Extensive and complete documentation must be submitted for obtaining a marketing authorization of an investigational medicinal product in the European Union, Japan, or the United States. One of the most critical of the documents submitted as part of the Common Technical Document, masterpiece of a marketing authorization application, is the Clinical Study Report, which represents the integrated full report of efficacy and safety data for an individual study of a therapeutic or diagnostic agent. The content and format of a Clinical Study Report is recommended by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline E3 on Structure and Content of Clinical Study Reports, which was approved in 1996. Some of the studies conducted during product development may ultimately not contribute to the evaluation of the effectiveness of a product for a specific indication. In these cases, abbreviated Clinical Study Reports are required to be submitted to the regulatory authorities. However, the ICH E3 guideline only provides information on the structure and content of full Clinical Study Reports. A guideline issued by the Food and Drug Administration of the United States in 1999 is the only document available from a regulatory authority that recommends which sections can be included in an abbreviated Clinical Study Report. This article describes which sections have to be included in abbreviated Clinical Study Reports written during clinical development of new medicinal products for human use.
Marketing authorizations for investigational medicinal products: common technical document and clinical study reports
Extensive and complete documentation must be presented for a marketing authorization of an investigational medicinal product (IMP) for human use in the European Union (EU), Japan, or the United States. The documentation to be submitted to the regulatory authorities has to prove the quality, safety, and efficacy of the new drug.
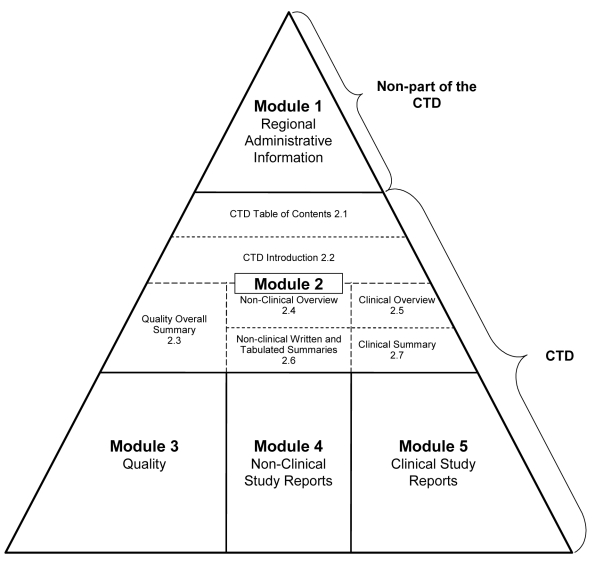
Under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), the involved parties (EU, Japan, and the United States) have joined their efforts to establish common reporting standards and template formats. The compilation of these reports forms the basis of the Common Technical Document (CTD) (1,2) (Figure 1), which has become an internationally agreed format for the preparation of a well-structured presentation for applications to be submitted to regulatory authorities in the regions where ICH is applied (4). Sponsors should pay keen attention to the guidelines established by the ICH to insure a speedy and efficient review of their submissions.
Figure 1.
Schematic representation of the five modules in the Common Technical Document (CTD). Drawn from International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) M4E guideline on CTD (3).
One of the most critical documents submitted as part of a CTD is the Clinical Study Report (CSR), which represents the integrated full report of the efficacy and safety data for an individual study with a therapeutic or diagnostic agent. The different CSRs produced during the clinical development of a medicinal product are included in module 5 of the CTD (3). The particular location of a CSR within the CTD is determined by the primary objective of the study: eg, efficacy and safety, pharmacokinetics, or pharmacodynamics (Box 1). Each CSR should appear in only one section, and when the study has several objectives, it should be cross-referenced in the other sections. Moreover, the CSRs also have to be cross-referenced in module 2.5 of the CTD, ie the Clinical Overview, which provides a critical review of the clinical data included in the CTD (Figure 1).
Box 1. Basic structure of the Module 5 of the Common Technical Document (CTD) where Clinical Study Reports (CSR) are to be included according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH M4E [efficacy] guideline) (3).
• 5.1 Table of Contents of Module 5
• 5.2 Tabular Listing of all Clinical Studies
• 5.3 Clinical Study Reports
o 5.3.1 Reports of Biopharmaceutical Studies
o 5.3.2 Reports of Studies Pertinent to Pharmacokinetics using Human Biomaterials
o 5.3.3 Reports of Human Pharmacokinetic Studies
o 5.3.4 Reports of Human Pharmacodynamic Studies
o 5.3.5 Reports of Efficacy and Safety Studies
o 5.3.6 Reports of Post-Marketing Experience
o 5.3.7 Case Report Forms and Individual Patient Listings
• 5.4 Literature References
Content and format of clinical study report
The content and format of a CSR is guided by the ICH Guideline E3 on Structure and Content of CSRs, which was approved in 1996 (5). During the more than ten years of use of this guidance document, the regulatory authorities and the industry have worked to refine some of its ambiguities and redundancies, and have allowed the template to evolve into a well-structured, highly intuitive format for the presentation of clinical results. The CSR integrates the clinical and statistical descriptions, presentations, and analyses into a single integrated report, incorporating tables and figures. This integrated report not only outlines the original plan of the protocol, but also describes in more depth and explains any practices in the clinical trial that were different from those originally planned. By reading the CSR, one can understand why and how the study was conducted, the types of data collected and analyzed, and the nature and extent of the conclusions that may be drawn from the results. The results of pivotal clinical trials and human pharmacology investigations that contribute to the evaluation of effectiveness and safety for a proposed indication or that otherwise support information included in the product labeling are to be presented as a full CSR, which addresses all the elements of the template report described in the ICH E3 guideline (Table 1).
Table 1.
Structure of a full Clinical Study Report according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH E3) guideline (5,6)
| ICH E3 guideline | ||
|---|---|---|
| 1. Title Page |
||
| 2. Synopsis |
||
| 3. Table of Contents |
||
| 4. List of Abbreviations and Definitions of Terms |
||
| 5. Ethics |
||
| 6. Investigators and Study Administrative Structure |
||
| 7. Introduction |
||
| 8. Study Objectives |
||
| 9. Investigational Plan |
||
| 10. Study Patients |
||
| 11. Efficacy Evaluation |
||
| 12. Safety Evaluation |
||
| 13. Discussion and Overall Conclusions |
||
| 14. Tables, Figure and Graphs Referred to But Not Included in the Text |
||
| 15. Reference List |
||
| 16. Appendices |
||
| Appendices to be submitted always or upon request in the initial application dossier within each Clinical Study Report |
Always |
Upon request*
(48 h) |
| 16.1 Study Information |
||
| 16.1.1. Protocol and Protocol Amendments |
X |
|
| 16.1.2. Sample Case Report Form (CRF) |
X |
|
| 16.1.3. List of IECs† or IRBs‡-Representative Written Information for Patient and Sample Consent Form |
X |
|
| 16.1.4. List and Description of Investigators and Other Important Participants in the Study, including Brief Curriculum Vitae. |
X |
|
| 16.1.5. Signatures of Principal or Coordinating Investigator(s) or Sponsor’s Responsible Medical Officer |
X |
|
| 16.1.6. Listing of Patients Receiving Test Drug from Specific Batches |
X |
|
| 16.1.7. Randomization Scheme and Codes |
X |
|
| 16.1.8. Audit Certificates |
X |
|
| 16.1.9. Documentation of Statistical Methods |
X |
|
| 16.1.10. Documentation of Inter-laboratory Standardization Methods and Quality Assurance Procedures |
X |
|
| 16.1.11. Publications Based on the Study |
X |
|
| 16.1.12. Important Publications Referenced in the Report |
X |
|
| 16.2 Patient Data Listings |
||
| 16.2.1. Discontinued Patients |
X |
|
| 16.2.2. Protocol Deviations |
X |
|
| 16.2.3. Patients Excluded from the Efficacy Analysis |
X |
|
| 16.2.4. Demographic Data |
X |
|
| 16.2.5. Compliance and/or Drug Concentration Data |
X |
|
| 16.2.6. Individual Efficacy Response Data |
X |
|
| 16.2.7. Adverse Events (AEs) Listing (Each Patient) |
X |
|
| 16.2.8. Listing of Individual Laboratory Measurements by Patient |
X |
|
| 16.3 Case Report Forms |
||
| 16.3.1. CRFs of Deaths, Other Serious Adverse Events and Withdrawals for AEs. |
X |
|
| 16.3.2. Other CRFs Submitted. | X |
*A note/guideline of the Committee on Human Medicinal Products (CHMP) issued in June 2004 (6) indicates that, in agreement with the competent authorities, the applicant of a Marketing Authorization Application may omit part of the information to be submitted but with the proviso that complete documentation (ie, the full CSR or some appendices) will have to be provided forthwith upon request by the regulatory authorities at short notice (48 h).
†IEC – independent ethics committee.
‡IRB – institutional review boards.
An issue of concern among users of the template provided by the ICH E3 guideline is whether, or how much, is one allowed to deviate from the structure of the guideline. While the initial intent of the document was to serve as a report template, it has been interpreted by many pharmaceutical regulatory/writing departments as a required template format. While this approach ensures consistency in data presentation and contributes to the ready access of information by reviewers, it holds some limitations. For instance, pharmaceutical companies are often confused as to where to present the results of pharmacokinetic analyses. The template provides a section (number 11.4.4) entitled “Drug Dose, Drug Concentration, and Relationships to Response,” but many believe that a new, better-defined section should be included in the report. While there is no formal prohibition to alter the structure of the ICH E3 template, sponsors must consider the implications of following a different structure from the standpoint of consistency with other documents, integration of non-standard templates into electronic document management systems, ease of review, and regulatory agency expectations. Nevertheless, current overall experience shows that the ICH E3 guideline is a valuable tool for the comprehensive presentation of clinical data.
The CSR has to be written following a modular approach that includes a core report, which gives the information necessary for assessing the results of the study, and the appendices, which contain additional information such as protocol, protocol amendments, sample case report form, investigator-related information, technical statistical documentation, related publications, patient data listings, and related computer printouts from the clinical study database, among others. To allow navigation in the e-format for future electronic applications (the so-called electronic CTD or e-CTD, now applicable in the United States but yet in development in the EU), the CSR has to integrate all parts of the report in a unique document (eg, in portable document format [pdf] files): the core report (with the 15 main sections) and the appendices (section 16). Therefore, only-based-in-paper documents (eg, signature pages) have to be scanned and converted to electronic format.
Abbreviated clinical study reports
Two guidelines are available on the structure and content of CSRs (5) or on the appendices to be submitted in a Marketing Authorization Application for an IMP (6). However, one of the most polemic questions in this area of medical writing is how to reduce the content of a CSR, ie, how to produce an “abbreviated CSR” in some particular cases. During product development, studies may be conducted that ultimately do not contribute toward the evaluation of the effectiveness of a product for a specific indication. Abbreviated reports should be submitted for these studies, and also for studies for which the reviewer needs sufficient information to determine that the results do not cause any doubts about the effectiveness claims.
The ICH E3 guideline on structure and content of CSRs (5) indicates in its introduction that “depending on the regulatory authority’s review policy, abbreviated reports using summarized data or with some sections deleted, may be acceptable for uncontrolled studies or other studies not designed to establish efficacy (but a controlled safety study should be reported in full), for seriously flawed or aborted studies, or for controlled studies that examine conditions clearly unrelated to those for which a claim is made. However, a full description of safety aspects should be included in these cases. If an abbreviated report is submitted, there should be enough detail of design and results to allow the regulatory authority to determine whether a full report is needed. If there is any question regarding whether the reports are needed, it may be useful to consult the regulatory authority” (5). No more details are given on how to abbreviate the contents of the CSR and, therefore, this only constitutes a declaration of intent that would require further support by the corresponding regulatory authority, ie, the European Medicines Agency (EMEA) in the European Union or the Food and Drug Administration (FDA) in the United States.
The ICH M4E guideline on the Common Technical Document (CTD) (3) indicates that “abbreviated reports can be provided for some studies.” However, as was already mentioned above, ICH E3 provides no description of the content of an abbreviated report. The only relevant information on the content of an abbreviated CSR that is available in the ICH E3 is that such an abbreviated report should contain all the safety information included in a full report.
Only the FDA seems to have promoted a guideline to solve the question of abbreviated CSRs, with no further initiatives from European regulatory authorities. A guidance for industry was issued in the United States and circulated in 1999 by the FDA (7). This guideline, although not strictly supported from a European regulatory point of view, currently forms the only basis available for defining in a direct and clear way the structure and content of abbreviated CSRs. In agreement with what is defined in the ICH E3 (approved in 1996, ie, three years before this FDA guideline), abbreviated reports should be submitted for studies that are not intended to contribute to the evaluation of product effectiveness or provide definitive information on clinical pharmacology, but about which the reviewer needs sufficient information to determine that the study results do not, in fact, cast doubts on the effectiveness claims or the description of the clinical pharmacology. Abbreviated reports should contain all the safety information included in a full report. This FDA guideline is the only official document available from a regulatory authority that recommends which sections may be included in an abbreviated CSR (Box 2). Briefly, this guideline recommends to drastically reduce the contents of sections 9 (Investigational Plan), 10 (Study Patients), and 11 (Efficacy Evaluation). In the case of the investigational plan, the information should be directed to the overall study design features and to the changes in the planned analyses. With respect to the study patients, only disposition is recommended to be included. Finally, section 11 (Efficacy Evaluation) may be replaced by a summary of the efficacy evaluation (preferably in table form) with enough information for the reviewer to determine whether the study results are germane to the overall evaluation of effectiveness and to use in the review of the integrated analysis of effectiveness, if necessary (including means, confidence intervals, P values, standard errors, etc). Section 11.4.1 (Analysis of Efficacy) as per the ICH E3 format (5) may be used, if appropriate. Any additional information pertinent to the evaluation of safety should also be included.
Box 2. Sections to be included in an abbreviated Clinical Study Report: based on an Food and Drug Administration (FDA) guideline (7). Any additional information pertinent to the evaluation of safety should also be included.
• Section 1 – Title Page
• Section 2 – Synopsis
• Section 3 – Table of Contents for the Individual Clinical Study Report
• Section 4 – List of Abbreviations and Definitions of Terms
• Section 9.1 – Overall Study and Design and Plan: Description
• Section 9.8 – Changes in the Conduct of the Study or Planned Analyses
• Section 10.1 – Disposition of Patients
• Section 12 – Safety Evaluation
• Section 13 – Discussion and Overall Conclusions
• Section 14 – Tables, Figures and Graphs Referred to but not Included in the Text
• Section 16.1.1 – Protocol and Protocol Amendments
• Section 16.1.2 – Sample Case Report Forms (unique pages only)
• Section 16.3.1 – Case Report Forms for Deaths, Other Serious Adverse Events and Withdrawals for Adverse Events (submit under item 12)
• Section 16.4 – Individual Patient Data Listings for Safety Data. Individual patient listings of efficacy data are not necessary.
• A summary of the efficacy evaluation (suggested to be primarily in table form). The summary should contain enough information for the reviewer to determine whether the study results are germane to the overall evaluation of effectiveness and in the review of the integrated analysis of effectiveness, if necessary (including means, confidence, intervals, P values, standard errors, etc). Section 11.4.1 of International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E3 format may be used, if appropriate.
Conclusion
Several guidelines applicable to the clinical development of IMPs for human use (eg, ICH M4E, ICH E3) define abbreviated clinical study reports as useful in cases where the study is not pivotal for claiming the effectiveness of a product in a Marketing Authorization Application or when the study was discontinued early due to, for instance, lack of efficacy. However, scant information is available on which sections are required to be included in these abbreviated clinical study reports. The single guideline available was issued in 1999 in the United States by the FDA, but no similar initiatives appear to have been produced in the European setting. While waiting regulatory initiatives in Europe to promote a definitive guideline on the content and structure of abbreviated clinical study reports (eagerly expected for a long time by pharmaceutical companies and other biomedical centers involved in drug development), our recommendation is to follow the FDA guideline in those cases where, according to the local regulatory authorities, an abbreviated report could be submitted.
Competing interests
All three authors work as medical writers for Pharmaceutical Industry in Spain but have no relevant financial interests in this manuscript.
References
- 1.Molzon JA. The International Conference on Harmonization common technical document – global submission format? Food Drug Law J. 2005;60:447–51. [PubMed] [Google Scholar]
- 2.Todic M. Dossier for marketing authorization in the European union. Bosn J Basic Med Sci. 2003;3:56–60. doi: 10.17305/bjbms.2003.3572. [DOI] [PubMed] [Google Scholar]
- 3.CPMP/ICH. 2887/99. ICH M4E. Common technical document for the registration of pharmaceuticals for human use-efficacy. Clinical overview and clinical summary of module 2. Module 5: clinical study reports. London: European Medicines Agency; 2003. [Google Scholar]
- 4.Molzon J. The common technical document: the changing face of the New Drug Application. Nat Rev Drug Discov. 2003;2:71–4. doi: 10.1038/nrd990. [DOI] [PubMed] [Google Scholar]
- 5.CPMP/ICH. 137/95. ICH topic E3. Structure and Content of Clinical Study Reports. London: European Medicines Agency; 1996. [Google Scholar]
- 6.CHMP/EWP. 2998/03. Note for guidance on the inclusion of appendices to clinical study reports in marketing authorization applications. London: European Medicines Agency; 2004. [Google Scholar]
- 7.U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). Guidance for Industry: submission of abbreviated reports and synopses in support of marketing applications. Rockville (MD): Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 1999. [Google Scholar]