Abstract
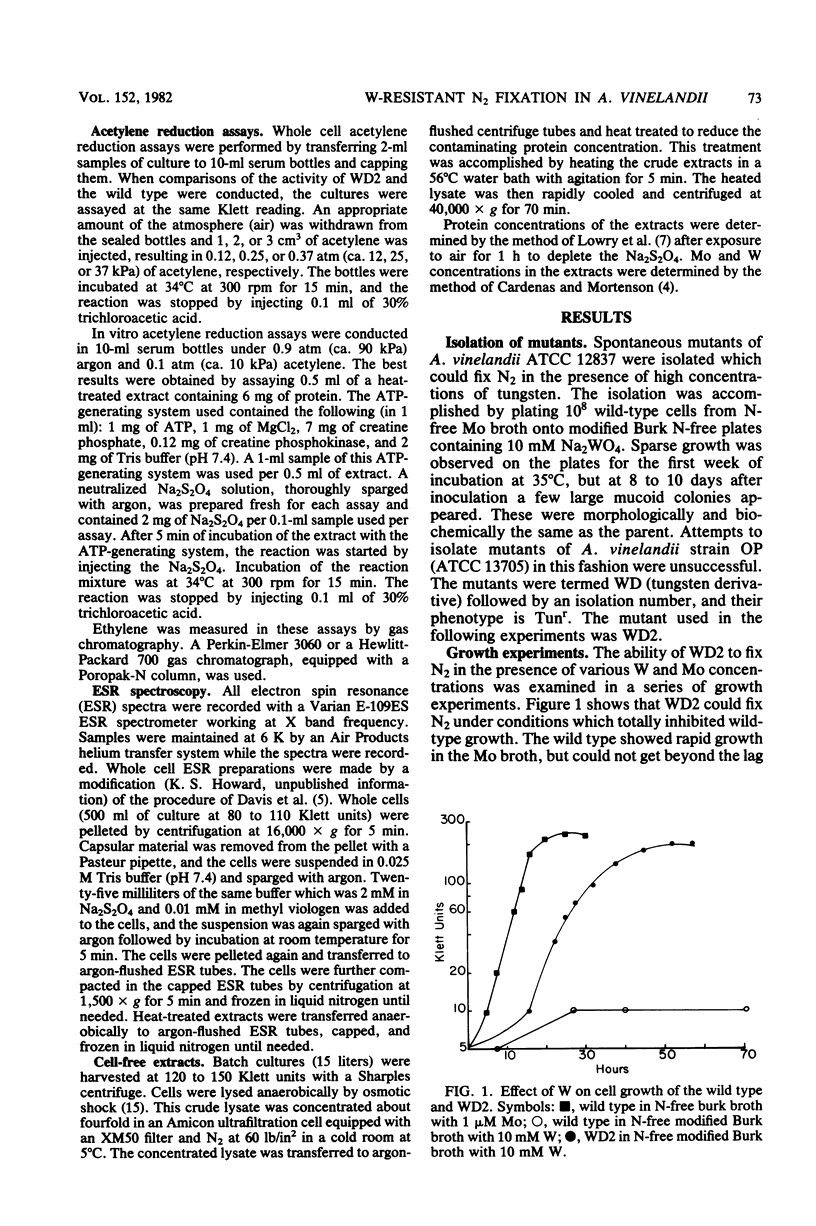
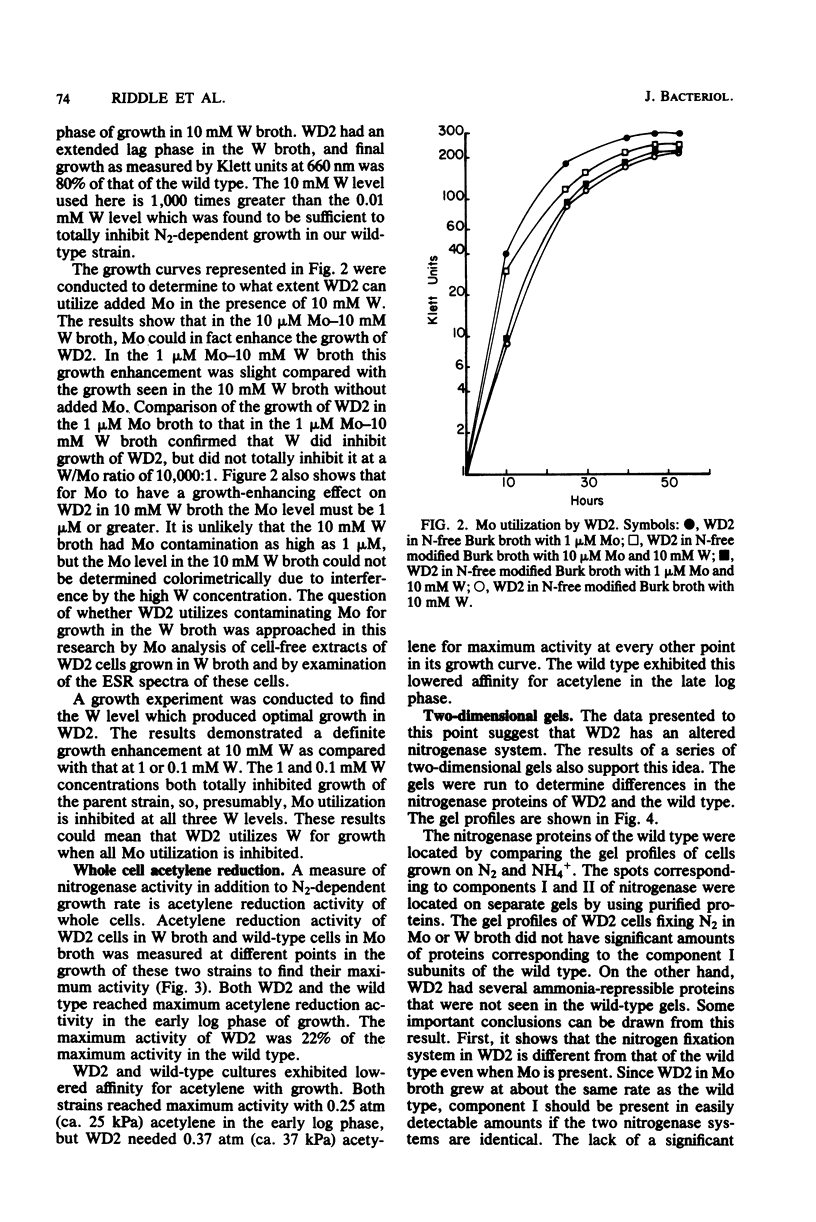
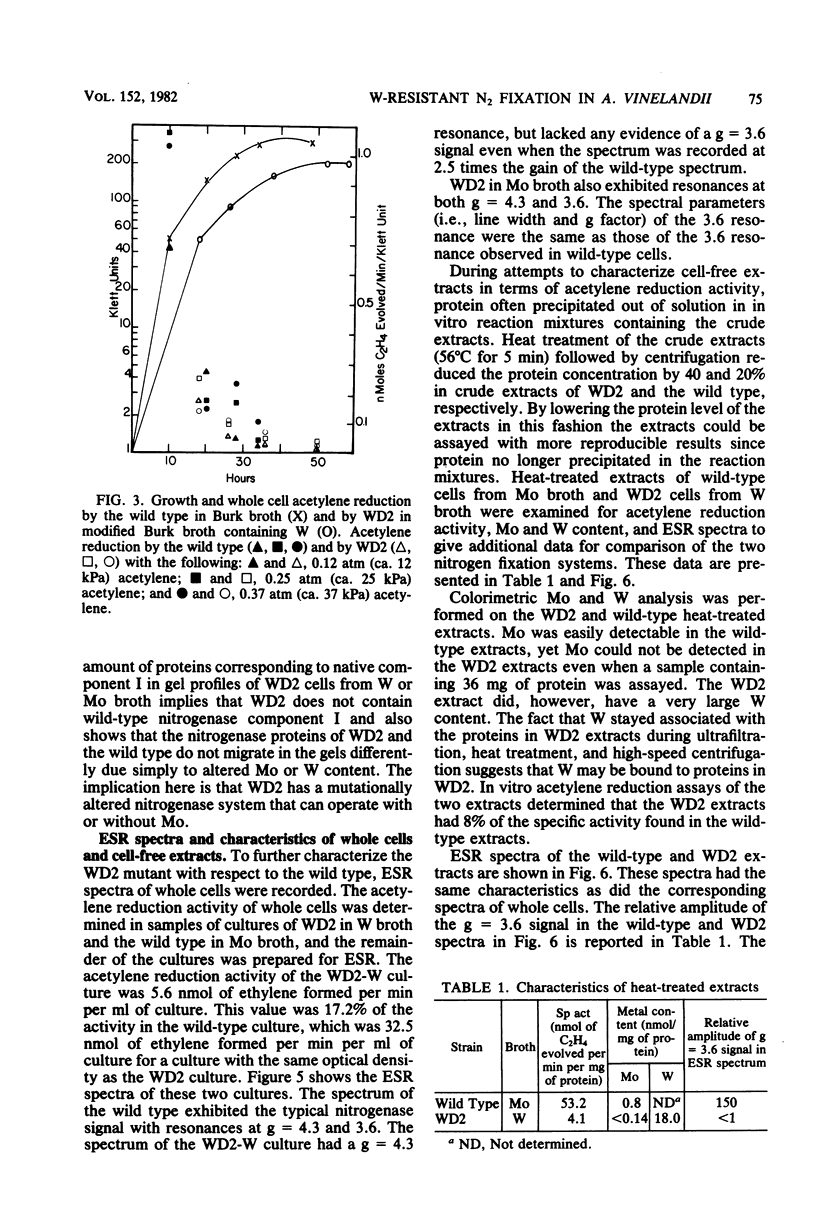
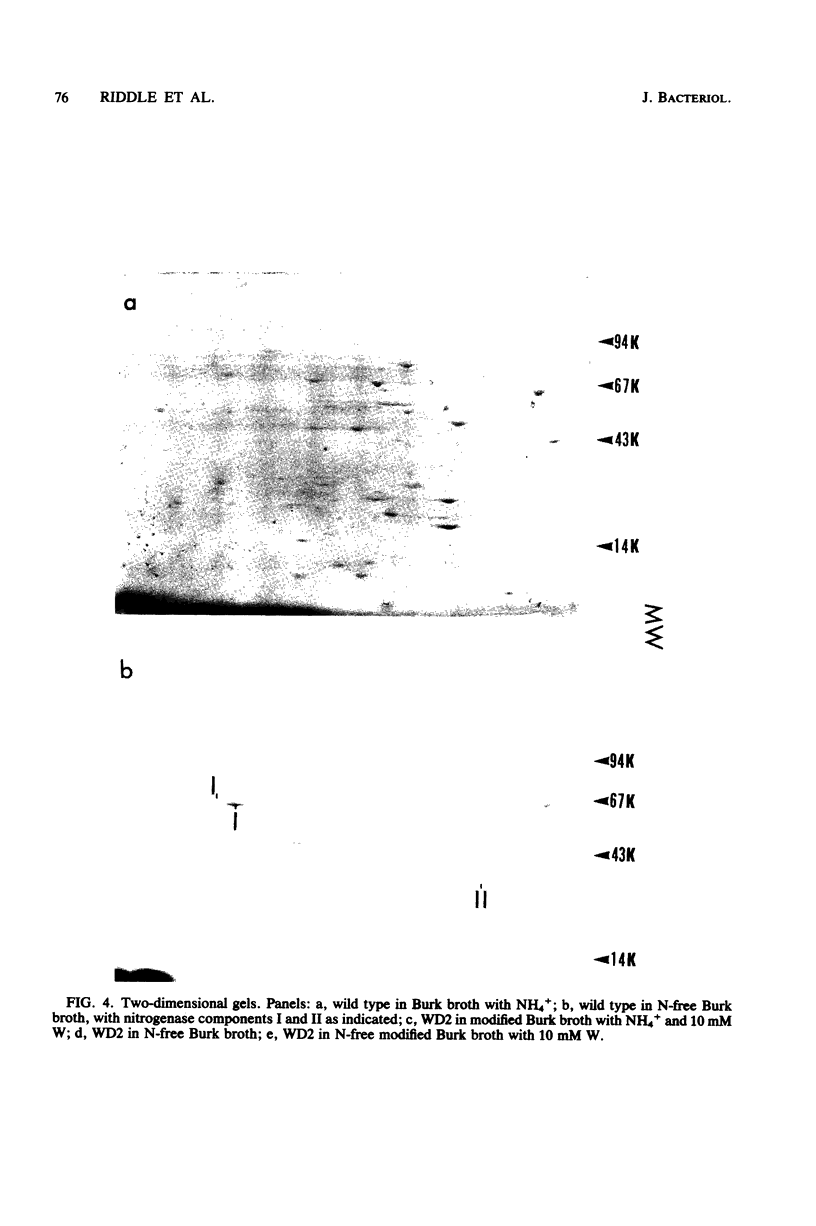
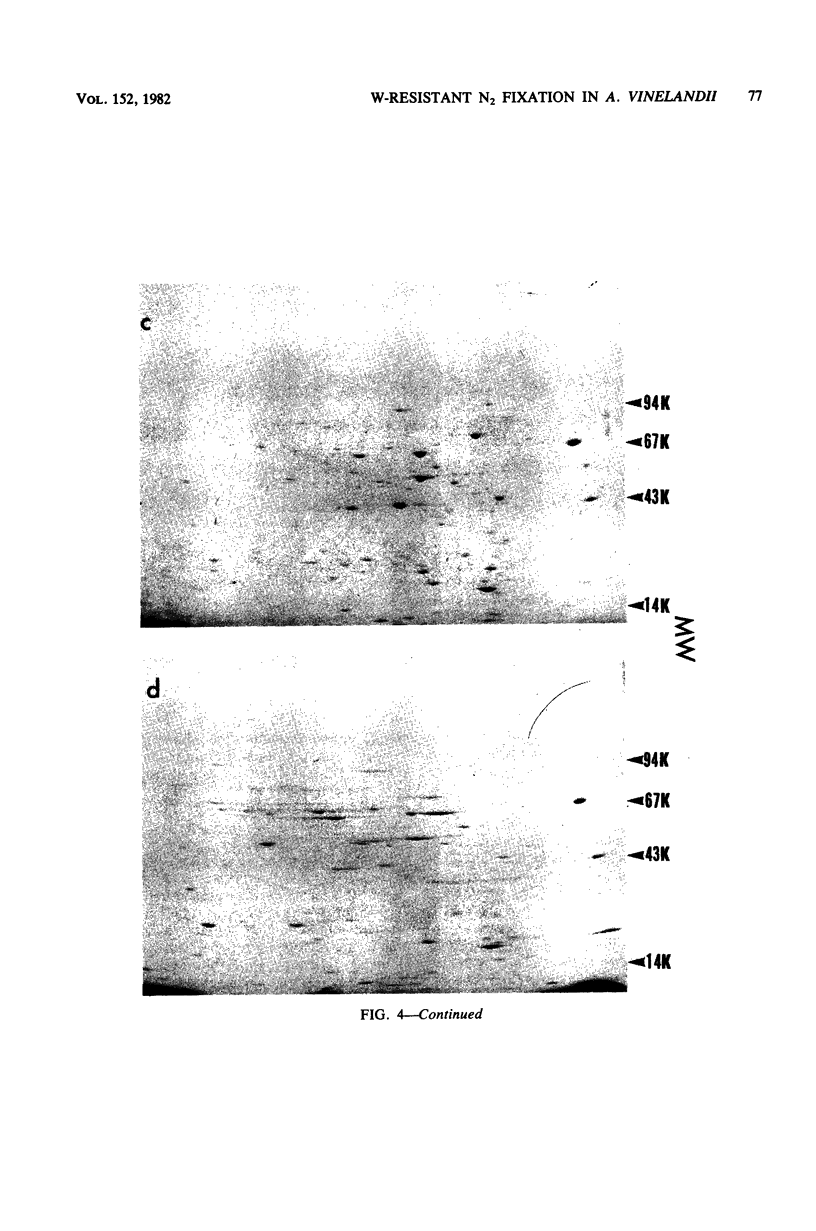
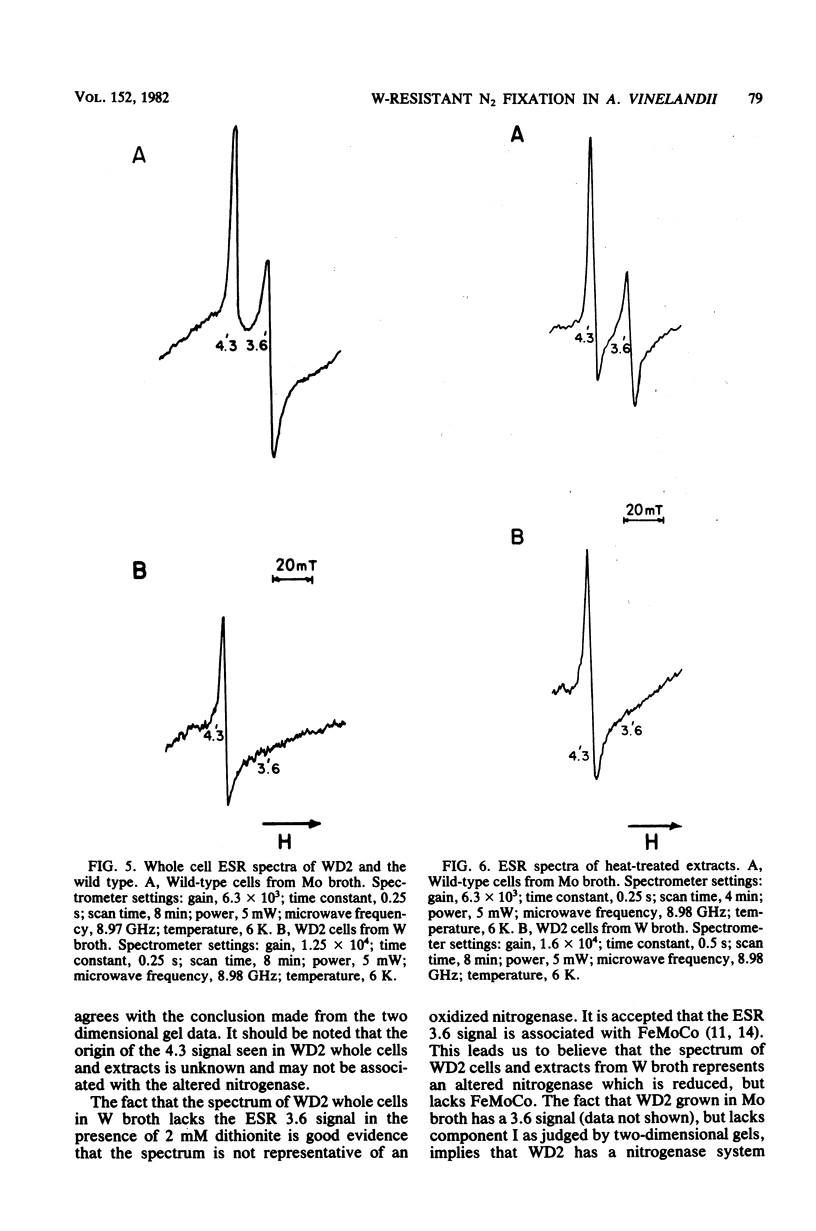
Mutants of Azotobacter vinelandii ATCC 12837 were isolated which could fix N2 in the presence of high tungsten concentrations. The most studied of these mutants (WD2) grew well in N-free modified Burk broth containing 10 mM W, whereas the wild type would not grow in this medium. WD2 would also grow in Burk N-free broth at about the same rate as the wild type. WD2 in broth containing W exhibited 22% of the whole cell acetylene reduction activity of the wild type in broth containing Mo and showed a lowered affinity for acetylene. Two-dimensional gel electrophoresis experiments showed that N2-fixing cells of WD2 from broth containing W or Mo did not produce significant amounts of component I of native nitrogenase protein. Electron spin resonance spectra of whole cells and cell-free extracts of WD2 from broth containing W lacked any trace of the g = 3.6 resonance associated with FeMoCo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Smith G. M., Kostel P. J., McKenna C. E. Tungsten incorporation into Azotobacter vinelandii nitrogenase. FEBS Lett. 1973 Feb 1;29(3):219–221. doi: 10.1016/0014-5793(73)80023-7. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A. EFFECT OF TUNGSTATE ON THE UPTAKE AND FUNCTION OF MOLYBDATE IN AZOTOBACTER AGILIS. J Bacteriol. 1961 Jul;82(1):130–134. doi: 10.1128/jb.82.1.130-134.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas J., Mortenson L. E. Determination of molybdenum and tungsten in biological materials. Anal Biochem. 1974 Aug;60(2):372–381. doi: 10.1016/0003-2697(74)90244-9. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagatani H. H., Brill W. J. Nitrogenase V. The effect of Mo, W and V on the synthesis of nitrogenase components in Azotobacter vinelandii. Biochim Biophys Acta. 1974 Aug 7;362(1):160–166. doi: 10.1016/0304-4165(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Klevickis S., Brill W. J. In vitro activation of inactive nitrogenase component I with molybdate. J Bacteriol. 1981 Jan;145(1):248–256. doi: 10.1128/jb.145.1.248-256.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBRISH S. A., MARR A. G. Location of enzymes in Azotobacteragilis. J Bacteriol. 1962 Jan;83:158–168. doi: 10.1128/jb.83.1.158-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Singh H. N., Vaishampayan A., Singh R. K. Evidence for the involvement of a genetic determinant controlling functional specificity of group VI B elements in the metabolism of N2 and NO-3 in the blue-green alga Nostoc muscorum. Biochem Biophys Res Commun. 1978 Mar 15;81(1):67–74. doi: 10.1016/0006-291x(78)91631-5. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI H., NASON A. Tungstate as competitive inhibitor of molybdate in nitrate assimilation and in N2 fixation by Azotobacter. Biochim Biophys Acta. 1957 Feb;23(2):433–435. doi: 10.1016/0006-3002(57)90351-7. [DOI] [PubMed] [Google Scholar]