Abstract
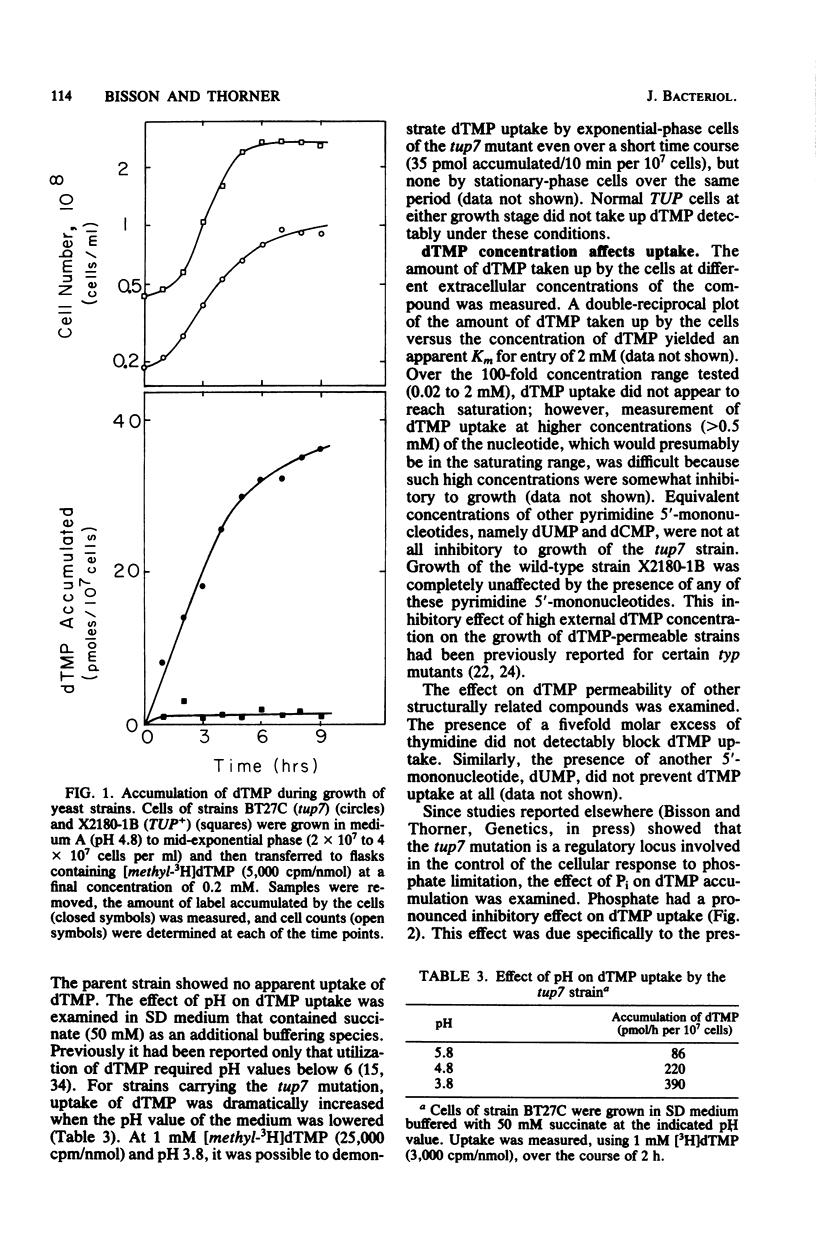
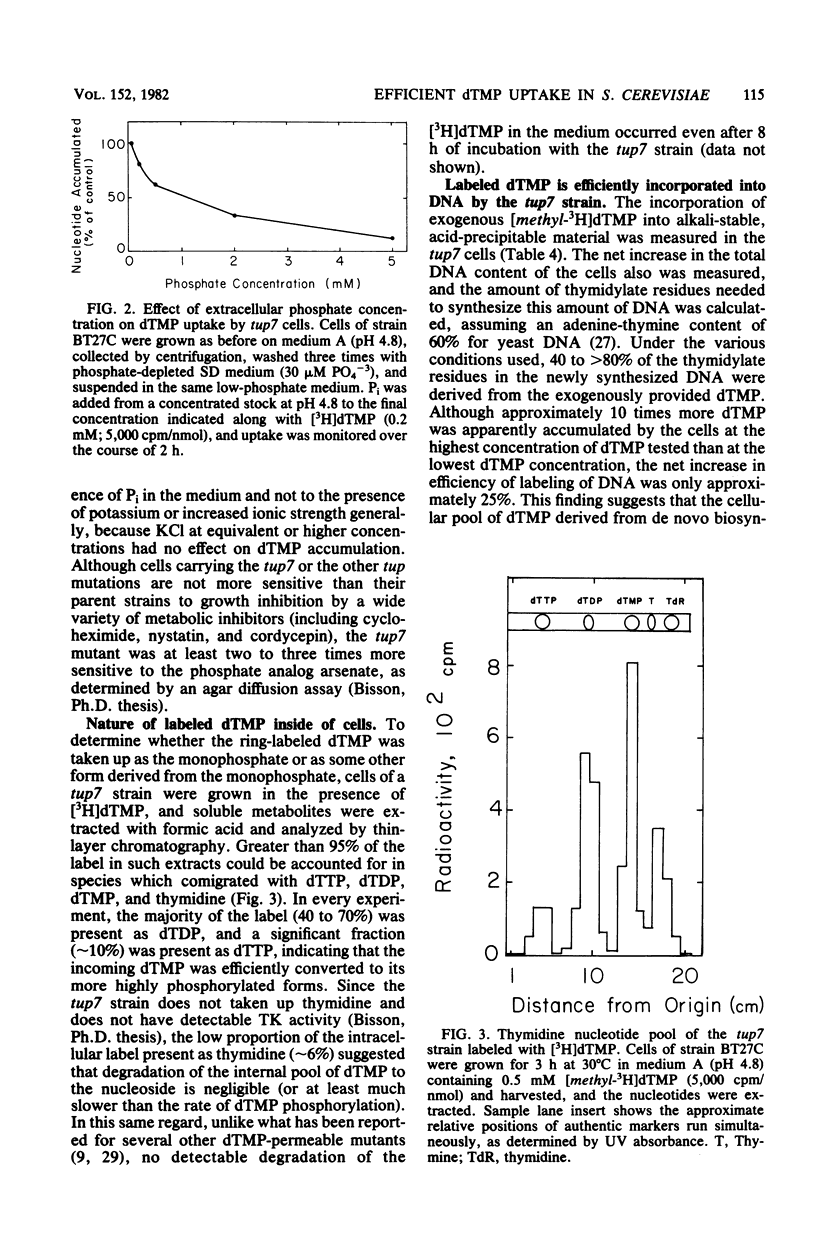
The rate and extent of entry of dTMP were measured in strains of Saccharomyces cerevisiae carrying two new tup mutations (tup5 and tup7) and most of the other tup mutations which have been reported previously by others. The tup7 mutation allowed dramatically greater accumulation of dTMP than any of the other mutations tested. Specific labeling of DNA by [CH3-3H]dTMP, fate of the dTMP pool inside of the cells, and degradation of the dTMP in the culture medium were investigated in strains carrying the tup7 mutation. The extracellular dTMP was not appreciably degraded, and that accumulated intracellularly was readily phosphorylated to dTDP and dTTP. Under optimum labeling conditions, 60 to 80% of the total thymidylate residues in newly synthesized DNA were derived from the exogenously provided dTMP, even in the absence of a block in de novo dTMP biosynthesis. An apparent Km for entry of 2 mM dTMP was found. The tup7 mutation increased permeability to dTMP (and some other 5'-mononucleotides), but did not affect uptake of nucleosides and purine and pyrimidine bases. Uptake of dTMP could be almost completely inhibited by moderate concentrations of Pi. These findings and other observations suggest that entry of dTMP in strains carrying the tup7 mutation is mediated by a permease whose function in normal cells is the transport of Pi.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay B. J., Little J. G. Genetic damage during thymidylate starvation in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Mar 20;160(1):33–40. doi: 10.1007/BF00275116. [DOI] [PubMed] [Google Scholar]
- Bisson L., Thorner J. Thymidine 5'-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol. 1977 Oct;132(1):44–50. doi: 10.1128/jb.132.1.44-50.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst-Pauwels G. W., Peters P. H. Effect of the medium pH and the cell pH upon the kinetical parameters of phosphate uptake by yeast. Biochim Biophys Acta. 1977 May 2;466(3):488–495. doi: 10.1016/0005-2736(77)90341-8. [DOI] [PubMed] [Google Scholar]
- Brendel M. A simple method for the isolation and characterization of thymidylate uptaking mutants in Saccharomyces cerevisiae. Mol Gen Genet. 1976 Aug 19;147(2):209–215. doi: 10.1007/BF00267573. [DOI] [PubMed] [Google Scholar]
- Brendel M., Fäth W. W. Isolation and characterization of mutants of Saccharomyces cerevisiae auxotrophic and conditionally auxotrophic for 5'-dTMP. Z Naturforsch C. 1974 Nov-Dec;29(11-12):733–738. doi: 10.1515/znc-1974-11-1214. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Exogenous thymidine 5'-monophosphate as a precursor for DNA synthesis in yeast. Mol Gen Genet. 1973 Nov 22;126(4):337–348. doi: 10.1007/BF00269443. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Kinetics and genetic control of the incorporation of thymidine monophosphate in yeast DNA. Mol Gen Genet. 1972;117(1):39–44. doi: 10.1007/BF00268835. [DOI] [PubMed] [Google Scholar]
- Brendel M., Langjahr U. G. "Thymineless death" in a strain of Saccharomyces cerevisiae auxotrophic for deoxythymidine-5'-monophosphate. Mol Gen Genet. 1974;131(4):351–358. doi: 10.1007/BF00264865. [DOI] [PubMed] [Google Scholar]
- Budd M., Mortimer R. K. Repair of double-strand breaks in a temperature conditional radiation-sensitive mutant of Saccharomyces cerevisiae. Mutat Res. 1982 Jan;103(1):19–24. doi: 10.1016/0165-7992(82)90080-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer D. R., Goldthwaite C. D., Zinker S., Lam K. B., Storm E., Hirschberg R., Blamire J., Finkelstein D. B., Marmur J. Studies on nuclear and mitochondrial DNA of Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1974;38:17–29. doi: 10.1101/sqb.1974.038.01.005. [DOI] [PubMed] [Google Scholar]
- Fäth W. W., Brendel M., Laskowski W., Lehmann-Brauns E. Economizing DNA-specific labelling by deoxythymidine-5'-monophosphate in Saccharomyces cerevisiae. Mol Gen Genet. 1974;132(4):335–345. doi: 10.1007/BF00268573. [DOI] [PubMed] [Google Scholar]
- Game J. C. Yeast cell-cycle mutant cdc21 is a temperature-sensitive thymidylate auxotroph. Mol Gen Genet. 1976 Aug 2;146(3):313–315. doi: 10.1007/BF00701257. [DOI] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- HEIDELBERGER C., KALDOR G., MUKHERJEE K. L., DANNEBERG P. B. Studies on fluorinated pyrimidines. XI. In vitro studies on tumor resistance. Cancer Res. 1960 Jul;20:903–909. [PubMed] [Google Scholar]
- Jannsen S., Lochmann E. -R., Megnet R. Specific incorporation of exogenous thymidine monophosphate into DNA in Saccharomyces cerevisiae. FEBS Lett. 1970 Jun 1;8(3):113–115. doi: 10.1016/0014-5793(70)80239-3. [DOI] [PubMed] [Google Scholar]
- Langjahr U. G., Hartmann E. M., Brendel M. Nucleic acid metabolism in yeast. I. Inhibition of RNA and DNA synthesis by high concentrations of exogenous deoxythymidine 5'-monophosphate in 5'-dTMP low requiring strains. Mol Gen Genet. 1975 Dec 30;143(1):113–118. doi: 10.1007/BF00269428. [DOI] [PubMed] [Google Scholar]
- Laskowski W., Lehmann-Brauns E. Mutants of Saccharomyces able to grow after inhibition of thymidine phosphate synthesis. Mol Gen Genet. 1973 Sep 12;125(3):275–277. doi: 10.1007/BF00270749. [DOI] [PubMed] [Google Scholar]
- Little J. G., Haynes R. H. Isolation and characterization of yeast mutants auxotrophic for 2'-deoxythymidine 5'-monophosphate. Mol Gen Genet. 1979 Jan 10;168(2):141–151. doi: 10.1007/BF00431440. [DOI] [PubMed] [Google Scholar]
- McNeil J. B., Friesen J. D. Expression of the Herpes simplex virus thymidine kinase gene in Saccharomyces cerevisiae. Mol Gen Genet. 1981;184(3):386–393. doi: 10.1007/BF00352510. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Ludescher R. D., Walter S. K. Production of petites by cell cycle mutants of Saccharomyces cerevisiae defective in DNA synthesis. Mol Gen Genet. 1979 Jan 31;169(2):189–194. doi: 10.1007/BF00271670. [DOI] [PubMed] [Google Scholar]
- Price C. W., Fuson G. B., Phaff H. J. Genome comparison in yeast systematics: delimitation of species within the genera Schwanniomyces, Saccharomyces, Debaryomyces, and Pichia. Microbiol Rev. 1978 Mar;42(1):161–193. doi: 10.1128/mr.42.1.161-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDERATH K., RANDERATH E. ION-EXCHANGE CHROMATOGRAPHY OF NUCLEOTIDES ON POLY-(ETHYLENEIMINE)-CELLULOSE THIN LAYERS. J Chromatogr. 1964 Oct;16:111–125. doi: 10.1016/s0021-9673(01)82445-6. [DOI] [PubMed] [Google Scholar]
- Remer S., Sherman A., Kraig E., Haber J. E. Suppressor of deoxythmidine monophosphate uptake in Saccharomyces cerevisiae. J Bacteriol. 1979 May;138(2):638–641. doi: 10.1128/jb.138.2.638-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomans G. M., Blasco F., Borst-Pauwels G. W. Cotransport of phosphate and sodium by yeast. Biochim Biophys Acta. 1977 May 16;467(1):65–71. doi: 10.1016/0005-2736(77)90242-5. [DOI] [PubMed] [Google Scholar]
- Weimberg R. Polyphosphate levels in nongrowing cells of Saccharomyces mellis as determined by magnesium ion and the phenomenon of "Uberkompensation". J Bacteriol. 1975 Mar;121(3):1122–1130. doi: 10.1128/jb.121.3.1122-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine 5'-monophosphate into DNA in vivo. Methods Cell Biol. 1975;11:295–302. doi: 10.1016/s0091-679x(08)60330-1. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore P. J., Parry J. M. Division delay and DNA degradation after mutagen treatment of the yeast, Saccharomyces cerevisiae. Mol Gen Genet. 1976 Jun 15;145(3):287–291. doi: 10.1007/BF00325825. [DOI] [PubMed] [Google Scholar]


