Abstract
Temporal filtering of afferent information is an intrinsic component of the processing of numerous types of sensory information. To date, no temporal filtering mechanism has been identified for nociceptive information. The phenomenon of offset analgesia, the disproportionately large decrease in perceived pain following slight decreases in noxious thermal intensity, however, suggests the existence of such a mechanism. To test the hypothesis that a temporal filtering mechanism is engaged during noxious stimulus offset, subjects rated heat pain intensity while stimulus fall rates were varied from −0.5 to −5.0°C/s. In the absence of a temporal filtering mechanism, pain intensity would be expected to decrease in direct proportion to the stimulus fall rate. However, psychophysical fall rates were considerably faster than stimulus fall rates, such that subjects reported no pain while stimulus temperatures were clearly within the noxious range (47.2°C). In addition, paired noxious stimuli were presented simultaneously to determine if offset analgesia evoked by one stimulus could inhibit pain arising from a separate population of primary afferent neurons. Pain ratings were significantly lower than those reported from two constant 49°C stimuli when offset analgesia was induced proximal to, but not distal to, a second noxious stimulus. These asymmetric spatial interactions are not readily explained by peripheral mechanisms. Taken together, these findings indicate that offset analgesia is mediated in part by central mechanisms and reflect a temporal filtering of the sensory information that enhances the contrast of dynamic decreases in noxious stimulus intensity.
Keywords: Temporal Filter, Signal Processing, Analgesia, Offset Analgesia, Psychophysics, Pain
INTRODUCTION
The filtering of afferent information is a critical dimension of sensory processing. In the somatosensory modality, spatial filtering is well documented, with spatial sharpening being accomplished by lateral inhibition for innocuous tactile information and diffuse noxious inhibitory control (DNIC) for noxious information (Mountcastle VB, 1968; Le Bars et al., 1979b, 1979a; Le Bars et al., 1992; Gardner EP, 2000). However, temporal filtering of nociceptive information is poorly understood despite the fact that profound temporal transformations of nociceptive information occur within both primary afferent neurons and central nervous system neurons. In many cases, these temporal transformations consist of progressive amplification of nociceptive afferent responses to fixed noxious stimuli, such as with windup and long term potentiation (LTP) (Mendell and Wall, 1965; Mendell, 1966; Woolf, 1996). Conversely, inhibitory mechanisms could increase the perceived temporal contrast of stimuli as they decrease over time by reducing post-stimulus after-responses. Accordingly, such post-stimulus inhibition could function as a temporal sharpening filter.
The recently identified phenomenon of offset analgesia could reflect such an inhibitory temporal sharpening mechanism. Offset analgesia is defined by disproportionately large decreases in perceived pain intensity following incremental decreases in stimulus temperature (Grill and Coghill, 2002). For example, given a three temperature stimulus (49°C [5s], 50°C [5s], 49°C [20s]) the 1°C decrease in stimulus temperature from 50°C to 49°C evokes a transient analgesia where pain ratings were significantly lower than those evoked by a constant 49°C stimulus (Grill and Coghill, 2002). These decreases in pain ratings following 1°C change in temperature were sufficiently large to be statistically indistinguishable from those following a 15°C decrease from 50°C to 35°C. Thus, subjects were incapable of discriminating between a 1°C and a 15°C decrease in stimulus temperature. Importantly, this analgesia is time-locked to the transient decrease in stimulus intensity.
Offset analgesia remains a poorly understood phenomenon and its functional significance remains unclear. Grill and Coghill (2002) proposed that it may act as a temporal contrast enhancement mechanism that amplifies the perception of decreases in stimulus energy and could therefore enhance escape behaviors. In order to directly test this hypothesis, we varied the stimulus fall rates of brief noxious thermal stimuli to determine if the perceptual experience during stimulus offset reflects a temporal transformation in noxious stimulus intensity. Temporal sharpening would be defined by psychophysical ratings that fall faster than would be predicted by stimulus fall rates. Conversely, temporal smoothing would be defined by psychophysical ratings that decrease more slowly than would be predicted by stimulus fall rates.
No data currently address which sites in the nervous system subserve offset analgesia. To determine if offset analgesia is centrally mediated, two thermal probes were applied simultaneously to separate skin regions to evaluate whether offset analgesia-inducing stimuli from one probe could modulate pain intensity evoked by stimulation of a spatially distinct population of primary afferents.
METHODS
Subjects
All subjects were healthy volunteers between the ages of 23 and 36 and had no history of chronic pain or any neurological disorder. Data were collected in two separate experiments. In the first experiment, data from the one and three temperature paradigms were collected from thirteen subjects. In the second experiment, data from the paired stimulus paradigm were collected from ten subjects. No subjects participated in both experiments. Subjects were asked not to take any analgesics within 48 hours of the study. One subject was disqualified from the first experiment for taking an asthma medication (Advair diskus) that contained corticosteroids and a Beta2-adenergic agonist. Written informed consent was obtained from all subjects. The Institutional Review Board (IRB) at Wake Forest University School of Medicine approved all procedures used in this experiment.
Thermal Stimulation and Pain Assessment
A 16mm × 16mm peltier device (Medoc TSAII, Ramat Yishai, Israel) was attached to the ventral surface of the dominant forearm of the subject by a Velcro strap. The thermal probe was maintained at a baseline of 35°C (approximately skin temperature).
Subjects were trained to rate their perceived pain intensity using a computerized continuous visual analog scale (VAS) (15cm in length, verbal anchors of “no pain sensation” and “most pain sensation imaginable”) (Price et al., 1983; Price et al., 1989; Price et al., 1994; Koyama et al., 2004). Subjects were specifically instructed to only rate pain, not temperature, on the VAS. Training consisted of four blocks of eight stimuli each. Stimuli presented were between 35–49°C (rise and fall rate 6°C/s) and lasted for five seconds.
Temperature data from the thermal probe and VAS data from the subject were sampled at 100Hz. The apparatus that we used to record VAS ratings provides a high signal to noise ratio. The average standard deviation of the VAS signal during rest is 0.0023±0.0007 VAS units. For all experimental trials, psychophysical ratings less than one VAS unit were not included in the data analysis. This occurred at a greater frequency for 48°C stimuli than it did for 50°C stimuli. For all stimulus conditions, a minimum of a 60 second interstimulus interval was maintained in order to reduce sensitization and/or habituation. Additionally, the thermal probe was moved following each stimulus to a completely distinct, yet adjacent area of skin. Recently Quevedo et al (in press) demonstrated that pain sensitivity did not vary with location on the ventral forearm. Thus, the use of different sites along the proximal-distal axis of the ventral forearm is likely to minimally influence psychophysical responses.
One-Temperature Paradigm
This paradigm was used to test the hypothesis that offset analgesia functions as a temporal sharpening mechanism that increases the detectability of slow decreases in noxious stimulus intensity. In this paradigm, subjects were presented with a one temperature stimulus of either 48 or 50°C, and the rate at which the probe returned to baseline was varied between trials (−0.5, −1.0, −2.0, −3.5, and −5.0°C/s). Rise rates were held constant at 5°C/s and plateau times were 5 seconds. This paradigm was employed to determine if there is a critical fall rate needed for activation of offset analgesia. Each fall rate was presented four times per stimulus intensity (48 and 50°C) per subject in random order. Three parameters were extracted from continuous VAS ratings for analysis. First, the latency for subjects to respond with a decreased VAS rating was calculated by subtracting the time at which the stimulus temperature started to fall from the time at which VAS ratings started to fall. Second, the temperature at which subjects first reported a pain intensity rating of zero following stimulus offset was calculated. Finally, VAS fall rates were calculated based on the starting time derived from the initial decrease in VAS ratings and an ending time of when subjects first reported VAS ratings of zero (Fig. 1). These three parameters for each stimulus fall rate (−0.5, −1.0, −2.0, and −3.5°C/s) were compared to those from stimuli with a fall rate of −5.0°C/s with repeated measures analysis of variance (ANOVA). This was performed separately for both stimulus temperatures (48°C and 50°C).
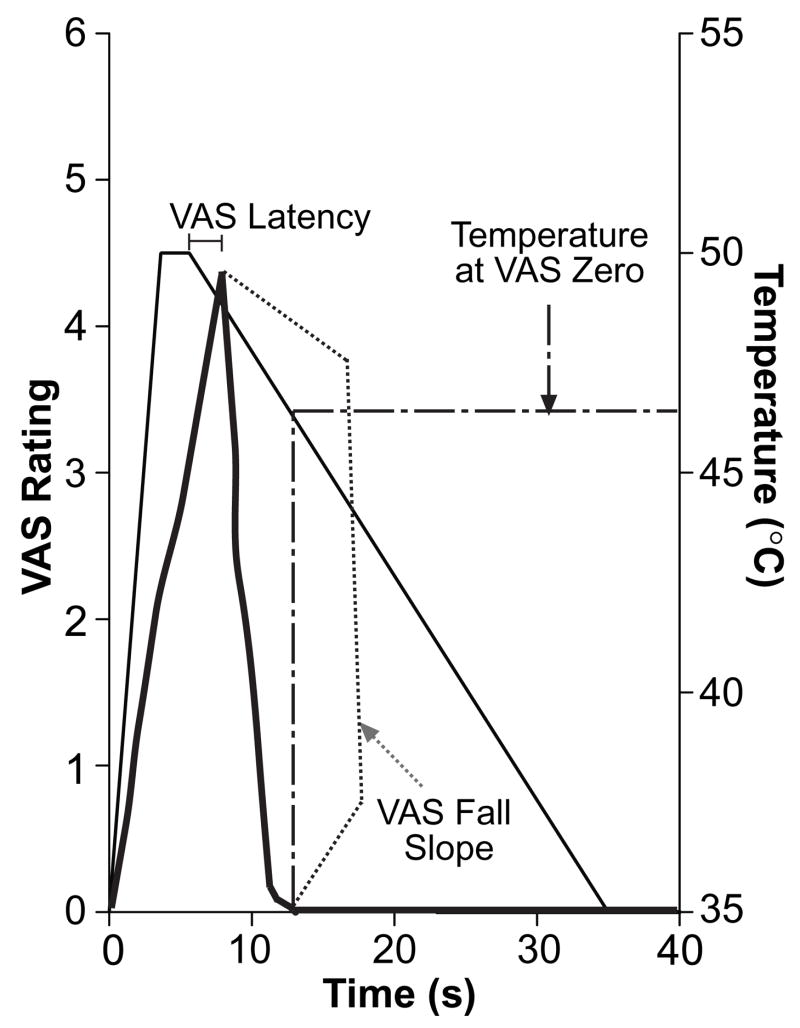
Figure 1. End Points of the One Temperature Paradigm.
Typical VAS ratings of pain (thick, solid line) are superimposed on stimulus temperature (thin, solid line). The VAS fall slope (dotted line) was derived from the VAS ratings alone, while the VAS latency and the temperature at VAS zero (dot-dash line) were calculated using both VAS and stimulus temperature.
Considerable evidence indicates that pain intensity increases with the rise rate of a noxious thermal stimulus (Bessou and Perl, 1969; Croze and Duclaux, 1978; Yarnitsky and Ochoa, 1990; Yarnitsky et al., 1992). However, little information describes the relationship between the rate of temperature change and the rate of perceptual change. Thus, in order to determine if VAS fall rates are linearly related to temperature fall rates, we first compared actual VAS fall rates to those expected from a linear relationship with temperature change. The expected VAS fall rates were calculated by dividing subjects’ peak VAS ratings for each stimulus by the time it took for the stimulus to return to approximate pain threshold (43°C). Then, each expected VAS fall rate was directly contrasted with the corresponding observed VAS fall rate using two-way ANOVA.
During steady-state stimulation, the relationship between the magnitude of a physical stimulus (φ) and its perceived intensity (Ψ) is described best by a power function (ψ = κφβ) and not by a linear function ((Stevens, 1957, 1970; Buchsbaum and Stevens, 1971; Price et al., 1983; Price et al., 1994). The value of this exponent (β) varies between different types of stimulus energy. To determine if this relationship is altered during dynamic decreases in stimulus intensity, we calculated β and κ (constant) from the average continuous VAS rating during the falling phase of the one temperature stimuli and during the plateau phase of the training stimuli (Price et al., 1994). These variables were calculated using ψ= κ (Temp−35°C)β.
Three-Temperature Paradigm
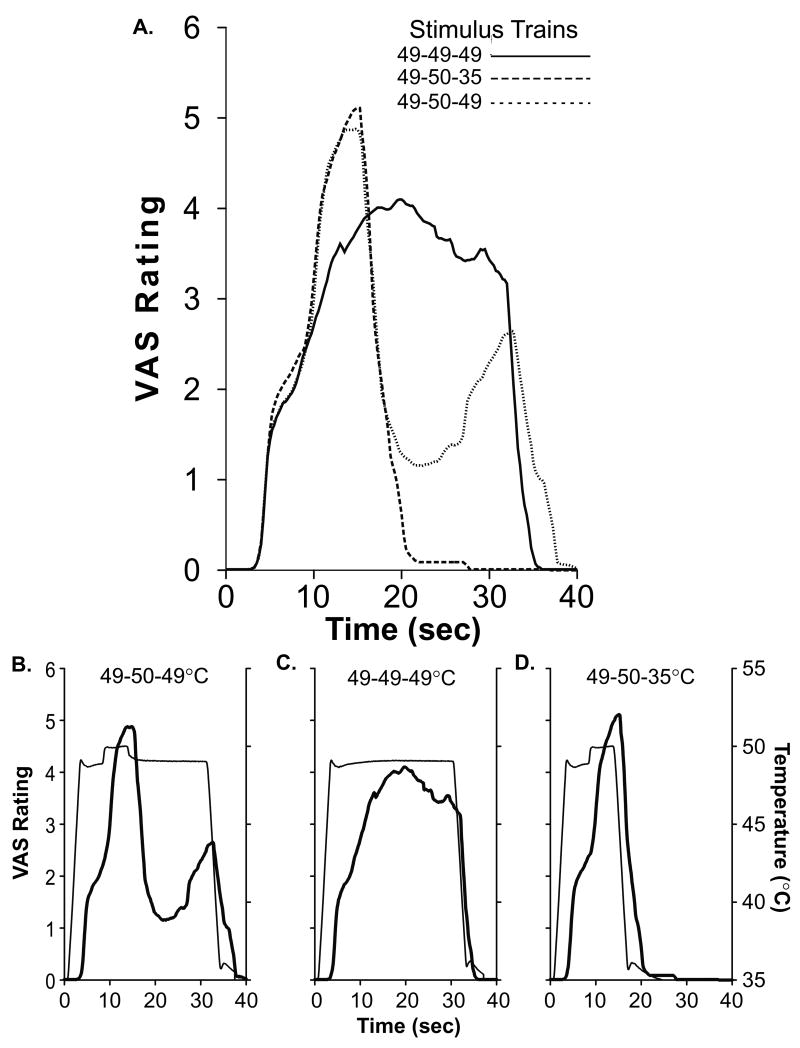
This paradigm was used to quantify the magnitude and time-course of offset analgesia and consisted of a three-temperature stimulus train (49°C [5s], 50°C [5s], 49°C [20s]) (Grill and Coghill, 2002). The 1°C temperature decrease (−6.0°C/s) between the second temperature (T2) and the third temperature (T3) initiated offset analgesia. Three-temperature control trains (49°C [5s], 50°C [5s], 35°C [20s]) and constant temperature stimuli (49°C [30s]) were also presented to subjects in order to assess the magnitude of offset analgesia relative to habituation. During stimulation, subjects were asked to rate pain intensity in real-time for each of the three conditions (experimental, constant, and control). Each condition was presented three times per subject in random order.
The time and magnitude (0.01 second resolution) of the lowest VAS rating following the transition from T2 to T3 until end of the T3 stimulus was first extracted from the continuous data. Next, VAS ratings of the three-temperature control condition (49°C[5s]-50°C[5s]-35°C[20]) and of the constant 49°C condition (49°C[30s]) were obtained from the exact same time points as the minimum values of the three-temperature trains. These three values were contrasted using a within subjects ANOVA. The average magnitude, and duration of analgesia was also calculated from these data.
Paired Stimulus Paradigm
This paradigm was used to determine if offset analgesia-inducing stimuli at one site could modulate pain intensity from a second, spatially distinct stimulus. This paradigm was designed to test two hypotheses. First, when two probes deliver stimuli that evoke offset analgesia, does the observed analgesia exhibit summation when compared to offset analgesia evoked by only one probe? Second, does offset analgesia induced at one probe modulate reported pain intensity evoked by constant thermal noxious stimuli from a different probe? If both probes were separated sufficiently so that different populations of primary afferents are activated, then either hypothesis would provide evidence for the involvement of central mechanisms in the mediation of offset analgesia.
Two 16mm × 16mm probes were placed 50mm apart on the ventral skin of the subject’s dominant forearm and moved between trials. Six different stimulus pairs were presented to subjects in this paradigm (Table 1). These six pairs of stimuli fall into three categories; matched, unmatched, and control. Condition 1 consisted of two matched constant 49°C stimuli lasting 30 seconds each. Condition 2 paired two matched three-temperature stimulus trains (49°C [5s]-50°C [5s]-49°C [20]). Condition 3 consisted of two unmatched stimuli, one constant 49°C stimulus and one three-temperature train. In Condition 3a, the proximal probe delivered the three-temperature stimulus and the distal probe delivered the constant 49°C stimulus. Condition 3b was simply the opposite. Lastly, two control conditions were used to ensure that tactile information from two probes on the skin did not disrupt offset analgesia. Control stimuli were paired with a distal 35°C stimulus. Control 1 was a constant 49°C stimulus and Control 2 was a three-temperature stimulus. Subjects were asked to rate overall pain intensity for both probes on a continuous VAS scale. Each paired stimulus was presented 4 times in random order.
Table 1. Stimuli for Paired Stimulus Paradigm.
49 = 49°C stimulus for 30s
35 = 35°C stimulus for 30s
3T = 49°C for 5 s, followed by 50°C for 5 seconds, followed by 49°C for 20s
| Condition | Proximal Probe | Distal Probe | Abbreviated | |
|---|---|---|---|---|
| Matched Paired Stimuli | 1 | 49 | 49 | 49_49 |
| 2 | 3T | 3T | 3T_3T | |
| Unmatched Paired Stimuli | 3a | 3T | 49 | 3T_49 |
| 3b | 49 | 3T | 49_3T | |
| Control Stimuli | Control 1 | 49 | 35 | 49_35 |
| Control 2 | 3T | 35 | 3T_35 |
The minimum VAS rating following decrease from T2 to T3 until the end of T3 was identified for each condition involving a three-temperature stimulus. For paired stimuli that consisted of purely constant thermal stimuli (49_49 and 49_35), the minimum VAS rating from the corresponding time period was extracted from the continuous data. The minimum VAS ratings from three-temperature trials were contrasted with those from the constant thermal trials (Condition 2 (3T_3T), 3a (3T _49), & 3b (49_3T) vs. Condition 1 (49_49), Condition 3a (3T_49) vs. Condition 3b (49_3T), and Condition 3a (3T _49) & 3b (49_3T) vs. Control 1 (49_35)) using ANOVA. Percent analgesia was also calculated for each three-temperature condition. This percent analgesia was computed by dividing the minimum VAS ratings from three-temperature stimulus trains by the minimum VAS ratings elicited by constant thermal stimuli from the same time period.
RESULTS
Training Data
Subjects showed they could properly use a continuous VAS scale to rate thermal stimulus intensity differences as small as 1°C (Fig. 2). When presented with different thermal stimuli in pseudo-random order, subjects were able to consistently rate pain intensities for each respective temperature (Overall, F(7, 2)=10.15, p<0.001, 35°C vs. 43°C, F(1, 8)=6.82, p<0.03; 43°C vs. 44°C, F(1, 8)=1.62, p<0.24; 44°C vs. 45°C, F(1, 8)=2.35, p<0.16; 45°C vs. 46°C, F(1, 8)=5.45, p<0.05; 46°C vs. 47°C, F(1, 8)=7.71, p<0.02; 47°C vs. 48°C, F(1,8)=16.05, p<0.01; 48°C vs. 49°C, F(1, 8)=5.62, p<0.05). It has been shown previously that subjects can more accurately rate temperature differences at higher noxious stimulus intensities (Bushnell et al., 1983). Note that as stimulus temperature increases, peak VAS ratings increase exponentially according to Price’s application of Steven’s power law (Fig. 2, right, (Stevens, 1957, 1970; Buchsbaum and Stevens, 1971; Price et al., 1983; Price et al., 1994).
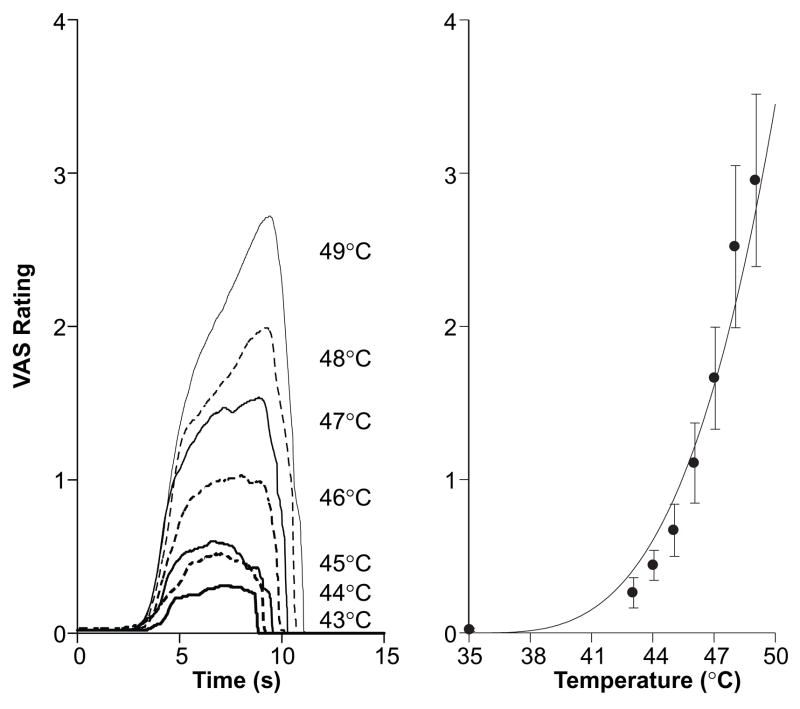
Figure 2. Subjects Distinguish Differences in Perceived Pain Intensity using the Continuous VAS.
During training sessions, subjects were presented with stimuli between 35°C–49°C. In the left panel, continuous VAS ratings (averaged across all subjects) from 5s stimuli are shown by stimulus temperature. In the right panel, average peak VAS ratings are shown for each stimulus temperature. Note that as stimulus temperature increases, peak VAS ratings increase exponentially (fit line) according to Price’s application of Steven’s power law (Stevens, 1957, 1970; Price et al., 1983, 1994).
One-Temperature Paradigm
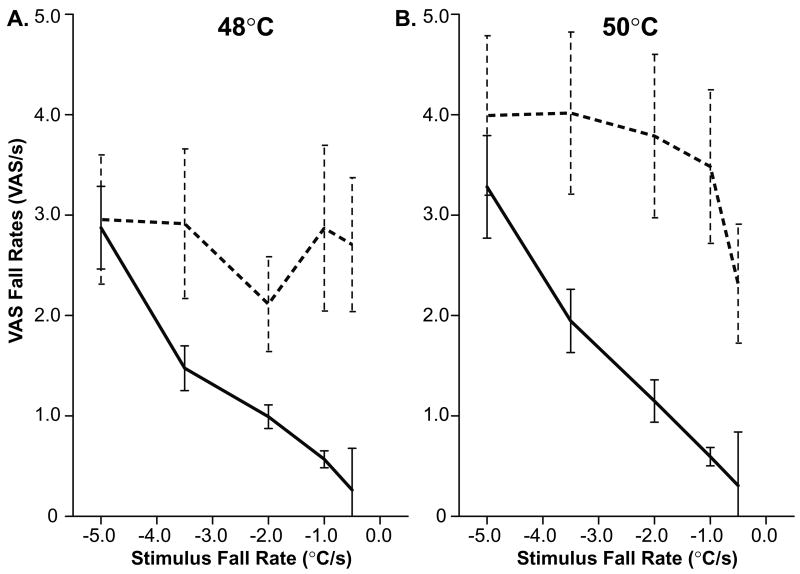
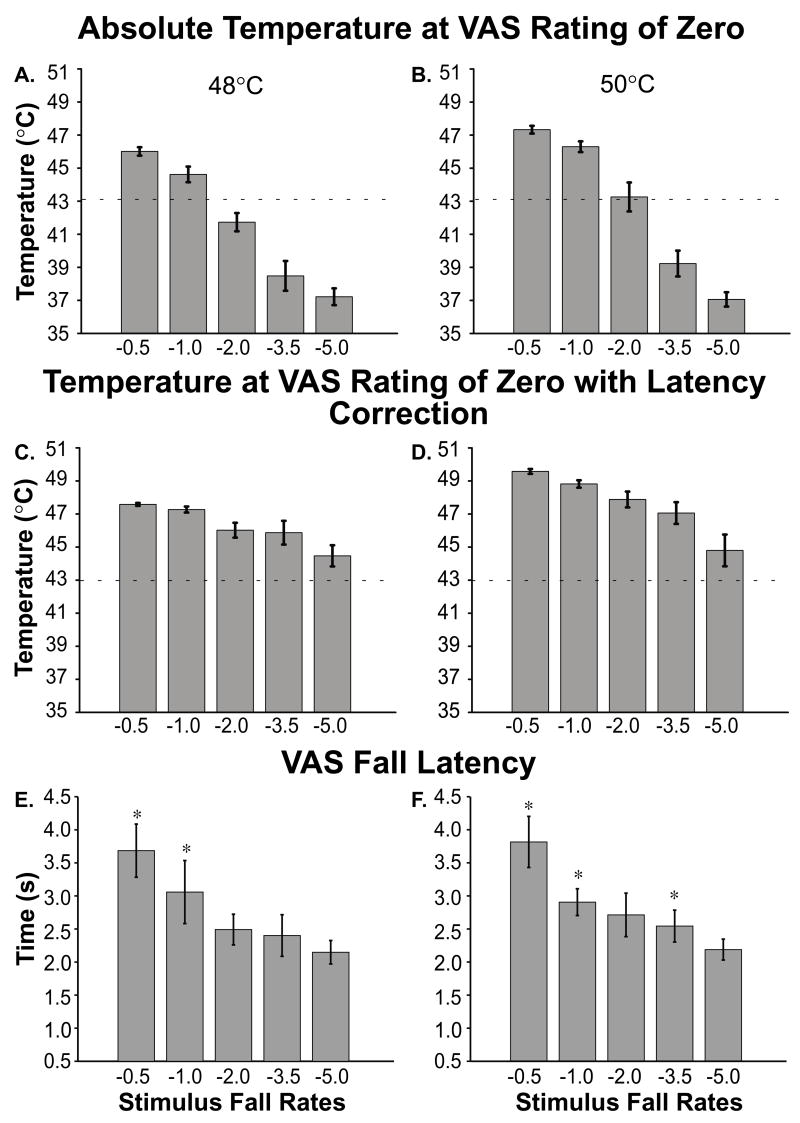
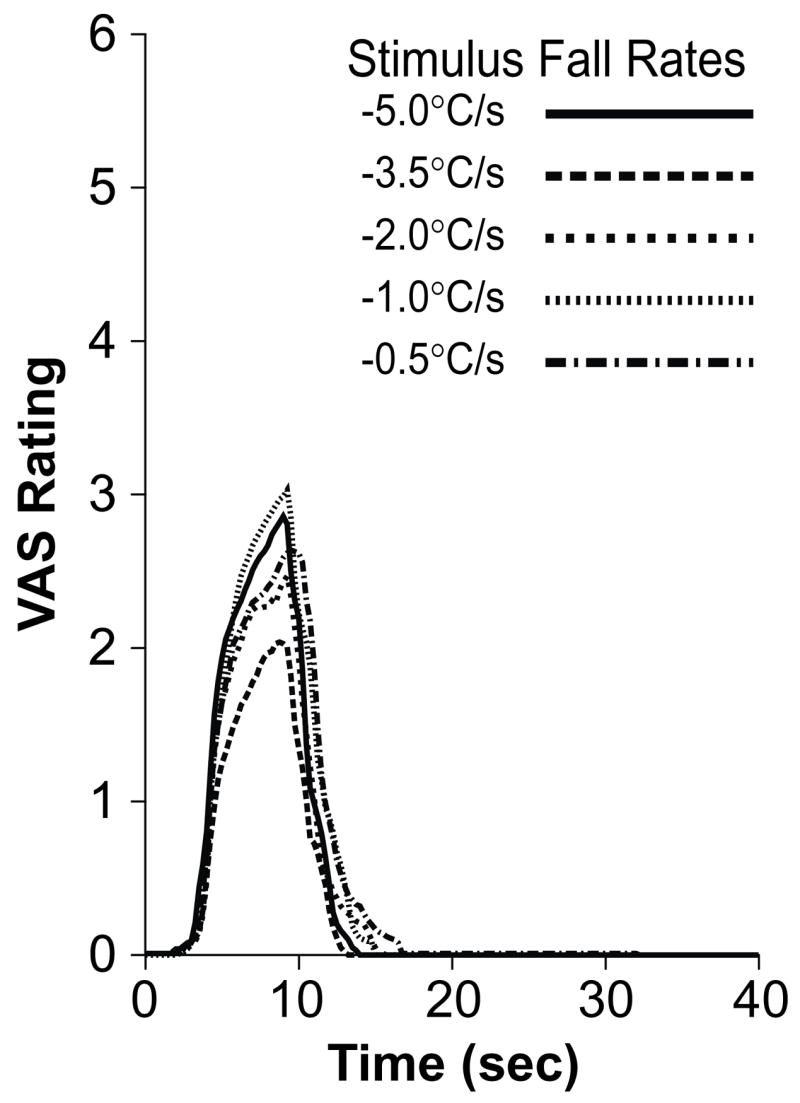
When subjects were presented with stimuli with fall rates ranging from −5°C/s to −0.5°C/s, VAS ratings fell at a rate which was not reliably dependent on stimulus fall rate (F(4, 28)=1.62, p<0.48 and F(4, 44)=5.79, p<0.08; ANOVA for 48°C and 50°C, respectively. Fig. 3a & 4). When the VAS fall rate for each stimulus fall rate was contrasted with that from a stimulus with a −5.0°C/s fall rate (Fig. 3b), no significant difference was observed in nearly, every case, for the fall rates of the 48°C stimuli (−3.5°C/s, F(1, 7)=0.68, p<0.44; −2.0°C/s, F(1, 7)=2.76, p<0.14; −1.0°C/s, F(1, 7)=0.00, p<0.99; −0.5°C/s, F(1, 7)=1.65, p<0.240; Fig. 4) or 50°C stimuli (−3.5°C/s, F(1, 11)=0.01, p<0.91, Fig. 3c; −2.0°C/s, F(1, 11)=0.47, p<0.51, Fig. 3d; −1.0°C/s, F(1, 11)=2.81, p<0.12, Fig. 3e) with the exception of −0.5°C/s (F(1, 11)=12.28, p<0.01, Fig. 3f).
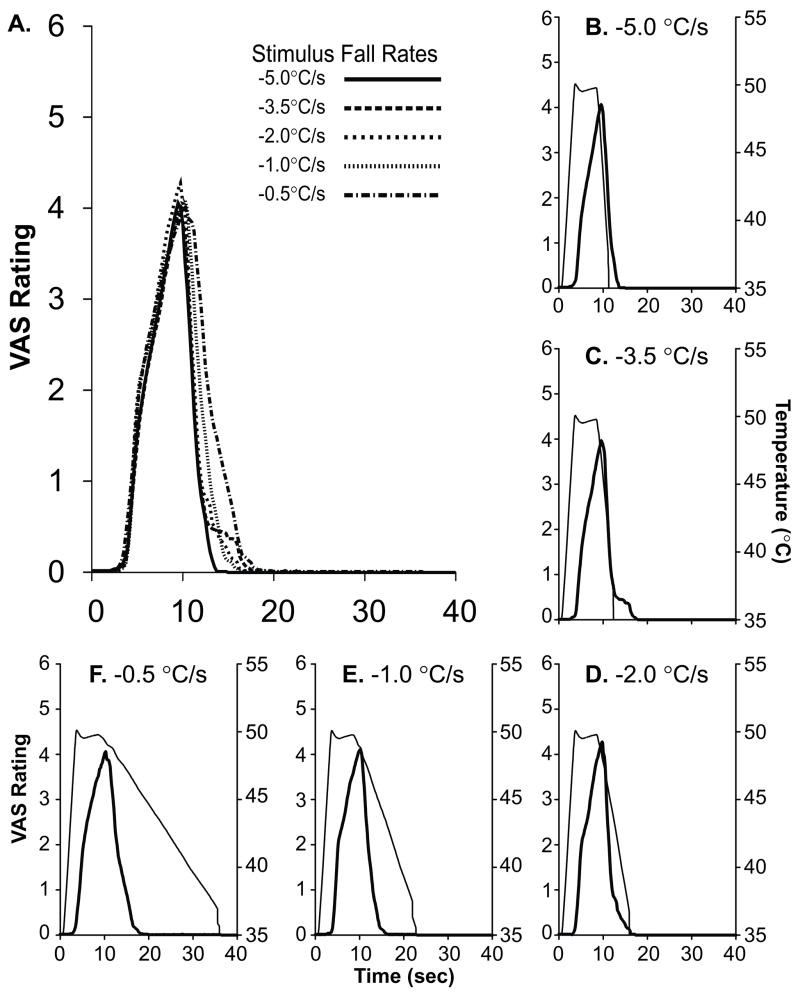
Figure 3. Psychophysical Fall Rates are Largely Independent of Temperature Fall Rates.
The mean continuous VAS ratings reported from each five second 50°C stimulus (stimulus fall rates varied from −0.5° C/s to −5.0° C/s) are shown together (overlay, A). Psychophysical ratings (thick line, averaged across all subjects) from each stimulus condition are shown in the insets (b–f) with their corresponding stimulus temperature trace (thin line).
Figure 4. Psychophysical Fall Rates For 48°C Stimuli.
Psychophysical ratings (averaged across all subjects) for each stimulus fall rate are plotted together for 48°C stimuli. As was the case for 50°C stimuli (Fig. 3), VAS fall rates were largely independent of stimulus fall rates for 48°C stimuli.
Actual VAS fall rates were significantly faster than those predicted by a linear relationship between psychophysical fall rate and temperature fall rate (F(5, 35)=8.79, p<0.0001 and F(5, 55)=12.05, p<0.0001; 48°C (Fig. 5a) and 50°C (Fig. 5b), Overall ANOVA, respectively.). Significant differences between expected VAS fall rates and actual VAS fall rates were found at nearly every stimulus fall rate at both 48°C and 50°C (Table 2). Thus, offset analgesia enhances the perception of slowly decreasing noxious thermal stimuli that would, otherwise, be difficult to detect. However, at the fastest temperature fall rate (−5.0°C/s) actual and expected VAS fall rates began to converge. Stimulus fall rates approaching −5.0°C/s may represent the fastest stimulus fall rates that subjects can accurately evaluate. At the fastest stimulus fall rates conduction and processing delays are comparable to the duration of the stimulus fall and therefore likely preclude a meaningful evaluation of fall rate.
Figure 5. Actual Psychophysical Fall Rates (dashed lines) are Significantly More Rapid than Expected Fall Rates (solid lines).
Expected fall rates were modeled using a linear relationship between the stimulus fall rate and perceived intensity fall rate. These expected data were plotted against observed VAS fall rates (averaged across subjects) for both 48°C and 50°C stimuli (a and b, respectively). Note that due to offset analgesia there is very little change in VAS fall rate regardless of stimulus fall rate. Additionally, at the fastest fall rate (−5.0°C/s) actual and expected fall rates begin to converge.
Table 2.
Decreases in VAS ratings are not linearly related to decreases in stimulus temperature. Actual VAS fall rates are significantly faster then those that would be predicted if pain intensity were linearly related to stimulus temperature. Abbrev. Degrees of Freedom (DOF).
| Stimulus Temperature | Stimulus Fall Rate (°C/s) | Exp. VAS Fall Rate (VAS/s) | Act. VAS Fall Rate (VAS/s) | p-value |
|---|---|---|---|---|
| 48°C DoF (1,7) | −5.0 | −2.87 | −2.96 | p<0.767 |
| −3.5 | −1.48 | −2.91 | p<0.037 | |
| −2.0 | −0.99 | −2.11 | p<0.062 | |
| −1.0 | −0.57 | −2.87 | p<0.011 | |
| −0.5 | −0.26 | −2.70 | p<0.009 | |
| 50°C DoF (1,11) | −5.0 | −3.28 | −3.99 | p<0.266 |
| −3.5 | −1.95 | −4.02 | p<0.008 | |
| −2.0 | −1.15 | −3.79 | p<0.003 | |
| −1.0 | −0.59 | −3.48 | p<0.002 | |
| −0.5 | −0.31 | −2.32 | p<0.004 |
The relationship of perceived peak pain intensity and stimulus intensity during the plateau phase of a noxious stimulus differs profoundly from that during different stimulus fall rates (Table 3). The exponent describing this relationship for stimulus plateau (5 seconds) is 3.412 (Fig. 2, right panel). At slow stimulus fall rates (i.e. −0.5°C/s) the exponent is quite large (14.059) but gets progressively smaller as the fall rate increases. When a noxious thermal stimulus decreased rapidly toward pain threshold, stimulus-response curves were shallower than those during the plateau phase. This was likely due to conduction/evaluation delays limiting psychophysical response speed. However, when the stimulus decreased slowly, stimulus-response curves were considerably steeper than those during plateau phase. This change in the stimulus-response slope indicates that small changes in stimulus intensity are amplified during slowly decreasing thermal stimuli.
Table 3. The relationship between perceived intensity and stimulus intensity is altered during the stimulus fall.
During slowly decreasing stimuli, stimulus-response curves were considerably steeper than those during plateau phase. Such increases in the slope indicate that small changes in stimulus intensity are amplified during slowly decreasing thermal stimuli. During more rapid decreases in stimulus temperature, stimulus-response curves grew more shallow than those during the plateau phase, an effect likely due to conduction/evaluation delays.
| β(Exponent) | κ(Constant) | |
|---|---|---|
| Stimulus Plateau | 3.412 | −8.004 |
| Stimulus Fall Rate -0.5°C/s | 14.059 | −35.525 |
| Stimulus Fall Rate -1.0°C/s | 9.014 | −21.833 |
| Stimulus Fall Rate -2.0°C/s | 1.788 | −3.709 |
| Stimulus Fall Rate -3.5°C/s | 0.973 | −1.038 |
| Stimulus Fall Rate -5.0°C/s | 0.484 | 0.287 |
For stimuli with plateau temperatures of 48°C and 50°C, subjects reported “no pain sensation at all” while the thermal probe was at suprathreshold temperatures (Fig 6 a & b). In the case of 48°C stimuli, pain ratings returned to zero while the stimulus temperature was 46.0°C for −0.5°C/s stimuli, and 44.6°C for −1.0°C/s stimuli (Fig. 6a). For stimuli with a plateau temperature of 50°C, pain ratings returned to zero while stimulus temperature was still 47.2°C for −0.5°C/s stimuli and 46.5°C for −1.0°C/s stimuli (Fig. 6b). In the training session (Fig. 2), these stimulus temperatures would evoke pain ratings of approximately 1.7 and 1.4 VAS units, respectively. When corrected for response latencies, the temperatures at which pain ratings first returned to zero were greater than 43°C for all stimuli (Fig. 6 c & d). The psychophysical response latencies for both 48°C and 50°C stimuli are also shown in Fig. 6e and 6f, respectively. Response latencies from −0.5°C/s, −1.0°C/s, −2.0°C/s, and −3.5°C/s were significantly different from those from −5.0°C/s stimuli (F(4, 28)=61.52, p<0.001 and F(4, 44)=81.76, p<0.001 for 48°C (Fig. 6e) and 50°C (Fig. 6f), respectively).
Figure 6. Temperatures at which VAS Ratings Returned to Zero and VAS Fall Latencies.
The absolute stimulus intensity (no latency correction) when subjects first reported VAS ratings of zero is shown for 48°C and 50°C (a and b, respectively; means ± SEM). These data are shown with latency correction in c and d (means ± SEM). The latency taken for subjects to first respond to an initial decrease in stimulus intensity is shown for 48°C and 50°C (e and f, respectively; means ± SEM). The dashed line (a–d) denotes approximate pain threshold (a–d). * denotes statistically significant differences (p<0.05).
Three-Temperature Paradigm
In the 20 seconds following a 1°C decrease in stimulus temperature from 50°C to 49°C, minimum pain intensity ratings were 72.2 ± 0.3% (mean±SEM) lower than minimum pain ratings evoked by constant thermal stimulation at 49°C (F(1, 11)=13.99, p<0.01; ANOVA. Fig. 7a). This analgesia lasted approximately 15 seconds. The decrease in pain intensity ratings evoked by this 1°C decrease in stimulus temperature (Fig. 7d) was sufficiently large to be statistically indistinguishable from the decrease in pain intensity ratings evoked by a 14°C decrease in stimulus temperature from 50° to 35°C (F(1, 11)=2.57, P<0.14; Fig. 7b).
Figure 7. The Magnitude and Duration of Offset Analgesia.
The continuous VAS ratings (averaged across all subjects) from each condition are shown together (overlay, a). In b-d, mean psychophysical ratings (thick lines) of each condition are shown at the bottom of the figure with their corresponding stimulus temperature trace (thin lines).
Paired Stimulus Paradigm
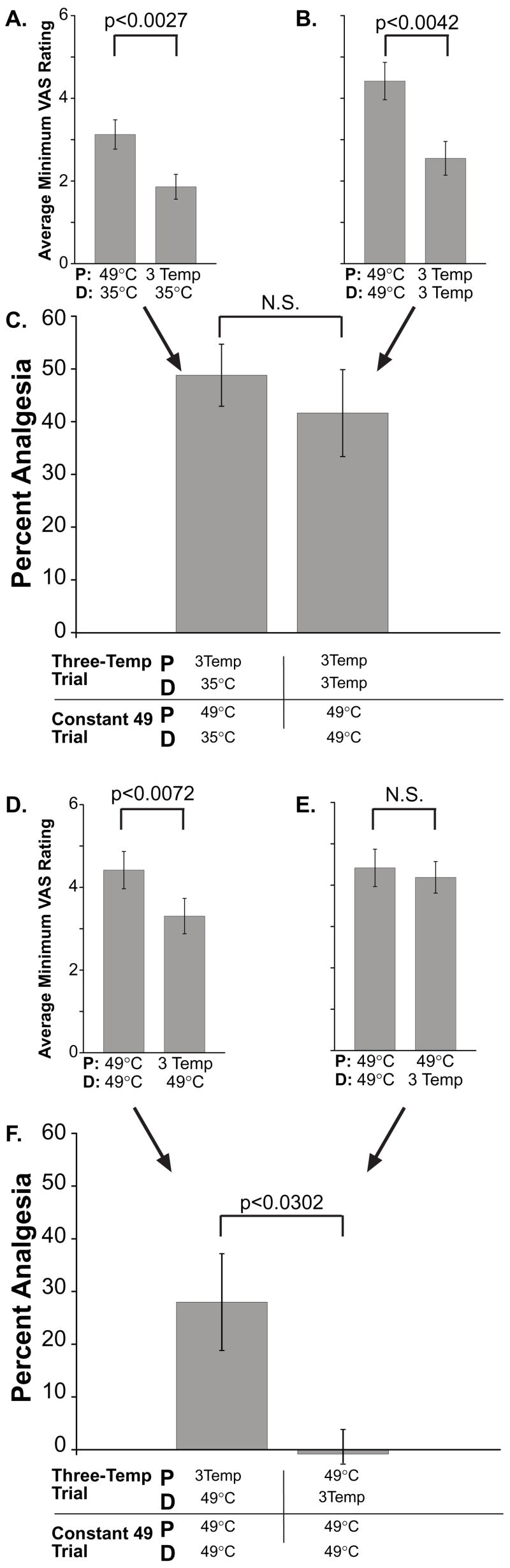
Spatial interactions between multiple noxious stimuli have long been known to be mediated, in part, by processes within the central nervous system (Price et al., 1989). In the present investigation, spatial summation of pain was observed when two probes were applied to the skin (49_49, Fig. 8B vs. 49_35, Fig. 8A; F(1, 8)=9.88, p<0.013 and 3T_3T, Fig. 8B vs. 3T_35, Fig. 8A; F(1,8)=8.76, p<0.02). If offset analgesia were centrally mediated, then offset analgesia evoked from two distinct areas might exhibit potentiation when contrasted with offset analgesia evoked from one smaller skin region (Fig. 8a–c). Pain ratings during control 2 (3T_35), following the T2 to T3 temperature decrease were 48.8 ± 5.9% lower than those from constant 49°C trial (Control 1, 49_35). This difference in minimum VAS ratings was statistically reliable (F(1, 8)=18.34, p<0.01, ANOVA; Fig. 8a). When offset analgesia was activated simultaneously by two probes (Condition 3a, 3T_3T) minimum VAS ratings following the T2 to T3 temperature shift were 41.6 ±8.2% less than pain ratings reported from two constant 49°C stimuli (Condition 3b, 49_49; F(1, 8)=15.6, p<0.01; Fig. 8b). Although offset analgesia was reliably activated when initiated by two probes, there was no potentiation of analgesia (F(1, 8)=1.04, p<0.34; Fig. 8a–c).
Figure 8. Interactions of Spatially Remote Stimuli with Offset Analgesia.
The minimum VAS ratings during the time period following the change from 50°C (T2) to 49°C (T3) were averaged across all subjects (A, B, D, & E, means ± SEM) for both the three temperature stimulus trains and from time matched locations for constant 49°C stimuli. Percent analgesia was calculated by dividing the minimum VAS from the three temperature stimulus train by the minimum VAS from the constant 49°C for each series within subjects (C & F, means, ± SEM). A–C. Offset analgesia evoked simultaneously at two sites (b) does not summate beyond that evoked at one site (a) since the percent analgesia (c) was not different between conditions. D–F. Offset analgesia evoked by a proximal stimulus inhibits pain evoked by a distal noxious stimulus (d). However, a proximal noxious stimulus abolishes offset analgesia evoked at a distal location (e). Examination of the percent analgesia confirms these spatial interactions are asymmetric (f). Since these complex spatial interactions cannot be readily explained by a peripheral mechanism, offset analgesia must be, in part, mediated by central mechanisms. Abbreviations: 49°C - 49°C 30 second stimulus. 35°C-35°C 30 second stimulus. 3Temp - 49°C for 5 seconds, followed by 50°C for 5 seconds, followed by 49°C for 20 seconds. D and P represent Distal and proximal, respectively. N.S. symbolizes “not significant.”
If offset analgesia were mediated within the central nervous system, it would also be expected that offset analgesia evoked at one area of skin would modulate pain evoked by a distinct stimulus at a remote area of skin. When subjects were presented with a proximal three temperature train paired with a distal constant 49°C stimulus (Condition 3a), VAS ratings following the T2 to T3 temperature decrease were statistically lower than two matched constant 49°C stimuli (F(1, 8)=12.84, p<0.02; Fig. 8d). Surprisingly, the same effect was not observed when a proximal 49°C stimulus was paired with a distal three temperature stimulus train (Condition 3b, F(1, 8)=0.92, p<0.37; Fig. 8e). In fact, minimum VAS ratings reported from condition 3a were significantly lower than those reported from condition 3b (F(1, 8)=7.72, p<0.02, ANOVA). It is important to note that stimulus energy deposition into the skin is identical in these two conditions. The percent analgesia was also significantly different between these two conditions when contrasted with matched constant 49°C stimuli (F(1, 8)=6.91, p<0.03; Fig. 8f). These data indicate that offset analgesia can modify perceived pain intensity from a remote distal stimulus and that offset analgesia can be abolished by a remote proximal noxious stimulus.
DISCUSSION
Spatio-temporal filtering is a ubiquitous component of signal processing across sensory modalities (Rose, 1986; Fortune and Rose, 2001) including vision (Hess and Snowden, 1992; Hosoya et al., 2005), audition (Sullivan, 1986; Frisina et al., 1994; Alder and Rose, 1998), olfaction (Sachse and Galizia, 2002; Wilson and Laurent, 2005; Rajan et al., 2006), gustation (Katz et al., 2001), and somatosensation (Mendell, 1966; Le Bars et al., 1992; Gabernet et al., 2005). To date, spatio-temporal filtering of nociceptive information has remained a poorly understood and minimally addressed aspect of basic pain mechanisms. In the present investigation, profound temporal transformations of nociceptive information were observed during decreasing stimulus intensities. The perceived rates of decreases in pain were far more rapid than those that would be predicted by the rates of decreases in stimulus temperatures (Fig. 5). Moreover, subjects perceived changes in stimulus intensity at a rate that was independent from the actual fall rate of the noxious thermal stimulus (Fig. 3 & 4). Taken together with observations of robust analgesia lasting approximately 15 seconds after a 1°C decrease in noxious stimulus intensity (Fig. 7), these data suggest that offset analgesia functions as an edge enhancement filter. Edge enhancement is commonly discussed in the processing of visuo-spatial information where contrast is enhanced by darkening and lightening opposing areas adjacent to the edge. In the case of offset analgesia, temporal contrast is enhanced by suppressing afferent nociceptive information following the temporal edge. This temporal edge enhancement appears to be activated in a nearly binary fashion, since offset analgesia was observed over a ten-fold range of temperature fall rates. This effect may result from the active engagement of central neural circuits or the intrinsic response properties of the primary afferents processing this information.
Perceptual Transformation of Decreases in Noxious Stimulus Intensity
Several studies have investigated neural and psychophysical responses to dynamic increases in noxious heat. Humans can detect incremental increases in noxious heat as small as 0.2°C (Robinson et al., 1983). In primates, wide-dynamic-range neural responses are correlated with the detection of 0.1–0.3°C increases in noxious heat (Maixner et al., 1986). Yet, little is known about perception of dynamic decreases in noxious heat.
Multiple, converging lines of evidence indicate that noxious thermal information is dramatically transformed during stimulus offset. First, the fall rates of perceived pain intensity were substantially faster than those that would be predicted from the stimulus temperature fall rates (Fig. 5, Table 2). Only at the fastest temperature fall rate (−5.0°C/s) did observed VAS fall rates begin to converge with those that would be predicted from stimulus fall rates. Second, not only were the fall rates of perceived pain intensity faster than predicted, they were also largely independent of stimulus fall rate (Fig. 3 and 4). Stimulus fall rates of −1.0, −2.0, and −3.5°C/s evoked pain intensity fall rates that were statistically indistinguishable from those evoked by the fastest stimulus fall rate (−5.0°C/s, Fig. 3 & 4). Only the slowest stimulus fall rate (−0.5°C/s) evoked perceived pain intensity fall rates which were slightly, yet reliably, slower than those evoked by stimulus fall rates of −5.0°C/s (Fig. 3f). Third, during slow temperature fall rates, the slope of the stimulus-response relationship of psychophysical ratings and stimulus intensity is much larger than that of fast temperature fall rates. Finally, during the slowest temperature fall rates, subjects’ pain ratings returned to zero while stimulus temperatures were clearly within the noxious range (Fig. 2, 3a–f, 4, and 6a & 6b). This result is particularly surprising, since the stimuli with the slowest fall rates were also the longest, and therefore, had the greatest energy deposition into the skin. When the time course of psychophysical ratings was corrected for conduction delays and evaluation times (i.e. response latency), subjects reported pain intensity ratings of zero for all stimulus fall rates while stimuli were still within the noxious range (i.e. > 43°C, Fig. 7b). Taken together these findings confirm that stimulus-response relationships are dynamically altered during decreases in stimulus intensity. Furthermore, the alteration of the time course of the percept from the time course of the stimulus provides clear evidence that offset analgesia serves as a filtering mechanism by temporally sharpening changes in noxious stimulus intensities.
Innocuous thermal information may be temporally sharpened in an analogous fashion. When a stimulus is maintained at an innocuously warm or cool temperature and then returned to skin temperature, paradoxical cooling or warming is reported (Greenspan et al., 1993; Harrison and Davis, 1999; Susser et al., 1999; Campero et al., 2001; Green and Pope, 2003; Davis et al., 2004). As such, these paradoxical sensations produce amplified awareness of temperature changes.
Potential Mechanisms of Offset Analgesia
As stimulus duration increases, decreases in pain intensity reported from constant 49°C stimuli likely result from primary afferent fatigue and adaptation (LaMotte and Campbell, 1978; LaMotte et al., 1983). However, pain intensity ratings following the T2–T3 shift were 77.6% lower than those of constant 49°C stimuli. Moreover, offset analgesia lasts for approximately 7.5 seconds before pain ratings begin to increase toward their expected values (Fig. 7a). These observations indicate that offset analgesia is distinct from primary afferent fatigue or adaptation (Treede, 1995, 1999).
Offset analgesia most likely involves a central component since complex spatial interactions were observed during the paired stimulus paradigm (Fig. 8d–f). When induced by a proximal stimulus, offset analgesia was able to modulate pain intensity evoked by a discrete distal stimulus. However, offset analgesia was not able to modulate pain from a proximal stimulus and was instead abolished. These asymmetric spatial interactions are not readily explained by peripheral mechanisms since the two heat probes were positioned 5cm apart and therefore activated distinct afferent fiber populations (Torebjork, 1974; LaMotte and Campbell, 1978; Van Hees and Gybels, 1981; Jorum et al., 1989; Raja et al., 1999). Moreover, it is unlikely that these asymmetric spatial interactions result from differential sensitivity of the forearm. When single 49°C stimuli are applied systematically along the length of the arm, pain intensity ratings do not reliably change according to proximal-distal stimulus location (Quevedo and Coghill, 2004, in press). However, during paired thermal stimulation, pain arising from distal sites is reduced (Quevedo and Coghill, 2004, in press). This finding is consistent with the distally directed analgesia following the T2 to T3 temperature decrease in the present investigation. The complex spatial interaction identified in this study shows that offset analgesia is modulated by the presence of other noxious stimuli within the same body region.
Offset analgesia appears to involve central mechanisms that produce analgesia in a spatially localized manner, since spatial summation of offset analgesia was not observed when two probes presented offset analgesia-inducing stimuli simultaneously. Inhibitory interconnections of the spinal substantia gelatinosa are highly selective and could support a highly localized inhibition of afferent information. For example, islet cells in the substantia gelatinosa have been demonstrated to provide a spatially restricted pathway through which large diameter C-fiber afferents can inhibit small diameter C-fiber effects on central cells (Lu and Perl, 2003). Supraspinal descending modulatory mechanisms involving the periaqueductal gray also may be involved (Mayer et al., 1971; Millan, 2002) since preliminary functional imaging studies have identified a transient activation of this region following incremental decreases in stimulus intensity (Strigo et al., 2004; Yelle et al., 2006b; Yelle et al., 2006a). In addition, ON/OFF cells in the medullary nucleus raphe magus (NRM) (Fields et al., 1983; Fields et al., 1991; Gao and Mason, 2000) could contribute to the activation of offset analgesia. Localized effects of endogenous pain control mechanisms have been identified in studies of placebo analgesia (Price et al., 1999). Strong and weak placebo manipulations applied to distinct yet closely adjacent sites on the forearm produced substantial and moderate reductions, respectively, in reported pain relative to a third control site. Offset analgesia is distinct from diffuse noxious inhibitory control (DNIC) since it is time-locked to the offset of a noxious stimulus whereas DNIC is activated by the onset of a remote noxious stimulus (Le Bars et al., 1979a, 1979b; Le Bars et al., 1992).
Disruption of Temporal Filtering of Nociceptive Information
Neuropathic pain patients often report that noxious mechanical and thermal stimuli elicit painful sensations that outlast the stimulus duration (Noordenbos, 1959; Lindblom and Tegner, 1985). Also, both normal subjects following heat/capsaicin treatment and pain patients with mechanical allodynia experience painful aftersensations when stroked with a cotton swab (Gottrup et al., 2003). Such aftersensations suggest that the temporal filtering of afferent information is substantially altered as a consequence of experimental allodynia and the pathophysiological changes that occur in the presence of chronic pain.
The microcircuitry of the dorsal horn and other CNS regions important in nociceptive processing remains poorly understood, but may be critically altered during chronic pain states (Sugimoto et al., 1990; Moore et al., 2002; Scholz et al., 2005). Spatio-temporal filtering can provide a conceptual framework for understanding the functional significance of excitatory and inhibitory interactions of this circuitry. Finally, elucidation of the temporal filtering mechanism that supports offset analgesia may reveal novel targets for the treatment of chronic pain.
Acknowledgments
Special thanks Matthew Belford for building our continuous VAS and to Peggy Mason for her helpful suggestion regarding experimental design. Research supported by DA20168 and NS39426.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marc D. Yelle, Wake Forest School of Medicine, Dept. of Neurobiology and Anatomy, Medical Center Blvd., Winston-Salem, NC 27157, myelle@wfubmc.edu
June M. Rogers, Wake Forest School of Medicine, Dept. of Neurobiology and Anatomy, Medical Center Blvd., Winston-Salem, NC 27157
Coghill Robert C., Wake Forest School of Medicine, Dept. of Neurobiology and Anatomy, Medical Center Blvd., Winston-Salem, NC 27157, rcoghill@wfubmc.edu, 336.716.0302 (Office), 336.716.4534 (Fax).
References
- Alder TB, Rose GJ. Long-term temporal integration in the anuran auditory system. Nat Neurosci. 1998;1:519–523. doi: 10.1038/2237. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M, Stevens SS. Neural events and psychophysical law. Science. 1971;172:502. doi: 10.1126/science.172.3982.502. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Taylor MB, Duncan GH, Dubner R. Discrimination of innocuous and noxious thermal stimuli applied to the face in human and monkey. Somatosens Res. 1983;1:119–129. doi: 10.3109/07367228309144544. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze S, Duclaux R. Thermal pain in humans: influence of the rate of stimulation. Brain Res. 1978;157:418–421. doi: 10.1016/0006-8993(78)90053-7. [DOI] [PubMed] [Google Scholar]
- Davis KD, Pope GE, Crawley AP, Mikulis DJ. Perceptual illusion of “paradoxical heat” engages the insular cortex. J Neurophysiol. 2004;92:1248–1251. doi: 10.1152/jn.00084.2004. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci. 2001;24:381–385. doi: 10.1016/s0166-2236(00)01835-x. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Walton JP, Karcich KJ. Dorsal cochlear nucleus single neurons can enhance temporal processing capabilities in background noise. Exp Brain Res. 1994;102:160–164. doi: 10.1007/BF00232448. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Serotonergic Raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol. 2000;84:1719–1725. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- Gardner EPKE. Touch. In: Kandel ERSJ, Jessell TM, editors. Principles of Neural□Science. 4. McGraw-Hill; 2000. pp. 451–471. [Google Scholar]
- Gottrup H, Kristensen AD, Bach FW, Jensen TS. Aftersensations in experimental and clinical hypersensitivity. Pain. 2003;103:57–64. doi: 10.1016/s0304-3959(02)00415-3. [DOI] [PubMed] [Google Scholar]
- Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Taylor DJ, McGillis SL. Body site variation of cool perception thresholds, with observations on paradoxical heat. Somatosens Mot Res. 1993;10:467–474. doi: 10.3109/08990229309028851. [DOI] [PubMed] [Google Scholar]
- Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol. 2002;87:2205–2208. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- Harrison JL, Davis KD. Cold-evoked pain varies with skin type and cooling rate: a psychophysical study in humans. Pain. 1999;83:123–135. doi: 10.1016/s0304-3959(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Snowden RJ. Temporal properties of human visual filters: number, shapes and spatial covariation. Vision Res. 1992;32:47–59. doi: 10.1016/0042-6989(92)90112-v. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- Jorum E, Lundberg LE, Torebjork HE. Peripheral projections of nociceptive unmyelinated axons in the human peroneal nerve. J Physiol. 1989;416:291–301. doi: 10.1113/jphysiol.1989.sp017761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Nicolelis MA. Dynamic and multimodal responses of gustatory cortical neurons in awake rats. J Neurosci. 2001;21:4478–4489. doi: 10.1523/JNEUROSCI.21-12-04478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Koyama T, Kroncke AP, Coghill RC. Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. Pain. 2004;107:256–266. doi: 10.1016/j.pain.2003.11.007. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. J Neurophysiol. 1983;50:1–26. doi: 10.1152/jn.1983.50.1.1. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979a;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979b;6:305–327. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Villanueva L, Bouhassira D, Willer JC. Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol Fiziol Eksp Ter. 1992:55–65. [PubMed] [Google Scholar]
- Lindblom U, Tegner R. Thermal sensitivity in uremic neuropathy. Acta Neurol Scand. 1985;71:290–294. doi: 10.1111/j.1600-0404.1985.tb03202.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Dubner R, Bushnell MC, Kenshalo DR, Jr, Oliveras JL. Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys perceive the intensity of noxious heat stimuli. Brain Res. 1986;374:385–388. doi: 10.1016/0006-8993(86)90435-x. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses Of Single Dorsal Cord Cells To Peripheral Cutaneous Unmyelinated Fibres. Nature. 1965;206:97–99. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VBD-SI. Neural mechanisms in somesthesia. In: VB M, editor. Medical Physiology. 12. St. Louis: Mosby; 1968. pp. 1372–1423. [Google Scholar]
- Noordenbos W. Pain. Amsterdam: Elsevier; 1959. [Google Scholar]
- Price DD, McHaffie JG, Larson MA. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 1989;62:1270–1279. doi: 10.1152/jn.1989.62.6.1270. [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Quevedo AS, Coghill RC. Spatial interactions between multiple painful stimuli. American Pain Society Annual Meeting; 2004. p. 32. [Google Scholar]
- Quevedo AS, Coghill RC. An illusion of proximal radiation of pain due to distally directed inhibition. J Pain. doi: 10.1016/j.jpain.2006.09.003. in press. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Ringkamp M, Campbell JN. Peripheral Neural Mechanisms of Nociception. In: Wall PD, Melzack R, editors. Textbook of Pain. 4. Edinburgh: Churchill Livingstone; 1999. pp. 11–57. [Google Scholar]
- Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Torebjork HE, LaMotte RH. Psychophysical detection and pain ratings of incremental thermal stimuli: a comparison with nociceptor responses in humans. Brain Res. 1983;274:87–106. doi: 10.1016/0006-8993(83)90523-1. [DOI] [PubMed] [Google Scholar]
- Rose G. A temporal-processing mechanism for all species? Brain Behav Evol. 1986;28:134–144. doi: 10.1159/000118698. [DOI] [PubMed] [Google Scholar]
- Sachse S, Galizia CG. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol. 2002;87:1106–1117. doi: 10.1152/jn.00325.2001. [DOI] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS. On the psychophysical law. Psychol Rev. 1957;64:153–181. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- Stevens SS. Neural events and the psychophysical law. Science. 1970;170:1043–1050. doi: 10.1126/science.170.3962.1043. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Backlund H, Olausson H, Bennett GJ, Bushnell MC. Neural Mechanisms of Offset Analgesia. 34th Society for Neuroscience Annual Meeting.2004. [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- Sullivan WE. Processing of acoustic temporal patterns in barn owls and echolocating bats: similar mechanisms for the generation of neural place representations of auditory space. Brain Behav Evol. 1986;28:109–121. doi: 10.1159/000118696. [DOI] [PubMed] [Google Scholar]
- Susser E, Sprecher E, Yarnitsky D. Paradoxical heat sensation in healthy subjects: peripherally conducted by A delta or C fibres? Brain. 1999;122(Pt 2):239–246. doi: 10.1093/brain/122.2.239. [DOI] [PubMed] [Google Scholar]
- Torebjork HE. Afferent C units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiol Scand. 1974;92:374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Treede RD. Peripheral acute pain mechanisms. Ann Med. 1995;27:213–216. doi: 10.3109/07853899509031961. [DOI] [PubMed] [Google Scholar]
- Treede RD. Transduction and transmission properties of primary nociceptive afferents. Ross Fiziol Zh Im I M Sechenova. 1999;85:205–211. [PubMed] [Google Scholar]
- Van Hees J, Gybels J. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry. 1981;44:600–607. doi: 10.1136/jnnp.44.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–108. [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Studies of heat pain sensation in man: perception thresholds, rate of stimulus rise and reaction time. Pain. 1990;40:85–91. doi: 10.1016/0304-3959(90)91055-N. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Simone DA, Dotson RM, Cline MA, Ochoa JL. Single C nociceptor responses and psychophysical parameters of evoked pain: effect of rate of rise of heat stimuli in humans. J Physiol. 1992;450:581–592. doi: 10.1113/jphysiol.1992.sp019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelle MD, Kraft RA, Coghill RC. Supraspinal mechanisms associated with offset analgesia. 25th American Pain Society Annual Meeting; San Antonio, TX USA. 2006a. [Google Scholar]
- Yelle MD, Kraft R, Oshiro Y, Coghill RC. Neural correlates to offset analgesia. 12th Organization for Human Brain Mapping Annual Meeting; Florence, Italy. 2006b. [Google Scholar]