presence of ectopic postembryonic neuroblasts, which proliferate and give rise to excess neurons (Peterson et al. 2002).
(b) Quiescence and reactivation of Drosophila neuroblasts
With the exception of the mushroom body and lateral neuroblasts, all neuroblasts within the Drosophila embryo have ceased to proliferate by late embryogenesis (Hartenstein et al. 1987; Green et al. 1993; Younossi-Hartenstein et al. 1996). It is at this point that each neuroblast makes the choice between apoptosis and entering into a mitotic dormancy, termed ‘quiescence’ (White et al. 1994; Prokop et al. 1998; Peterson et al. 2002; it is unknown whether some embryonic neuroblasts terminally differentiate). During larval life, quiescent neuroblasts reactivate, to resume proliferation, giving rise to the vast majority of neurons found in the adult CNS (Truman & Bate 1988; Hofbauer & Campos-Ortega 1990; Prokop & Technau 1991; Ito & Hotta 1992). The molecular mechanisms that govern the transitions between, and maintenance of, the quiescent and the proliferative states are just beginning to be understood.
In the adult mammalian brain, neural stem cells in the subventricular and subgranular zones allow limited neurogenesis to occur throughout the life of the animal (reviewed by Doetsch 2003). It has been shown that the stem cell population present in the subventricular zone is not significantly affected when proliferating cells are killed with antimitotic treatments (Morshead et al. 1994; Doetsch et al. 1999), suggesting that these neural stem cells, like Drosophila neuroblasts, may pass through a quiescent state. However, it is also possible that the subventricular zone stem cells are largely refractory to these antimitotic treatments, because they progress through the cell cycle extremely slowly. Whether these cells enter into a distinct quiescent state, or merely cycle slowly, is an intriguing question that awaits resolution.
(c) Entry into quiescence
As mentioned previously, isolated embryonic neuroblasts appear to faithfully recapitulate their division patterns in vitro (Huff et al. 1989; Brody & Odenwald 2000). If this is indeed the case, it may be that their final temporal state is quiescence, and that this state is regulated by an intrinsic (intra-clonal) neuroblast ‘clock’. Alternatively, it is known that neuroblasts of the VNC become smaller with each successive division so that, by the end of their embryonic proliferation, they are indistinguishable from the surrounding cells (Hartenstein et al. 1987). Quiescence could therefore be triggered when a neuroblast's volume drops below a critical point. It has been shown that nucleo-cytoplasmic ratio plays an important role in controlling early embryonic cell division (Edgar et al. 1986) and gene expression (Yasuda et al. 1991). In agreement with this are experiments in grasshopper, in which the mitotic spindle is mechanically shifted during the anaphase of neuroblast divisions. These experiments demonstrate that resultant cells with a volume over a critical level will always develop as neuroblasts, whereas cells with a volume below a critical level will always develop as GMCs (long cell cycle and a single division; Yamashiki & Kawamura 1986; Doe et al. 1998).
The postembryonic lineages of all thoracic VNC neuroblasts have been defined, and many neuroblasts have been matched with their embryonic identity (Truman et al. 2004). Therefore, the entire life history of many neuroblasts is known, including whether they quiesce or reactivate. The isolated culture of identified embryonic neuroblasts might make it possible to ascertain whether neuroblasts do indeed recapitulate their in vivo division patterns in vitro, and whether the entry into quiescence can be completed in a clonally autonomous fashion, without external signals. Such a system might also help to discover the factors that govern the exit from quiescence.
(d) Neuroblast reactivation
The first stage of neuroblast reactivation is enlargement (Truman & Bate 1988), which is a prerequisite for re-entry into mitosis (figure 6c; Britton & Edgar 1998). Neuroblast enlargement is dependent on nutrition, specifically the presence of amino acids in the diet (Britton & Edgar 1998). It was shown in vitro that the fat body produces an as-yet-unidentified mitogen in response to nutritional amino acids (Britton & Edgar 1998). In starved larvae, in which the mitogen is not produced and hence neuroblasts never enlarge, the overexpression of G1⇒S-positive regulators is unable to reactivate quiescent neuroblasts (Britton & Edgar 1998).
The second stage of reactivation is entry into S phase (Truman & Bate 1988). Several factors have been found that govern the initial G1⇒S transition of reactivating neuroblasts. The anachronism (ana) gene is expressed in a subset of glial cells that neighbour neuroblasts, and codes for a secreted glycoprotein of unknown biochemical function (Ebens et al. 1993). Loss of Ana function results in precocious neuroblast reactivation, with neuroblasts entering their first S phase around 8 h early. Thus, the Ana protein appears to function as a quiescence maintenance factor (Ebens et al. 1993).
Another factor involved in this G1⇒S transition is the product of the terribly reduced optic lobes (trol) gene (Datta 1995). In trol loss-of-function mutants, neuroblast reactivation is severely retarded. The neuroblasts enlarge, but never enter their first S phase. The effect is most evident in the VNC, where almost no neuroblasts reactivate (Datta 1995). The proliferation defect in trol mutants can be rescued by the overexpression of cyclin E (Caldwell & Datta 1998). cyclin E expression is lower in the trol mutant larval CNS, suggesting that trol neuroblasts may be arrested in G1 due to a lack of Cyclin E (Caldwell & Datta 1998). The ana/trol double mutant displays the ana mutant phenotype of precocious neuroblast reactivation (Datta 1995). This demonstrates that Trol acts downstream of Ana to bypass or inactivate Ana's repressive effect on the cell cycle (Datta 1995).
trol encodes Drosophila Perlecan, a heparan sulphate proteoglycan (Voigt et al. 2002; Park et al. 2003). In mammals, Perlecan is a component of basal membranes that interacts with other extracellular matrix proteins, growth factors and receptors, to regulate cellular signalling (Voigt et al. 2002; Park et al. 2003). Loss-of-function mutations in hedgehog (hh) and branchless (bnl, an FGF homologue) dominantly enhance the neuroblast proliferation defect in a sensitized trol mutant background (Park et al. 2003). Both Hh and human FGF-2 have been shown to physically interact with Trol in coimmunoprecipitation studies, and the addition of human FGF-2 to trol mutant brains in culture can restore wild-type levels of neuroblast proliferation (Park et al. 2003). Interestingly, it is known that FGF can also act as a mitogen for adult mammalian neural stem cells (reviewed by Doetsch 2003). It has also been reported that during the first larval instar, when neuroblasts reactivate, trol is expressed over the surface of the larval brain (Park et al. 2003). This would place Trol in close proximity to the superficially located neuroblasts. Thus, Trol may function to locally modulate Hh and FGF signalling in a manner critical to the reactivation of neuroblasts.
Another factor implicated in neuroblast reactivation is the steroid hormone ecdysone (20-hydroxyecdysone). In Drosophila, ecdysone is vital to the regulation of many developmental transitions (Riddiford 1993). It has been shown that ecdysone can support in vivo levels of neuroblast reactivation in explant culture of the larval CNS (Datta 1999). The expression of the EcR-B1 ecdysone receptor becomes detectable in neuroblasts during the middle of the second larval instar (Truman et al. 1994), by which time many neuroblasts have already begun to divide (Truman & Bate 1988). Hence, if ecdysone is involved in reactivation, as opposed to being involved in the maintenance of proliferation, it must be via an intermediate cell type or through an undetectable level of ecdysone receptor expression in neuroblasts. Alternatively, ecdysone might act on a subset of neuroblasts that re-enter mitosis later in development, perhaps driving them into M phase. In the abdominal segments of the VNC, there is a prolonged pause between the S and the M phase (Truman & Bate 1988), indicating that, for some neuroblasts, there could be an important G2-associated reactivation checkpoint.
(e) Larval proliferation of abdominal neuroblasts is stopped by a pulse of Abdominal-A
What stops postembryonic proliferation after neuroblasts have been reactivated? It is unknown whether a transcription factor cascade similar to that found in embryonic neuroblasts is present during postembryonic stages. Furthermore, it seems unlikely that nucleo-cytoplasmic ratio plays a role, as postembryonic neuroblasts do not decrease in size with each division. However, the end of abdominal neuroblast proliferation is one aspect of larval neurogenesis that has begun to be understood. It has been shown that during the third larval instar, abdominal neuroblasts undergo programmed cell death (Bello et al. 2003). When an abdominal neuroblast is made deficient for the three proapoptotic genes, head involution defective, grim and reaper, it persists throughout larval life, generating many more progeny than normal. A pulse of Abd-A, operating upstream of these genes, is required in the dividing neuroblast immediately before apoptosis occurs. This is in contrast to the apoptosis of abdominal neuroblasts in the embryo, where Abd-A is expressed early on, and leads to apoptosis in a less direct manner (Bello et al. 2003).
(f) Grainyhead regulates various aspects of postembryonic neuroblasts behaviour
The transcription factor Grainyhead (Grh) is an excellent marker for postembryonic neuroblasts, since its expression starts late in embryogenesis, and is then maintained throughout larval stages (Bray et al. 1989; Prokop et al. 1998). A loss-of-function mutant for the neuroblast-specific isoforms of Grh causes lethality during postembryonic stages (Uv et al. 1997). In such mutants, the abdominal larval neuroblasts produce a larger number of progeny, whereas the thoracic neuroblasts proliferate less (Almeida & Bray 2005; Cenci & Gould 2005). Intriguingly, it has also been reported that grh mutant neuroblasts prematurely exit quiescence (Cenci & Gould 2005). It has been shown that Grh is necessary for abdominal neuroblasts to respond to (and maintain) the pulse of Abd-A and enter into apoptosis (Cenci & Gould 2005). How then is Grh regulating these different aspects of neuroblast behaviour? What are the targets of this transcription factor in the CNS? One candidate for a direct target of Grh is DE-cadherin. DE-cadherin expression is significantly lower in grh mutant neuroblasts, and Grh-binding sites have been identified in the DE-cadherin flanking sequences (Almeida & Bray 2005). It is thought that DE-cadherin mediates neuroblast–glia interactions that are required for proper neuroblast proliferation (Dumstrei et al. 2003). The elucidation of further Grh targets and how these targets might vary in a segment-specific way to control the different behaviours of thoracic and abdominal neuroblasts will be of great interest.
(g) A postembryonic neuroblast niche?
In a variety of systems, it has been demonstrated that regulatory microenvironments surrounding stem cells are critically important for their survival and proper activity (Fuchs et al. 2004). This has given rise to the concept of the stem cell niche. In the adult mammalian brain, the subventricular and subgranular zones contain such specialized niches, maintaining neural stem cell populations (reviewed by Doetsch 2003).
In the larval Drosophila brain, each individual neuroblast and its progeny are separated from other cells into a compartment formed by glial cell processes (Dumstrei et al. 2003). Furthermore, as mentioned previously, Ana and Trol are secreted by subsets of cells (most probably glia) in close proximity to neuroblasts (Ebens et al. 1993; Park et al. 2003). When the interaction between neuroblasts and surrounding glia is disrupted by expressing a dominant negative form of DE-cadherin, neuroblasts appear in normal numbers, but their proliferation is greatly reduced (Dumstrei et al. 2003). Thus, it appears probable that there is a larval neuroblast niche, in which glial cells play an integral role.
A deeper understanding of neuroblast behaviour, and what mechanisms are conserved with vertebrates, will enable the Drosophila CNS to act as a powerful model system for studying stem cell proliferation.
Acknowledgments
We apologize to our many colleagues whose work we were unable to include in this review. We thank members of the Brand laboratory for their helpful discussions and comments on the manuscript. This work was funded by a programme grant from the Wellcome Trust to A.H.B.
Footnotes
One contribution of 14 to a Theme Issue ‘Stem cells and brain repair’.
References
- Akiyama-Oda Y, Hosoya T, Hotta Y. Asymmetric cell division of thoracic neuroblast 6–4 to bifurcate glial and neuronal lineage in Drosophila. Development. 1999;126:1967–1974. doi: 10.1242/dev.126.9.1967. [DOI] [PubMed] [Google Scholar]
- Almeida M.S, Bray S.J. Regulation of post-embryonic neuroblasts by Drosophila grainyhead. Mech. Dev. 2005;122:1282–1293. doi: 10.1016/j.mod.2005.08.004. doi:10.1016/j.mod.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo J.M, Tramontin A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. doi:10.1038/35067582 [DOI] [PubMed] [Google Scholar]
- Arama E, Dickman D, Kimchie Z, Shearn A, Lev Z. Mutations in the beta-propeller domain of the Drosophila brain tumor (brat) protein induce neoplasm in the larval brain. Oncogene. 2000;19:3706–3716. doi: 10.1038/sj.onc.1203706. doi:10.1038/sj.onc.1203706 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Simpson P. Choosing a cell fate: a view from the Notch locus. Trends Genet. 1991;7:403–408. doi: 10.1016/0168-9525(91)90264-q. [DOI] [PubMed] [Google Scholar]
- Ashraf S.I, Ip Y.T. The Snail protein family regulates neuroblast expression of inscuteable and string, genes involved in asymmetry and cell division in Drosophila. Development. 2001;128:4757–4767. doi: 10.1242/dev.128.23.4757. [DOI] [PubMed] [Google Scholar]
- Bardin A.J, Le Borgne R, Schweisguth F. Asymmetric localization and function of cell-fate determinants: a fly's view. Curr. Opin. Neurobiol. 2004;14:6–14. doi: 10.1016/j.conb.2003.12.002. doi:10.1016/j.conb.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Barros C.S, Phelps C.B, Brand A.H. Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev. Cell. 2003;5:829–840. doi: 10.1016/s1534-5807(03)00359-9. doi:10.1016/S1534-5807(03)00359-9 [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Gotta M. Heterotrimeric G proteins and regulation of size asymmetry during cell division. Curr. Opin. Cell Biol. 2005;17:658–663. doi: 10.1016/j.ceb.2005.10.002. doi:10.1016/j.ceb.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Bello B.C, Hirth F, Gould A.P. A pulse of the Drosophila Hox protein abdominal-A schedules the end of neural proliferation via neuroblast apoptosis. Neuron. 2003;37:209–219. doi: 10.1016/s0896-6273(02)01181-9. doi:10.1016/S0896-6273(02)01181-9 [DOI] [PubMed] [Google Scholar]
- Bello B, Reichert H, Hirth F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development. 2006;133:2639–2648. doi: 10.1242/dev.02429. doi:10.1242/dev.02429 [DOI] [PubMed] [Google Scholar]
- Berger C, Pallavi S.K, Prasad M, Shashidhara L.S, Technau G.M. A critical role for cyclin E in cell fate determination in the central nervous system of Drosophila melanogaster. Nat. Cell Biol. 2005;7:56–62. doi: 10.1038/ncb1203. doi:10.1038/ncb1203 [DOI] [PubMed] [Google Scholar]
- Bernardoni R, Kammerer M, Vonesch J.L, Giangrande A. Gliogenesis depends on glide/gcm through asymmetric division of neuroglioblasts. Dev. Biol. 1999;216:265–275. doi: 10.1006/dbio.1999.9511. doi:10.1006/dbio.1999.9511 [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro D.S, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. doi:10.1038/nrn874 [DOI] [PubMed] [Google Scholar]
- Betschinger J, Knoblich J.A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 2004;14:R674–R685. doi: 10.1016/j.cub.2004.08.017. doi:10.1016/j.cub.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich J.A. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 2006;124:1241–1253. doi: 10.1016/j.cell.2006.01.038. doi:10.1016/j.cell.2006.01.038 [DOI] [PubMed] [Google Scholar]
- Biehs B, Francois V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–2934. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- Blaschke A.J, Weiner J.A, Chun J. Programmed cell death is a universal feature of embryonic and postnatal neuroproliferative regions throughout the central nervous system. J. Comp. Neurol. 1998;396:39–50. doi: 10.1002/(sici)1096-9861(19980622)396:1<39::aid-cne4>3.0.co;2-j. doi:10.1002/(SICI)1096-9861(19980622)396:1<39::AID-CNE4>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- Bossing T, Udolph G, Doe C.Q, Technau G.M. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev. Biol. 1996;179:41–64. doi: 10.1006/dbio.1996.0240. doi:10.1006/dbio.1996.0240 [DOI] [PubMed] [Google Scholar]
- Bowman S.K, Neumuller R.A, Novatchkova M, Du Q, Knoblich J.A. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell. 2006;10:731–742. doi: 10.1016/j.devcel.2006.05.005. doi:10.1016/j.devcel.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin. Cell Dev. Biol. 1998;9:591–597. doi: 10.1006/scdb.1998.0262. doi:10.1006/scdb.1998.0262 [DOI] [PubMed] [Google Scholar]
- Bray S.J, Burke B, Brown N.H, Hirsh J. Embryonic expression pattern of a family of Drosophila proteins that interact with a central nervous system regulatory element. Genes Dev. 1989;3:1130–1145. doi: 10.1101/gad.3.8.1130. [DOI] [PubMed] [Google Scholar]
- Britton J.S, Edgar B.A. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- Broadus J, Doe C.Q. Extrinsic cues, intrinsic cues and microfilaments regulate asymmetric protein localization in Drosophila neuroblasts. Curr. Biol. 1997;7:827–835. doi: 10.1016/s0960-9822(06)00370-8. doi:10.1016/S0960-9822(06)00370-8 [DOI] [PubMed] [Google Scholar]
- Broadus J, Fuerstenberg S, Doe C.Q. Staufen-dependent localization of prospero mRNA contributes to neuroblast daughter-cell fate. Nature. 1998;391:792–795. doi: 10.1038/35861. doi:10.1038/35861 [DOI] [PubMed] [Google Scholar]
- Brody T, Odenwald W.F. Programmed transformations in neuroblast gene expression during Drosophila CNS lineage development. Dev. Biol. 2000;226:34–44. doi: 10.1006/dbio.2000.9829. doi:10.1006/dbio.2000.9829 [DOI] [PubMed] [Google Scholar]
- Brody T, Odenwald W.F. Cellular diversity in the developing nervous system: a temporal view from Drosophila. Development. 2002;129:3763–3770. doi: 10.1242/dev.129.16.3763. [DOI] [PubMed] [Google Scholar]
- Buescher M, Yeo S.L, Udolph G, Zavortink M, Yang X, Tear G, Chia W. Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable-mediated asymmetry of ganglion mother cells. Genes Dev. 1998;12:1858–1870. doi: 10.1101/gad.12.12.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher M, Hing F.S, Chia W. Formation of neuroblasts in the embryonic central nervous system of Drosophila melanogaster is controlled by SoxNeuro. Development. 2002;129:4193–4203. doi: 10.1242/dev.129.18.4193. [DOI] [PubMed] [Google Scholar]
- Cai Y, Chia W, Yang X. A family of snail-related zinc finger proteins regulates two distinct and parallel mechanisms that mediate Drosophila neuroblast asymmetric divisions. EMBO J. 2001;20:1704–1714. doi: 10.1093/emboj/20.7.1704. doi:10.1093/emboj/20.7.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yu F, Lin S, Chia W, Yang X. Apical complex genes control mitotic spindle geometry and relative size of daughter cells in Drosophila neuroblast and pI asymmetric divisions. Cell. 2003;112:51–62. doi: 10.1016/s0092-8674(02)01170-4. doi:10.1016/S0092-8674(02)01170-4 [DOI] [PubMed] [Google Scholar]
- Caldwell M.C, Datta S. Expression of cyclin E or DP/E2F rescues the G1 arrest of trol mutant neuroblasts in the Drosophila larval central nervous system. Mech. Dev. 1998;79:121–130. doi: 10.1016/s0925-4773(98)00178-6. doi:10.1016/S0925-4773(98)00178-6 [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J, Hartenstein V. Springer; Heidelberg, Germany: 1997. The embryonic development of Drosophila melanogaster. [Google Scholar]
- Campuzano S, Modolell J. Patterning of the Drosophila nervous system: the achaete–scute gene complex. Trends Genet. 1992;8:202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Cenci C, Gould A.P. Drosophila grainyhead specifies late programmes of neural proliferation by regulating the mitotic activity and Hox-dependent apoptosis of neuroblasts. Development. 2005;132:3835–3845. doi: 10.1242/dev.01932. doi:10.1242/dev.01932 [DOI] [PubMed] [Google Scholar]
- Ceron J, Gonzalez C, Tejedor F.J. Patterns of cell division and expression of asymmetric cell fate determinants in postembryonic neuroblast lineages of Drosophila. Dev. Biol. 2001;230:125–138. doi: 10.1006/dbio.2000.0110. doi:10.1006/dbio.2000.0110 [DOI] [PubMed] [Google Scholar]
- Choksi S.P, Southall T, Bossing T, Edoff K, de Wit E, van Steensel B, Micklem G, Brand A.H. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev. Cell. 2006;11:775–789. doi: 10.1016/j.devcel.2006.09.015. doi:10.1016/j.devcel.2006.09.015 [DOI] [PubMed] [Google Scholar]
- Cleary M.D, Doe C.Q. Regulation of neuroblast competence: multiple temporal identity factors specify distinct neuronal fates within a single early competence window. Genes Dev. 2006;20:429–434. doi: 10.1101/gad.1382206. doi:10.1101/gad.1382206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R.A, Von Ohlen T. vnd/Nkx, ind/Gsh, and msh/Msx: conserved regulators of dorsoventral neural patterning? Curr. Opin. Neurobiol. 2000;10:63–71. doi: 10.1016/s0959-4388(99)00049-5. doi:10.1016/S0959-4388(99)00049-5 [DOI] [PubMed] [Google Scholar]
- Cubas P, de Celis J.F, Campuzano S, Modolell J. Proneural clusters of achaete–scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 1991;5:996–1008. doi: 10.1101/gad.5.6.996. [DOI] [PubMed] [Google Scholar]
- Datta S. Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system. Development. 1995;121:1173–1182. doi: 10.1242/dev.121.4.1173. [DOI] [PubMed] [Google Scholar]
- Datta S. Activation of neuroblast proliferation in explant culture of the Drosophila larval CNS. Brain Res. 1999;818:77–83. doi: 10.1016/s0006-8993(98)01292-x. doi:10.1016/S0006-8993(98)01292-X [DOI] [PubMed] [Google Scholar]
- Desai A.R, McConnell S.K. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Doe C.Q. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development. 1992;116:855–863. doi: 10.1242/dev.116.4.855. [DOI] [PubMed] [Google Scholar]
- Doe C.Q, Chu-LaGraff Q, Wright D.M, Scott M.P. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–464. doi: 10.1016/0092-8674(91)90463-9. doi:10.1016/0092-8674(91)90463-9 [DOI] [PubMed] [Google Scholar]
- Doe C.Q, Fuerstenberg S, Peng C.Y. Neural stem cells: from fly to vertebrates. J. Neurobiol. 1998;36:111–127. doi:10.1002/(SICI)1097-4695(199808)36:2<111::AID-NEU2>3.0.CO;2-4 [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. doi:10.1016/j.gde.2003.08.012 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo J.M, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl Acad. Sci. USA. 1999;96:11 619–11 624. doi: 10.1073/pnas.96.20.11619. doi:10.1073/pnas.96.20.11619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J. Neurosci. 2003;23:3325–3335. doi: 10.1523/JNEUROSCI.23-08-03325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebens A.J, Garren H, Cheyette B.N, Zipursky S.L. The Drosophila anachronism locus: a glycoprotein secreted by glia inhibits neuroblast proliferation. Cell. 1993;74:15–27. doi: 10.1016/0092-8674(93)90291-w. doi:10.1016/0092-8674(93)90291-W [DOI] [PubMed] [Google Scholar]
- Edgar B.A, Kiehle C.P, Schubiger G. Cell cycle control by the nucleo-cytoplasmic ratio in early Drosophila development. Cell. 1986;44:365–372. doi: 10.1016/0092-8674(86)90771-3. doi:10.1016/0092-8674(86)90771-3 [DOI] [PubMed] [Google Scholar]
- Egger B, Boone J.Q, Stevens N.R, Brand A.H, Doe C.Q. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Dev. 2007;2 doi: 10.1186/1749-8104-2-1. doi:10.1186/1749-8104-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E.L. Conservation of dorsal-ventral patterning in arthropods and chordates. Curr. Opin. Genet. Dev. 1996;6:424–431. doi: 10.1016/s0959-437x(96)80063-3. doi:10.1016/S0959-437X(96)80063-3 [DOI] [PubMed] [Google Scholar]
- Ferguson E.L, Anderson K.V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. doi:10.1016/0092-8674(92)90514-D [DOI] [PubMed] [Google Scholar]
- Francois V, Solloway M, O'Neill J.W, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Frank D.J, Edgar B.A, Roth M.B. The Drosophila melanogaster gene brain tumor negatively regulates cell growth and ribosomal RNA synthesis. Development. 2002;129:399–407. doi: 10.1242/dev.129.2.399. [DOI] [PubMed] [Google Scholar]
- Freeman M.R, Doe C.Q. Asymmetric Prospero localization is required to generate mixed neuronal/glial lineages in the Drosophila CNS. Development. 2001;128:4103–4112. doi: 10.1242/dev.128.20.4103. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. doi:10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- Fuse N, Hisata K, Katzen A.L, Matsuzaki F. Heterotrimeric G proteins regulate daughter cell size asymmetry in Drosophila neuroblast divisions. Curr. Biol. 2003;13:947–954. doi: 10.1016/s0960-9822(03)00334-8. doi:10.1016/S0960-9822(03)00334-8 [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. Genesis of the Drosophila peripheral nervous system. Trends Genet. 1989;5:251–255. doi: 10.1016/0168-9525(89)90097-8. doi:10.1016/0168-9525(89)90097-8 [DOI] [PubMed] [Google Scholar]
- Giesen K, Hummel T, Stollewerk A, Harrison S, Travers A, Klämbt C. Glial development in the Drosophila CNS requires concomitant activation of glial and repression of neuronal differentiation genes. Development. 1997;124:2307–2316. doi: 10.1242/dev.124.12.2307. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. doi:10.1016/S0896-6273(03)00497-5 [DOI] [PubMed] [Google Scholar]
- Green P, Hartenstein A.Y, Hartenstein V. The embryonic development of the Drosophila visual system. Cell Tissue Res. 1993;273:583–598. doi: 10.1007/BF00333712. doi:10.1007/BF00333712 [DOI] [PubMed] [Google Scholar]
- Grosskortenhaus R, Pearson B.J, Marusich A, Doe C.Q. Regulation of temporal identity transitions in Drosophila neuroblasts. Dev. Cell. 2005;8:193–202. doi: 10.1016/j.devcel.2004.11.019. doi:10.1016/j.devcel.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Rudloff E, Campos-Ortega J.A. The pattern of proliferation of the neuroblasts in the wild-type embryo of Drosophila melanogaster. Roux's Arch. Dev. Biol. 1987;196:473–485. doi: 10.1007/BF00399871. doi:10.1007/BF00399871 [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl Acad. Sci. USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. doi:10.1073/pnas.0308600100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata J, Nakagoshi H, Nabeshima Y, Matsuzaki F. Asymmetric segregation of the homeodomain protein Prospero during Drosophila development. Nature. 1995;377:627–630. doi: 10.1038/377627a0. doi:10.1038/377627a0 [DOI] [PubMed] [Google Scholar]
- Hofbauer A, Campos-Ortega J.A. Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Roux's Arch. Dev. Biol. 1990;198:264–274. doi: 10.1007/BF00377393. doi:10.1007/BF00377393 [DOI] [PubMed] [Google Scholar]
- Holley S.A, Jackson P.D, Sasai Y, Lu B, De Robertis E.M, Hoffmann F.M, Ferguson E.L. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. doi:10.1038/376249a0 [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y. glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82:1025–1036. doi: 10.1016/0092-8674(95)90281-3. doi:10.1016/0092-8674(95)90281-3 [DOI] [PubMed] [Google Scholar]
- Huff R, Furst A, Mahowald A.P. Drosophila embryonic neuroblasts in culture: autonomous differentiation of specific neurotransmitters. Dev. Biol. 1989;134:146–157. doi: 10.1016/0012-1606(89)90085-7. doi:10.1016/0012-1606(89)90085-7 [DOI] [PubMed] [Google Scholar]
- Ikeshima-Kataoka H, Skeath J.B, Nabeshima Y, Doe C.Q, Matsuzaki F. Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature. 1997;390:625–629. doi: 10.1038/37641. doi:10.1038/37641 [DOI] [PubMed] [Google Scholar]
- Isshiki T, Pearson B, Holbrook S, Doe C.Q. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521. doi: 10.1016/s0092-8674(01)00465-2. doi:10.1016/S0092-8674(01)00465-2 [DOI] [PubMed] [Google Scholar]
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-q. doi:10.1016/0012-1606(92)90270-Q [DOI] [PubMed] [Google Scholar]
- Izumi Y, Ohta N, Itoh-Furuya A, Fuse N, Matsuzaki F. Differential functions of G protein and Baz-aPKC signaling pathways in Drosophila neuroblast asymmetric division. J. Cell Biol. 2004;164:729–738. doi: 10.1083/jcb.200309162. doi:10.1083/jcb.200309162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Ohta N, Hisata K, Raabe T, Matsuzaki F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat. Cell Biol. 2006;8:586–593. doi: 10.1038/ncb1409. doi:10.1038/ncb1409 [DOI] [PubMed] [Google Scholar]
- Jones B.W, Fetter R.D, Tear G, Goodman C.S. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. doi:10.1016/0092-8674(95)90280-5 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt J.A, Brand A.H. Asymmetric cell division: microtubule dynamics and spindle asymmetry. J. Cell Sci. 2002;115:2257–2264. doi: 10.1242/jcs.115.11.2257. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt J.A, Davidson C.M, Brown N.H, Brand A.H. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nat. Cell Biol. 2000;2:7–12. doi: 10.1038/71323. doi:10.1038/71323 [DOI] [PubMed] [Google Scholar]
- Kambadur R, Koizumi K, Stivers C, Nagle J, Poole S.J, Odenwald W.F. Regulation of POU genes by castor and hunchback establishes layered compartments in the Drosophila CNS. Genes Dev. 1998;12:246–260. doi: 10.1101/gad.12.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M.I, Okabe M, Hiromi Y. seven-up controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev. Cell. 2005;8:203–213. doi: 10.1016/j.devcel.2004.12.014. doi:10.1016/j.devcel.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Knoblich J.A, Jan L.Y, Jan Y.N. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377:624–627. doi: 10.1038/377624a0. doi:10.1038/377624a0 [DOI] [PubMed] [Google Scholar]
- Kraut R, Campos-Ortega J.A. inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol. 1996;174:65–81. doi: 10.1006/dbio.1996.0052. doi:10.1006/dbio.1996.0052 [DOI] [PubMed] [Google Scholar]
- Kuchinke U, Grawe F, Knust E. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr. Biol. 1998;8:1357–1365. doi: 10.1016/s0960-9822(98)00016-5. doi:10.1016/S0960-9822(98)00016-5 [DOI] [PubMed] [Google Scholar]
- Kurzik-Dumke U, Phannavong B, Gundacker D, Gateff E. Genetic, cytogenetic and developmental analysis of the Drosophila melanogaster tumor suppressor gene lethal(2)tumorous imaginal discs (1(2)tid) Differentiation. 1992;51:91–104. doi: 10.1111/j.1432-0436.1992.tb00685.x. doi:10.1111/j.1432-0436.1992.tb00685.x [DOI] [PubMed] [Google Scholar]
- Lee C.Y, Robinson K.J, Doe C.Q. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006a;439:594–598. doi: 10.1038/nature04299. doi:10.1038/nature04299 [DOI] [PubMed] [Google Scholar]
- Lee C.Y, Wilkinson B.D, Siegrist S.E, Wharton R.P, Doe C.Q. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev. Cell. 2006b;10:441–449. doi: 10.1016/j.devcel.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Li L, Vaessin H. Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev. 2000;14:147–151. [PMC free article] [PubMed] [Google Scholar]
- Li P, Yang X, Wasser M, Cai Y, Chia W. Inscuteable and Staufen mediate asymmetric localization and segregation of prospero RNA during Drosophila neuroblast cell divisions. Cell. 1997;90:437–447. doi: 10.1016/s0092-8674(00)80504-8. doi:10.1016/S0092-8674(00)80504-8 [DOI] [PubMed] [Google Scholar]
- Liu T.H, Li L, Vaessin H. Transcription of the Drosophila CKI gene dacapo is regulated by a modular array of cis-regulatory sequences. Mech. Dev. 2002;112:25–36. doi: 10.1016/s0925-4773(01)00626-8. doi:10.1016/S0925-4773(01)00626-8 [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, Koizumi K, Hama C, Yoshioka T, Nabeshima Y. Cloning of the Drosophila prospero gene and its expression in ganglion mother cells. Biochem. Biophys. Res. Commun. 1992;182:1326–1332. doi: 10.1016/0006-291x(92)91878-t. doi:10.1016/0006-291X(92)91878-T [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, Ohshiro T, Ikeshima-Kataoka H, Izumi H. Miranda localizes Staufen and Prospero asymmetrically in mitotic neuroblasts and epithelial cells in early Drosophila embryogenesis. Development. 1998;125:4089–4098. doi: 10.1242/dev.125.20.4089. [DOI] [PubMed] [Google Scholar]
- Maurange C, Gould A.P. Brainy but not too brainy: starting and stopping neuroblast divisions in Drosophila. Trends Neurosci. 2005;28:30–36. doi: 10.1016/j.tins.2004.10.009. doi:10.1016/j.tins.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Mettler U, Vogler G, Urban J. Timing of identity: spatiotemporal regulation of hunchback in neuroblast lineages of Drosophila by Seven-up and Prospero. Development. 2006;133:429–437. doi: 10.1242/dev.02229. doi:10.1242/dev.02229 [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. doi:10.1242/dev.01173 [DOI] [PubMed] [Google Scholar]
- Morshead C.M, Reynolds B.A, Craig C.G, McBurney M.W, Staines W.A, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. doi:10.1016/0896-6273(94)90046-9 [DOI] [PubMed] [Google Scholar]
- Noctor S.C, Martinez-Cerdeno V, Ivic L, Kriegstein A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. doi:10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Novotny T, Eiselt R, Urban J. Hunchback is required for the specification of the early sublineage of neuroblast 7–3 in the Drosophila central nervous system. Development. 2002;129:1027–1036. doi: 10.1242/dev.129.4.1027. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–596. doi: 10.1038/35046087. doi:10.1038/35046087 [DOI] [PubMed] [Google Scholar]
- Overton P.M, Meadows L.A, Urban J, Russell S. Evidence for differential and redundant function of the Sox genes Dichaete and SoxN during CNS development in Drosophila. Development. 2002;129:4219–4228. doi: 10.1242/dev.129.18.4219. [DOI] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds M.M, Caldwell M.C, Johns M, Nayak M, Welsh C.J, McDermott S, Datta S. Drosophila Perlecan modulates FGF and Hedgehog signals to activate neural stem cell division. Dev. Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. doi:10.1016/S0012-1606(02)00019-2 [DOI] [PubMed] [Google Scholar]
- Parmentier M.L, Woods D, Greig S, Phan P.G, Radovic A, Bryant P, O'Kane C.J. Rapsynoid/partner of inscuteable controls asymmetric division of larval neuroblasts in Drosophila. J. Neurosci. 2000;20:RC84. doi: 10.1523/JNEUROSCI.20-14-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson B.J, Doe C.Q. Regulation of neuroblast competence in Drosophila. Nature. 2003;425:624–628. doi: 10.1038/nature01910. doi:10.1038/nature01910 [DOI] [PubMed] [Google Scholar]
- Pearson B.J, Doe C.Q. Specification of temporal identity in the developing nervous system. Annu. Rev. Cell Dev. Biol. 2004;20:619–647. doi: 10.1146/annurev.cellbio.19.111301.115142. doi:10.1146/annurev.cellbio.19.111301.115142 [DOI] [PubMed] [Google Scholar]
- Peng C.Y, Manning L, Albertson R, Doe C.Q. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. doi:10.1038/35046094 [DOI] [PubMed] [Google Scholar]
- Peterson C, Carney G.E, Taylor B.J, White K. reaper is required for neuroblast apoptosis during Drosophila development. Development. 2002;129:1467–1476. doi: 10.1242/dev.129.6.1467. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich J.A. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. doi:10.1038/35050550 [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau G.M. The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Development. 1991;111:79–88. doi: 10.1242/dev.111.1.79. [DOI] [PubMed] [Google Scholar]
- Prokop A, Bray S, Harrison E, Technau G.M. Homeotic regulation of segment-specific differences in neuroblast numbers and proliferation in the Drosophila central nervous system. Mech. Dev. 1998;74:99–110. doi: 10.1016/s0925-4773(98)00068-9. doi:10.1016/S0925-4773(98)00068-9 [DOI] [PubMed] [Google Scholar]
- Read T.A, Hegedus B, Wechsler-Reya R, Gutmann D.H. The neurobiology of neurooncology. Ann. Neurol. 2006;60:3–11. doi: 10.1002/ana.20912. doi:10.1002/ana.20912 [DOI] [PubMed] [Google Scholar]
- Rhyu M.S, Jan L.Y, Jan Y.N. Asymmetric distribution of Numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. doi:10.1016/0092-8674(94)90112-0 [DOI] [PubMed] [Google Scholar]
- Riddiford L.M. Hormones and Drosophila development. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; New York, NY: 1993. pp. 899–939. [Google Scholar]
- Sasai Y. Identifying the missing links: genes that connect neural induction and primary neurogenesis in vertebrate embryos. Neuron. 1998;21:455–458. doi: 10.1016/s0896-6273(00)80554-1. doi:10.1016/S0896-6273(00)80554-1 [DOI] [PubMed] [Google Scholar]
- Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich J.A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. doi:10.1016/S0092-8674(01)00521-9 [DOI] [PubMed] [Google Scholar]
- Schmid H, Rickert C, Bossing T, Vef O, Urban J, Technau G.M. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev. Biol. 1997;189:186–204. doi: 10.1006/dbio.1997.8660. doi:10.1006/dbio.1997.8660 [DOI] [PubMed] [Google Scholar]
- Schmid A, Chiba A, Doe C.Q. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126:4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- Schober M, Schaefer M, Knoblich J.A. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature. 1999;402:548–551. doi: 10.1038/990135. doi:10.1038/990135 [DOI] [PubMed] [Google Scholar]
- Shen C.P, Jan L.Y, Jan Y.N. Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell. 1997;90:449–458. doi: 10.1016/s0092-8674(00)80505-x. doi:10.1016/S0092-8674(00)80505-X [DOI] [PubMed] [Google Scholar]
- Siegrist S.E, Doe C.Q. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. doi:10.1016/j.cell.2005.09.043 [DOI] [PubMed] [Google Scholar]
- Siegrist S.E, Doe C.Q. Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development. 2006;133:529–536. doi: 10.1242/dev.02211. doi:10.1242/dev.02211 [DOI] [PubMed] [Google Scholar]
- Siller K.H, Cabernard C, Doe C.Q. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 2006;8:594–600. doi: 10.1038/ncb1412. doi:10.1038/ncb1412 [DOI] [PubMed] [Google Scholar]
- Singh S.K, Hawkins C, Clarke I.D, Squire J.A, Bayani J, Hide T, Henkelman R.M, Cusimano M.D, Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. doi:10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- Skeath J.B, Carroll S.B. Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development. 1992;114:939–946. doi: 10.1242/dev.114.4.939. [DOI] [PubMed] [Google Scholar]
- Skeath J.B, Carroll S.B. The achaete–scute complex: generation of cellular pattern and fate within the Drosophila nervous system. FASEB J. 1994;8:714–721. doi: 10.1096/fasebj.8.10.8050670. [DOI] [PubMed] [Google Scholar]
- Skeath J.B, Thor S. Genetic control of Drosophila nerve cord development. Curr. Opin. Neurobiol. 2003;13:8–15. doi: 10.1016/s0959-4388(03)00007-2. doi:10.1016/S0959-4388(03)00007-2 [DOI] [PubMed] [Google Scholar]
- Spana E.P, Doe C.Q. The Prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development. 1995;121:3187–3195. doi: 10.1242/dev.121.10.3187. [DOI] [PubMed] [Google Scholar]
- Spana E.P, Doe C.Q. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron. 1996;17:21–26. doi: 10.1016/s0896-6273(00)80277-9. doi:10.1016/S0896-6273(00)80277-9 [DOI] [PubMed] [Google Scholar]
- Spana E.P, Kopczynski C, Goodman C.S, Doe C.Q. Asymmetric localization of Numb autonomously determines sibling neuron identity in the Drosophila CNS. Development. 1995;121:3489–3494. doi: 10.1242/dev.121.11.3489. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A, Levine M. Dorsal gradient networks in the Drosophila embryo. Dev. Biol. 2002;246:57–67. doi: 10.1006/dbio.2002.0652. doi:10.1006/dbio.2002.0652 [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. doi:10.1038/35102174 [DOI] [PubMed] [Google Scholar]
- Truman J.W, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev. Biol. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-x. doi:10.1016/0012-1606(88)90067-X [DOI] [PubMed] [Google Scholar]
- Truman J.W, Taylor B.J, Awad T.A. Formation of the adult nervous system. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; New York, NY: 1993. pp. 1245–1275. [Google Scholar]
- Truman J.W, Talbot W.S, Fahrbach S.E, Hogness D.S. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development. 1994;120:219–234. doi: 10.1242/dev.120.1.219. [DOI] [PubMed] [Google Scholar]
- Truman J.W, Schuppe H, Shepherd D, Williams D.W. Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development. 2004;131:5167–5184. doi: 10.1242/dev.01371. doi:10.1242/dev.01371 [DOI] [PubMed] [Google Scholar]
- Udolph G, Urban J, Rusing G, Luer K, Technau G.M. Differential effects of EGF receptor signalling on neuroblast lineages along the dorsoventral axis of the Drosophila CNS. Development. 1998;125:3291–3299. doi: 10.1242/dev.125.17.3291. [DOI] [PubMed] [Google Scholar]
- Udolph G, Rath P, Chia W. A requirement for Notch in the genesis of a subset of glial cells in the Drosophila embryonic central nervous system which arise through asymmetric divisions. Development. 2001;128:1457–1466. doi: 10.1242/dev.128.8.1457. [DOI] [PubMed] [Google Scholar]
- Uemura T, Shepherd S, Ackerman L, Jan L.Y, Jan Y.N. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58:349–360. doi: 10.1016/0092-8674(89)90849-0. doi:10.1016/0092-8674(89)90849-0 [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau G.M. Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development. 2003a;130:3621–3637. doi: 10.1242/dev.00533. doi:10.1242/dev.00533 [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau G.M. Segment polarity and DV patterning gene expression reveals segmental organization of the Drosophila brain. Development. 2003b;130:3607–3620. doi: 10.1242/dev.00532. doi:10.1242/dev.00532 [DOI] [PubMed] [Google Scholar]
- Urbach R, Schnabel R, Technau G.M. The pattern of neuroblast formation, mitotic domains and proneural gene expression during early brain development in Drosophila. Development. 2003;130:3589–3606. doi: 10.1242/dev.00528. doi:10.1242/dev.00528 [DOI] [PubMed] [Google Scholar]
- Uv A.E, Harrison E.J, Bray S.J. Tissue-specific splicing and functions of the Drosophila transcription factor grainyhead. Mol. Cell. Biol. 1997;17:6727–6735. doi: 10.1128/mcb.17.11.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessin H, Grell E, Wolff E, Bier E, Jan L.Y, Jan Y.N. prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell. 1991;67:941–953. doi: 10.1016/0092-8674(91)90367-8. doi:10.1016/0092-8674(91)90367-8 [DOI] [PubMed] [Google Scholar]
- Van De Bor V, Giangrande A. Notch signaling represses the glial fate in fly PNS. Development. 2001;128:1381–1390. doi: 10.1242/dev.128.8.1381. [DOI] [PubMed] [Google Scholar]
- Vincent S, Vonesch J.L, Giangrande A. Glide directs glial fate commitment and cell fate switch between neurones and glia. Development. 1996;122:131–139. doi: 10.1242/dev.122.1.131. [DOI] [PubMed] [Google Scholar]
- Voigt A, Pflanz R, Schafer U, Jackle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev. Dyn. 2002;224:403–412. doi: 10.1002/dvdy.10120. doi:10.1002/dvdy.10120 [DOI] [PubMed] [Google Scholar]
- Von Ohlen T, Doe C.Q. Convergence of dorsal, dpp, and Egfr signaling pathways subdivides the Drosophila neuroectoderm into three dorsal-ventral columns. Dev. Biol. 2000;224:362–372. doi: 10.1006/dbio.2000.9789. doi:10.1006/dbio.2000.9789 [DOI] [PubMed] [Google Scholar]
- Wang H, Chia W. Drosophila neural progenitor polarity and asymmetric division. Biol. Cell. 2005;97:63–74. doi: 10.1042/BC20040064. doi:10.1042/BC20040064 [DOI] [PubMed] [Google Scholar]
- White K, Kankel D.R. Patterns of cell division and cell movement in the formation of the imaginal nervous system in Drosophila melanogaster. Dev. Biol. 1978;65:296–321. doi: 10.1016/0012-1606(78)90029-5. doi:10.1016/0012-1606(78)90029-5 [DOI] [PubMed] [Google Scholar]
- White K, Grether M.E, Abrams J.M, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. doi:10.1126/science.8171319 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Huttner W.B. Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mech. Dev. 2003;120:1297–1309. doi: 10.1016/j.mod.2003.06.003. doi:10.1016/j.mod.2003.06.003 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. doi:10.1038/990128 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. doi:10.1083/jcb.150.6.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse E, Hersperger E, Shearn A. Growth, metastasis, and invasiveness of Drosophila tumors caused by mutations in specific tumor suppressor genes. Dev. Genes Evol. 1998;207:542–550. doi: 10.1007/s004270050145. doi:10.1007/s004270050145 [DOI] [PubMed] [Google Scholar]
- Yamashiki N, Kawamura K. Microdissection studies on the polarity of unequal division in grasshopper neuroblasts. I. Subsequent divisions in neuroblast-type cells produced against the polarity by micromanipulation. Exp. Cell Res. 1986;166:127–138. doi: 10.1016/0014-4827(86)90513-6. doi:10.1016/0014-4827(86)90513-6 [DOI] [PubMed] [Google Scholar]
- Yasuda G.K, Baker J, Schubiger G. Temporal regulation of gene expression in the blastoderm Drosophila embryo. Genes Dev. 1991;5:1800–1812. doi: 10.1101/gad.5.10.1800. [DOI] [PubMed] [Google Scholar]
- Younossi-Hartenstein A, Nassif C, Green P, Hartenstein V. Early neurogenesis of the Drosophila brain. J. Comp. Neurol. 1996;370:313–329. doi: 10.1002/(SICI)1096-9861(19960701)370:3<313::AID-CNE3>3.0.CO;2-7. doi:10.1002/(SICI)1096-9861(19960701)370:3<313::AID-CNE3>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- Yu F, Morin X, Cai Y, Yang X, Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. doi:10.1016/S0092-8674(00)80676-5 [DOI] [PubMed] [Google Scholar]
- Yu F, Cai Y, Kaushik R, Yang X, Chia W. Distinct roles of Galphai and Gbeta13F subunits of the heterotrimeric G protein complex in the mediation of Drosophila neuroblast asymmetric divisions. J. Cell Biol. 2003;162:623–633. doi: 10.1083/jcb.200303174. doi:10.1083/jcb.200303174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Wang H, Qian H, Kaushik R, Bownes M, Yang X, Chia W. Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev. 2005;19:1341–1353. doi: 10.1101/gad.1295505. doi:10.1101/gad.1295505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Skeath J.B. The Sox-domain containing gene Dichaete/fish-hook acts in concert with vnd and ind to regulate cell fate in the Drosophila neuroectoderm. Development. 2002;129:1165–1174. doi: 10.1242/dev.129.5.1165. [DOI] [PubMed] [Google Scholar]