Abstract
We report the case of a 22-year-old woman who presented with acute onset flaccid quadriparesis. Physical examination showed mild pallor with cervical and axillary lymphadenopathy, hepatomegaly, and bilateral smooth enlarged kidneys. Neurological examination revealed lower motor neuron muscle weakness in all the four limbs with hyporeflexia and normal sensory examination. Laboratory investigations showed anemia, severe hypokalemia, and metabolic acidosis. Urinalysis showed a specific gravity of 1.010, pH of 7.0, with a positive urine anion gap. Ultrasound revealed hepatosplenomegaly with bilateral enlarged smooth kidneys. Renal biopsy was consistent with the diagnosis of non-Hodgkin lymphoma (B cell type). Metabolic acidosis, alkaline urine, and severe hypokalemia due to excessive urinary loss in our patient were suggestive of distal renal tubular acidosis. Renal involvement in lymphoma is usually subclinical and clinically overt renal disease is rare. Diffuse lymphomatous infiltration of the kidneys may cause tubular dysfunction and present with hypokalemic paralysis.
Renal lymphoma occurs most often as a part of a multi-systemic disseminated lymphoma or as a primary involvement which is extremely rare. Incidence of renal involvement in patients with lymphoma has been reported to be 30-40% in several autopsy series. However, renal involvement at an initial presentation has been found in only 2.7-6% of the cases. It mostly appears in the form of insidious renal failure but cases of acute renal failure due to lymphoma have been described. We report a case of diffuse lymphomatous infiltration of the kidney with initial presentation as renal tubular acidosis and hypokalemic paralysis.
Case report
A 22-year-old single woman presented with acute onset flaccid quadriparesis. The weakness was not associated with myalgia, involuntary movements, tingling, or paresthesias. She complained of fluctuating low grade fever lasting for a month, which was undocumented, with no other significant medical, surgical, drug, or family history. Physical examination showed mild pallor with cervical and axillary lymphadenopathy, hepatomegaly, and bilateral smooth enlarged kidneys. Neurological examination revealed lower motor neuron muscle weakness in all the four limbs, which was much more marked in the proximal than in the distal group of muscles, with hyporeflexia and normal sensory examination. The initial laboratory investigations showed anemia with severe hypokalemia, with normal serum creatinine and liver function tests (Table 1).
Table 1.
Laboratory findings in the patient with renal tubular acidosis and hypokalemic paralysis
| Values at |
||||
|---|---|---|---|---|
| Laboratory measurement | admission | day 3 | day 10* | Reference range |
| Hemoglobin (g/L) |
63 |
81 |
83 |
120-160 |
| Total leukocyte count (109/L) |
7.78 |
10.3 |
9.7 |
4.5-11 |
| Differential cell count (%): |
||||
| polymorphonuclear leukocytes |
67 |
64 |
62 |
|
| lymphocytes |
33 |
34 |
36 |
|
| eosinophils |
2 |
2 |
||
| Platelet count (109/L) |
140 |
190 |
221 |
150-350 |
| Serum creatinine (µmol/L) |
88.4 |
61.88 |
87.5 |
70-120 |
| Sodium (mmol/L) |
138 |
135 |
137 |
136-145 |
| Potassium (mmol/L) |
1.5 |
3 |
2.6 |
3.5-5.0 |
| Calcium (mmol/L) |
2.25 |
2.5 |
2.4 |
2.15-2.58 |
| Phosphorus (mmol/L) |
1.13 |
1.29 |
1.19 |
0.81-1.45 |
| Uric acid (mmol/L) | 416.5 | 380.8 | 368.9 | 179-476 |
*The day before chemotherapy.
Arterial blood gas analysis indicated metabolic acidosis. Urinalysis revealed a specific gravity of 1.010, pH of 7.0, with a positive urine anion gap. X-ray chest was normal, with no evidence of mediastinal lymphadenopathy. Electrocardiogram and echocardiogram were normal. The patient was given intravenous potassium chloride to treat severe hypokalemia, which led to a rapid improvement of muscular weakness. Oral potassium supplement was continued in view of persistent hypokalemia. Abdominal ultrasound showed hepatosplenomegaly, normal pancreas, and retroperitoneum, and grossly enlarged kidneys (15 cm in size bilaterally) with no evidence of hydronephrosis. Magnetic resonance imaging (Figure 1) revealed hepatosplenomegaly and bilateral enlarged smooth kidneys with loss of corticomedullary differentiation and poor enhancement, which occurred due to loss of normal anatomical delineation secondary to infiltration by lymphoma with retroperitoneal lymphadenopathy. Renal biopsy (Figure 2) revealed normal morphology of the glomeruli with compression of the tubules and tubular atrophy. The interstitium was densely infiltrated by a diffuse infiltrate of atypical lymphoid cells, which were positive for B-cell antigens CD19 and CD20. Excision biopsy of cervical lymph nodes revealed onion skinning of blood vessels with eosinophils infiltrating the lymph node tissue. Lymphocytes showed no pleomorphism or atypia and the lymph node architecture was relatively preserved. No conclusive opinion was made. Subsequently, bone marrow examination (Figure 3) showed a collection of atypical lymphoid cells surrounded by cells of erythroid and myeloid lineage series in both central and paratrabecular distribution. The final diagnosis of acute B cell lymphoblastic lymphoma (stage IV) with hypokalemic paralysis due to renal tubular acidosis (secondary to lymphomatous infiltration of kidneys) was made. Allopurinol treatment was initiated and the patient was also given systemic chemotherapy according to the cyclophosphamide, doxorubicin (Adriamycin), vincristine (Oncovin), and prednisone protocol (CHOP). She developed resistant ventricular tachycardia leading to cardiac arrest a day after receiving chemotherapy, which was presumably related to persistent severe hypokalemia.
Figure 1.
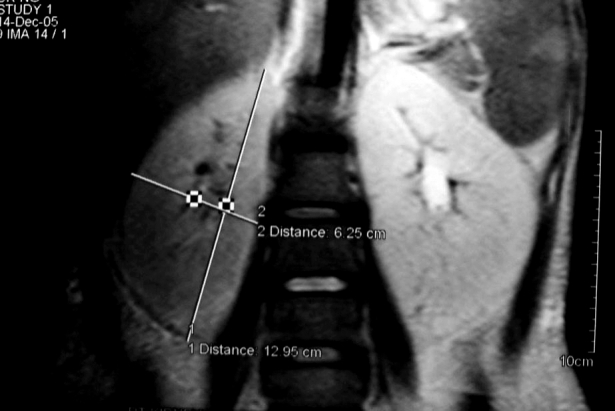
Magnetic resonance imaging scan showing bilateral enlarged smooth kidneys with loss of corticomedullary differentiation.
Figure 2.
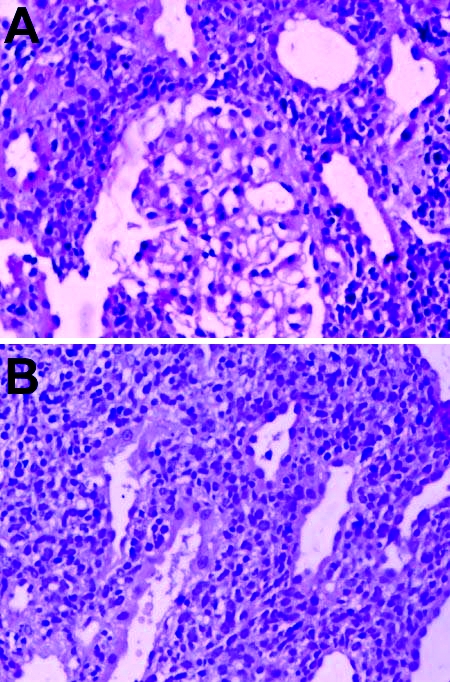
(A) Low power view of renal biopsy showed normal glomeruli with dense infiltrate of atypical lymphoid cells in the interstitium. (B) High power view of renal biopsy showed compression of tubules with tubular atrophy. There was a dense and diffuse infiltrate of atypical lymphoid cells which strongly expressed CD 19 and CD 20 antigens (B-cells).
Figure 3.
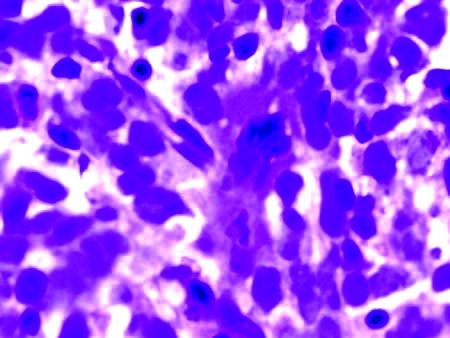
Bone marrow aspirate examination showing the collection of atypical lymphoid cells. Immunohistochemically, these cells were positive for B-cell markers.
Discussion
The presentation of our patient with severe hypokalemic paralysis and renal tubular acidosis consequent to diffuse lymphomatous infiltration of the kidneys is exceptional. Involvement of kidney by lymphomatous process occurs in 30%-40% of cases of lymphoma, if the disease is left untreated (1). On the other hand, primary renal lymphoma is extremely rare, accounting for 0.7% of all extranodal lymphomas in North America and 0.1% of all malignant lymphomas in Japan (2). Primary renal lymphoma is a controversial and infrequent disease. Some authors believe that since there is no lymphoid tissue in kidneys, primary renal lymphoma is unlikely. However, others claim that primary renal lymphoma does occur and also suggested the criteria for diagnosis of this entity (3). Most patients with primary renal lymphoma have no clinical evidence of renal involvement. Extrarenal signs and symptoms of lymphoma are frequently present (4,5). Tissue diagnosis can usually be made by lymph node or bone marrow biopsy in most patients with widespread lymphoma and renal involvement. It is believed that kidney biopsy is the most expeditious and direct way to establish the underlying diagnosis of renal masses. In our case the presence of lymphadenopathy, hepatosplenomegaly, and bone marrow involvement suggests the involvement of kidneys secondary to infiltration by a lymphomatous process. However, late presentation in a case of primary renal lymphoma may also present with systemic involvement.
Interstitial infiltration by hematologic malignancies is usually bilateral, diffuse, and more prominent in the cortex. Lymphoma may involve the kidney by multinodular or diffuse infiltration or occasionally by the presence of a large solitary tumor. Renal involvement in lymphoma can manifest as nephrotic syndrome or it may present as acute renal failure (4-8). In other patients, it may be asymptomatic or present with anasarca, urinary tract infections, or uremic symptoms (8). Renal failure in lymphoma may be due to obstruction or due to indirect effects such as hypercalcemia, glomerulonephritis, volume depletion, or infections leading to sepsis. Various factors in lymphoma alone or in combination lead to renal failure (4-6). When it does occur, affected patients generally present with relatively acute renal failure and normal urinary sediment.
Direct effects of infiltration were seen in one third of cases in a study reported by Khanna et al (8). The prognosis in patients presenting with renal involvement is usually poor with most patients dying within 9 months of presentation (7).
The presence of massively enlarged kidneys and histopathologic evidence of diffuse lymphocytic infiltration in our case points to the involvement of kidneys by lymphoma as the cause of renal tubular dysfunction. Renal infiltration by lymphoma presenting as quadriparesis due to hypokalemia secondary to renal tubular acidosis has not been described. Dense infiltration of kidneys by lymphoma may cause compression of tubular lumen and result in tubular atrophy and necrosis, leading to renal tubular dysfunction. It is important to be aware of this potential complication in patients with advanced non-Hodgkin lymphoma.
Reference
- 1.Tandon P, Krishnani N. Acute renal failure in lymphoma of the kidney. Indian J Pathol Microbiol. 1993;36:61–4. [PubMed] [Google Scholar]
- 2.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–60. doi: 10.1002/1097-0142(197201)29:1<252::AID-CNCR2820290138>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Malbrain ML, Lambrecht GL, Daelemans R, Lins RL, Hermans P, Zachée P. Acute renal failure due to bilateral lymphomatous infiltrates. Primary extranodal non-Hodgkin's lymphoma (p-EN-NHL) of the kidneys: does it really exist? Clin Nephrol. 1994;42:163–9. [PubMed] [Google Scholar]
- 4.Glicklich D, Sung MW, Frey M. Renal failure due to lymphomatous infiltration of the kidneys. Report of three new cases and review of the literature. Cancer. 1986;58:748–53. doi: 10.1002/1097-0142(19860801)58:3<748::AID-CNCR2820580323>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 5.Obrador GT, Price B, O'Meara Y, Salant DJ. Acute renal failure due to lymphomatous infiltration of the kidneys. J Am Soc Nephrol. 1997;8:1348–54. doi: 10.1681/ASN.V881348. [DOI] [PubMed] [Google Scholar]
- 6.Sellin L, Friedl C, Klein G, Waldherr R, Rump LC, Weiner SM. Acute renal failure due to a malignant lymphoma infiltration uncovered by renal biopsy. Nephrol Dial Transplant. 2004;19:2657–60. doi: 10.1093/ndt/gfh201. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh L, Muehrcke RC. Nephrotic syndrome: a prodrome to lymphoma. Ann Intern Med. 1970;72:379–82. doi: 10.7326/0003-4819-72-3-379. [DOI] [PubMed] [Google Scholar]
- 8.Khanna UB, Almeida AF, Bhivandkar MG, Shah BV, Mittal BV, Kinare SG, et al. Renal involvement in hematological malignancies. J Assoc Physicians India. 1985;33:565–8. [PubMed] [Google Scholar]


