Abstract
The aim of this investigation was to develop novel oil-in-water (o/w) nanoemulsions containing saquinavir (SQV), an anti-HIV protease inhibitor, for enhanced oral bioavailability and brain disposition. SQV was dissolved in different types of edible oils rich in essential polyunsaturated fatty acids (PUFA) to constitute the internal oil phase of the nanoemulsions. The external phase consisted of surfactants Lipoid®-80 and deoxycholic acid dissolved in water. The nanoemulsions with an average oil droplet size of 100-200 nm, containing tritiated [3H]-SQV, were administered orally and intravenously to male Balb/c mice. The SQV bioavailability as well as distribution in different organ systems was examined. SQV concentrations in the systemic circulation administered in flax-seed oil nanoemulsions were 3-fold higher as compared to the control aqueous suspension. The oral bioavailability and distribution to the brain, a potential sanctuary site for HIV, were significantly enhanced with SQV delivered in nanoemulsion formulations. In comparing SQV in flax-seed oil nanoemulsion with aqueous suspension, the maximum concentration (Cmax) and the area-under-the-curve (AUC) values were found to be 5-fold and 3-fold higher in the brain, respectively, suggesting enhanced rate and extent of SQV absorption following oral administration of nanoemulsions. The results of this study show that oil-in-water nanoemulsions made with PUFA-rich oils may be very promising for HIV/AIDS therapy, in particular, for reducing the viral load in important anatomical reservoir sites.
Keywords: Oil-in-water nanoemulsions, biodistribution, HIV/AIDS, protease inhibitor, reservoir sites, oral administration, CNS delivery
1. Introduction
Acquired immunodeficiency syndrome (AIDS) is a debilitating disease caused by human immunodeficiency virus (HIV). More than 25 years have elapsed since the first discovery of HIV-1 as a causative agent for AIDS. Currently, HIV/AIDS represent one of the deadliest worldwide epidemics, with significant social, economical, and political challenge. According to the December, 2005 World Health Organization's estimates of AIDS epidemic, 38 million adults and 2.3 million children are infected with the virus across the globe. Also in 2005, over 3.0 million individuals died due to HIV/AIDS world-wide, with over 2.4 million in sub-Saharan Africa (http://www.avert.org/worldstats.htm). There have been significant accomplishments in the past 25 years in terms of greater emphasis on disease prevention, technologies for diagnosis, and development of innovative therapeutic strategies (Gallo, 2006). At present, there are over 20 different anti-retroviral drugs approved in the United States under the general classes of nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors (PI), and fusion inhibitors (FI) (Chearskul et al., 2006).
Highly-active anti-retroviral therapy (HAART) strategy involves the use of combination anti-retroviral agents for synergistic therapeutic outcomes. With the adoption of HAART, the average survival of HIV/AIDS patients has increased from less than 1 year to over 10 years (Frezzini, Leao, and Porter, 2005, Holtgrave, 2005). Despite the success of HAART in the clinics, HIV/AIDS therapy is far from optimal. One of the major problems in the chronic treatment is the fact that the viral particles are able to reside in cellular and anatomical sites in the body following replication and remain viable even when there are adequate drug concentrations in the blood (Schrager and D'Souza, 1998, Chun, 2000). Examples of cellular reservoirs include T-lymphocytes, monocytes, and macrophages, while the major anatomical reservoirs include central nervous system (CNS), lymph notes, liver, spleen, lungs, and the genitals (Vyas, Shah, and Amiji, 2006). Poor drug availability in the cellular and anatomical reservoirs is affected by expression of efflux transporters (e.g., P-glycoprotein), presence of drug metabolizing enzymes (e.g., cytochrome P-450), poor permeability properties, non-targeted distribution, and rapid clearance. The reduced bioavailability and short residence of anti-retroviral agents at these viral reservoir sites have profound impact on the clinical management of the disease. The overall consequence is that upon discontinuation of therapy or when drug resistance develops, HIV is able to re-seed the systemic circulation and continue to propagate the infection (Clarke, White, and Weber, 2000, Kulkovsky and Bray, 2006).
Saquinavir (SQV, Invirase®), the first HIV-protease inhibitor to be marketed for the treatment of HIV/AIDS, is a peptide derivative and a transition-state mimetic of the Phe-Pro peptide bond (King et al., 2004). It competitively inhibits HIV-1 and HIV-2 protease-mediated cleavage of the gag and pol polyproteins, thus preventing the post-translational processing required for virus maturation and spread. Although SQV has a very potent anti-HIV activity in vitro (IC50 of 20 nM), it is currently not indicated as a single agent. In addition, when SQV is used in combination therapy protocols, the oral daily dose ranges from 1,200 mg to 3,400 mg (Figgitt and Plosker, 2000). This is due to the fact that oral bioavailability of SQV from the conventional gelatin capsule formulation is only 4-5%. SQV is a substrate for P-glycoprotein efflux transporter on the enterocytes and is also metabolized by the cytochrome P-450 enzyme system locally in the gastrointestinal tract and upon first pass effect (Kandanearatchi, Williams, and Everall, 2003, Shah and Amiji, 2006). In addition, SQV is not adequately transported into the CNS or other anatomical reservoir sites.
In order to enhance the availability and distribution of anti-retroviral agents, like SQV, to cellular and anatomical reservoir sites, we have proposed that nanotechnology-based drug delivery systems could provide a unique strategic advantage (Vyas, Shah, and Amiji, 2006). Using biodegradable poly(ethylene oxide)-modified poly(epsilon-caprolactone)-based nanoparticles of less than 200 nm in diameter, we showed enhanced delivery and prolonged residence of SQV in THP-1 monocytes/macrophage cells (Shah and Amiji, 2006b). Additionally, we observed that when the nanoemulsions were made with oils rich in polyunsaturated fatty acids (PUFA), paclitaxel was efficiently solubilized in the oil droplet and there was significant enhancement in the drug absorption across the gastro-intestinal tract following oral administration (Tiwari and Amiji, 2006). Moreover, with the nanoemulsions made with pine-nut oil, which is rich in alpha- and gamma-linolenic acid, an example of omega-3 fatty acid with 18 carbon and 3 double bonds, and stabilized with Lipoid-80® and sterylamine, there was significant enhancement in the delivery of paclitaxel across the blood-brain barrier in mice (results not published).
In order to enhance delivery of SQV to anatomical reservoirs, in the present study, we have formulated the drug in different nanoemulsions made with oils rich in PUFA. These oil-in-water nanoemulsions with the oil droplet size of 100-200 nm were made either with flax-seed oil or safflower oil. Flax-seed oil contains up to 57% by weight of linolenic acid, an example of omega-3 fatty acid, and 17% by weight linoleic acid, an example of omega-6 fatty acid with 18 carbons and 2 double bonds. Safflower oil, on the other hand, contains up to 73% by weight of linoleic acid (Boles et al., 2005). To examine oral bioavailability and distribution to vital organs including the brain, SQV was incorporated in the nanoemulsions and administered orally to conscious Balb/c mice. Intravenous administration was also carried out to determine the relative bioavailability values of SQV following oral administration in different formulations. Control preparation of SQV was made as aqueous suspension containing all of the other ingredients (e.g., surfactants) except the oils.
2. Materials and Methods
2.1 Materials
SQV base was purchased from Aapin Chemicals Limited (Abingdon, United Kingdom). Tritiated [3H]-SQV, with an activity of 250 μCi in 250 μl ethyl alcohol, was purchased from Moravek Biochemicals (Brea, CA, USA). PUFA-containing pure flax-seed and safflower oils were kindly provided by Jedwards International, Inc. (Quincy, MA, USA). Egg phosphatidylcholine (Lipoid® E80) was purchased from Lipoid GMBH (Ludwigshafen, Germany). Deoxycholic acid was purchased from Sigma Chemicals (St. Louis, MO, USA). Deionized distilled water (Barnsted/Thermolyne, Dubuque, IA, USA) was used for the preparation of the nanoemulsions and other aqueous solutions.
2.2. Preparation of the Nanoemulsions and Aqueous Suspension Formulations
2.2.1. Preparation of [3H]-SQV-Containing Nanoemulsions
SQV nanoemulsions, containing a final concentration of 400 μg/mL of the therapeutic agent, were prepared by adding SQV solution (50% w/w stock solution in dehydrated ethanol) to 1 ml of either flax-seed oil or safflower oil. The weight ratio of radiolabeled (i.e., [3H]-SQV) to unlabeled drug was maintained constant at 0.023:1 by weight.
The oil-drug mixture was stirred to homogenously distribute the drug and allow the ethanol to completely evaporate. The aqueous phase was prepared using deionized distilled water (4 mL) containing 120 mg of egg phosphatidylcholine (Lipoid E80®) and 40 mg of deoxycholic acid. The aqueous phase was also mixed to insure complete dissolution of all additives. Subsequently, both the oil phase and the aqueous phase were independently heated to 70°C on a hot-plate.
The oil phase was gradually added to the aqueous phase with constant stirring. The resultant mixture containing both oil and aqueous phase was sonicated for 10 minutes using the Vibra Cell VC 505 probe sonicator (Sonics and Material Inc., Newtown, CT, USA). The probe sonicator was adjusted at 21% amplitude and 50% duty cycle. The resulting stable dispersion was uniform and milky-white in color. Following sonication, the nanoemulsions were kept on a hot-plate under stirring condition and the temperature was maintained at 60°C to remove any residual ethanol and then allowed to cool to room temperature. SQV-containing nanoemulsions were filtered through a 0.45 μm membrane filter and stored at 4°C in the dark.
2.2.3. Preparation of SQV Aqueous Suspension
Since SQV base is not readily soluble in water, we have compared the bioavailability from the nanoemulsion preparation with an aqueous suspension formulation that was made with all of the other constituents except the oils. The aqueous suspension was prepared by mixing the 3H-labeled and unlabeled drug in ethanol with deionized distilled water containing egg phosphatidylcholine (Lipoid E80®) and deoxycholic acid at the same proportions as was used for the nanoemulsion formulations. The suspension was sonicated for 10 minutes using the Vibra Cell VC 505 probe sonicator to reduce the particle size in the nanometer range.
2.3. Characterization of the Nanoemulsions
2.3.1. Particle Size Analysis
The hydrodynamic oil droplet diameter in the control and SQV-containing nanoemulsions was measured using a light scattering method with Brookhaven Instruments Corporation's (Holtville, NY, USA) 90Plus ZetaPALS system. Approximately 50 μL of the nanoemulsion formulations were diluted to 5 mL using deionized distilled water in a disposable zeta cells. The observations were recorded at 90° light scattering angle and temperature was maintained at 25°C. During the measurement, average particle count rate was maintained between 50 and 500 kcps (Meyer, 2006).
2.3.2. Measurements of Surface Charge
ZetaPALS instrument was also used for the surface charge (zeta potential) measurements of the blank and SQV-loaded control suspension and nanoemulsions. The measurements were carried out with diluted nanoemulsion formulations as described above. The refractive index was kept at 1.33 and the viscosity at 1.0 cps to mimic the values for pure water. Zeta potential values were determined from the electrophoretic mobility of the oil droplets using a in-built software, which uses the Helmholtz-Smoluchowski equation (Meyer, 2006, Shah and Amiji, 2006a).
2.3.3. Transmission Electron Microscopy (TEM)
The morphology of the oil droplets in the nanoemulsion formulations was visualized with TEM analysis. TEM analysis was also important in order to visualize any precipitation of the drug upon addition of the aqueous phase. Control and SQV-containing nanoemulsions (50 μL) were added to 200-mesh formwar-coated copper TEM sample holders (EM Sciences, Hatfield, PA, USA). The samples were then negatively-stained with 50 μL of 1.5% (w/v) phosphotungstic acid for 10 minutes at room temperature. Excess liquid was blotted with a piece of Whatman filter paper. The TEM samples were observed with JEOL 100-X transmission electron microscope (Peabody, MA, USA) equipped with 20 μm aperture at 67 kV. The acquired digital images were processed with Adobe Photoshop® software.
2.4. Biodistribution Study Following Oral and Intravenous Administration
2.4.1. Animal Protocol for Oral and Intravenous Administration
The experimental protocol involving use of radioactive material in animals was approved by the Institutional Animal Care and Use Committee, the Radiation Safety Committee, and the Office of Environmental Health and Safety at Northeastern University (Boston, MA). Male Balb/c mice of approximately 10-weeks age, weighing 28-30 g, were purchased from Charles River Laboratory (Wilmington, MA). The animals were housed in a climate-controlled environment with full access to food and water. Prior to any experimentation, the animals were allowed to acclimate for at least 48 hours.
For the oral absorption and biodistribution study of SQV aqueous suspension (control) and the nanoemulsions formulations, the animals were fasted for 24 hours. They were then randomly divided into three groups to receive [3H]-SQV in aqueous suspension, flax-seed oil nanoemulsion, or safflower oil nanoemulsion formulations. Each conscious animal was administered with 0.5 mL (200 μg of SQV) of the control aqueous suspension or nanoemulsion formulations containing 1 μCi of radioactivity by oral gavage. After 1, 2, 4, 8, 12, and 24 hours post-administration, a group of 4 animals per time point, lightly anesthetized with isoflurane, were sacrificed by cervical decapitation.
In a separate series of experiments, the [3H]-SQV in aqueous suspension and nanoemulsion formulations were also administered intravenously (i.v.) via the tail vein in isoflurane-anasthesized male Balb/c mice. For these studies, the formulations were made such that 200 μg of total SQV and 1 μC of radioactivity was incorporated in 100 μL of the injectable aqueous suspension or nanoemulsion formulations. At specific time points, a group of 4 anesthetized mice were sacrificed by cervical decapitation.
Following oral and i.v. drug administration, blood was rapidly collected by cardiac puncture and placed with anticoagulants. Brain, lung, heart, liver, spleen, kidneys, stomach, and intestine were collected and processed for analysis of SQV concentrations.
2.4.2 Analysis of SQV Absorption and Disposition
Radioactivity in the blood and isolated tissues was used to determine the concentration of SQV following oral and i.v. administration. The harvested tissues were weighed and rapidly homogenized using a Fisher PowerGen-125 homogenizer to prepare a 10% (w/v) tissue homogenate in deionized distilled water. One-mL of the tissue homogenate was added to a scintillation vial. Blood and tissue homogenates were then digested with 1 mL of Scintigest® solution (Fisher Scientific, Pittsburgh, PA, USA) and incubated for 2 hours at 50°C. The samples were then decolorized with 200 μL of 30% (v/v) hydrogen peroxide by incubating at 50°C for an additional 30 minutes. To the decolorized samples, 10 mL of ScintiSafe® Econo-1 scintillation cocktail ((Fisher Scientific) was added and they were allowed to quench for 4 hours in the dark. Radioactivity analysis was performed with a Packard Instrument's Tri-Carb 1600TR liquid scintillation analyzer (Downers Grove, IL) after appropriate calibration with tritium standards. The counts-per-minute (CPM) values were converted into μCi of radioactivity using appropriate calibration curves. The radioactivity values were then converted into the amount of saquinavir or its equivalent (saquinavir and/or its metabolites) by multiplying with the total dose of saquinavir administered and divided by the amount of radioactive dose.
2.4.3. Non-Compartmental Pharmacokinetic Analysis
Non-compartmental pharmacokinetic analysis of SQV following oral and i.v. administration in blood and various harvested tissues was performed with WinNonlin®, version 5.0 software package (Pharsight Corporation, Mountain View, CA, USA) Pharmacokinetic parameters such as the maximum plasma concentration (Cmax), time to reach maximum concentration (Tmax), half-life (t1/2), mean residence time (MRT), and the area-under-the-curve from zero to infinity (AUC0→∞) were calculated. Since the same dose was administered orally and i.v., the plasma and brain bioavailability values (F) of SQV in the nanoemulsion formulations were determined relative to the aqueous suspension according to the following equation:
2.5. Data Analysis
All the values are reported as mean ± SEM from at least four independent experiments. The statistical differences between the groups were tested using student's t-test and, with more than two groups, ANOVA was used to compare results. Experimental results were considered statistically significant at 95% confidence (i.e., p<0.05).
3. Results
3.1. Preparation and Characterization of the Nanoemulsions
SQV-containing nanoemulsions were prepared using oils rich in PUFA by the ultra sonication method. Ultrasound energy has been successfully used in reducing the oil droplet size of the nanoemulsions to below 200 nm (Jafari, 2006). In these studies, as shown in Table 1, the average oil droplet diameters of the SQV-containing nanoemulsions were in the range of 100-200 nm. Blank nanoemulsions, prepared in the absence of the drug, also had similar oil droplet size of about 200 nm in diameter. Ultrasound was also used here to reduce the particle size of solid drug precipitate in the control aqueous suspension to approximately 300 nm in diameter. In addition, we have also examined the change in the oil droplet particle size as a function of various centrifugation cycles as an accelerated stability indicating system. There was no change in particle size of the oil droplets in these nanoemulsions upon centrifugation at up to 2,000 rpm for 20 minutes as well as on storage at 4°C in the dark for up to two months.
Table 1.
Particle Size and Surface Charge Measurements of the Control Suspension and Nanoemulsions Formulations of Saquinavira
| Formulations | Particle Size
(nm) |
Zeta Potential
(mV) |
|---|---|---|
| Control Aqueous Suspension | 311.2 ± 17.4b | -9.42 ± 4.28 |
| Blank Flax-Seed Oil Nanoemulsion | 176.6 ± 18.2 | -39.56 ± 3.67 |
| Saquinavir-Containing Flax-Seed Oil Nanoemulsion | 218.0 ± 13.9 | -43.28 ± 3.79 |
| Blank Safflower Oil Nanoemulsion | 217.4 ± 11.6 | -45.62 ± 4.29 |
| Saquinavir- Containing Safflower Oil Nanoemulsion | 140.0 ± 12.6 | -49.55 ± 5.02 |
Particle size and surface charge measurements of the aqueous suspension and nanoemulsion formulations using the ZetaPALS instrument.
Mean ± S.E. (n = 3).
Table 1 also shows the surface charge of the various types of nanoemulsions with and without added SQV. Both flax-seed and safflower oil nanoemulsions showed net negative zeta potential values of approximately -40 mV. In this study, we used egg phosphatidylcholine (Lipoid® E-80) as the primary emulsifier for stabilization of the oil droplets. Manufacturer's specification (Lipoid, Ludwigshafen, Germany) suggests that Lipoid® E-80 is a mixture of phospholipids from egg yolk sources with the major constituent being phosphatidylcholine. Lipoid® 80 also contains a small fraction of zwitterionic and neutral phospholipids such as phosphatidylserine, phosphatidic acid, phosphatidylglycerol, and phosphatidylinositol that would contribute a net negative charge at neutral pH. More importantly, deoxycholic acid was included as a co-surfactant in the nanoemulsion preparation in order to enhance oral absorption. Deoxycholic acid can increase membrane fluidity (Zhao and Hirst, 1990) and inhibit P-glycoprotein function in the intestinal lumen enterocytes (Lo and Huang, 2000).
With a pKa of 6.5, significant fraction of the deoxycholic acid molecules would be ionized at neutral pH. Adsorbed deoxycholic acid at the oil-water interface would clearly impart a significant net negative charge to the nanoemulsions. Inclusion of SQV did not alter the surface charge of the nanoemulsion. This observation corroborated our earlier findings that hydrophobic drug molecules were well distributed in the oil phase of the emulsion and did not migrate to the interface (Tiwari and Amiji, 2006).
In order to observe the physical properties of the oil droplets in the nanoemulsions, TEM analysis was carried out with negatively stained samples. As shown in Figure 1, phosphotungstic acid-stained oil droplets were clearly visible and the droplet size correlated well with the results from particle size analysis using ZetaPALS light scattering instrument. In addition, the morphology of the droplet was spherical and there was no evidence of SQV precipitation in either the oil phase or the aqueous phase with the concentrations that were incorporated in both flax-seed and safflower oil nanoemulsions. Also, the radioactive substance purity was determined and was found to be stable for at least 48 hours at 37°C in PBS.
Figure 1.
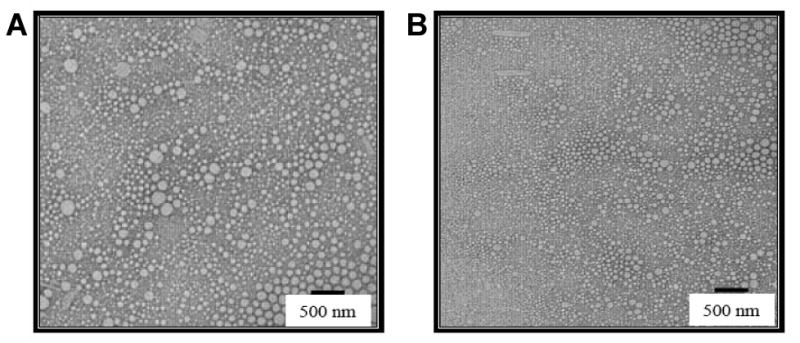
Transmission electron micrographs of the blank (A) and saquinavir-containing (B) nanoemulsions. Both of the nanoemulsions were prepared with flax-seed oil. The scale bar represents a distance of 500 nm.
3.2. Plasma and Brain SQV Concentrations Following Oral and Intravenous Administration
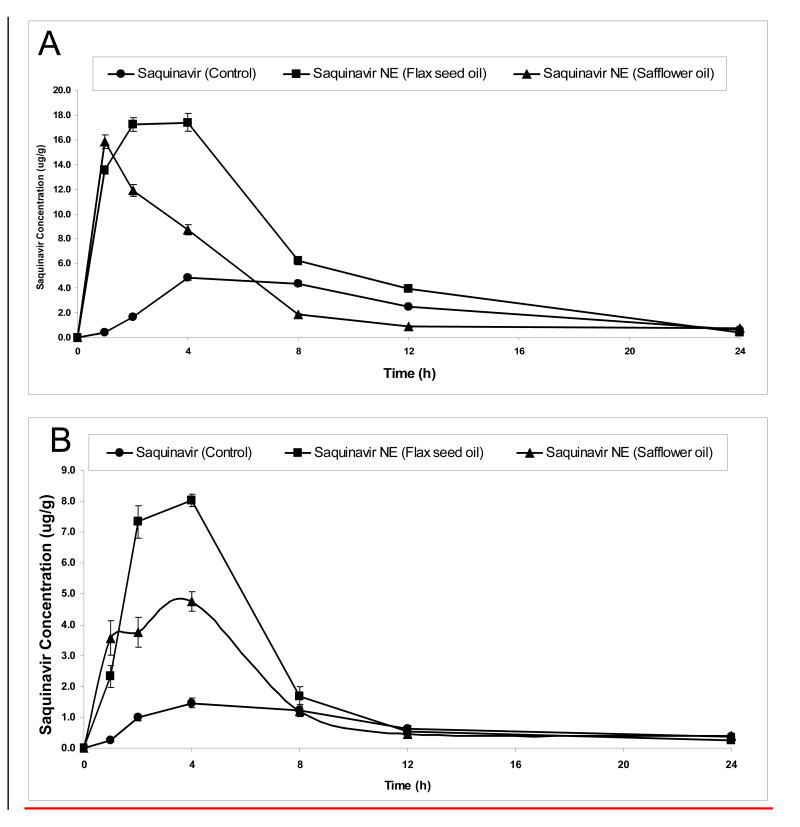
In order to evaluate the enhanced bioavailability and distribution pattern in the body following oral administration, control aqueous suspension and the nanoemulsion formulations containing [3H]-SQV were administered to conscious male Balb/c mice. Figure 2 shows the plasma and brain concentration versus time profile of SQV following oral administration in aqueous suspension (SSoral), flax-seed oil nanoemulsion (SFNoral), and safflower oil nanoemulsion (SSNoral). These results clearly show that there were higher plasma and brain SQV concentrations when administered orally in the nanoemulsions as compared to the aqueous suspension. SFNoral was more effective in enhancing oral absorption and availability of SQV in the plasma as well as in the brain. The average plasma concentrations of 17.4 μg/gm and 11.8 μg/gm of SQV were observed after 2 hours following SFNoral and SSNoral, respectively. In contrast, the plasma concentration of SSoral after 2 hours was only 1.64 μg/gm (Figure 2A). In addition, brain concentrations of SQV were also significantly higher (p<0.05) for SFNoral and SSNoral compared to SSoral. After 2 hours of oral administration, the average SQV concentrations in the brain were 7.4 μg/gm with SFNoral, 3.8 μg/gm with SSNoral, and 0.98 μg/gm with SSoral (Figure 2B). Plasma and brain SQV concentrations when administered with the nanoemulsions remained higher than with aqueous suspension for up to 8 hours.
Figure 2.
Plasma and brain saquinavir concentration versus time profiles following oral administration of the drug in aqueous suspension or nanoemulsion formulations to Balb/c mice. The plots represent plasma concentrations versus time following oral administration (A) and brain concentrations versus time following oral administration (B). The Balb/c mice was dosed with 0.50 mL of the control aqueous suspension or nanoemulsion formulations by oral gavage. The administered dose contained 1 μCi radioactivity as tritiated [3H]-saquinavir.
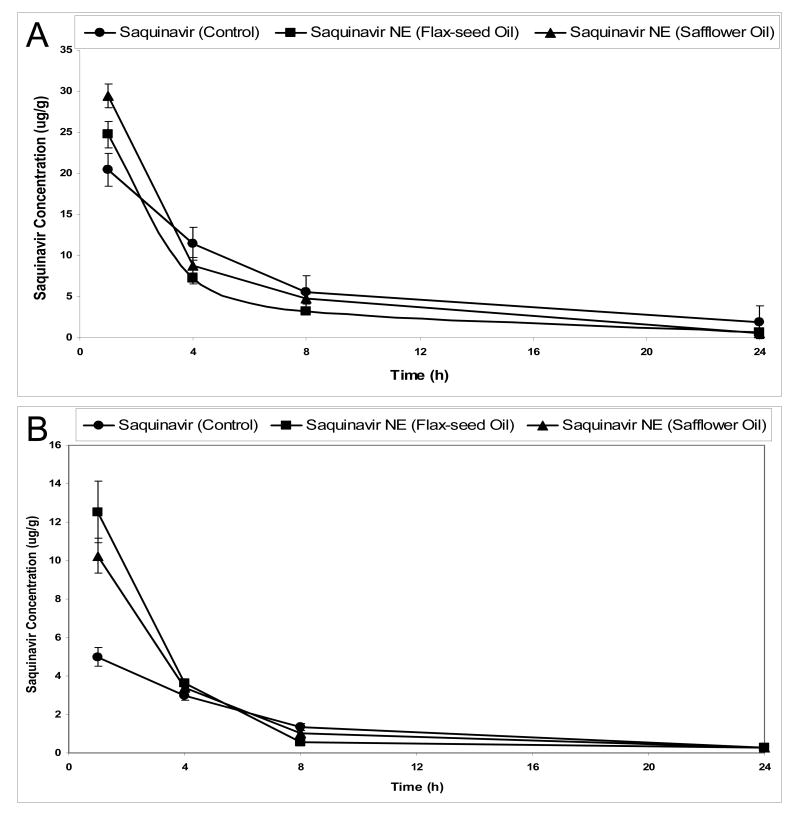
In order to determine the effectiveness of oral administration relative to i.v. delivery, in Figure 3, we show the results of i.v. SQV administration in the aqueous suspension (SSiv) and in the nanoemulsion formulations (SFNiv and SSNiv). At 1 hour post i.v. administration, SQV concentration in the plasma was 24.8 μg/gm with SFNiv, 29.4 μg/gm with SSNiv, and 20.4 μg/gm with SSiv formulation (Figure 3A). However, the brain SQV concentrations were significantly higher (p <0.05) when administered i.v. in the nanoemulsions as compared to the aqueous suspension. Brain SQV concentration after 1 hour, for instance, was 12.6 μg/gram with SFNiv, 10.2 μg/gm with the SSNiv, and 5.2 μg/gm with the SSiv.
Figure 3.
Plasma and brain saquinavir concentration versus time profiles following intravenous administration of the drug in aqueous suspension or nanoemulsion formulations to Balb/c mice. The plots represent plasma concentrations versus time following oral administration (A) and and brain concentrations versus time following intravenous administration (B). The Balb/c mice were dosed with 0.10 mL of the control aqueous suspension or nanoemulsion formulations by the intravenous tail vein injection. Each administered dose contained 1 μCi radioactivity as tritiated [3H]-saquinavir.
3.3 Pharmacokinetic Analysis Following Oral and Intravenous Administration
Non-compartmental pharmacokinetic analysis was carried to determine the plasma and brain parameters following oral and intravenous administration. In Table 2, we show the results of pharmacokinetic analysis in the plasma following oral and i.v. administration of SQV in aqueous suspension and in the nanoemulsion formulations. Significantly higher Cmax values were observed for emulsion formulations compared to control formulation by both the route. Mean Tmax values were 1 hr following i.v. and 4 hr following oral for all the formulations except for SSNoral (i.e. 1 hr). In case of i.v., since the highest concentrations (Cmax) were observed at first time point, i.e. 1 hour, this was the time considered as Tmax. The clearance (Cl) and volume-of-distribution (Vdss) values were higher for the oral formulations as compared to the i.v. in case of control and safflower oil nanoemulsion treated groups. However, an opposite trend was observed in the case of flax-seed oil nanoemulsion treated animals. MRT in plasma was found to be lowest in case of SFNoral.
Table 2.
Pharmacokinetics Parameters Upon Oral and Intravenous Administration of the Control Suspension and Nanoemulsion Formulations of [3H]-Saquinavir in the Plasma Compartment in Balb/c Micea
| Pharmacokinetic Parameter | Saquinavir Control Suspension | Saquinavir in Flax-Seed Oil Nanoemulsion | Saquinavir in Safflower Oil Nanoemulsion | |||
|---|---|---|---|---|---|---|
| i.v. | Oral | i.v. | Oral | i.v. | Oral | |
| Cmax (μg/gm) | 20.44 ± 1.6 | 4.84 ± 0.3 | 24.76 ± 1.3b | 17.40 ± 0.4b | 29.42 ± 1.1b | 15.86 ± 0.6b |
| Tmax (h) | 1.00 ± 0.1 | 4.00 ± 0.2 | 1.00 ± 0.1 | 4.00 ± 0.5 | 1.00 ±0.05 | 1.00 ± 0.2 |
| T1/2 (h) | 5.57 ± 0.74 | 5.93 ± 0.6 | 6.14 ± 0.8 | 3.86 ± 0.4 | 4.63 ±0.3b | 4.99 ± 0.3 |
| AUC0→∞h(μg/g) | 141.63 ± 5.5 | 59.75 ± 4.7 | 100.68 ± 4.6b | 217.8 ± 6.8b,c | 127.21 ± 6.8b | 84.83 ± 5.4b |
| Fd (%) | 100 ± 5.0 | 42.19 ± 0.3 | 100 ± 2.0 | 216.33 ± 3.0 | 100 ± 2.5 | 66.69 ± 2.0 |
| Cl (lit/hr) | 1.41 ± 0.2 | 3.35 ± 0.1 | 1.99 ± 0.2 | 0.92 ± 0.1 | 1.57 ± 0.2 | 2.36 ± 0.2 |
| Vdss (lit) | 9.97 ± 0.4 | 30.02 ± 0.3 | 10.61 ± 0.3 | 3.88 ± 0.2 | 8.19 ± 0.4 | 16.43 ± 0.4 |
Each value is a mean ± S.E. of four independent experiments.
Significantly higher (p<0.05) compared to saquinavir control suspension.
Significantly higher (p<0.05) compared to saquinavir nanoemulsions in safflower oil.
Comparisons were made to its i.v. counterpart.
Table 3 shows the pharmacokinetic parameters of SQV following administration in the aqueous suspension and the nanoemulsion formulations in the brain. Significantly higher Cmax values were observed for SFN compared to SS and SSN for both the route of administration. Tmax of 4 hours was observed following oral administration in all the groups. Similar kind of trend observed in brain compartment to plasma compartment. The CL and Vdss values were higher for oral compared to i.v. in case of control and safflower oil Nanoemulsion treated groups, however reverse in case of SFN treated animals.
Table 3.
Pharmacokinetics Parameters Upon Oral and Intravenous Administration of the Control Suspension and Nanoemulsion Formulations of [3H]-Saquinavir in the Brain Compartment in Balb/c Micea
| Pharmacokinetics Parameter | Saquinavir Control Suspension | Saquinavir in Flax-Seed Oil Nanoemulsion | Saquinavir in Safflower Oil Nanoemulsion | |||
|---|---|---|---|---|---|---|
| i.v. | Oral | i.v. | Oral | i.v. | Oral | |
| Cmax (μg/gm) | 5.00 ± 0.3 | 1.46 ± 0.1 | 12.52 ± 0.5b,c | 8.04 ± 0.3b,c | 5.12 ± 0.5b | 2.38 ± 0.5b |
| Tmax (h) | 1.00 ± 0.1 | 4.00 ± 0.3 | 1.00 ± 0.1 | 4.00 ± 0.35 | 1.00 ± 0.1 | 4.00 ± 0.3 |
| T1/2 (h) | 5.15 ± 0.7 | 9.92 ± 0.5 | 4.76 ± 0.61b | 4.48 ± 0.35b,c | 4.43 ± 0.3b | 6.45 ± 0.8b |
| AUC0→∞ h(μg/g) | 35.41 ± 1.8 | 21.82 ± 2.0 | 45.97 ± 2.45b | 51.65 ± 1.98b,c | 41.92 ± 3.5b | 38.76 ± 1.7b |
| Fd (%) | 100 ± 3.0 | 61.62 ± 2.0 | 100 ± 2.0 | 112.36 ± 3.0 | 100 ± 2.5 | 92.4 ± 2.0 |
| CL (lit/hr) | 5.65 ± 0.3 | 9.17 ± 0.4 | 5.65 ± 0.2 | 3.87 ± 0.2 | 4.7 ± 0.3 | 5.16 ± 0.2 |
| Vdss (lit) | 42.48 ± 0.3 | 123.24 ± 0.5 | 44.98 ± 0.3 | 23.37 ± 0.4 | 31.05 ± 0.3 | 49.07 ± 0.4 |
Each value is a mean ± S.E. of four independent experiments.
Significantly higher (p<0.05) compared to saquinavir control suspension.
Significantly higher (p<0.05) compared to saquinavir nanoemulsions in safflower oil.
Comparisons were made to its i.v. counterpart.
To compare the relative availability of SQV in the brain following oral administration to that of i.v. administration, we have determined the ratio of concentrations in the brain at different time points from 1 hour to 24 hours post-administration. As shown in Table 4, the brain oral/i.v. concentration ratios with flax-seed oil nanoemulsion were 2.2 and 2.9 at 4 hours and 8 hours, respectively. With safflower oil nanoemulsions, the ratios were 0.70 and 1.2 at 4 hours and 8 hours, respectively. Aqueous suspension was not very effective in brain delivery following oral administration and, as such, the concentration ratios were 0.49 and 0.91 at 4 hours and 8 hours, respectively.
Table 4.
Ratio of Brain Concentrations of [3H]-Saquinavir Following Oral and Intravenous Administration in the Control Suspension and Nanoemulsion Formulations in Balb/c Micea
| Formulation and Route of Administration | Compartments | 1 h | 4 h | 8 h | 24 h |
|---|---|---|---|---|---|
| Control Suspension | Brain Oral/Brain Intravenous | 0.05 ± 0.02 | 0.49 ± 0.08 | 0.91 ± 0.06 | 1.36 ± 0.21 |
| Saquinavir Nanoemulsion in Flax-Seed Oil | Brain Oral/Brain Intravenous | 0.19 ± 0.05b | 2.22 ± 0.07b,c | 2.90 ± 0.11b,c | 0.93 ± 0.11b |
| Saquinavir Nanoemulsion in Safflower Oil | Brain Oral/Brain Intravenous | 0.35 ± 0.09b | 0.70 ± 0.12b | 1.18 ± 0.16b | 1.54 ± 0.08b |
Each value is a mean ± S.E. of four independent experiments.
Significantly higher (p<0.05) compared to saquinavir control suspension.
Significantly higher (p<0.05) compared to saquinavir nanoemulsions in safflower oil.
3.4 Relative Bioavailabilities in the Plasma and Brain
The relative bioavailability values of SQV in the plasma and brain following oral and i.v. administration in the aqueous suspension and the nanoemulsion formulations normalized based on the AUC values of the aqueous suspension formulation administered i.v. were also calculated are shown in Table 5. SFN showed significant improvement in the bioavailability of the drug in plasma and brain upon oral administration. As compared to the average availability of 42.19% of the drug in aqueous suspension upon oral administration, the flax-seed oil nanoemulsion and safflower oil nanoemulsion afforded the average relative bioavalabilities of 108.1% and 59.73%, respectively. Most importantly, oral administration of SQV in flax-seed (86.69%) and safflower oil (63.6%) nanoemulsions provided significant improvement (p<0.05) in average availability to the brain as compared to the aqueous suspension (39.5%). Upon i.v. administration, the average relative bioavalabilities of SQV in the plasma with flax-seed oil and safflower oil nanoemulsions were 71.09% and 89.82%, respectively, as compared to the aqueous suspension formulation. Additionally, following i.v. administration, flax-seed oil and safflower oil nanoemulsions provided average relative bioavalabilities of 364.52% and 141.97%, respectively, in the brain.
Table 5.
Relative Bioavailabilities of [3H]-Saquinavir Upon Administration in Control Suspension and Nanoemulsion Formulations to Balb/c Micea
| Formulation | I.V. | ORAL | ||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| Saquinavir Control Suspension | 100 ± 5.0 | 100 ± 3.0 | 42.19 ± 4.0 | 39.5 ± 2.0 |
| Saquinavir in Flax-Seed Oil Nanoemulsion | 71.09 ± 3.0 | 364.52 ± 4.5b,c | 108.1 ± 4.5 | 86.69 ± 3.0 |
| Saquinavir in Safflower Oil Nanoemulsion | 89.82 ± 4.5 | 141.97 ± 4.0b | 59.73 ± 2.5 | 63.6 ± 3.5 |
Each value is a mean ± S.E. of four independent experiments.
Significantly higher (p<0.05) compared to saquinavir control suspension.
Significantly higher (p<0.05) compared to saquinavir nanoemulsions in safflower oil.
4. Discussion
Although significant advances have occurred in HIV/AIDS therapy over the last 25 years, the challenge remains to completely eradicate the infection, especially from latent viral particles in cellular and anatomical reservoir sites in the body (Obaru and Mitsuya). Many of the current therapeutic strategies, including the use of HAART, do not provide adequate drug concentrations and sufficient residence at these sites using conventional delivery systems. Lack of efficient drug absorption and distribution has significant clinical ramification, especially in the development of multidrug resistance in HIV/AIDS. As such, there is a critical need to develop novel delivery systems for HIV/AIDS therapeutics that can enhance solubility, permeability, oral bioavailability, and distribution to reservoir sites (Sonza and Crowe, 2001, Vyas, Shah, and Amiji, 2006).
In this study, we examined the potential of oral SQV administration using oil-in-water nanoemulsion systems, where the oils were rich in essential omega-3 and omega-6 PUFA. Since the body does not produce these essential fatty acids, we hypothesized that PUFA-rich edible plant-seed oils, such as flax-seed and safflower oils, would be preferentially absorbed and distributed. Additionally, the use of nano-sized delivery mechanism would further enhance the absorption and distribution to important reservoir sites, such as the CNS (Tiwari and Amiji, 2006). Previous studies from our group have shown that deoxycholic acid-containing pine-nut oil nanoemulsion can enhance oral absorption of paclitaxel, a hydrophobic anticancer therapeutic agent, which is also a substrate for P-glycoprotein (Tiwari and Amiji, 2006). We speculated that the presence of deoxycholic acid in the nanoemulsion would probably enhance oral absorption based on structural similarities to chylomicrons or by inhibiting P-glycoprotein efflux. Bypassing the normal absorption route which results in “first pass effect” and utilizing the lymphatic transport pathway would also have favorable results in terms of effectiveness in HIV as the lymphatic system is known to be a major viral reservoir (Hirunpanich, 2005).
In other studies, the control formulation of SQV is usually prepared by dissolving in organic solvent. We wanted to avoid the influence of organic solvents on oral absorption and brain transport and, as such, have used the aqueous nano-sized suspension of the drug as control formulation. In formulation of the nanoemulsions, we included SQV in the oil phase using an ethanolic stock solution. Based on gross observations as well as more detailed TEM analysis, we did not see any precipitation of the drug upon addition of the aqueous phase. This means that the SQV was encapsulated in the oil droplet and preferred to remain in the oil phase upon addition of water. The encapsulation of SQV and protection afforded by the oil droplets is expected to further improve the drug availability upon oral administration by minimizing degradation or metabolism.
In this study, we have used radioactive drug equivalent per weight to evaluate pharmacokinetic behavior of the drug in the body. Though the HPLC method is used to determine unchanged saquinavir, the complete tissue distribution profiling is not possible as the drug levels are low and below the level of detection by HPLC and were for that reason determined as radioactive (Huisman et al., 2001). Though radioactivity in plasma is accounted for the drug and its metabolites, however, in the tissue of interest, i.e. brain, the level of unchanged drug can be successfully measured as radioactive, since the metabolites can not cross the blood brain barrier. Also, since the comparative pharmacokinetic evaluations (Cmax, Tmax, Cls, Vdss, bioavailability etc.) were made by the two routes for the all formulations, contributions of metabolites towards the findings will be negligible. In another study, it was shown that SQV clearance from plasma was rapid and seemed to be P-glycoprotein independent at doses similar to those used in this study (Huisman et al., 2001). These authors also found that there was no significant difference in the plasma concentrations when the levels were measured by both high performance liquid chromatography and radioactivity.
Comparison of pharmacokinetic parameters in the plasma and brain show higher Cmax and AUC values when SQV was administered in the nanoemulsions as compared to the aqueous suspension formulation for both the routes shows the potential role of developed formulations (Tiwari and Amiji, 2006). When plasma and brain bioavailability values of orally delivered formulations were compared to the similar formulations administered by i.v. route, significant enhancements were observed in case of developed nanoemulsions compared to control formulation. This may be attributed to the drug in the soluble form and role of emulsions in directed the drug to the lymphatics (O'Driscoll, 2003). In addition, SQV administration in flax-seed oil containing nanoemulsions results in significantly higher (p<0.05) Cmax and AUC values in the brain as compared to the safflower oil nanoemulsions. Also, plasma and brain bioavailability values were significantly higher for orally administered flax-seed oil nanoemulsion compared to its i.v. administration, shows significant contribution of lymphatic drug transport. This is again confirmed by lower Cl and Vdss values in case of SFNoral compared to SFNi.v.
Comparative bioavailability was achieved for SFN following oral delivery to i.v. administered control suspension. The low F values in plasma for SFNi.v and SSNi.v as compared to SSiv may be attributed to the higher tissue distributions. This is confirmed by the significant enhancement in brain uptake following i.v. administration in nanoemulsion formulation. The significantly higher bioavailability in the brain following SFNi.v. and SFNoral as compared to SSNi.v. and SSNoral may be due to the higher concentrations of omega-3 fatty acid (Edmond, 2001) in the flax-seed oil as compared to safflower oil. Flax-seed oil contains 58% omega-3 and 14% omega-6, while safflower oil up to 75% omega-6 fatty acids. Selective brain uptake of essential PUFA has been established by several reported studies (Taogoshi et al., 2005). For instance, studies have shown that linolenic and linoleic acids, which are 18-carbon monocarboxylic acids with three and two cis-double bonds, respectively were imported in the brain, while oleic acid containing 18 carbons and one cis-double bond was not (Edmond, 2001). These results suggest exquisite selectivity in the transport of essential PUFA across the blood-brain barrier. Several proteins involved in facilitated fatty acid transport (e.g., fatty acid transport protein, fatty acid binding protein and very-long-chain acyl-coenzyme A synthetase) have been found in brain (Kemin, 2002).
5. Conclusions
In this investigation, we evaluated the enhancement in oral bioavailability and brain distribution of SQV, an anti-HIV PI, using PUFA-rich oil containing nanoemulsion formulations. Both flax-seed and safflower oil-containing nanoemulsions, formulated with deoxycholic acid, improved the oral bioavailability and brain uptake of SQV as compared to the aqueous suspension formulation. Overall, the results of this study show tremendous promise of nanoemulsions, made with PUFA-rich oils, for enhancing oral bioavailability and efficient brain delivery of anti-HIV drugs. This strategy can potentially be extremely useful in patients infected with HIV/AIDS for specific delivery to hard-to-reach viral reservoir sites.
Acknowledgments
The authors are thankful to Dr. Robert Campbell, Dr. Richard Deth, and Mr. William Fowle of Northeastern University (Boston, MA), for access to experimental facilities used in this study. JEdwards International Inc., (Quincy, MA) is gratefully acknowledged for providing gift samples of flax-seed and safflower oils.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AIDS epidemic update - December 2005. Global HIV/AIDS estimates, end of 2006. World AIDS Day. AVERT. http://www.avert.org/worldstats.htm(Accessed on November 29, 2006)
- Boles JA, Kott RW, Hatfield PG, Bergman JW, Flynn CR. Supplemental safflower oil affects the fatty acid profile, including conjugated linoleic acid, of lamb. J Anim Sci. 2005;83:2175–81. doi: 10.2527/2005.8392175x. [DOI] [PubMed] [Google Scholar]
- Chearskul P, Rongkavilit C, Al-Tatari H, Asmar B. New antiretroviral drugs in clinical use. Indian J Pediatr. 2006;73:335–41. doi: 10.1007/BF02825828. [DOI] [PubMed] [Google Scholar]
- Chun TW, Davey R, Ostrowski M, Engel D, Mullins J, Lane C, Fauci A. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- Clarke JR, White NC, Weber JN. HIV compartmentalization: pathogenesis and clinical implications. AIDS Rev. 2000;2:15–22. [Google Scholar]
- Edmond J. Esssential polyunsaturated fatty acids and the barrier to the brain: the components of a model for transport. J Mol Neurosci. 2001;16:181–193. doi: 10.1385/JMN:16:2-3:181. [DOI] [PubMed] [Google Scholar]
- Figgitt DP, Plosker GL. Saquinavir soft-gel capsule: an updated review of its use in the management of HIV infection. Drugs. 2000;60:481–516. doi: 10.2165/00003495-200060020-00016. [DOI] [PubMed] [Google Scholar]
- Frezzini C, Leao JC, Porter S. Current trends of HIV disease of the mouth. J Oral Pathol Med. 2005;34:513–31. doi: 10.1111/j.1600-0714.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- Gallo RC. A reflection on HIV/AIDS research after 25 Years. Retrovirology. 2006;3:72. doi: 10.1186/1742-4690-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtgrave DR. Causes of the decline in AIDS deaths, United States, 1995-2002: prevention, treatment or both? Int J STD AIDS. 2005;16:777–81. doi: 10.1258/095646205774988109. [DOI] [PubMed] [Google Scholar]
- Huisman MT, Smit JW, Wiltshire HR, Hoetelmans RM, Beijnen JH, Schinkel AH. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol Pharmacol. 2001;59:806–13. doi: 10.1124/mol.59.4.806. [DOI] [PubMed] [Google Scholar]
- Jafari SM, Yinghe He, Bhandari B. Nano-Emulsion production by sonication and microfluidization - a comparison. International Journal of Food Properties. 2006;9:475–485. [Google Scholar]
- Kandanearatchi A, Williams B, Everall IP. Assessing the efficacy of highly active antiretroviral therapy in the brain. Brain Pathol. 2003;13:104–10. doi: 10.1111/j.1750-3639.2003.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemin Q, Marni H, Richard JD. Long-chain polyunsaturated fatty acid accretion in brain. Curr Opinion Clin Nutrit Metab Care. 2002;5:133–138. doi: 10.1097/00075197-200203000-00003. [DOI] [PubMed] [Google Scholar]
- King JR, Wynn H, Brundage R, Acosta EP. Pharmacokinetic enhancement of protease inhibitor therapy. Clin Pharmacokinet. 2004;43:291–310. doi: 10.2165/00003088-200443050-00003. [DOI] [PubMed] [Google Scholar]
- Kulkovsky J, Bray S. HAART-persistent HIV-1 latent reservoirs: their origin, mechanisms of stability, and potential strategies for eradication. Curr HIV Res. 2006;4:199–208. doi: 10.2174/157016206776055084. [DOI] [PubMed] [Google Scholar]
- Lo YL, Huang JD. Effects of sodium deoxycholate and sodium caprate on the transport of epirubicin in human intestinal epithelial Caco-2 cell layers and everted gut sacs of rats. Biochem Pharmacol. 2000;59:665–72. doi: 10.1016/s0006-2952(99)00377-9. [DOI] [PubMed] [Google Scholar]
- Meyer S, Berrut S, Goodenough TIJ, Rajendram VS, Pinfield VJ, Povey MJW. A comparative study of ultrasound and laser light diffraction techniques for particle size determination in dairy beverages. Meas Sci Technol. 2006;17:289–297. [Google Scholar]
- O'Driscoll CM. Intestinal lmphatic targeting of drugs. STP Pharma Sci. 2003;13:17–25. [Google Scholar]
- Obaru K, Mitsuya H. Anti-HIV drugs and drug delivery system. Nippon Rinsho. 1998;56:769–75. [PubMed] [Google Scholar]
- Schrager LK, D'Souza MP. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. J Am Med Assoc. 1998;280:67–71. doi: 10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- Shah LK, Amiji MM. Intracellular Delivery of Saquinavir in Biodegradable Polymeric Nanoparticles for HIV/AIDS. Pharm Res. 2006a;23:2638–45. doi: 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- Sonza S, Crowe SM. Reservoirs for HIV infection and their persistence in the face of undetectable viral load. AIDS Patient Care & STDs. 2001;15:511–518. doi: 10.1089/108729101753205676. [DOI] [PubMed] [Google Scholar]
- Taogoshi T, Nomura A, Murakami T, Nagai J, Takano M. Transport of prostaglandin E1 across the blood-brain barrier in rats. J Pharm Pharmacol. 2005;57:61–6. doi: 10.1211/0022357055173. [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Amiji MM. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol. 2006;6:3215–21. doi: 10.1166/jnn.2006.440. [DOI] [PubMed] [Google Scholar]
- Hirunpanich V, Katagi J, Sethabouppha B, Sato H. Demonstration of docosahexanoic acid as a bioavailability enhancer for CYP3A substrates: In vitro and In vivo evidence using cyclosporine in rats. Drug Metab Disp. 2005;34:305–310. doi: 10.1124/dmd.105.007088. [DOI] [PubMed] [Google Scholar]
- Vyas TK, Shah L, Amiji MM. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. Expert Opin Drug Deliv. 2006;3:613–28. doi: 10.1517/17425247.3.5.613. [DOI] [PubMed] [Google Scholar]
- Zhao DL, Hirst BH. Comparison of bile salt perturbation of duodenal and jejunal isolated brush-border membranes. Digestion. 1990;47:200–7. doi: 10.1159/000200498. [DOI] [PubMed] [Google Scholar]