Abstract
Aim
To define sensitive and reliable Doppler parameters for measurements in the superior mesenteric artery and mural arteries of affected bowel loops used in the assessment of Crohn’s disease activity.
Methods
We performed cross-sectional study at a tertiary care setting in Zagreb, Croatia, between January 2001 and March 2005. We measured arterial flow in the superior mesenteric artery and affected bowel wall in 138 patients with Crohn’s disease (74 with active, 64 with inactive disease) and 67 healthy volunteers. The disease activity was determined by the clinical examination, Crohn’s disease activity index, and standard laboratory tests. Superior mesenteric artery color and pulsed Doppler parameters were peak systolic velocity, end-diastolic velocity, resistance index, mean velocity flow, cross-sectional area, and flow volume. When gut mural vessels were identified, we performed spectral analysis of mural arteries by pulsed Doppler, with a measurement of resistance index.
Results
The measurements in the superior mesenteric artery showed statistically and clinically significant difference in flow volume in active group, compared with inactive and control groups (C±Q = 564 ± 263 mL/min for active, 421 ± 157 for inactive and 416 ± 248 for control group). Affected bowel loops analysis showed significant difference between inactive and active Crohn’s disease group in wall thickness (3.1 ± 1.4 vs 5.0 ± 1.8 mm, P<0.001, Mann-Whitney test) while all participants from control group had thickness below 2mm. Intensity of color Doppler signals was different for all groups (P<0.001, χ2 test) with the highest level of hyperemia in the active group. Resistance index measurements of mural arteries in bowel wall revealed differences between all three groups (0.61 ± 0.05 in active group, 0.71 ± 0.05 in the inactive group and 0.80 ± 0.11 in the control group, P<0.001, Kruskal-Wallis test).
Conclusion
Intensity of color Doppler signals and resistance index measurements of mural arteries in the thickened bowel wall can be used as quantitative diagnostic tool in the assessment of Crohn’s disease activity.
Most studies have found high resolution bowel ultrasound to be a useful tool in the management of Crohn’s disease (1). Another useful tool was Doppler ultrasound, which is used for detection of complications, assessing disease activity, and reduction of patients' radiation exposure (2-4).
Several tests and parameters are used to evaluate the disease activity and to guide therapeutic options (5,6). These include Crohn’s disease activity index (CDAI); laboratory findings (white cell count, C-reactive protein, erythrocyte sedimentation rate, alpha-1 antitrypsin clearance rate in feces, etc); endoscopy, scintigraphy, conventional x-ray examinations, computed tomography, and magnetic resonance imaging. Assessment of disease activity is a major clinical problem, which can lead to undertreatment or overtreatment of patients, because there is no absolute reference method to assess disease activity. The increase in mesenterial blood flow in Crohn’s disease can be demonstrated by mesenteric angiography and recently by Doppler ultrasound. Doppler ultrasound could be an ideal method, because it is a non-invasive, non-ionizing, safe, and reproducible technique. Therefore, Doppler ultrasound has received an increasing interest in the investigations of splanchnic hemodynamics in Crohn’s disease, where hypervascularity exists (7,8).
In the last decade, assessment of Crohn’s disease activity by Doppler ultrasound has been mainly based on the measurements in superior mesenteric artery. Some authors emphasize the resistance index in the superior mesenteric artery (9,10) as a parameter that can assess activity in Crohn’s disease, while others emphasize maximum flow volume (8,11). A few studies have analyzed local mesenterial flow in affected bowel loops. Vascularization, characterized by increased blood flow, in these loops is increased in the active form of Crohn’s disease, which can be depicted by Doppler ultrasound. Hyperemia of the inflammable gut wall was mostly described by color Doppler and sometimes by power Doppler (12,13). Pulsed Doppler spectral analysis, as an objective method, was described in a few studies (14,15).
The purpose of this study was to define Doppler ultrasound parameters of the superior mesenteric artery and dilated mural arteries of thickened bowel wall for the assessment of Crohn’s disease activity.
Patients and methods
This cross-sectional study was performed at a tertiary care setting (Zagreb University Hospital Center, Zagreb, Croatia) between January 2001 and March 2005. Ethical committee of the Zagreb University School of Medicine gave an approval for the study.
Patients
Arterial flow in the superior mesenteric artery and affected bowel wall was measured in a total of 205 examinees. There were 138 patients with Crohn’s disease (74 with active and 64 with inactive or quiescent disease) and 67 healthy volunteers as control group (Table 1).
Table 1.
Demographic characteristics of patients with Crohn’s disease (n = 138) and control group (n = 67)
| Parameter |
No. of patients |
||
|---|---|---|---|
| active (n = 74) | inactive(n = 64) | control (n = 67) | |
| Age: |
|||
| ≤20 |
24 |
7 |
15 |
| 21-40 |
33 |
35 |
26 |
| 41-60 |
16 |
14 |
20 |
| ≥61 |
1 |
8 |
6 |
| Sex: |
|||
| male |
38 |
34 |
35 |
| female | 36 | 30 | 32 |
The disease activity was determined by clinical examination and anamnestic data, Crohn’s disease activity index (CDAI), and standard laboratory tests (C-reactive protein, erythrocyte sedimentation rate) (5,6). Disease was classified as active if the CDAI was ≥150 and inactive with CDAI<150. Moderate activity CDAI ranges from 150-450 and strong activity CDAI ranges above 450. By these methods, patients were divided into active and inactive group at the moment of the sonographic exam. Patients with any previous extensive surgical procedures on the small bowel or colon (right hemicolectomy and ileotransversoanastomosis) were excluded from this study. Additional complementary examinations (endoscopy, x-ray examination, and scintigraphy) were evaluated by a strictly clinical-diagnostic procedure to define extension and activity of the disease in a limited number of patients.
Measurements
All of our patients were examined with Logic 500 PRO scanner (General Electric, Milwaukee, WI, USA), using convex abdominal probe 2-5 MHz, and linear probe 6-9 MHz, with triplex Doppler. Examination was performed after an overnight fasting in the supine position, with an average duration of 45 minutes. The results were evaluated by two radiologists together, so inter-observer variability was not recorded. Intra-observer variability was minimized by averaging a large number of measurements, especially in mural arteries. Both radiologists were blinded to the patients' medical history, clinical laboratory data, and the results of the other tests.
We first performed Doppler spectral analysis in the superior mesenteric artery. The superior mesenteric artery was studied in its long axis in the sagittal plane. The sampling cursor was placed 2-3 cm from the origin, proximal to any arterial side branch. The calipers were chosen for cross-sectional area measurement with maximum zoom function. The insonation angle was 45-60°. The measurements were taken after the optimal Doppler signal was recognized. Each measurement was recorded three times from three different cardiac cycles and averaged for each patient to minimize random error (16). The following superior mesenteric artery Doppler parameters were evaluated: peak systolic velocity (PSV), end-diastolic velocity (EDV), resistance index, mean velocity flow (MV), cross-sectional area (CSA), and flow volume. Flow volume (mL/min) was automatically calculated by the Doppler instrument, from the equation MV (cm/s) × CSA (cm2) (Figure 1).
Figure 1.
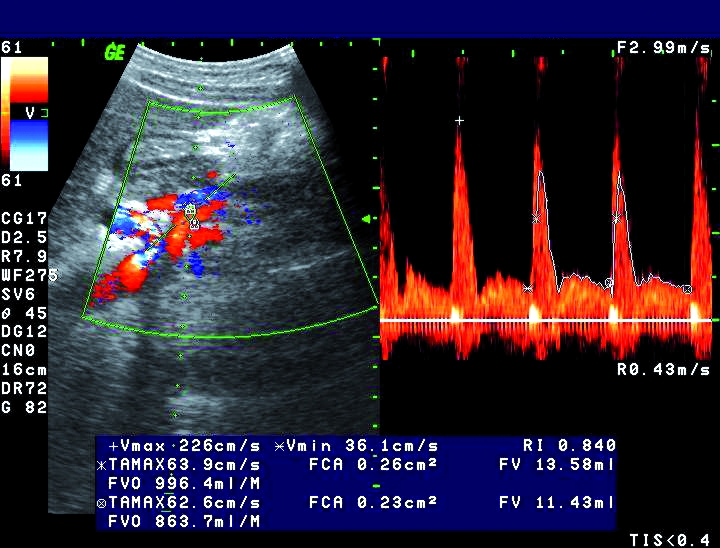
Color and pulsed Doppler measurements of flow volume in the superior mesenteric artery of a patient with active Crohn’s disease. Flow volume is higher than 850 mL/min.
After superior mesenteric artery measurements, the entire abdomen was first examined by conventional B-mode ultrasound, with special attention paid to the terminal ileum. We looked for signs of the bowel wall thickening with diminished peristaltic movement in the terminal ileum or in some other thickened bowel segment in the small bowel and colon. Thickening of the bowel wall of more than 2.5 mm was considered to be significant.
Color Doppler examinations of the affected gut were balanced to detect low flow rates. Color gain was adjusted dynamically during examinations to maximize gut wall vessels vascularization and to avoid excessive artificial color noise. Hyperemia of the bowel wall was subjectively divided into four grades as follows: 0 – no color signal, 1 – weak or scattered color signals, 2 – multiple color signals, and 3 – hypervascularity with abscess formation. When gut wall vessels were identified, we performed spectral analysis of arterial spectrum by pulsed Doppler, with the measurement of PSV, EDV, and resistance index. In each examination we sampled at least 3-12 mural arterial spectra and calculated mean Doppler parameters for each patient (Figures 2-4).
Figure 2.
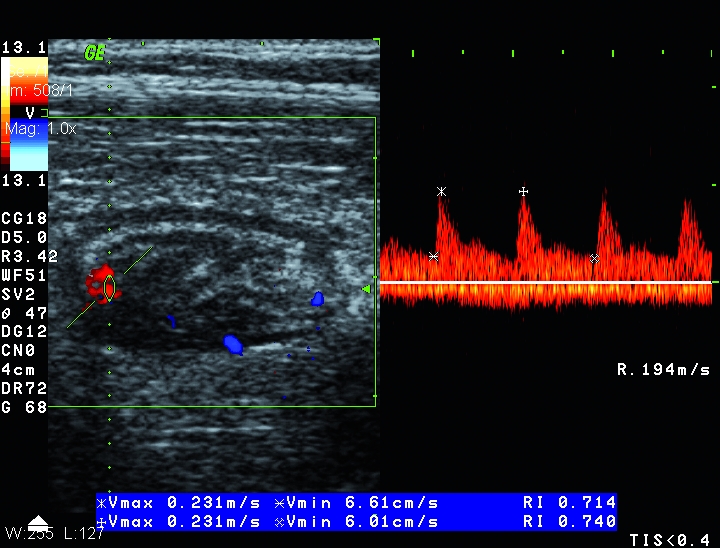
Color and pulsed Doppler measurements in thickened bowel wall of a patient with inactive Crohn’s disease. Weak Doppler signals (grade 1) and resistance index higher than 0.70.
Figure 4.
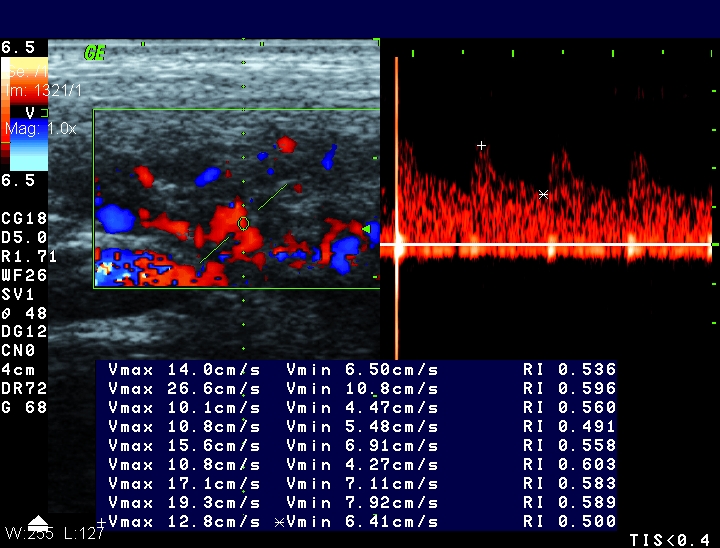
Pulsed Doppler measurements in thickened wall of the terminal ileum (the same patient as in Figure 3). Resistance index was lower than 0.60.
Figure 3.
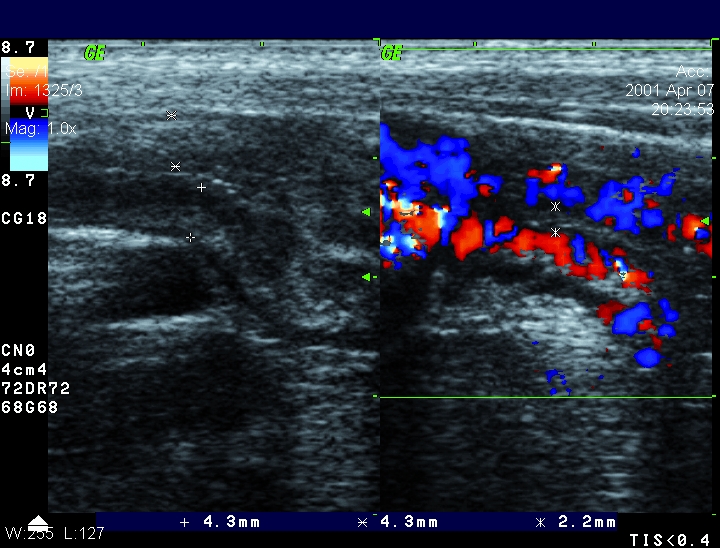
B-mod ultrasound and color Doppler of the terminal ileum of a patient with active Crohn’s disease. Wall thickening higher than 4 mm and strong hyperemia (grade 3).
Statistical analysis
Kolmogorov-Smirnov test was used to test the normality of distribution of all measured variables and showed that data were non-normally distributed. Therefore, non-parametric procedures were used in all the analyses. The results are reported as median ± interquartile range. Differences between parameters in the two groups were tested by Mann-Whitney test. Differences between the parameters in the three groups were tested by parametric Kruskal-Wallis and Mann-Whitney as a post-hoc procedure. Differences in the distribution of qualitative variables were tested by χ2 test. Correlation between the values in superior mesenteric artery and thickened bowel wall was analyzed using Spearman ρ coefficient.
Results
The measurements in the superior mesenteric artery in the three groups showed no significant difference in PSV. Significant differences were found for all other parameters (MV, CSA, EDV, resistance index, and flow volume). However, all differences were too small to be considered clinically significant, except higher flow volume in the active group (Table 2).
Table 2.
Doppler parameter values (median ± interquartile range) in the superior mesenteric artery in patients with active and inactive Crohn’s disease and control group
| Crohn’s disease |
||||
|---|---|---|---|---|
| Doppler parameters* | active (n = 63) | inactive (n = 60) | control (n = 67) | P† |
| PSV (m/s) |
1.4 ± 0.7 |
1.6 ± 0.6 |
1.4 ± 0.8 |
0.295 |
| EDV (m/s) |
0.24 ± 0.10‡ |
0.22 ± 0.08 |
0.24 ± 0.07 |
<0.001 |
| RI |
0.839 ± 0.036§ |
0.856 ± 0.033 |
0.860 ± 0.060 |
0.003 |
| MV (cm/s) |
39.1 ± 15.4 |
33.1 ± 13.3║ |
33.5 ± 17.7 |
0.035 |
| CSA (cm2) |
0.24 ± 0.05¶ |
0.21 ± 0.04 |
0.20 ± 0.07 |
0.001 |
| FVO (mL/min) | 564 ± 263** | 421 ± 157 | 416 ± 248 | <0.001 |
*Abbreviations: PSV – peak systolic velocity; EDV – end-diastolic velocity; RI – resistance index; MV – mean velocity flow; CSA – cross-sectional area; FVO – flow volume.
†Kruskal-Wallis test.
‡Significant difference from control group (P = 0.004, Mann-Whitney test).
§Significant difference from inactive and control group (P = 0.033 and P = 0.002 respectively, Mann-Whitney test).
║Significant difference from inactive group (P = 0.004, Mann-Whitney test).
¶Significant difference from inactive and control group (P<0.001 and P = 0.023 respectively, Mann-Whitney test).
**Significant difference from inactive and control group (P<0.001 and P = 0.002 respectively, Mann-Whitney test).
The second part of the examination included high resolution Doppler ultrasound of inflammable intestinal segment. In the control group there was no thickening or hyperemia (grade 0) of the bowel wall. Gut wall was maximum 2 mm thick with peristaltic activity. Only in 15 examinees we detected some intestinal branches of the superior mesenteric artery (Table 3).
Table 3.
Measurements of wall thickness, color Doppler hyperemia, and resistance index in thickened bowel wall in patients with active and inactive Crohn’s disease and control group
| Crohn’s disease |
||||
|---|---|---|---|---|
| Doppler ultrasound parameters | active (n = 74) | inactive (n = 64) | control (n = 67) | P |
| Wall thickness* (mm) |
5.0 ± 1.8 |
3.1 ± 1.4 |
<2 |
<0.001* |
| Color hyperemia (grade): |
||||
| 0 |
0 |
16 |
67 |
<0.001‡ |
| 1 |
6 |
45 |
0 |
|
| 2 |
56 |
3 |
0 |
|
| 3 |
12 |
0 |
0 |
|
| Resistance index* | 0.61 ± 0.05 | 0.71 ± 0.05§ | 0.80 ± 0.11║ | <0.001¶ |
*Median ± interquartile range.
†Mann-Whitney test for comparison of active and inactive group.
‡χ2 test.
§48 examinees.
║15 examinees.
¶Significant differences between all three groups (Kruskal-Wallis test and Mann-Whitney test as pot hoc, P<0.001 for all).
In the group of patients with inactive Crohn’s disease we found median thickening ±Q of the gut wall of 3.1 ± 1.4 mm, without peristaltic activity. On color Doppler, 61 out of 64 patients with inactive Crohn’s disease showed weak or scattered color signals (grade 0, 1) and only 3 of them showed moderate signals. Median resistance index±Q for 48 patients (those with pulsed Doppler analysis) in inactive group was 0.71 ± 0.05; with a range of 0.65-0.87. In 74 patients with active Crohn’s disease we found affected, non-compressible loops with thickened wall (5.0 ± 1.8 mm), and clearly visible wall hyperemia (grades 2, 3) in all but 6 patients (grade 1). The spectral analysis showed low resistance arterial spectra with median resistance index±Q 0.61 ± 0.05; range 0.52-0.67 (Table 3).
Bowel wall thickness measurement showed a significant difference between inactive and active Crohn’s disease group (P<0.001, Mann-Whitney test). Color Doppler analysis showed a significant difference between all groups (P<0.001, χ2 test). The analysis of affected bowel loops showed a significant difference between all three groups by measuring resistance index in the gut wall (P<0.001, Kruskal-Wallis and Mann-Whitney as post hoc; Table 3).
We tested the correlation between the pulsed Doppler parameters (PSV, EDV, resistance index) in the superior mesenteric artery and affected bowel wall in 122 patients with Crohn’s disease, Spearman ρ coefficient. Resistance index values showed low but significant correlation (Spearman ρ coefficient, ρ = 0.29, P<0.050).
Discussion
Our results showed that Doppler measurements in the superior mesenteric artery are not reliable in routine clinical practice, although we found higher flow volume in the superior mesenteric artery in active Crohn’s disease. We obtained low significant correlation between resistance index in superior mesenteric artery and affected bowel wall in 122 patients with Crohn’s disease. The most accurate Doppler parameter in Crohn’s disease activity assessment was the estimation intensity of color Doppler signals and resistance index measurements of mural arteries in thickened bowel wall.
Recent data (7,8) have shown that superior mesenteric artery flow was increased in active Crohn’s disease. Similar results, with higher values of flow volume or lower resistance index, were recorded in different studies (9-11). In several studies (11,17,18) van Oostayen et al found no difference in flow volume between inactive and control group, although it was found between control and active group. They emphasize the “gray zone” of flow volume in the superior mesenteric artery between 450 and 600 mL/min, where an overlap can occur between the control group, as well as mildly active Crohn’s disease patients, and inactive Crohn’s disease patients, which is similar to the findings of our study. Patients with active Crohn’s disease in the “gray zone” cannot be discriminated from inactive and control group by measuring flow volume alone. Hare et al (19) have assumed that the flow volume threshold value between the normal and higher values in superior mesenteric artery was 500 mL/min. Our study also showed similar results; median flow volume was 564 ± 263 mL/min in active Crohn’s disease patients, 421 ± 157 mL/min in inactive Crohn’s disease patients, and 416 ± 248 mL/min in the control group. Byrne et al (20) have evaluated similar Doppler parameters in the superior mesenteric artery and found three significantly different parameters in active and inactive Crohn’s disease group. We found very similar results; the most sensitive parameter was flow volume, but only in the active group. Other parameters, such as CSA, EDV, and resistance index did show significant differences, but with more overlaps between all three groups and clinically irrelevant magnitude. We agree with Byrne et al (20) that Doppler measurements in the superior mesenteric artery are not reliable in routine clinical practice.
Maconi et al (21) have concluded that a hyperdynamic mesenteric circulation exists in Crohn’s disease, which however, does not reflect either the clinical or the biochemical activity of the disease, but seems to be more linked to other Crohn’s disease characteristics, such as maximum bowel thickness and anatomical location. It is obvious that hyperdynamic mesenteric flow exists in Crohn’s disease, but it is still a problem to express this excess of vascular circulation with a sensitive Doppler parameter which can discriminate active, inactive, and control group. We believe that such a sensitive parameter can be resistance index in mural arteries in thickened bowel wall.
We tested the correlation between resistance index in the superior mesenteric artery and affected bowel wall in 122 patients with Crohn’s disease. We compared the same Doppler parameter, in the same patients, but on different mesenterial vascular segments. Low, but significant correlation of resistance index values was noticed. We found this result particularly important, because it shows a connection between arterial flow in the superior mesenteric artery and mural arteries in the gut wall. The connection proves that an increased flow in the superior mesenteric artery occurs because of arterial hyperemia that appears with Crohn’s disease inflammation activity in a thickened gut wall.
We think that Doppler evaluation of thickened bowel wall (>2.5 mm) is much more important for the assessment of Crohn’s disease activity. The analysis of affected bowel segments is possible only with high resolution probe. Doppler ultrasound diagnostic difficulties can arise in the initial stage of Crohn’s disease, when pathomorphological changes affect mucosa. In advanced stages, inflammation penetrates more deep into submucosa and muscular layer. Therefore, we agree that endoscopy and conventional radiology are more accurate in mucosal changes, whereas Doppler ultrasound and computed tomography are more accurate in gut wall pathological changes (2,3).
We found significantly higher values of bowel wall thickness in active than inactive Crohn’s disease. The cause of thickening in the active group is a combination of edema, muscular spasm, and submucosal cellular inflammation. In inactive Crohn’s disease bowel wall can also be thick, due to fibrosis in chronic stage. Some authors believe that gut wall thickness measurements are crucial for assessment of Crohn’s disease activity (22-24). Futagami et al (23) defined ultrasound activity index on the basis of two parameters – gut wall thickness and stratification. Mayer et al (24) defined volume of inflammable gut wall on the basis of gut wall thickness and longitudinal extension of diseased segment. It is important to stress that these authors used only gray-scale ultrasound. In our opinion, the measurement of gut wall thickness is just a first step in the assessment of Crohn’s disease activity.
Increased mural blood flow on colored and power Doppler was described by several authors in active Crohn’s disease (4,12,13,25). Because of intense neovascularity there is an increase in color Doppler signal in and around the gut wall. Spalinger et al (26) studied the correlation of gut wall hypervascularity in Crohn’s disease with clinical disease activity by combining two criteria – vessel density on color Doppler and wall thickness. They found a significant difference in gut wall thickness and color Doppler signals between active and inactive Crohn’s disease, like we did in our research. They also found that hypervascularity and gut wall thickness higher than 5 mm indicated active Crohn’s disease. We found fair activity in patients with wall thickness less than 5 mm. Potential limitation for color Doppler studies is an estimation and not a direct count of vessels present in the area examined, because a single tortuous vessel may result in several color Doppler signals.
To the best of our knowledge, the work of Esteban et al (15) is the only study with pulsed Doppler spectral analysis in mural arteries in the thickened bowel wall. The authors distinguished active and inactive Crohn’s disease patients by measuring resistance index in mural arteries. Similar to our results, there was no thickening or hyperemia of the bowel wall in the control group. Both studies found the correlation of lower wall thickness with weak color Doppler signals in inactive Crohn’s disease and higher wall thickness with more color Doppler signals in active Crohn’s disease. We also found significant wall thickening in several patients with inactive Crohn’s disease with weak color Doppler signals due to fibrosis and scarring. Both studies found a highly significant difference in resistance index measurements in inactive and active group. We explained this resistance index difference as a result of mural arterial vasodilatation and vascular resistance decrease in correlation with neovascularity and gut wall inflammation. Since we had a higher number of patients with mural arteries resistance index values in our study, we propose borderline resistance index values to be about 0.65-0.67, because upper range resistance index for active group was 0.67 and lower range resistance index for inactive group was 0.65.
A problem can occur in the investigation of bowel segments in inactive Crohn’s disease. Forty eight of 64 inactive patients had walls thicker than 3 mm, scattered Doppler color signal, and good conditions for spectral analysis (pulsed Doppler measurements). Sixteen patients with inactive Crohn’s disease had borderline wall thickness, no color signal, and therefore no possibility for pulsed Doppler measurements. In patients with stabile remission, affected bowel loops were hard to image with Doppler ultrasound, perhaps because of the initial weak activity or treatment effect. In the detection of Crohn’s disease by ultrasound, these patients would be false negative, and we would not be able to differentiate them from the control group. In literature these hard detectable segments of bowel are called “burned out segments” (27). Detection of inactive segments and signal visualization can be improved with contrast-enhanced bowel ultrasonography (28,29). Contrast-enhanced ultrasound assessment of bowel wall vascularity is, however, a semiquantitative method and is open to subjective interpretation. Further technological improvements are expected to provide quantitative assessment (30,31). Therefore, we consider that resistance index measurement of mural arteries is important for an objective assessment of bowel wall vascularity.
In some cases, we found mesenterial vascular structure (vasa recta or intestinal artery branches) adjacent to the thickened bowel wall. Eleven patients with hypervascular mesenterium belong to the active group (data not shown), which correlates with “comb sign” described in literature (32). We did not notice this sign in inactive group. Spectral analysis of mesenterial branches showed high resistance arterial spectra (data not shown), opposite from mural low arterial spectra in the same patients, which is controversial. Therefore, we advise to measure mural arterial spectra deep in the wall, as far as possible from the serosal border. In our research Doppler ultrasound vascularity assessment in reactive enlarged lymph nodes and solid part of abscess was not examined, because our primary focus was vascularity in thickened bowel wall.
In further studies, use of contrast-enhanced bowel ultrasonography, especially in inactive group, with resistance index mural arteries measure, could provide valuable quantitative assessment of the mural vascularity. Our study showed the measurement of intensity of color Doppler signals and resistance index of mural arteries in thickened bowel wall to be simple and quantitative diagnostic tool for the assessment of Crohn’s disease activity. Therefore, the possibility of Doppler ultrasound to assess treatment response in further follow-up clinical studies is promising.
References
- 1.Valette PJ, Rioux M, Pilleul F, Saurin JC, Fouque P, Henry L. Ultrasonography of chronic inflammatory bowel diseases. Eur Radiol. 2001;11:1859–66. doi: 10.1007/s003300101065. [DOI] [PubMed] [Google Scholar]
- 2.Tarjan Z, Toth G, Gyorke T, Mester A, Karlinger K, Mako EK. Ultrasound in Crohn's disease of the small bowel. Eur J Radiol. 2000;35:176–82. doi: 10.1016/S0720-048X(00)00240-0. [DOI] [PubMed] [Google Scholar]
- 3.Rioux M, Gagnon J. Imaging modalities in the puzzling world of inflammatory bowel disease. Abdom Imaging. 1997;22:173–4. doi: 10.1007/s002619900165. [DOI] [PubMed] [Google Scholar]
- 4.Maconi G, Radice E, Greco S, Bianchi Porro G. Bowel ultrasound in Crohn's disease. Best Pract Res Clin Gastroenterol. 2006;20:93–112. doi: 10.1016/j.bpg.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Kjeldsen J, Schaffalitzky de Muckadell OB. Assessment of disease severity and activity in inflammatory bowel disease. Scand J Gastroenterol. 1993;28:1–9. doi: 10.3109/00365529309096037. [DOI] [PubMed] [Google Scholar]
- 6.Tromm A, Tromm CD, Huppe D, Schwegler U, Krieg M, May B. Evaluation of different laboratory tests and activity indices reflecting the inflammatory activity of Crohn's disease. Scand J Gastroenterol. 1992;27:774–8. doi: 10.3109/00365529209011182. [DOI] [PubMed] [Google Scholar]
- 7.Maconi G, Imbesi V, Bianchi Porro G. Doppler ultrasound measurement of intestinal blood flow inflammatory bowel disease. Scand J Gastroenterol. 1996;31:590–3. doi: 10.3109/00365529609009132. [DOI] [PubMed] [Google Scholar]
- 8.Erden A, Cumhur T, Olcer T. Superior mesenteric artery Doppler waveform changes in response to inflammation of the ileocecal region. Abdom Imaging. 1997;22:483–6. doi: 10.1007/s002619900243. [DOI] [PubMed] [Google Scholar]
- 9.Bolondi L, Gaiani S, Brignola C, Campieri M, Rigamonti A, Zironi G, et al. Changes in splanchnic hemodynamics in inflammatory bowel disease. Non-invasive assessment by Doppler ultrasound flowmetry. Scand J Gastroenterol. 1992;27:501–7. doi: 10.3109/00365529209000112. [DOI] [PubMed] [Google Scholar]
- 10.Giovagnorio F, Diacinti D, Vernia P. Doppler sonography of the superior mesenteric artery in Crohn's disease. AJR Am J Roentgenol. 1998;170:123–6. doi: 10.2214/ajr.170.1.9423614. [DOI] [PubMed] [Google Scholar]
- 11.Van Oostayen JA, Wasser MN, van Hogezand RA, Griffioen G, de Roos A. Activity of Crohn disease assessed by measurement of superior mesenteric artery flow with Doppler US. Radiology. 1994;193:551–4. doi: 10.1148/radiology.193.2.7972778. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Lees WR. Color doppler imaging in inflammatory bowel disease. Gut. 1989;30:A.1480–1. doi: 10.1136/gut.30.5.569. [DOI] [Google Scholar]
- 13.Heyne R, Rickes S, Bock P, Schreiber S, Wermke W, Lochs H. Non-invasive evaluation of activity in inflammatory bowel disease by power Doppler sonography. Z Gastroenterol. 2002;40:171–5. doi: 10.1055/s-2002-22325. [DOI] [PubMed] [Google Scholar]
- 14.Sjekavica I, Barbarić-Babić V, Krznarić Ž, Čikara I, Štern-Padovan R. Comparison of mesenteric arterial blood flow in the superior mesenteric artery and affected bowel loops by Doppler US in the assessment of Crohn's disease activity: preliminary results. Eur Radiol Suppl. 2003;6:122. [Google Scholar]
- 15.Esteban JM, Maldonado L, Sanchiz V, Minguez M, Benages A. Activity of Crohn's disease assessed by color Doppler ultrasound analysis of the affected loops. Eur Radiol. 2001;11:1423–8. doi: 10.1007/s003300000770. [DOI] [PubMed] [Google Scholar]
- 16.Zoli M, Merkel C, Sabba C, Sacerdoti D, Gaiani S, Ferraioli G, et al. Interobserver and inter-equipmental variability of echo-Doppler sonographic evaluation of the superior mesenteric artery. J Ultrasound Med. 1996;15:99–106. doi: 10.7863/jum.1996.15.2.99. [DOI] [PubMed] [Google Scholar]
- 17.Van Oostayen JA, Wasser MN, van Hogezand RA, Griffioen G, Biemond I, Lamers CB, et al. Doppler sonography evaluation of superior mesenteric artery flow to assess Crohn's disease activity: correlation with clinical evaluation, Crohn's disease activity index, and alpha 1-antitrypsin clearance in feces. AJR Am J Roentgenol. 1997;168:429–33. doi: 10.2214/ajr.168.2.9016220. [DOI] [PubMed] [Google Scholar]
- 18.Van Oostayen JA, Wasser MN, van Hogezand RA, Griffioen G, Lamers CB, de Roos A. Diagnosis of Crohn's ileitis and monitoring of disease activity: value of Doppler ultrasound of superior mesenteric artery flow. Am J Gastroenterol. 1998;93:88–91. doi: 10.1111/j.1572-0241.1998.088_c.x. [DOI] [PubMed] [Google Scholar]
- 19.Hare C, Hassan MT, Bartram CI, Forbes A. Superior mesenteric artery Doppler flow: a valuable indicator of disease activity in Crohn's disease. Gastroenterology. 1996;110:A.921. [Google Scholar]
- 20.Byrne MF, Farrell MA, Abass S, Fitzgerald A, Varghese JC, Thornton F, et al. Assessment of Crohn's disease activity by Doppler sonography of the superior mesenteric artery, clinical evaluation and the Crohn's disease activity index: a prospective study. Clin Radiol. 2001;56:973–8. doi: 10.1053/crad.2001.0794. [DOI] [PubMed] [Google Scholar]
- 21.Maconi G, Parente F, Bollani S, Imbesi V, Ardizzone S, Russo A, et al. Factors affecting splanchnic haemodynamics in Crohn's disease: a prospective controlled study using Doppler ultrasound. Gut. 1998;43:645–50. doi: 10.1136/gut.43.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haber HP, Busch A, Ziebach R, Stern M. Bowel wall thickness measured by ultrasound as a marker of Crohn's disease activity in children. Lancet. 2000;355:1239–40. doi: 10.1016/S0140-6736(00)02092-4. [DOI] [PubMed] [Google Scholar]
- 23.Futagami Y, Haruma K, Hata J, Fujimura J, Tani H, Okamoto E, et al. Development and validation of an ultrasonographic activity index of Crohn's disease. Eur J Gastroenterol Hepatol. 1999;11:1007–12. doi: 10.1097/00042737-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Mayer D, Reinshagen M, Mason RA, Muche R, von Tirpitz C, Eckelt D, et al. Sonographic measurement of thickened bowel wall segments as a quantitative parameter for activity in inflammatory bowel disease. Z Gastroenterol. 2000;38:295–300. doi: 10.1055/s-2000-14875. [DOI] [PubMed] [Google Scholar]
- 25.Jakab Z, Cserepes E, Tulassay Z. Development and validation of an ultrasonographic activity index of Crohn's disease. Eur J Gastroenterol Hepatol. 2000;12:1355–6. [PubMed] [Google Scholar]
- 26.Spalinger J, Patriquin H, Miron MC, Marx G, Herzog D, Dubois J, et al. Doppler US in patients with Crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217:787–91. doi: 10.1148/radiology.217.3.r00dc19787. [DOI] [PubMed] [Google Scholar]
- 27.Holzknecht N, Helmberger T, von Ritter C, Gauger J, Faber S, Reiser M. MRI of the small intestine with rapid MRI sequences in Crohn disease after enteroclysis with oral iron particles. Radiologe. 1998;38:29–36. doi: 10.1007/s001170050320. [in German] [DOI] [PubMed] [Google Scholar]
- 28.Kratzer W, von Tirpitz C, Mason R, Reinshagen M, Adler G, Moller P, et al. Contrast-enhanced power Doppler sonography of the intestinal wall in the differentiation of hypervascularized and hypovascularized intestinal obstructions in patients with Crohn's disease. J Ultrasound Med. 2002;21:149–57. doi: 10.7863/jum.2002.21.2.149. [DOI] [PubMed] [Google Scholar]
- 29.Di Sabatino A, Fulle I, Ciccocioppo R, Ricevuti L, Tinozzi FP, Tinozzi S, et al. Doppler enhancement after intravenous levovist injection in Crohn's disease. Inflamm Bowel Dis. 2002;8:251–7. doi: 10.1097/00054725-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 30.De Pascale A, Garofalo G, Perna M, Priola S, Fava C. Contrast-enhanced ultrasonography in Crohn's disease. Radiol Med (Torino) 2006;111:539–50. doi: 10.1007/s11547-006-0049-9. [DOI] [PubMed] [Google Scholar]
- 31.Scholbach T, Herrero I, Scholbach J. Dynamic color Doppler sonography of intestinal wall in patients with Crohn disease compared with healthy subjects. J Pediatr Gastroenterol Nutr. 2004;39:524–8. doi: 10.1097/00005176-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Lee SS, Ha HK, Yang SK, Kim AY, Kim TK, Kim PN, et al. CT of prominent pericolic or perienteric vasculature in patients with Crohn's disease: correlation with clinical disease activity and findings on barium studies. AJR Am J Roentgenol. 2002;179:1029–36. doi: 10.2214/ajr.179.4.1791029. [DOI] [PubMed] [Google Scholar]


