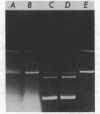
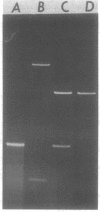
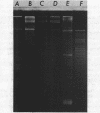
Abstract
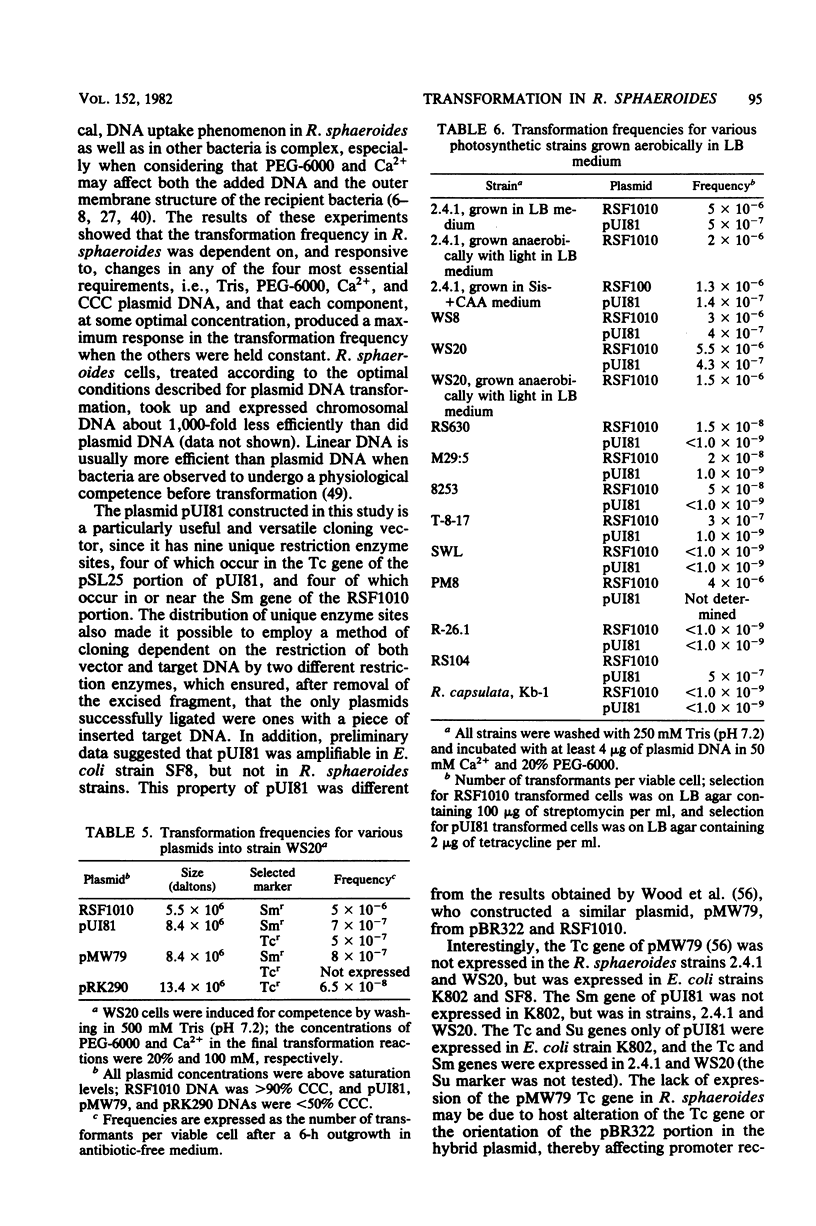
A broad-host-range cloning vector, pUI81, was constructed in vitro from plasmids RSF1010 and pSL25 (a pBR322 derivative) and used to assay for transformation in Rhodopseudomonas sphaeroides. Washing cells with 500 mM Tris was an effective means of inducing competence for DNA uptake. Transformation frequencies as high as 10(-5) (transformants per viable cell) have been achieved by incubating Tris-treated cells with plasmid DNA, 100 mM CaCl2, and 20% polyethylene glycol 6000. Maximum frequencies were obtained when recipient cells were spread onto selective media after a 6.5-h outgrowth period in antibiotic-free medium. The structure (open circular versus closed, covalent circular), size, and concentration of plasmid DNA all significantly affected the transformation frequency. Four different plasmids, all small and suitable as cloning vectors, have been introduced by transformation into several different R. sphaeroides strains. Recombinant DNA carried on small, nonconjugative plasmids with broad host ranges can now be directly transferred to R. sphaeroides by this method.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso G. L., Arrigó D. M., Terradas de Fermani S. Effect of tris(hydroxymethyl)aminomethane on isolated sarcoplasmic reticulum vesicles. Arch Biochem Biophys. 1979 Nov;198(1):131–136. doi: 10.1016/0003-9861(79)90403-x. [DOI] [PubMed] [Google Scholar]
- Ansari A. A., Baig M. A., Malling H. V. In vivo germinal mutation detection with "monospecific" antibody against lactate dehydrogenase-X. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7352–7356. doi: 10.1073/pnas.77.12.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boni L. T., Stewart T. P., Alderfer J. L., Hui S. W. Lipid-polyethylene glycol interactions: I. Induction of fusion between liposomes. J Membr Biol. 1981;62(1-2):65–70. doi: 10.1007/BF01870200. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Sistrom W. R., Zaugg W. S. The role of a reaction center in photochemical activities of bacterial chromatophores. Biochim Biophys Acta. 1965 Jul 22;102(2):341–348. doi: 10.1016/0926-6585(65)90123-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Jackson D. A., DeVries A. J. Biochemical construction of specific chimeric plasmids from ColE1 DNA and unfractionated Escherichia coli DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3838–3842. doi: 10.1073/pnas.73.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. Genetic transformation in Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jan;70(1):84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G., Oelze J. Organization and differentiation of membranes of phototrophic bacteria. Adv Microb Physiol. 1981;22:1–92. doi: 10.1016/s0065-2911(08)60325-2. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Fornari C. S., Kaplan S. Entrapment of a bacterial plasmid in phospholipid vesicles: potential for gene transfer. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3348–3352. doi: 10.1073/pnas.76.7.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantotti B. V., Patil S. S., Mandel M. Genetic transformation of Pseudomonas phaseolicola by R-factor DNA. Mol Gen Genet. 1979 Jul 2;174(1):101–103. doi: 10.1007/BF00433310. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Chan R., Costerton J. W. Citrate-tris(hydroxymethyl)aminomethane-mediated release of outer membrane sections from the cell envelope of a deep-rough (heptose-deficient lipopolysaccharide) strain of Escherichia coli O8. J Bacteriol. 1981 Mar;145(3):1386–1396. doi: 10.1128/jb.145.3.1386-1396.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn S. P., Cohen L. K., Gardner J. F., Kaplan S. Characterization of a site-specific restriction endonuclease from Rhodopseudomonas sphaeroides. J Bacteriol. 1979 May;138(2):505–509. doi: 10.1128/jb.138.2.505-509.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi-Meyrueis C., Fodor K., Schaeffer P. Polyethyleneglycol-induced transformation of Bacillus subtilis protoplasts by bacterial chromosomal DNA. Mol Gen Genet. 1980;179(3):589–594. doi: 10.1007/BF00271749. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Kaplan S. Plasmid transfer and expression in Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1978 Apr 15;187(1):229–234. doi: 10.1016/0003-9861(78)90028-0. [DOI] [PubMed] [Google Scholar]
- Mylroie J. R., Friello D. A., Chakrabarty A. M. Transformation of Pseudomonas putida with chromosomal DNA. Biochem Biophys Res Commun. 1978 May 15;82(1):281–288. doi: 10.1016/0006-291x(78)90606-x. [DOI] [PubMed] [Google Scholar]
- Nagahari K., Sakaguchi K. RSF1010 plasmid as a potentially useful vector in Pseudomonas species. J Bacteriol. 1978 Mar;133(3):1527–1529. doi: 10.1128/jb.133.3.1527-1529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M., Bowen A. R. High-frequency chromosome transfer in Rhodopseudomonas sphaeroides promoted by broad-host-range plasmid RP1 carrying mercury transposon Tn501. J Bacteriol. 1981 Jul;147(1):110–117. doi: 10.1128/jb.147.1.110-117.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Saunders V. A., Saunders J. R., Bennett P. M. Extrachromosomal deoxyribonucleic acid in wild-type and photosynthetically incompetent strains of Rhodopseudomonas spheroides. J Bacteriol. 1976 Mar;125(3):1180–1187. doi: 10.1128/jb.125.3.1180-1187.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Walker M. A., Marrs B. L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J Biol Chem. 1980 Mar 25;255(6):2427–2432. [PubMed] [Google Scholar]
- Sistrom W. R. Transfer of chromosomal genes mediated by plasmid r68.45 in Rhodopseudomonas sphaeroides. J Bacteriol. 1977 Aug;131(2):526–532. doi: 10.1128/jb.131.2.526-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Danner D. B., Deich R. A. Genetic transformation. Annu Rev Biochem. 1981;50:41–68. doi: 10.1146/annurev.bi.50.070181.000353. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Plasmid transfer in Haemophilus influenzae. J Bacteriol. 1979 Aug;139(2):520–529. doi: 10.1128/jb.139.2.520-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W. T., Pemberton J. M. Transformation of Rhodopseudomonas sphaeroides with deoxyribonucleic acid isolated from bacteriophage R phi 6P. J Bacteriol. 1980 Jul;143(1):43–49. doi: 10.1128/jb.143.1.43-49.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W. T., Pemberton J. M. Viral R plasmid Rphi6P: properties of the penicillinase plasmid prophage and the supercoiled, circular encapsidated genome. J Bacteriol. 1978 Jul;135(1):207–214. doi: 10.1128/jb.135.1.207-214.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediaminetetra-acetate and surface active agents. J Gen Microbiol. 1967 Sep;48(3):391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- Wood D. O., Hollinger M. F., Tindol M. B. Versatile cloning vector for Pseudomonas aeruginosa. J Bacteriol. 1981 Mar;145(3):1448–1451. doi: 10.1128/jb.145.3.1448-1451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]