Abstract
Aim
To evaluate and compare effects of 48-week treatment with rosiglitazone and metformin on insulin resistance in patients infected with Human Immunodeficiency Virus (HIV) receiving highly active antiretroviral therapy (HAART), containing a protease inhibitor.
Methods
Randomized prospective controlled clinical trial enrolled 90 male patients infected with HIV and having impaired glucose tolerance and insulin resistance (fasting insulin concentration >20 mIU/L). The patients were randomly assigned into three groups (each 30 patients); the first group receiving 4 mg rosiglitazone once a day, the second group receiving 500 mg metformin two times a day, and the third group serving as control without hypoglycemic treatment. The primary efficacy parameters were fasting plasma glucose and insulin levels compared between baseline and week. Data on insulin resistance and beta cell function were analyzed by the Homeostasis Model Assessment (HOMA).
Results
After 48 weeks of treatment, the fasting insulin concentration (±standard deviation) in rosiglitazone group significantly declined from 39.0 ± 3.35 to 19.7 ± 3.99 mIU/L (P<0.001; 49% decrease) and in metformin group from 40.3 ± 2.29 to 29.2 ± 2.82 mIU/L (P<0.001; 27% decrease). HOMA indicated that rosiglitazone significantly reduced insulin resistance from 11.3 ± 1.03 to 4.0 ± 0.95 (P<0.001), compared with metformin which reduced it from 11.9 ± 0.73 to 5.7 ± 0.62 (P<0.001). Insulin resistance was significantly lower in the rosiglitazone group after 48 weeks (P<0.001). Metformin significantly improved beta cell function (from 257.3 ± 21.91 to 707.4 ± 207.32; P<0.001), as did rosiglitazone (from 261.3 ± 27.98 to 403.3 ± 162.50; P<0.001), but the improvement in the metformin group was significantly better (P<0.001). However, metformin was more efficient in improving beta cell function (from 257.3 ± 21.91 to 707.4 ± 207.32) than rosiglitazone (from 261.3 ± 27.98 to 403.3 ± 162.50) .
Conclusions
Both rosiglitazone and metformin were effective and well tolerated in HIV patients, treated with protease inhibitor-containing HAART. Rosiglitazone significantly more reduced insulin resistance, while beta cell function was significantly better in patients on metformin. Both drugs may be considered as an appropriate therapy, with rosiglitazone being a better alternative in treating insulin resistance in this patient population.
ClinicalTrials.gov trial registration number: NCT00483392.
The introduction of Highly Active Antiretroviral Therapy (HAART) has significantly improved the quality of life and life expectancy of patients infected with Human Immunodeficiency Virus (HIV). However, the use of HIV protease inhibitor (PI)-containing HAART regimens is associated with insulin resistance, dyslipidaemia, and lipodystrophy syndrome (1). Although the incidence for PI-associated, new-onset diabetes mellitus has been in the range of 1-7% (2,3), the prevalence of glucose intolerance and insulin resistance may be substantially higher (4,5). Murata et al (6) reported that PI drugs block the transport of glucose via the glucose transporter-4 (GLUT-4), thus giving a direct demonstration of the pathogenetic role of PIs in the development of iatrogenic diabetes mellitus. Recent data have demonstrated that these may predispose to early coronary artery disease, accelerating cardiovascular mortality in HIV patients (7,8).
Treatment with insulin sensitizers can ameliorate PI-associated insulin resistance (9). Rosiglitazone maleate, a member of the thiazolidinedione class of antidiabetic agents, targets insulin resistance by binding to the transcription factor peroxisome proliferator-activated receptor-gamma, promoting the synthesis of glucose transporters which are involved in insulin-mediated glucose transport in peripheral tissues (10,11). In contrast, metformin hydrochloride promotes glucose lowering by reducing hepatic glucose production and gluconeogenesis and by enhancing peripheral glucose uptake (12-14). The aim of the study was to compare the effects of treatment with rosiglitazone and metformin on insulin resistance in HIV patients receiving PI-containing HAART.
Patients and methods
Patients
The study population consisted of male patients with documented HIV infection and stable PI-containing HAART regimen. During the period from August 2004 and October 2005, 95 patients were recruited in our Diabetes Center at University Medical Centre Ljubljana. Patients were eligible based on the following inclusion criteria: documented HIV infection, age between 18 and 60 years, stable PI-containing HAART regimen for at least 12 months prior to study enrolment, impaired glucose tolerance (IGT) confirmed by Oral Glucose Tolerance Test (OGTT) with insulin resistance characterized by fasting insulin concentration greater than 20 mIU/L. The exclusion criteria were the following: heart failure (New York Heart Association [NYHA] I-IV), liver or kidney diseases, elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) above two times upper normal range or creatinine above 150 mmol/L or patients already taking insulin or oral hypoglycemic therapy.
Study design
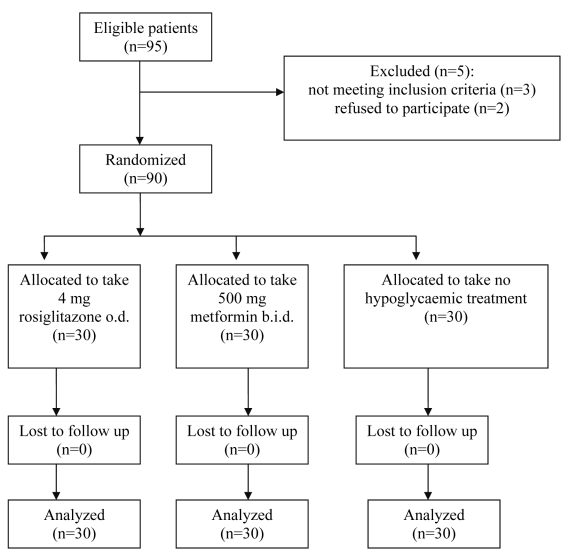
This was a prospective, randomized, open-label, controlled study comparing two different treatments for insulin resistance induced by HAART therapy in HIV-infected patients. Patients were receiving a protease inhibitor (indinavir, nelfinavir, lopinavir, ritonavir) and two nucleoside analogues (lamivudine, stavudine, zidovudine, or didanosine) as a part of the HAART regimen. Three patients did not meet the inclusion criteria (fasting insulin concentration below 20 mIU/L) and 2 patients refused to participate. Ninety eligible patients were randomly divided into three groups: group one was assigned to receive rosiglitazone maleate, 4 mg once per day (rosiglitazone group, n = 30), group two metformin hydrochloride, 500 mg twice a day (metformin group, n = 30) and group three was not given any hypoglycemic treatment (control group, n = 30) (Figure 1). The control group did not take any hypoglycemic treatment, so the number of pills patients were taking was different in the three groups. Randomization was performed using random number generated through a computer program (40 numbers were generated for each group). The numbers were put in similar envelops and, after obtaining elevated glucose and insulin results, the whole procedure was explained to the patients and, if agreed, they were allowed to choose a single envelope. The randomization process, drug prescription, and allocation key were kept by one author who did not have any role in the patient follow-up or outcome assessment. Allocation key was opened after obtaining the statistical results. All subjects gave their written informed consent before entering the study, which was conducted in accordance with the Declaration of Helsinki and approved by the Slovenian Ethical Committee, under the number 94/06/04 and the title “Treatment of hyperglycaemia and insulin resistance in HIV infected patients.”
Figure 1.
Flow of the patients through the study. Abbreviations: o.d. – once a day; b.i.d. – two times a day.
Laboratory measurements
Plasma HIV RNA concentrations (HIV viral load) were determined by the Amplicor HIV-1 Monitor assay (Roche Molecular Systems, Branchburg, NJ, USA); results below the limit of detection were assigned a value of <20 copies/mL plasma. CD4-lymphocytes counts (CD4 cell count) were measured by three-color flow cytometry and hematology analyzers.
Normal and impaired glucose tolerance were defined according to 2004 American Diabetes Association guidelines (15) – fasting blood glucose of less than 6.1 mmol/L and 6.1-6.9 mmol/L, respectively, or blood glucose 2 hours after glucose-tolerance test (75 g) of less than 7.8 mmol/L and 7.8-11.0 mmol/L, respectively. For purposes of clarity, patients with impaired fasting glucose (6.1-6.9 mmol/L) were defined as having impaired glucose tolerance. Fasting plasma glucose was measured by the hexokinase method with an Olympus Corp. Analyzer (New Hyde Park, NY, USA). Basal insulin levels were determined using a commercial radioimmunoassay method (Boehringer, Mannheim, Germany).
Follow-up and outcome
Patients were seen at the diabetes clinic every 3-6 months during the period of 48 weeks. At each follow-up visit, glucose laboratory measurements were performed, compliance with the medications was obtained, and possible side effects were determined. Detailed clinical examinations and all laboratory tests were performed at baseline and after 48 weeks. The primary outcome measures were fasting plasma glucose and insulin levels, which were used to calculate insulin resistance and beta cell function by HOMA.
Statistical analysis
Efficacy analyses were performed for the intention to treat population, defined as all randomized patients who had at least one on therapy value. The safety parameters were assessed based on data collected at week 48. Estimates of insulin resistance and beta cell function were derived from fasting glucose and insulin concentrations using the HOMA, a mathematical model that relates fasting blood glucose and insulin levels to insulin resistance (IR) and beta cell function (BCF): IR = [fasting insulin (mIU/L) × fasting glucose (mmol/L)]/22.5; and BCF = [20 × fasting insulin (mIU/L)]/[fasting glucose (mmol/L) - 3.5]. HOMA estimates of insulin resistance and beta cell function were validated by comparison with the results of glucose clamp studies (16). Estimates of insulin resistance and beta cell function were calculated for all patients at baseline and after 48 weeks. Treatment groups were compared with baseline and between groups, using analysis of variance. Significance of difference between means of groups before and after treatment was analyzed within each parameter by Tukey HSD test. Statistical analyses were performed using the Statistical Package for the Social Sciences, version 14.0 (SPSS, Inc., Chicago, IL, USA). A P value of <0.05 was considered to indicate statistical significance.
Results
Patient population
Ninety male patients infected with HIV and receiving PI-containing HAART regimen were included in this prospective study and completed the 48 weeks protocol. Table 1 shows the means of clinical characteristics of the patients in each treatment group at baseline. Mean age ± standard deviation (SD) of the study population was 42.3 ± 10.7 years, mean duration of HIV infection was 7.4 ± 1.9 years, whereas mean duration of HAART therapy was 3.1 ± 1.6 years. There was a significant difference in duration of HIV infection between the groups (P<0.001). The means of all clinical characteristics of the patients in each treatment group at baseline and after 48 week protocol are shown in Table 2. All three groups matched for weight, body mass index (BMI), viral load, and CD4 cell counts at baseline and after treatment. However, there was a significant difference in BMI (P<0.020) between the rosiglitazone and metformin group at week 48. The difference in viral load (P<0.004) confirmed with two way ANOVA and consecutively analyzed with Tukey HSD test, confirmed that the differences between the groups after 48 weeks were not truly significant due to the higher baseline value in metformin than in rosiglitazone (P = 0.015) and control (P = 0.007) group. The compliance with the medications in all treatment groups (protease inhibitor, nucleoside analogues, metformin, rosiglitazone) was optimal and there were no lost patients during the follow up and all 90 patients completed the study protocol.
Table 1.
Baseline clinical characteristics (mean ± standard deviation) in patients receiving rosiglitazone, metformin, and no hypoglycemic treatment
| Treatment with |
||||
|---|---|---|---|---|
| Characteristic (years) | rosiglitazone (n = 30) | metformin (n = 30) | control (n = 30) | P* |
| Age |
41.5 ± 11.4 |
42.1 ± 9.8 |
43.4 ± 10.9 |
0.782 |
| Duration of HIV infection |
8.4 ± 1.4 |
7.8 ± 2.7 |
6.0 ± 1.9 |
<0.001 |
| Duration of treatment | 3.4 ± 1.2 | 3.1 ± 2.1 | 2.8 ± 1.6 | 0.386 |
*One way ANOVA.
Table 2.
Clinical characteristics (mean ± standard deviation) in patients receiving rosiglitazone, metformin, and no hypoglycemic treatment*
| Treatment with |
P† |
|||||
|---|---|---|---|---|---|---|
| Characteristic | rosiglitazone (n = 30) | metformin (n = 30) | control (n = 30) | time | treatment | interaction |
| Weight (kg): |
0.691 |
0.453 |
0.074 |
|||
| baseline |
71.4 ± 8.6 |
72.8 ± 6.4 |
73.1 ± 8.4 |
|||
| week 48 |
75.2 ± 7.3 |
70.6 ± 4.9 |
72.8 ± 7.6 |
|||
| BMI (kg/m2): |
1.000 |
0.020 |
0.146 |
|||
| baseline |
24.2 ± 3.6 |
23.8 ± 2.8 |
24.7 ± 3.1 |
|||
| week 48 |
25.4 ± 3.1 |
22.9 ± 2.4 |
24.4 ± 2.9 |
|||
| CD4 cell count (cells/mm3): |
0.183 |
0.454 |
0.449 |
|||
| baseline |
380 ± 100 |
364 ± 211 |
315 ± 119 |
|||
| week 48 |
395 ± 140 |
370 ± 221 |
388 ± 105 |
|||
| HIV RNA (copies/mL): |
0.829 |
0.004 |
0.691 |
|||
| baseline |
7450 ± 4291 |
9686 ± 3265 |
7850 ± 3851 |
|||
| week 48 | 8190 ± 3765 | 9725 ± 4157 | 7430 ± 2791 |
*Abbreviations: BMI – body mass index; P time – P value for main factor time in two way ANOVA (between baseline and week 48); P treatment – P value for main factor treatment in two way ANOVA (between different treatments); P interaction – P value for interaction of both factors in two way ANOVA.
†Two way ANOVA.
Adverse events
Both drugs were well tolerated and, although there were a few clinically minor adverse events, they did not lead to discontinuation of the treatment in any of the patients. Four subjects in the rosiglitazone group complained about mild headaches and 6 subjects in the metformin group had temporary mild gastrointestinal problems. No elevation of liver enzymes was found and no case of edema was reported in the rosiglitazone group.
Glycemic control
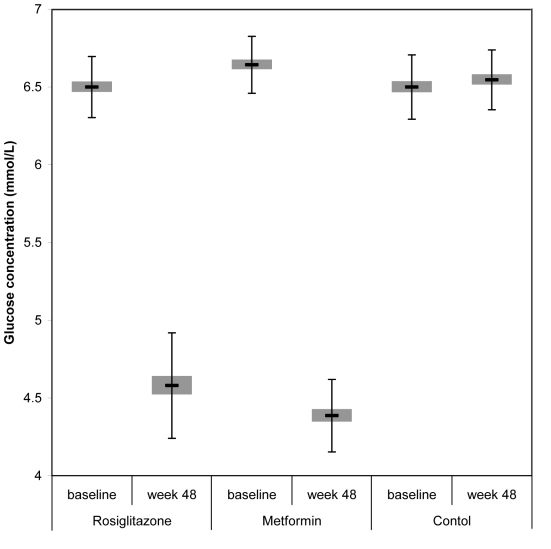
After 48 weeks of treatment mean fasting plasma glucose concentration in rosiglitazone group decreased from 6.5 ± 0.20 to 4.6 ± 0.34 mmol/L (P<0.001) and in metformin group from 6.6 ± 0.18 to 4.4 ± 0.23 mmol/L (P<0.001), compared with 6.5 ± 0.21 mmol/L in the control group (P<0.001). There was also a significant change between rosiglitazone and metformin group at the end of the study (P = 0.015) (Table 3, Figure 2).
Table 3.
Changes in study variables (means ± standard deviation) between baseline and 48th week in patients receiving rosiglitazone, metformin, and no hypoglycemic treatment
| Variable | Treatment with rosiglitazone (n = 30) | P rosiglitazone/metformin* | Treatment with metformin (n = 30) | control (n = 30) | P* control/rosiglitazone | P* control/metformin |
|---|---|---|---|---|---|---|
| Fasting glucose (mmol/L): |
||||||
| baseline (t = 0) |
6.5 ± 0.20 |
0.156 |
6.6 ± 0.18 |
6.5 ± 0.21 |
1.000 |
0.156 |
| week 48 |
4.6 ± 0.34 |
0.015 |
4.4 ± 0.23 |
6.5 ± 0.19 |
<0.001 |
<0.001 |
|
P* |
<0.001 |
<0.001 |
0.971 |
|||
| Fasting insulin (mIU/l): |
||||||
| baseline (t = 0) |
39.0 ± 3.35 |
0.663 |
40.3 ± 2.29 |
39.0 ± 3.54 |
1.000 |
0.663 |
| week 48 |
19.7 ± 3.99 |
<0.001 |
29.2 ± 2.82 |
39.7 ± 3.35 |
<0.001 |
<0.001 |
|
P* |
<0.001 |
<0.001 |
0.962 |
|||
| Beta cell function: |
||||||
| baseline (t = 0) |
261.3 ± 28.0 |
1.000 |
257.3 ± 21.9 |
261.3 ± 28.8 |
1.000 |
1.000 |
| week 48 |
403.3 ± 162.5 |
<0.001 |
707.4 ± 207.3 |
261.7 ± 26.7 |
<0.001 |
<0.001 |
|
P* |
<0.001 |
<0.001 |
1.000 |
|||
| Insulin resistance: |
||||||
| baseline (t = 0) |
11.3 ± 1.03 |
0.102 |
11.9 ± 0.73 |
11.3 ± 1.10 |
1.000 |
0.103 |
| week 48 |
4.0 ± 0.95 |
<0.001 |
5.7 ± 0.62 |
11.6 ± 1.06 |
<0.001 |
<0.001 |
| P* | <0.001 | <0.001 | 0.845 | |||
*Repeated measures ANOVA (Post hoc Tukey HSD test).
Figure 2.
Mean fasting glucose levels at baseline and at week 48 in patients taking rosiglitazone, metformin, and no hypoglycemic treatment. Error bars indicate standard deviation.
Effect on fasting insulin levels
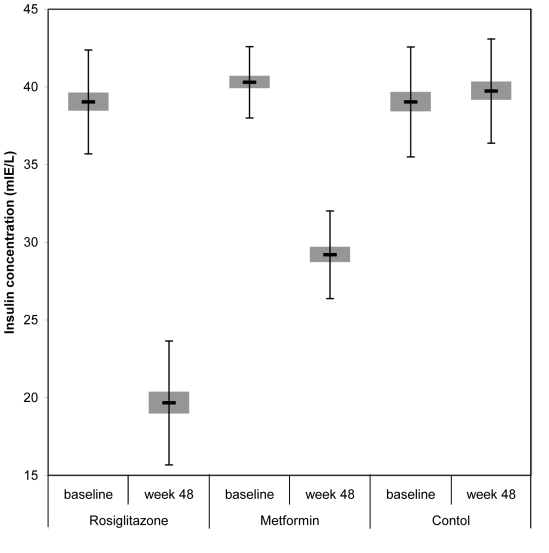
All the study groups had high basal fasting insulin levels, above the published normal range for the assay (5-20mIU/L). After 48 weeks of treatment, the fasting insulin concentration group decreased in rosiglitazone from 39.0 ± 3.35 to 19.7 ± 3.99 mIU/L (P<0.001) and in metformin group from 40.3 ± 2.29 to 29.2 ± 2.82 mIU/L (P<0.001). There was also a significant decrease in fasting insulin in rosiglitazone compared with metformin (P<0.001), and control group (P<0.001) (Table 3, Figure 3).
Figure 3.
Mean fasting insulin levels at baseline and at week 48 in patients taking rosiglitazone, metformin, and no hypoglycemic treatment. Error bars indicate standard deviation.
Effects on beta cell function and insulin resistance
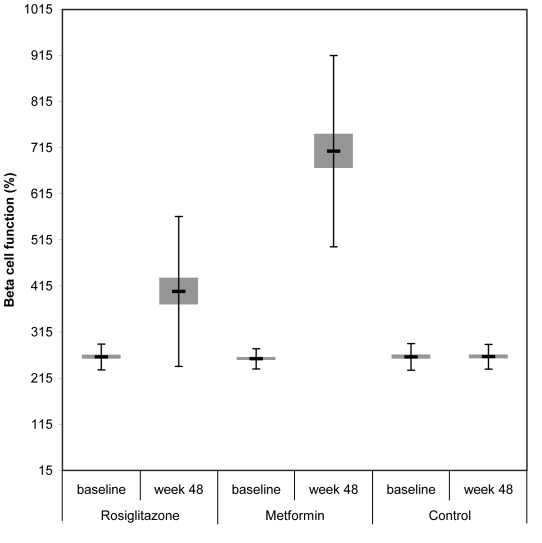
HOMA estimates showed a significant mean increase in beta cell function and reduction in insulin resistance among rosiglitazone and metformin treated patients. At 48 weeks patients treated with rosiglitazone showed an increase in estimated beta cell function from 261.3 ± 27.98 to 403.3 ± 162.50 (P<0.001). Also, patients on metformin showed an increase in beta cell function from 257.3 ± 21.91 to 707.4 ± 207.32 (P<0.001). The difference in the increase in beta cell function between rosiglitazone, metformin, and control group was statistically significant (P<0.001), indicating that metformin significantly better improved beta cell function than rosiglitazone (P<0.001) (Table 3, Figure 4).
Figure 4.
Beta cell function at baseline and at week 48 in patients taking rosiglitazone, metformin, and no hypoglycemic treatment. Error bars indicate standard deviation.
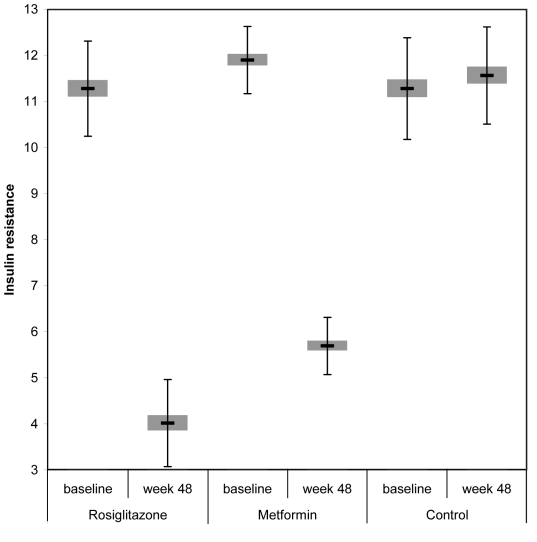
HOMA estimates of insulin resistance showed a reduction from 11.3 ± 1.03 to 4.0 ± 0.95 in rosiglitazone group (P<0.001) and 11.9 ± 0.73 to 5.7 ± 0.62 in metformin group (P<0.001), as well as a significant reduction for both groups compared with controls (P<0.001). In the control group, estimated insulin resistance increased after 48 weeks but was not statistically significant (P = 0.845). The decrease in insulin resistance was significantly lower in rosiglitazone group, as compared with metformin group (P<0.001) (Table 3, Figure 5).
Figure 5.
Insulin resistance at baseline and at week 48 in patients taking rosiglitazone, metformin, and no hypoglycemic treatment. Error bars indicate standard deviation.
Discussion
Abnormalities of glucose regulation, including impaired glucose tolerance and insulin resistance, are often seen among HIV infected patients receiving PI containing HAART regimens (17,18). Two studies involving HIV seronegative individuals have shown that HIV infection is not a prerequisite condition for the effects of PI on glucose metabolism (19,20). Insulin resistance occurs early after the initiation of PI-containing regimen in HIV infected patients. While diet and exercise remain the cornerstone of therapy for insulin resistance, pharmacological intervention is becoming an increasingly viable option (21). Rosiglitazone and metformin are currently under investigation in vivo as drugs with potential for improvement insulin resistance in HIV-infected patients with abnormal glucose homeostasis.
An investigation of 90 male patients in our study on PI-containing HAART who received metformin, rosiglitazone, or no hypoglycemic treatment found different effects on insulin resistance. Both drugs improved glycemic control, but the improvement was significantly better in metformin than in rosiglitazone group. They also varied in their ability to reduce insulin resistance. The decrease in fasting insulin was significantly higher in rosiglitazone group than in metformin group. In this study, HOMA estimates of beta cell function indicated that rosiglitazone reduced insulin resistance significantly better than metformin, while metformin improved beta cell function significantly better than rosiglitazone. The improvement in beta cell function observed in both therapies is probably secondary to the increased insulin sensitivity and the concomitant decrease in hyperglycemia.
The preliminary results with metformin in patients receiving effective PI therapy were encouraging, with treatment significantly reducing fasting insulin and glucose concentrations, but having no effect on percentage of body and visceral fat (22). Hadingan et al (23) conducted a randomized double-blind placebo-controlled trial of metformin in HIV-infected patients with abnormal glucose tolerance tests, and at three-month follow up found not only a significant reduction in insulin resistance but also a reduction in visceral fat and body weight in the treatment group compared with the placebo group. Concerning metformin, caution is important because of possible lactacidosis, complicating combination therapy with metformin and nucleoside analogues (14). However, limited data are also available on tolerability, safety, and efficacy of glitazone agents in HIV infection. Initial study with troglitazone showed improvements in insulin sensitivity in over a three-month period of the follow up (24). However, troglitazone has now been withdrawn due to concerns over liver function tests abnormalities (25). For newer agents like rosiglitazone, there are not enough data on their efficacy and tolerability in HIV infected patients. Sutinen et al (11) conducted a clinical controlled study, where patients with HAART-associated lipodystrophy received rosiglitazone or placebo for 24 weeks. Rosiglitazone had no effect on morphologic parameters; however, it decreased serum insulin concentrations and percentage of liver fat analyzed by spectroscopy. Gelato et al (26) also demonstrated improved insulin sensitivity and body fat distribution in HIV-infected patients treated with rosiglitazone. On the other hand, Feldt et al (27) found moderate clinical efficacy of rosiglitazone, showing a trend toward improved insulin sensitivity but unfavorable increase in serum cholesterol and triglycerides. Van Wijk et al (28) conducted a comparison of rosiglitazone and metformin for treating HIV lipodystrophy and indicated the importance of individual patient evaluation. Rosiglitazone may partly correct lipoatrophy, while metformin improves visceral fat accumulation, fasting lipid profile, and endothelial function.
We also observed a significant difference in BMI between rosiglitazone and metformin group after 48 weeks. This confirms a more favorable effect of metformin on body weight. Little is still known about safety and pharmacological interaction of antiretroviral drugs with metformin or rosiglitazone with antiretroviral drugs. According to our trial, both drugs were efficient and safe. Concerning drug interactions, we have not measured antiretroviral drug concentrations in the blood of our patient population, but there was no decrease in CD4 counts or increase in viral loads in the 48-week follow up. Since concomitant use of antiretroviral drugs and drugs used in the treatment of the metabolic complications of HIV is increasing, only well performed drug-drug interaction studies under steady state conditions for all drugs involved will give us a definite answer in terms of safety and efficacy of concomitant therapy.
Limitations of our study, such as relatively small sample size, short duration, measuring only surrogate end points without monitoring the lipid profiles and fat accumulation prevent us from extrapolating on the utility of rosiglitazone and metformin in the large population of HIV-infected patients.
However, rosiglitazone decreased insulin resistance significantly more than metformin. A recently published meta-analysis of studies of rosiglitazone (29), showing a significant increase in the risk of myocardial infarction and death from cardiovascular causes, has implied careful monitoring of patients receiving glitazones for insulin resistance.
Because of increased survival of HIV infected patients, PI-induced development of diabetes mellitus is of potential concern, as it is closely associated with an increased risk for cardiovascular disease. Insulin resistance may increase the risk of coronary heart disease among this population of patients. Taken together, our findings on metformin and rosiglitazone suggested their beneficial effect on abnormalities in glucose homeostasis and insulin resistance and could be recommended for the use in clinical practice. It remains to be demonstrated whether restoring insulin levels to normal in these patients will reduce the risk of coronary heart diseases. Metabolic effects of antiretroviral drugs, together with family histories of diabetes and/or hyperlipidemia, also need to be considered when planning strategies for sequential regimens for inhibiting HIV.
Acknowledgment
This work was supported by Slovenian Research Agency, Slovenia (Research project No.J3-6298).
References
- 1.Fantoni M, Del Borgo C, Autore C. Evaluation and management of metabolic and coagulative disorders in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2003;17(Suppl 1):S162–9. doi: 10.1097/00002030-200304001-00020. [DOI] [PubMed] [Google Scholar]
- 2.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050–6. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 3.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 4.Vigouroux C, Gharakhanian S, Salhi Y, Nguyęn TH, Adda N, Rozenbaum W, et al. Adverse metabolic disorders during highly active antiretroviral treatments (HAART) of HIV disease. Diabetes Metab. 1999;25:383–92. [PubMed] [Google Scholar]
- 5.Jain RG, Furfine ES, Pedneault L, White AJ, Lenhard JM. Metabolic complications associated with antiretroviral therapy. Antiviral Res. 2001;51:151–77. doi: 10.1016/S0166-3542(01)00148-6. [DOI] [PubMed] [Google Scholar]
- 6.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 7.Mooser V. Atherosclerosis and HIV in the highly active antiretroviral therapy era: towards an epidemic of cardiovascular disease? AIDS. 2003;17(Suppl 1):65–9. doi: 10.1097/00002030-200301030-00009. [DOI] [PubMed] [Google Scholar]
- 8.Behrens GM, Meyer-Olson D, Stoll M, Schmidt RE. Clinical impact of HIV-related lipodystrophy and metabolic abnormalities on cardiovascular disease. AIDS. 2003;17(Suppl 1):S149–54. doi: 10.1097/00002030-200304001-00018. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt HH. Where does insulin resistance in lipodystrophic HIV-1-positive subjects come from? AIDS. 2001;15:2187–8. doi: 10.1097/00002030-200111090-00015. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270:12953–6. doi: 10.1074/jbc.270.50.30221. [DOI] [PubMed] [Google Scholar]
- 11.Sutinen J, Häkkinen AM, Westerbacka J, Seppälä-Lindroos A, Vehkavaara S, Halavaara J, et al. Rosiglitazone in the treatment of HAART-associated lipodystrophy – a randomized double-blind placebo-controlled study. Antivir Ther. 2003;8:199–207. [PubMed] [Google Scholar]
- 12.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059–67. doi: 10.1210/jc.81.11.4059. [DOI] [PubMed] [Google Scholar]
- 13.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 14.Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338:265–6. doi: 10.1056/NEJM199801223380415. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–10. doi: 10.2337/diacare.27.2007.S5. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Grinspoon S. Mechanisms and strategies for insulin resistance in acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(Suppl 2):S85–90. doi: 10.1086/375885. [DOI] [PubMed] [Google Scholar]
- 18.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050–6. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- 19.Noor MA, Lo JC, Mulligan K, Schwarz JM, Halvorsen RA, Schambelan M, et al. Metabolic effects of indinavir in healthy HIV seronegative subjects. AIDS. 2001;15:F11–8. doi: 10.1097/00002030-200105040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. AIDS. 2002;16:F1–8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunfeld C. HIV protease inhibitors and glucose metabolism. AIDS. 2002;16:925–6. doi: 10.1097/00002030-200204120-00013. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Marc T, Touraine JL. Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS. 1999;13:1000–2. doi: 10.1097/00002030-199905280-00023. [DOI] [PubMed] [Google Scholar]
- 23.Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000;284:472–7. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 24.Walli R, Michl GM, Mühlbayer D, Brinkmann L, Goebel FD. Effects of troglitazone on insulin sensitivity in HIV-infected patients with protease inhibitor-associated diabetes mellitus. Res Exp Med (Berl) 2000;199:253–62. doi: 10.1007/s004330050123. [DOI] [PubMed] [Google Scholar]
- 25.Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000;95:272–6. doi: 10.1111/j.1572-0241.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 26.Gelato MC, Mynarcik DC, Quick JL, Steigbigel RT, Fuhrer J, Brathwaite CE, et al. Improved insulin sensitivity and body fat distribution in HIV-infected patients treated with rosiglitazone: a pilot study. J Acquir Immune Defic Syndr. 2002;31:163–70. doi: 10.1097/00126334-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 27.Feldt T, Oette M, Kroidl A, Goebels K, Fritzen R, Kambergs J, et al. Evaluation of safety and efficacy of rosiglitazone in the treatment of HIV-associated lipodystrophy syndrome. Infection. 2006;34:55–61. doi: 10.1007/s15010-006-5022-y. [DOI] [PubMed] [Google Scholar]
- 28.van Wijk JP, de Koning EJ, Cabezas MC, op't Roodt J, Joven J, Rabelink TJ, et al. Comparison of rosiglitazone and metformin for treating HIV lipodystrophy: a randomized trial. Ann Intern Med. 2005;143:337–46. doi: 10.7326/0003-4819-143-5-200509060-00009. [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]