Abstract
The authors measured food reinforcement, polymorphisms of the dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes, and laboratory energy intake in 29 obese and 45 nonobese humans 18–40 years old. Food reinforcement was greater in obese than in nonobese individuals, especially in obese individuals with the TaqI A1 allele. Energy intake was greater for individuals high in food reinforcement and greatest in those high in food reinforcement with the TaqI A1 allele. No effect of the DAT1 genotype was observed. These data show that individual differences in food reinforcement may be important for obesity and that the DRD2 genotype may interact with food reinforcement to influence energy intake.
Keywords: food reinforcement, energy intake, obesity, dopamine, dopamine receptor
Eating is a highly reinforcing activity (Wise, 2006), and there are individual differences in the reinforcing efficacy of food that may relate to differences in eating and energy intake (Epstein, Leddy, Temple, & Faith, in press). In the same way that the reinforcing efficacy of a drug is related to drug consumption (Bickel, Marsch, & Carroll, 2000), subjects who find food highly reinforcing may consume more energy in an ad libitum eating situation than those who are low in food reinforcement (Epstein et al., 2004a). If individual differences in food reinforcement are related to differences in energy intake, then obesity, which is characterized by excess energy intake, may be related to food reinforcement. Obese individuals may find food more reinforcing and may be more motivated to eat than normal weight individuals (Saelens & Epstein, 1996).
There have been limited experimental tests of the hypothesis that high levels of food reinforcement lead to greater energy intake and that obese persons are more motivated to obtain food than nonobese persons. Initial research showed that smokers high in food reinforcement consumed more food than smokers low in food reinforcement in an ad libitum snack food eating task (Epstein et al., 2004a). This study had two important limitations. First, the study used a questionnaire version of a food reinforcement task (Goldfield, Epstein, Davidson, & Saad, 2006) rather than directly measuring the amount of work subjects would do to gain access to food, which is the more sensitive methodology for measuring food reinforcement. Second, only smokers were studied, and smokers generally weigh less and therefore may consume less food than nonsmokers (Klesges, Meyers, Klesges, & LaVasque, 1989); further, smokers may have a different level of food reinforcement than nonsmokers. There has been one experimental test of the hypothesis that food is more reinforcing for obese than for lean persons (Saelens & Epstein, 1996). This study showed that obese young women responded more for access to food than leaner young women, but the study was small (N = 20) and did not include men or older subjects. Because men and women may differ in how food reinforcement relates to energy intake (Epstein et al., 2004a), it is important to study how individual differences in food reinforcement are related to obesity in both men and women.
The reinforcing value of food is related to activity of the dopaminergic system. Food consumption increases brain dopamine levels in animals (Hernandez & Hoebel, 1988, 1990) and humans (Small, Jones-Gotman, & Dagher, 2003). Modifying brain dopamine levels influences energy intake, with dopamine agonists reducing energy intake (Leddy et al., 2004) and dopamine antagonists increasing energy intake and body weight (Wellman, 2005). Individual differences in food reinforcement may be related to individual differences in dopaminergic activity. Dopamine activity is related to both the density of dopamine receptors and the amount of the dopamine transporter. Thus, one way to indirectly study individual differences in brain dopamine levels is by studying polymorphisms in dopamine receptor and transporter genes. For example, the presence of the TaqI A1 allele has been associated with a 30%–40% reduction in the density of the dopamine D2 receptor (DRD2) and weaker dopamine signaling (Jonsson et al., 1999; Pohjalainen et al., 1998; Ritchie & Noble, 2003). The 10-repeats allele (10R) of the dopamine transporter (DAT1) variable number of tandem repeats (VNTR) region (SLC6A3 3′-untranslated region) is related to increased expression of DAT1 protein, leading to greater dopamine reuptake and lower synaptic levels of dopamine (Heinz et al., 2000).
Preliminary data on the interaction of these dopamine genotypes as markers of individual differences in dopaminergic activation and food reinforcement showed that smokers, most of whom were nonobese, who were high in food reinforcement in combination with the TaqI A1 allele of the DRD2 or the absence of the 9R allele of DAT1 had increased energy intake (Epstein et al., 2004b). To our knowledge, this was the first demonstration that the interaction of a dopamine genotype and a food reinforcement phenotype influences energy intake. However, this interaction needs to be extended beyond nonobese smokers. Nicotine produces reliable increases in brain dopamine levels and reduces food intake (M. D. Li, Kane, & Konu, 2003; Miyata, Meguid, Fetissov, Torelli, & Kim, 1999). Long-term exposure to smoking up-regulates dopamine transporter activity (S. Li et al., 2004) and DRD2 (Bahk, Li, Park, & Kim, 2002), but it lessens the up-regulation of DRD2 by other factors (Janson, Hedlund, Fuxe, & von Euler, 1994). Therefore, it is possible that the interaction of the dopamine genotypes and the food reinforcement is the result of smoking’s influence on dopaminergic systems and may not replicate in nonsmokers.
The primary aim of this study was to test two hypotheses derived from reinforcement theory: whether obesity is related to individual differences in food reinforcement and whether individual differences in food reinforcement are related to differences in energy intake in an ad libitum eating task. A secondary aim of the study was to extend previous findings (Epstein et al., 2004b) on the interaction of food reinforcement with the DRD2 and DAT1 dopamine genotypes in nonsmokers to nonobese and obese nonsmokers. Another secondary aim was to compare food reinforcement and food hedonics as predictors of energy intake. Our preliminary data in smokers suggest that the reinforcing value of food is a stronger determinant of energy intake than food liking (Epstein, et al., 2004a) and we want to determine if the same is true for nonsmokers.
Method
Participants
Seventy-four participants were studied, 29 obese (body mass index [BMI] ≥ 30) and 45 nonobese (BMI < 30) nonsmoking adults between the ages of 18 and 40 (M = 25.7 years, SD = 7.0). Of the participants, 18.9% were minorities and 44.5% were male. The distribution of DRD2 genotypes was equal (presence of A1 allele = 37, absence of A1 allele = 37); distribution of the DAT1 genotypes was 31 participants with a 9R allele and 41 without a 9R allele. DAT1 genotypes were unable to be determined for 2 participants. Participants were excluded if they were taking medication associated with a loss of appetite, were smokers, had diabetes, had previously been diagnosed with an eating disorder, were allergic to ingredients in the study foods, were currently dieting, found the study foods aversive, or did not normally eat breakfast and lunch.
Procedures
Participants visited the laboratory for two sessions separated by 2–7 days (see Table 1). The first session involved completion of consent and demographic forms and the ad libitum snack-eating task; the second session involved the food reinforcement task. A subset of participants (n = 20) completed a third session 2–7 days later identical to the second session to determine the test–retest reliability of the food reinforcement phenotype. Experimental sessions were run during a typical lunch period, at least 3 hr post-prandial. All participants consumed the same breakfast each day and were provided with a 150-kcal preload (18% fat, 17% protein, 65% carbohydrates) to minimize the effects of hunger on food reinforcement. The inclusion of a standard preload increases the ability to show individual differences in food reinforcement (Reiss & Havercamp, 1996). At the beginning of each session, participants completed a same-day dietary recall to ensure adherence to the dietary instructions.
Table 1.
Study Design
| Session
|
|||
|---|---|---|---|
| Phase | 1 | 2 | 3 |
| N | 74 | 74 | 20 (of 74) |
| Breakfast | Same breakfast each day | ||
| Preload | Same 150-kcal preload each day | ||
| Lunch task | Ad libitum eating task | Reinforcement task (1) | Reinforcement task (2) |
Note. Sessions were 2–7 days apart.
Ad libitum eating task
The ad libitum food consumption task was presented as a taste test. Participants were presented 480–500-kcal servings of six palatable high-fat, high-carbohydrate, low-protein snack foods (percentages of fat, carbohydrates, and protein shown in parentheses): Lay’s Potato Chips (57%, 38%, 5%); Doritos (44%, 50.4%, 5.6%); M&M’s (38.8%, 57.4%, 3.8%); Twix (45%, 50.0%, 5.0%); Kit Kat (45.2%, 49.3%, 5.5%); and Butterfinger (35.2%, 60.3%, 4.5%). Water was provided ad libitum. Participants rated each food on a number of different characteristics using 9-point Likert-type scales, including pleasurability, sweetness, saltiness, blandness, flavorfulness, and bitterness. They were told that they could consume as little or as much of the food as they wished as long as they tasted each one so that they could rate its characteristics. Participants were then given three eating questionnaires to complete. Food from the taste test was left in the room, and participants were told they could continue to eat if they wished, as the food would be discarded after the session. When participants indicated that they were finished, they were asked to identify their favorite food from among the six and told that this was the food that would be used in the next session. Once the questionnaires were complete, participants’ cheek epithelial cells were collected using a buccal brush for DNA analysis, and participants were given a reminder for their next visit.
Food reinforcement task
The reinforcing value of food was measured by determining the number of responses on a concurrent schedule task that participants made for food or food alternatives. The experimental environment included two computer stations with a swivel chair in the middle. At one station participants could earn points toward food, and at the other station they could earn points for time to spend reading the Buffalo News. This alternative activity was provided to reduce the likelihood that participants would engage in responding out of boredom.
Participants earned access to food or the alternative on concurrent variable-ratio (VR) schedules of reinforcement. The reinforcement schedule for food was a progressive VR schedule with response requirements of 4, 8, 16, 32, 64, 128, 256, 512, 1,024, and so forth, on the average, for each point. After 5 points were earned, the participant received a 100-kcal portion of his or her preferred snack food selected during Session 1. The task was identical on the computer on which reading time could be earned, but the schedule remained at VR4 throughout, and after 5 points were earned, the participant received access to reading for 2 min. The reinforcement task was similar to a slot machine. When the left button on the mouse was pressed, three boxes containing different colored shapes revolved, and when all of the shapes matched in shape and color, the participant earned 1 point. Participants were instructed that the session would end when they no longer wished to earn points for access to food or reading. Water was provided ad libitum.
Laboratory environment
The laboratory was specially constructed for eating experiments. It was equipped with an air delivery system that circulated new air through each room approximately 10 times per hour. The laboratory rooms were also equipped with high-efficiency particulate air purifiers containing a carbon–permanganate–zeolite filter to remove airborne odorants.
Genotyping
DNA was extracted from the buccal samples using a commercially available genomic DNA quick preparation kit (Gentra Systems, Qiagen, Inc., Valencia, CA), yielding 20 μl of DNA at a concentration of 15–40 ng/μl. After DNA purification, each sample was assigned an accession number and stored at −20 °C for later analysis.
For detection of the TaqI A1 polymorphism in the DRD2 gene, a region of 304 base pairs (bp) was amplified. The primers first described by Grandy et al. (Grandy et al., 1989) were modified to sense 5′-CCC TTC CTG AGT GTC ATC A-3′ and antisense 5′-CGG CTG GCC AAG TTG TCT-3′. The presence of the amplicon was confirmed by electrophoresis on a 1% agarose gel. The restriction endonuclease TaqI A1 digests the 304-bp amplicon, and subsequently the fragments are separated on a 6% polyacrylamide gel by electrophoresis. The TaqI A1 polymorphism in the DRD2 gene at Position 32806 T to C creates a restriction site, resulting in partition of the 304 bp into a fragment of 177 and 127 bp. The A1/A1 or TT variant therefore is represented by an uncut amplicon of 304 bp; the A1/A2 or TC heterozygous form digests in three fragments of 304, 177, and 127 bp; and the A2/A2 or CC variant is characterized by two fragments of 177 and 127 bp. The DRD2 was coded for the allele patterns of A1/A1, A1/A2, and A2/A2. Analyses were performed combining the A1/A1 and A1/A2 patterns, comparing presence or absence of the A1 allele.
Analysis of DAT1 gene focused on the VNTR of a 40-bp sequence, which recurs 3 to 11 times. Primers first described by Vandenbergh et al. (Vandenbergh et al., 1992) were modified to sense 5′-GGT GTA GGG AAC GGC CTG AG-3′ and antisense 5′-CTG GAG GTC ACG GCT CAA GG-3′. The amplicon was analyzed on a 10% polyacrylamide gel by electrophoresis. The large increment of 40 bp provides distinct typing of the VNTRs. Each run of participant DNA samples included sequenced control DNA samples and a negative control. The DAT1 was coded for the allele patterns of 9R/9R, 9R/10R, and 10R/10R. Analyses were performed combining the 9R/9R and 9R/10R patterns, comparing presence or absence of the 9R allele.
Dietary recalls
At the beginning of each session, participants were asked to recall what foods and beverages they had consumed the day of the experiment to ensure adherence to the experimental protocol. Participants were guided through the recall process and recorded each food item recalled as well as the portion size, condiments, added fats, and added sugars. Measuring cups and spoons were provided to help the participants estimate portion sizes.
Demographics
A general demographics questionnaire was used to assess participants’ education status, race, and ethnicity.
Anthropometrics
Height (cm) and weight (lb) were measured using a Digi-Kit digital stadiometer (Digi-Kit, North Bend, WA) and a Tanita digital weight scale (Tanita, Arlington Heights, IL), and used to calculate BMI (kg/m2). Individuals were considered obese if their BMI was at least 30 kg/m2 and nonobese if their BMI was less than 30 kg/m2 (NHLBI Obesity Education Initiative Expert Panel, 1998).
Eating questionnaires
Participants completed the Three-Factor Eating Questionnaire (French, Jeffery, & Wing, 1994; Stunkard & Messick, 1985), the Questionnaire of Eating and Weight Patterns (Spitzer et al., 1992), and the Binge Eating Scale (Gormally, Black, Daston, & Rardin, 1982). The Three-Factor Eating Questionnaire has three subscales that assess dietary restraint, hunger, and disinhibition. The Questionnaire of Eating and Weight Patterns and Binge Eating Scale are used to assess binge eating disorder. Participants were classified as dietary restrained if they scored higher than 12 on the Dietary Restraint subscale of the Three-Factor Eating Questionnaire. Participants were identified as potentially having binge eating disorder if they scored higher than 27 on the Binge Eating Scale or were indicated as having the disorder by the Questionnaire on Eating and Weight Patterns. Any participant identified as potentially having binge eating disorder was required to complete the Eating Disorders Examination (Bryant-Waugh, Cooper, Taylor, & Lask, 1996), administered by trained personnel in an additional session. No participants met criteria for binge eating disorder.
Food liking, hunger, and fullness
Both before and after the food reinforcement task, participants rated how hungry they felt on a 100-mm visual analogue scale anchored by not hungry at all and extremely hungry, and how much they liked the snack food reinforcer on a 100-mm visual analogue scale anchored by not like at all and like very much.
Analytic Plan
Differences in participant characteristics by genotypes and by obesity were assessed using one-way analysis of variance for continuous variables and chi-square tests for categorical variables. Differences in patterns of operant responding for food were determined using hierarchical mixed-effects regression models (MRM), which allow for regression analyses to test predictors of repeated dependent measures (Hedeker & Gibbons, 2006). The first model used responding for each schedule of reinforcement as the dependent variable, with obesity, gender, age, and binge eating score as time-invariant independent predictors and schedule of reinforcement as the time-variant predictor. Both linear (Obesity × Schedule of Reinforcement) and quadratic (Obesity × Schedule × Schedule of Reinforcement) patterns were tested. Quadratic patterns were tested because the pattern of responding represents an increase in responding as the schedules increase, followed by a reduction in responding as the break point for each participant is reached. Age, gender, and binge eating score were tested as moderators of the obesity effect by interacting the potential moderator with obesity using methods recommended by Kraemer and colleagues (Kraemer, Frank, & Kupfer, 2006). The next step was to add the dopamine genotypes × schedules of reinforcement (including linear and quadratic terms) as predictors of responding in separate analyses and assess whether adding information on either genotype tested separately improved the fit of the model by using chi-square to test the change in the log-likelihood ratio. The final models tested the interaction of obesity and each of the two dopamine genotypes × schedules of reinforcement as predictors of responding. Thus, the first model tested whether obesity was related to responding for food, whereas the subsequent models tested whether knowledge about the genotype(s) improved the fit of the model.
Changes in hunger and food liking were tested using mixed analysis of covariance (ANCOVA), with obesity and the DRD2 and DAT1 genotypes as between-subjects variables, and time of assessment (pre- or posttask) as the within-subject variable. Variables that were different between groups, age and binge eating score, were used as covariates, in addition to gender. Linear contrasts were used to compare differences in the pattern of change over time. Differences in energy intake for food were determined using a factorial ANCOVA with food reinforcement and the DRD2 and DAT1 genotypes as the between-subjects variables, with age, binge eating, and sex as covariates. Responding at or below the VR32 schedule was used to differentiate low and high responding on the basis of the median of the distribution of responding. Contrasts were used to compare differences in energy intake by group. Outliers were determined using exploratory data analysis, and one outlier (greater than three standard deviations from the mean) for energy intake was removed from the analysis. Initial analyses used both genotypes to examine the interaction between the genotypes. Subsequent analyses were performed separately for each genotype, as there was no interaction between the genotypes, and to maximize the sample for each genotype because there were two DAT1 genotypes that could not be determined. Food reinforcement and food liking were assessed as determinants of energy intake using a multiple regression model including both of these predictors, as well as age, sex, and binge eating. The test–retest reliability of food reinforcement was assessed by a correlation of the break points in each measure.
Results
Participant Characteristics
There were no differences between the groups for education, percentage of minority participants, food pleasurability, or hunger (p > .05; see Table 2). There was an effect of weight status on age and binge eating score, where the obese individuals were older, F(1, 72) = 14.60, p < .0001, and scored higher on the Binge Eating Scale, F(1, 72) = 19.81, p < .0001, than the nonobese individuals. Participants with the A2/A2 genotype had higher binge eating scores, F(1, 72) = 4.79, p < .05, than those with the A1/A1 or A1/A2 genotype.
Table 2.
Participant Characteristics
| Obesity
|
DRD2 |
SLC6A3
|
||||
|---|---|---|---|---|---|---|
| Variable | Nonobese | Obese | A1/A1 & A1/A2 | A2/A2 | 9/9 & 9/10 | 10/10 |
| N (male/female) | 45 (22/23) | 29 (11/18) | 37 (15/22) | 37 (18/19) | 31 (15/16) | 41 (17/24) |
| Age (M ± SD) | 23.4 ± 5.4a | 29.3 ± 7.8a | 26.1 ± 7.2 | 25.4 ± 6.8 | 26.7 ± 7.3 | 24.8 ± 6.6 |
| BMI (kg/m2) (M ± SD) | 23.2 ± 2.8 | 34.5 ± 4.6a | 26.3 ± 6.7 | 29.0 ± 6.3 | 28.0 ± 6.2 | 27.0 ± 6.4 |
| Dietary restraint (M ± SD) | 5.8 ± 3.9 | 6.9 ± 4.2 | 6.4 ± 4.1 | 6.1 ± 3.9 | 6.9 ± 4.2 | 5.9 ± 3.9 |
| Binge Eating Scale (M ± SD) | 6.8 ± 5.5 | 13.8 ± 8.0a | 7.7 ± 6.5 | 11.4 ± 7.9b | 10.3 ± 6.5 | 8.9 ± 7.7 |
| Baseline hunger (M ± SD) | 53.3 ± 22.6 | 52.0 ± 26.2 | 50.4 ± 24.4 | 55.2 ± 23.4 | 52.9 ± 25.7 | 53.5 ± 23.1 |
| Average food liking (M ± SD) | 75.9 ± 22.9 | 74.3 ± 20.8 | 75.6 ± 20.5 | 75.0 ± 23.6 | 75.3 ± 20.8 | 74.8 ± 23.4 |
| Race: n (%) | ||||||
| Caucasian | 37 (82) | 22 (76) | 33 (89) | 26 (70) | 26 (84) | 31 (76) |
| Minority | 7 (16) | 6 (21) | 3 (8) | 10 (27) | 4 (13) | 9 (22) |
| Mixed race | 1 (2) | 1 (3) | 1 (3) | 1 (3) | 1 (3) | 1 (2) |
| Education: n (%) | ||||||
| High school | 28 (62) | 17 (59) | 20 (54) | 25 (68) | 18 (58) | 25 (61) |
| College degree | 17 (38) | 12 (41) | 17 (46) | 12 (32) | 13 (42) | 16 (39) |
| DRD2 n (%) | ||||||
| A1/A1 and A1/A2 | 25 (56) | 12 (41) | 14 (45) | 22 (54) | ||
| A2/A2 | 20 (44) | 17 (59) | 17 (55) | 19 (46) | ||
| SLC6A3 n (%) | ||||||
| 9/9 and 9/10 | 19 (43) | 12 (43) | 14 (39) | 17 (47) | ||
| 10/10 | 25 (57) | 16 (57) | 22 (61) | 19 (53) | ||
Note. Dietary restraint scores were derived from the Three-Factor Eating Questionnaire (Stunkard & Messick, 1985). DRD2 = dopamine D2 receptor; BMI = body mass index.
Significant differences between obese and nonobese, p < .001.
Significant differences between DRD2 genotypes, p < .001.
Weight Status, Genotype, and Food Reinforcement
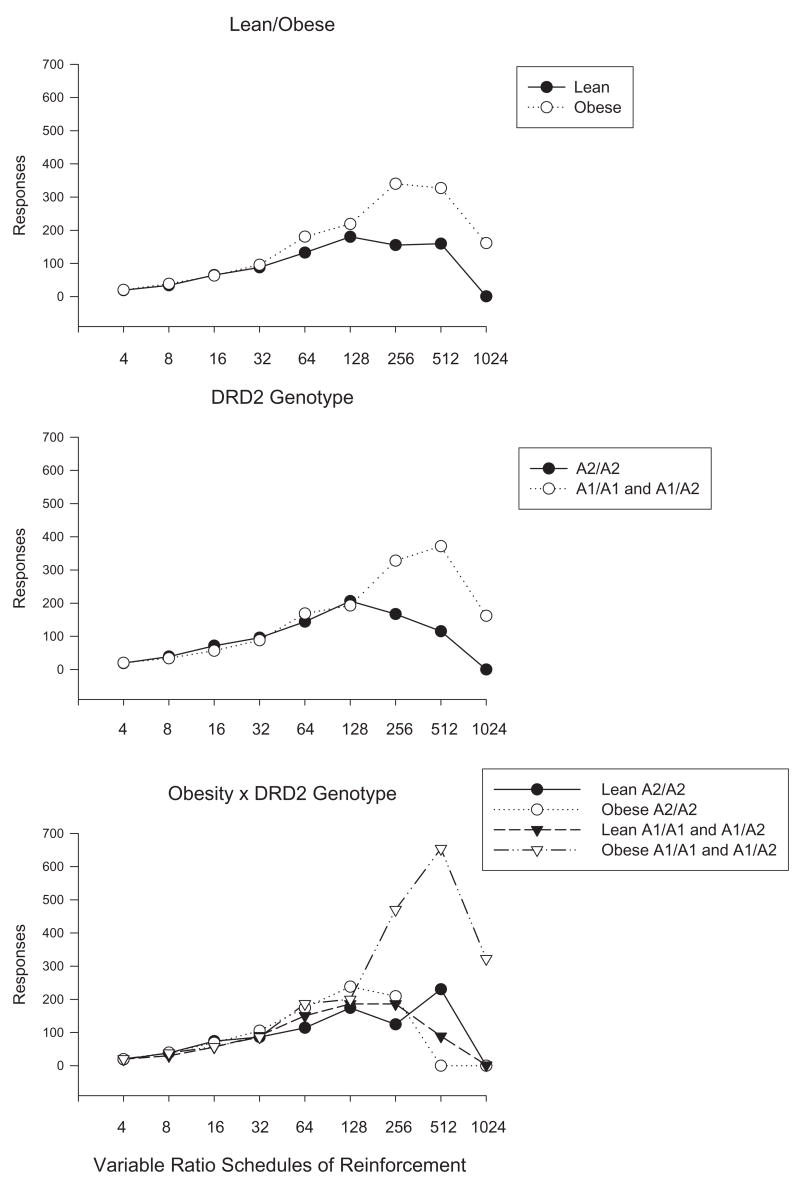
MRM showed that obesity was a significant predictor of responding for food for both linear (p = .004) and quadratic (p = .033) trends, which improved the fit of the basic model (χ2(3) = 15.49, p = .0014), controlling for age, gender, and binge eating status. Analyses did not show that these variables interacted with obesity to moderate responding for food. The relationships between obesity and responding for food across the schedules of reinforcement are shown in the top graph of Figure 1. The DRD2 genotype was related to linear (p = .022) but not quadratic responding for food (p = .009; middle graph, Figure 1), which improved the fit of the basic model (χ2(3) = 9.15, p = .027), as participants with the A1 allele responded more for food than those without the A1 allele. In addition, the DRD2 genotype interacted with obesity to predict linear (p < .001) and quadratic (p = .011) trends for responding for food, improving fit of the obesity × schedules model (χ2(5) = 25.70, p < .0001). These relationships, shown in the bottom graph of Figure 1, were explored by separate MRM analyses of participants with and without the A1 allele. Model fit was not improved by adding the obesity × schedule interaction for participants without the A1 allele (χ2(3) = 1.63, p > .05), but both linear (p < .001) and quadratic (p = .011) differences in responding were observed for obese and nonobese participants with the A1 allele, improving fit of the model (χ2(3) = 20.55, p < .0001), as obese subjects with the A1 allele responded more for food. Adding the DAT1 genotype × schedule interaction did not improve fit of the basic model, χ2(3) = 4.12, p > .05, and adding the interaction of DAT1 genotype × obesity × schedules did not improve the fit of the obesity × schedules model, χ2(5) = 9.32, p > .05.
Figure 1.
Differences in motivated responding for snack foods across variable ratio schedules of reinforcement for those who were obese (body mass index > 30) versus nonobese (top graph), for those with versus without the dopamine D2 receptor (DRD2) A1 allele (middle graph), and for the combination of obesity and the A1 allele (bottom graph). Mixed-effects regression models showed that obesity (p = .0014) and the A1 allele (p = .027) independently predicted responding for food. An interaction of obesity with the DRD2 genotype was observed, as obese participants with the A1 allele responded more for food than obese participants without the A1 allele (p < .0001), whereas there were no significant differences in responding for nonobese participants with or without the A1 allele.
Genotype, Food Reinforcement, and Energy Intake
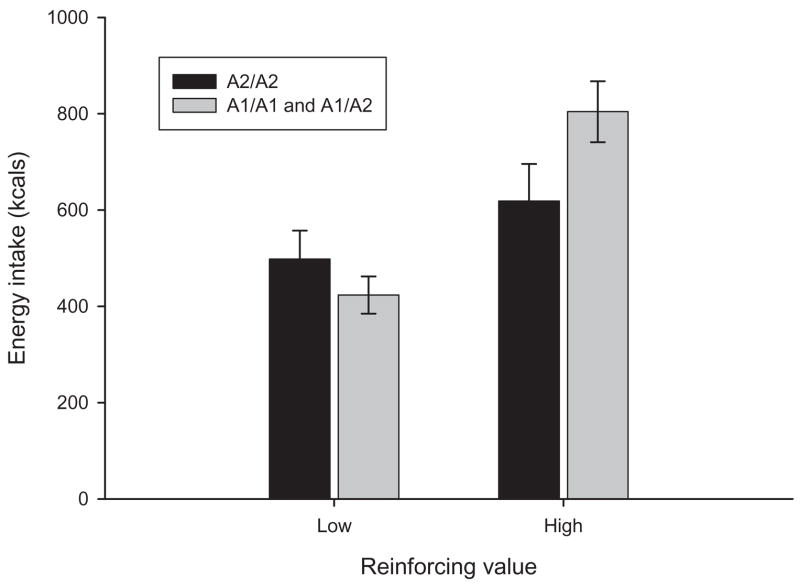
The results for the ad libitum energy intake task (Figure 2) showed a significant main effect for food reinforcement, F(1, 66) = 9.40, p = .003, as those with high levels of food reinforcement consumed more energy (686.6 ± 336.4 kcal, M ± SD) compared with those with low levels of food reinforcement (494.4 ± 194.5 kcal). In addition, an interaction between the food reinforcement phenotype and the DRD2 genotype, F(1, 66) = 6.22, p = .015, was observed. Contrasts showed that those high in food reinforcement with the A1 allele consumed more energy (774.4 ± 283.2 kcal) than those low in food reinforcement either with (430.1 ± 158.5 kcal, p = .0002) or without (558.7 ± 227.7 kcal, p = .0005) the A1 allele and those high in food reinforcement without the A1 allele (598.8 ± 362.1 kcal, p = .037). In analyses including the DAT1 genotype, no interaction of DAT1 genotype with food reinforcement (p = .92), main effect of DAT1 (p = .83), interaction between DRD2 and DAT1 (p = .96), or interaction of DRD2, DAT1, and food reinforcement (p = .83) was observed.
Figure 2.
Participants’ energy consumption by food reinforcement value and presence or absence of the A1 allele (p = .003). Participants high in food reinforcement with the A1 allele consumed more food than participants high in food reinforcement without the A1 allele (p = .037) and participants low in food reinforcement with (p < .0002) or without the A1 allele (p = .0005).
A significant decrease in hunger (51.9 ± 23.9 to 26.4 ± 23.1) was observed after food consumption, F(1, 64) = 9.89, p = .002, but no interaction of hunger reduction with weight status, or either of the dopamine genotypes, was found. No significant changes in food liking were observed over time (75.3 ± 22.0 to 63.8 ± 25.2) or by weight or genotype status.
Reinforcing Value of Food, Food Liking, and Energy Intake
Regression analysis showed that the reinforcing value of food was a significant predictor of energy intake (p = .002) but that self-reported liking of the favorite food was not (p = .72), controlling for sex, age, and binge eating scores. The univariate correlation between reinforcing value and energy intake was .40 (p = .0004), whereas the univariate correlation between liking and energy intake was .02 (p = .87).
Reliability of Study Measures
There was a significant positive correlation between the break points in the repeated measures of food reinforcement (n = 20, r = .80, p < .05). The regression line for predicting the break point in Session 2 from the break point in Session 1 had a slope of 1.00 and an intercept of −0.18.
Discussion
The results support the hypotheses that individuals higher in food reinforcement will consume more food in an ad libitum eating situation than those lower in food reinforcement and that obese individuals are higher in food reinforcement than those who are nonobese. These results are consistent with previous research in smokers showing that subjects high in food reinforcement consume more food than those lower in food reinforcement (Epstein et al., 2004b) and that subjects who find food more reinforcing consume more energy than those less motivated to eat (Johnson, 1974; Saelens & Epstein, 1996).
These results provide support for the importance of studying food reinforcement as a contributor to obesity. Food is a powerful reinforcer that can be as reinforcing as drugs of abuse (Hursh & Bauman, 1987). The motivation to eat and food reinforcement can be increased by food deprivation (Raynor & Epstein, 2003), as well as by food variety (Temple, Giacomelli, Roemmich, & Epstein, in press). Chronic food deprivation may sensitize the brain to increases in food reinforcement (Carr, 1996). Conceptualizing overeating as increased motivation to eat because of increased food reinforcement is similar to conceptualizations of drug abuse that focus on the positive reinforcing effects of drug self-administration in maintaining drug abuse (Bickel, Madden, & Petry, 1998; Bickel et al., 2000). This approach provides the opportunity to take advantage of a wealth of basic research that has provided new insights into how reinforcement may motivate behavior (Wise, 2006).
There are several directions for research on food reinforcement and obesity. One important question is whether individual differences in food reinforcement are risk factors for the development of obesity, or whether a history of positive energy balance that leads to obesity results in higher levels of food reinforcement. Prospective research on children is needed in which individual differences in food reinforcement are measured in lean children and children are followed over time. One of the biggest risk factors for the development of obesity is parental obesity (Garn & Clark, 1976; Whitaker, Wright, Pepe, Seidel, & Dietz, 1997), and one prediction is that individual differences in food reinforcement are shared between parents and children. Consistent with this hypothesis, researchers have recently shown a strong relationship between parent and child levels of food reinforcement in 50 families with overweight children (Epstein, Dearing, Temple, & Cavanaugh, 2007).
Understanding individual differences in food reinforcement sheds light on only one of the factors that influence eating, as eating represents a choice among behaviors (Epstein et al., in press), and to understand how people make the choice to eat, it is necessary to understand the alternatives that are available. One possibility is that the relative reinforcing value of eating is determined not only by the reinforcing value of food but also by the reinforcing value of alternatives to eating (Epstein et al., in press). We have previously shown that nonfood alternatives and less preferred foods can substitute for highly preferred foods when access to the preferred foods is reduced (Goldfield & Epstein, 2002). In order to better understand the role of food reinforcement as a risk factor for obesity, it may be necessary to understand the reinforcing value of alternatives to eating in obese people. It is possible that for some people the reinforcing value of food may not be pronounced but that these people do not have reliable alternatives to food reinforcement, which results in greater relative food reinforcement than reinforcement from alternatives to eating, which could result in overeating.
The results of this study replicate research indicating that the presence of the TaqI A1 allele interacts with obesity to influence food reinforcement and interacts with food reinforcement to influence energy intake (Epstein et al., 2004b). The approach we have taken focuses on theoretical advances in understanding the neurobiology of the reinforcement process and studies genotypes that relate to or may be markers of basic neurobiological processes. The presence of the TaqI A1 allele of the DRD2 is associated with a lower density of DRD2 (Jonsson et al., 1999; Pohjalainen et al., 1998; Ritchie & Noble, 2003), and both the TaqI A1 allele (Comings et al., 1993; Noble et al., 1994) and reduced density of DRD2 (Wang et al., 2001) have been associated with human obesity. Demonstrating that polymorphisms that are related to the density of DRD2 receptors can affect food reinforcement, obesity, and energy intake provides a mechanistic explanation for how dopaminergic activity may influence ingestive behavior and obesity risk. An important limitation of the present study is the small sample size and population admixture. Research designed to identify genes that are associated with or responsible for specific behaviors usually requires much larger sample sizes and control of population stratification that can arise when multiple racial and ethnic groups are studied, and usually involves tests of a large number of genes to provide data on the association between specific dopaminergic genes and food reinforcement.
A second limitation in the current study is that the TaqI A1 allele lies 10 kb downstream of the DRD2 gene and may reside in the coding region of a novel serine/threonine kinase gene (Neville, Johnstone, & Walton, 2004). It is therefore unlikely that polymorphisms in the TaqI A1 have a direct influence on DRD2 expression. It is possible that the TaqI A1 allele is in linkage disequilibrium with functional variants of DRD2 and, thus, indirectly affects DRD2 expression (Neville et al., 2004). The majority of studies that have examined associations between the TaqI A1 polymorphism and DRD2 density have shown significant reductions in DRD2 in individuals who carry at least one copy of the TaqI A1 (Jonsson et al., 1999; Pohjalainen et al., 1998; Ritchie & Noble, 2003), though this is not universally accepted (Laruelle, Gelernter, & Innis, 1998). Research showing that the TaqI A1 polymorphism is not contained within the DRD2 gene constrains attributing food reinforcement directly to the TaqI A1 polymorphism of DRD2 genotype. Additional research is needed to identify the polymorphisms that directly lead to changes in the number of DRD2 receptors, and to link reductions inDRD2 density with energy intake, food reinforcement, and weight status. Until these polymorphisms are identified, the TaqI A1 allele remains an indirect marker of DRD2 expression.
Synaptic dopamine levels are related to dopamine transporter activity (Heinz et al., 2000). In previous research, smokers who were homozygous for the 10R allele and had high food reinforcement levels also consumed more energy than smokers who carried at least one copy of the 9R allele or who had low food reinforcement levels (Epstein et al., 2004b). In the present study no interactions of this DAT1 genotype and food reinforcement phenotype were observed, and there was no main effect of the DAT1 genotype on energy intake. The differences in effects of the dopamine transporter may be due to the use of smokers in the previous study. Smoking has effects on the DAT1 genotype (S. Li et al., 2004), and a long history of smoking may have altered the dopamine transport system to increase sensitivity to food reinforcement.
With current technology it is difficult to assess dynamic changes in neurotransmitter activity in brains of behaving humans. Functional MRI studies can demonstrate changes in regional cerebral blood flow during cognitive tasks in areas of the brain known to contain dopamine receptors but cannot yield information about specific neurotransmitter activity (Holsen et al., 2005). Positron emission tomography (PET) scans provide the resolution to measure the density of available DRD2 using receptor-specific radioligands (Elsinga, Hatano, & Ishiwata, 2006). However, this measurement is sensitive to potential fluctuations in endogenous dopamine release that may occur as a result of the experimental manipulations and, thus, can differ depending on the context (Adler et al., 2000; Leyton et al., 2002; Volkow et al., 2002). In addition, raclopride, the most common radioligand used for the study of DRD2, is a DRD2 antagonist and, thus, may have its own influence on food reinforcement and impulsivity for reinforcers (Hsiao & Smith, 1995; Wade, de Wit, & Richards, 2000). An alternative approach to the direct measurement of dopaminergic activity is to measure individual differences in dopamine receptor genotypes as markers for dopaminergic activity in behaviorally active contexts. Postmortem analysis of frontal cortex and caudate nucleus (Ritchie & Noble, 2003) and PET studies have shown that the presence of at least one copy of the TaqI A1 allele is associated with a 30%–40% reduction in the density of DRD2 (Jonsson et al., 1999; Pohjalainen et al., 1998). Thus, it may be possible to use the genotype as a marker for DRD2 density and dopaminergic tone.
This study did not consider physical activity and energy expenditure, but there may be separate or complementary biological processes that interact with physical activity reinforcement phenotypes that may be associated with differences in energy expenditure. These patterns of behavioral and biological processes may be individual-difference characteristics, such that some people may be in positive energy balance owing to overeating whereas others may be in positive energy balance owing to relatively low energy expenditure and still others may combine overeating with low activity levels.
This study represents a novel, theoretically driven approach to the use of polymorphisms in genes that alter dopaminergic activity to study biological influences on complex behavioral processes. The interaction of DRD2 with other factors that control dopaminergic activity needs to be examined, along with how dopaminergic activity interacts with other neurotransmitters, such as opioids or serotonin. The results of this study have important implications for the development and treatment of obesity. The ability to characterize people as at risk for obesity on the basis of behavioral and neurobiological factors provides the opportunity to develop treatment programs that are tailored to individuals with specific patterns of risk factors. Current obesity prevention programs have not been successful (Swinburn, Gill, & Kumanyika, 2005), and the approach of focusing behavior change efforts on those at high risk may be better suited to prevention than less focused efforts at the general population, many of whom are at low risk. Obesity treatment programs generally provide a one-size-fits-all approach, with the same treatments for all participants. Weight loss and maintenance might be dramatically improved if treatment approaches would differentially focus on the individual characteristics of the participants. It is easy to conceptualize that a prevention or treatment strategy for someone who is high in food reinforcement would be very different from the strategy for someone who is low in food reinforcement but higher in activity reinforcement. The identification of individual differences in behavioral and neurobiological factors that are related to obesity may make the potential for individualizing prevention or treatment approaches to obesity a reality.
Acknowledgments
Leonard H. Epstein is a consultant to Kraft foods. The other authors do not have any potential conflicts of interest. This research was funded in part by a grant from the National Institute of Child Health and Human Development (R01 HD 39778) to Leonard H. Epstein.
References
- Adler CM, Elman I, Weisenfeld N, Kestler L, Pickar D, Breier A. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology. 2000;22:545–550. doi: 10.1016/S0893-133X(99)00153-0. [DOI] [PubMed] [Google Scholar]
- Bahk JY, Li S, Park MS, Kim MO. Dopamine D1 and D2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Progress in Neuropsychopharma-cology and Biological Psychiatry. 2002;26:1095–1104. doi: 10.1016/s0278-5846(02)00243-9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ, Petry NM. The price of change: The behavioral economics of drug dependence. Behavior Therapy. 1998;29:545–565. [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders. 1996;19:391–397. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochemical Research. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- Comings DE, Flanagan SD, Dietz G, Muhleman D, Knell E, Gysin R. The dopamine D2 receptor (DRD2) as a major gene in obesity and height. Biochemical Medicine and Metabolic Biology. 1993;50:176–185. doi: 10.1006/bmmb.1993.1059. [DOI] [PubMed] [Google Scholar]
- Elsinga PH, Hatano K, Ishiwata K. PET tracers for imaging of the dopaminergic system. Current Medicinal Chemistry. 2006;13:2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Temple JL, Cavanaugh MD. Food reinforcement and impulsivity in overweight children and their parents. 2007. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. doi: 10.1037/0033-2909.133.5.884. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jaroni JL, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiology & Behavior. 2004a;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jaroni JL, et al. The relationship between food reinforcement and dopamine genotypes on food intake in smokers. American Journal of Clinical Nutrition. 2004b;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- French SA, Jeffery RW, Wing RR. Food intake and physical activity: A comparison of three measures of dieting. Addictive Behaviors. 1994;19:401–409. doi: 10.1016/0306-4603(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Garn SM, Clark DC. Trends in fatness and the origins of obesity. Pediatrics. 1976;57:443–456. [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH. Can fruits and vegetables and activities substitute for snack foods? Health Psychology. 2002;21:299–303. [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH, Davidson M, Saad FG. Validation of a multiple choice questionnaire measure of the relative reinforcing value of food. Eating Behaviors. 2006;6:283–292. doi: 10.1016/j.eatbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behaviors. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, et al. The human dopamine D2 receptor gene is located on Chromosome 11 at q22–q23 and identifies a TaqI RFLP. American Journal of Human Genetics. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiology & Behavior. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Research Bulletin. 1990;25:975–979. doi: 10.1016/0361-9230(90)90197-8. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, et al. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao S, Smith GP. Raclopride reduces sucrose preference in rats. Pharmacology Biochemistry and Behavior. 1995;50:121–125. doi: 10.1016/0091-3057(95)00315-n. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Bauman RA. The behavioral analysis of demand. In: Green L, Kagel JH, editors. Advances in behavioral economics. Vol. 1. Norwood, NJ: Ablex; 1987. pp. 117–165. [Google Scholar]
- Janson AM, Hedlund PB, Fuxe K, von Euler G. Chronic nicotine treatment counteracts dopamine D2 receptor upregulation induced by a partial meso-diencephalic hemitransection in the rat. Brain Research. 1994;655:25–32. doi: 10.1016/0006-8993(94)91593-8. [DOI] [PubMed] [Google Scholar]
- Johnson WG. Effect of cue prominence and subject weight on human food-directed performance. Journal of Personality and Social Psychology. 1974;29:843–848. doi: 10.1037/h0036390. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, LaVasque MD. Smoking, body weight, and their effects on smoking behavior: A comprehensive review of the literature. Psychological Bulletin. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: Clinical, research, and policy importance. Journal of the American Medical Association. 2006;296:1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Gelernter J, Innis RB. D2 receptors binding potential is not affected by Taq1 polymorphism at the D2 receptor gene. Molecular Psychiatry. 1998;3:261–265. doi: 10.1038/sj.mp.4000343. [DOI] [PubMed] [Google Scholar]
- Leddy JJ, Epstein LH, Jaroni JL, Roemmich JN, Paluch RA, Goldfield GS, et al. The influence of methylphenidate on eating in obese men. Obesity Research. 2004;12:224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: A PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Konu O. Nicotine, body weight and potential implications in the treatment of obesity. Current Topics in Medical Chemistry. 2003;3:899–919. doi: 10.2174/1568026033452203. [DOI] [PubMed] [Google Scholar]
- Li S, Kim KY, Kim JH, Park MS, Bahk JY, Kim MO. Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in the rat midbrain. Neuroscience Letters. 2004;363:29–32. doi: 10.1016/j.neulet.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim HJ. Nicotine’s effect on hypothalamic neurotransmitters and appetite regulation. Surgery. 1999;126:255–263. [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- NHLBI Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obesity Research. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- Noble EP, Noble RE, Ritchie T, Syndulko K, Bohlman MC, Noble LA, et al. D2 dopamine receptor gene and obesity. International Journal of Eating Disorders. 1994;15:205–217. doi: 10.1002/1098-108x(199404)15:3<205::aid-eat2260150303>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Molecular Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Reiss S, Havercamp S. The sensitivity theory of motivation: Implications for psychopathology. Behaviour Research and Therapy. 1996;34:621–632. doi: 10.1016/0005-7967(96)00041-1. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochemistry Research. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. The reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Devlin MJ, Walsh B, Hasin D, Wing RR, Marcus MD, et al. Binge eating disorder: A multisite field trial of the diagnostic criteria. International Journal of Eating Disorders. 1992;11:191–203. [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Swinburn B, Gill T, Kumanyika S. Obesity prevention: A proposed framework for translating evidence into action. Obesity Reviews. 2005;6:23–33. doi: 10.1111/j.1467-789X.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- Temple JL, Giacomelli AM, Roemmich JN, Epstein LH. Dietary variety impairs habituation in children. Health Psychology. doi: 10.1037/0278-6133.27.1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, et al. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Current Drug Targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philosophical Transactions of the Royal Society, Series B. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]