Summary points
Treatment should improve patients’ symptoms and reduce progression and need for surgery while minimising harms and costs
Management includes observation, lifestyle modification, drug treatment, minimally invasive surgery, and surgical removal of prostate tissue
α blockers are the most effective drug for improving lower urinary tract symptoms and short term quality of life
Combination treatment (α blockers and 5α-reductase inhibitors) reduces progression of benign prostatic hyperplasia and need for surgery if the symptoms are moderate or severe and the prostate glands large, if taken for more than a year; adverse events increase
5α-reductase inhibitors may reduce risk of prostate cancer but may increase the risk of high grade disease
Transurethral resection of the prostate results in the greatest improvement in symptoms and flow rate, but adverse effects include the risk of surgery
Minimally invasive surgery can provide benefits comparable to transurethral resection of the prostate, with fewer serious adverse effects. Short term repeat intervention and urinary retention rates are higher than with transurethral resection of the prostate. Long term effect is unclear
Management of benign prostatic hyperplasia (BPH) is mainly directed at improving bothersome lower urinary tract symptoms. The vast majority of men with these symptoms initially present to primary care seeking information about the risks and benefits of available treatments. Few men require urgent referral to a specialist for additional diagnostic testing or management. This article provides evidence to guide primary care doctors in the treatment of men with lower urinary tract symptoms, with emphasis on BPH. A previous article discussed diagnosis.1
What treatment options exist?
Treatment goals are to improve bothersome symptoms, prevent symptom progression, and reduce longer term complications (including acute urinary retention, incontinence, recurrent urinary tract infections, renal insufficiency, and the need for surgery).2 3 Options include observation (watchful waiting); lifestyle management; modification of existing medications and/or management of coexisting medical conditions; prostate and bladder specific drug treatment; and major surgical and minimally invasive surgical treatments (box). Treatment choices are primarily determined by how severe and bothersome the symptoms are and by patient preferences for types of interventions based on their weighting of established effectiveness and adverse effects. Surgery provides the largest improvement in symptom score (on the American Urological Association’s international prostate symptom scale), with minimally invasive surgery producing greater changes in symptom relief than medical treatments.2 3
Treatment options for lower urinary tract symptoms
Observation or watchful waiting
No intervention needed beyond explanation and reassurance
Lifestyle modification
Reduce fluid or diuretic intake and/or modify behaviours to reduce the severity of symptoms and reduce the bothersome nature of the symptoms: avoid excess or night-time fluid intake, caffeine, and alcohol; void the bladder before long trips, meetings, or bed time
Treatment of comorbid contributing conditions
This may improve symptoms and result in reduced diuresis: improve the control of diabetes, adjust diuretic medications
Drug treatments
Use α antagonists to improve bladder and prostate smooth muscle tone; 5α-reductase inhibitors to reduce prostate volume; combination therapy with α antagonists plus 5α-reductase inhibitors; and anticholinergics (for symptoms of an overactive bladder) to decrease hypercontractility of the detrusor muscle
Major surgical treatments
Transurethral resection of the prostate: improves urinary symptoms and flow by “coring out the prostate” through the urethra using instrument with light source
Transurethral incision of the prostate: for small prostates, relieves urethral narrowing by using special instrument to make incisions in prostate through urethra
Other ablative surgical treatments use higher energy devices to resect or vaporise prostate tissue: transurethral laser prostatectomy (resection/enucleation); transurethral laser prostatectomy (vaporisation); bipolar transurethral resection of the prostate; transurethral electrovaporisation of the prostate; bipolar transurethral electrovaporisation of the prostate; transurethral vaporesection of the prostate; bipolar transurethral vaporesection of the prostate
Minimally invasive surgical treatments
These destroy prostate tissue using low energy devices through coagulative necrosis: transurethral microwave thermotherapy; transurethral needle ablation of the prostate; high intensity focused ultrasonography; transurethral ethanol ablation of the prostate; transurethral laser coagulation of the prostate; water thermotherapy
Lifestyle management
Many men are reassured to be told that lower urinary tract symptoms are common in ageing men, typically progress slowly over time, rarely result in urgent or life threatening complications, are not due to prostate cancer, and do not increase their risk of developing prostate cancer. Simple lifestyle modifications (box) or adjustment of medications that can worsen urinary symptoms (such as diuretics) can result in acceptable improvement.2,3,4 Participation in self help groups may also improve outcomes.5 6
Drug therapy
Monotherapy with α-1 selective adrenergic antagonists or 5α-reductase inhibitors
For men with moderate or severe symptoms that do not improve satisfactorily with lifestyle management, drug treatments for BPH can be effective (box), with an average reduction in the international prostate symptom scale (range 0 to 35) of three to six points from baseline. A four point change in the international prostate symptom score corresponds with a noticeable difference by patients and is used to assess the clinical significance of interventions or symptom progression.7 On the basis of this criterion, about 60% of men will notice an improvement of their symptoms with drug treatment.2,3,4
Systematic reviews of randomised controlled clinical trials evaluating effectiveness and adverse effects of drug treatments have shown that in the first year of treatment, α-1 selective adrenergic antagonists (α blockers) are more effective than 5α-reductase inhibitors in improving symptoms.2,3,4 All α blockers have similar efficacy in improving symptoms and urinary flow rate, and their effect is generally maximal within a month of treatment starting. In most men who respond to an α blocker and who tolerate it well initially, the drug continues to work and be well tolerated for many years.4 Head to head trials of α blockers are few, small, and have serious methodological limitations.2,3,4 8 9 Terazosin and doxazosin require dose titration to minimise adverse effects at the start (such as dizziness and syncope). Tamsulosin and alfuzosin do not require dose titration, but no convincing evidence exists that they cause fewer cardiovascular adverse effects—such as symptomatic hypotension—than other α blockers.2,3,4 8 9 Few data exist on the safety of α blockers in men taking drugs for erectile dysfunction; however, there is no absolute contraindication to their concomitant use.
Combination therapy
A combination of an α blocker and a 5α-reductase inhibitor has similar effects on quality of life to an α blocker alone in the first year and a half of treatment.10 Long term effectiveness of combination therapy on symptom progression and need for surgery depends on prostate size as assessed by digital rectal examination, ultrasonography, or level of prostate specific antigen. For men with moderate to severe symptoms and a large prostate (>40 g) on digital rectal examination or ultrasonography or a baseline level of prostate specific antigen of >4 ng/ml, combination therapy can prevent about two episodes of clinical progression per 100 men per year over four years of treatment. Effectiveness was considerably less (or non-existent) in men with smaller prostates.
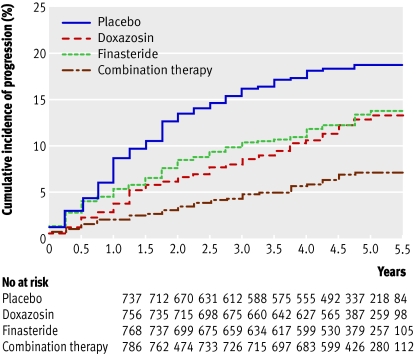
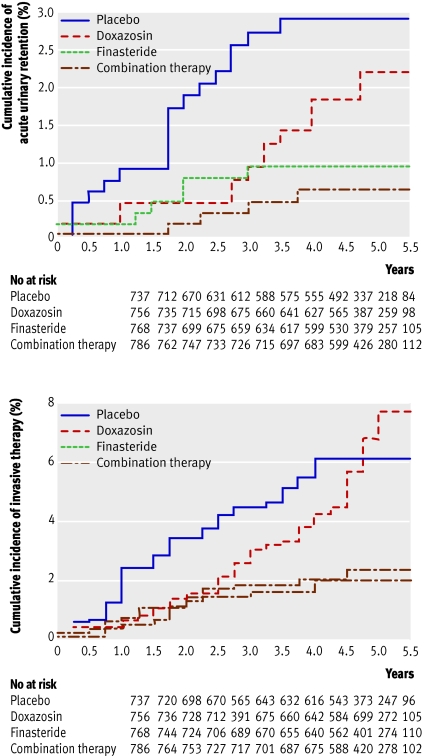
Most clinical progression is due to worsening symptoms rather than development of health threatening complications or need for surgery (figs 1 and 2). Disadvantages of the combination therapy described above compared with an α blocker alone include the need for treatment for more than a year before a difference in outcomes is usually noticed; the fact that most men will have no additional benefit; higher medication costs; and sexual side effects (from the 5α-reductase inhibitors), which occur in about four additional patients per 100.
Fig 1 Cumulative incidence of progression of benign prostatic hyperplasia in men with moderate to severe symptoms at baseline. Progression was defined by an increase of at least four points in the international prostate symptom score, acute urinary retention, incontinence renal insufficiency, or recurrent urinary tract infection
Fig 2 Cumulative incidence of acute urinary retention and invasive therapy for BPH. Adapted from McConnell et al
A possible side benefit of 5α-reductase inhibitors is their use as primary prevention of prostate cancer—regardless of the presence or severity of lower urinary tract symptoms—in men with levels of prostate specific antigen of <4 ng/ml.11 However, the risk of being diagnosed with high grade prostate cancer is increased. Whether this is a true increased presence of high grade disease or merely a histological or sampling artefact caused by reduction in prostate size owing to 5α-reductase inhibitors is not certain.10 If prostate specific antigen is measured with the aim of detecting prostate cancer, thresholds for “abnormal” values should be lowered because 5α-reductase inhibitors reduce the antigen values by about 50%.11 12
The decision on when to use combination therapy for lower urinary tract symptoms is complex and should ideally be based on informed, shared decision making between patients and providers that incorporates the above information on benefits and harms for the urinary symptoms as well as prevention of prostate cancer.
Phytotherapy
Numerous plant based products (phytotherapy) are commonly used for self treatment of lower urinary tract symptoms and can be prescribed in some European countries. Systematic reviews have suggested that both saw palmetto and Pygeum africanum provided modest improvement in urinary symptoms and flow.13 14 However, a recent high quality randomised trial found that saw palmetto was no more effective than placebo in men with BPH and moderate to severe symptoms.15 Ongoing trials are assessing long term effectiveness and safety of varying doses of both saw palmetto and Pygeum africanum.
Antimuscarinics for storage problems
For some men, symptoms of storage problems—such as urinary urgency (with or without urge incontinence), frequency, small urine volumes, and nocturia—in the absence of serious obstructive symptoms are predominant. Recently this symptom complex has been categorised as overactive bladder syndrome. For these men options such as bladder training, biofeedback, and antimuscarinic drugs may be useful either alone or in combination with treatment that is more specifically directed at benign prostatic enlargement.16 17 A systematic review of 56 trials found that antimuscarinics (oxybutynin, tolterodine, trospium, solifenacin, and darifenacin) were safe and efficacious in the treatment of overactive bladder syndrome. All antimuscarinics except immediate release oxybutynin were well tolerated.18 Dry mouth was the most commonly reported adverse event, and no drug was associated with an increase in any serious adverse event.
Antimuscarinics should be used with caution in men with severe obstructive or voiding symptoms as these patients may have high residual urine volumes (more than 150 ml) and antimuscarinics have a theoretical risk of precipitating a deterioration of voiding symptoms including urinary retention. The evidence for this risk, however, is weak.
What are the benefits and harms of surgery?
Evidence of effectiveness for minimally invasive surgical treatments comes from case series and randomised trials versus transurethral resection of the prostate, other minimally invasive treatments, sham procedures, and drug interventions.
If conservative management does not give sufficient symptom relief, the standard surgical option is transurethral resection of the prostate. This involves endoscopic removal of the inner (paraurethral) zones of the enlarged prostate using a diathermy loop. Although highly effective (average improvement in score at 16 months is 10 to 18 points from baseline) in relieving both symptoms and urodynamic obstruction, this surgical procedure requires an anaesthetic and a stay in hospital and carries a 5% risk of severe haemorrhage.2 3 19 Because of this, newer procedures using alternative energy sources (such as ultrasound, laser, or microwave) have been developed. Some do not require a general anaesthetic and can be carried out in an outpatient setting with fewer adverse effects. However, uncertainty remains about their long term clinical and cost effectiveness.
Current standard
Transurethral resection of the prostate has been the standard method of surgical management of clinical BPH for 50 years. In recent times the procedure accounted for more than 90% of prostatectomies for BPH, although now the proportion is only 60-80% because of new minimally invasive procedures.20 Improvements in endoscope design, diathermy units, and bladder irrigation have reduced operating time and risk of major morbidity.20 In respect of symptoms associated with BPH, transurethral resection of the prostate provides a consistent, long lasting, high level of improvement in quality of life and peak urine flow rate.2 19
New technology
Newer surgical options for BPH can be broadly divided into “minimally invasive” and “tissue ablative” treatments. Minimally invasive surgical treatments do not remove tissue but cause in situ coagulative necrosis through low energy heating devices (40-80°C). These include: transurethral microwave therapy, radiofrequency transurethral needle ablation, and transurethral or interstitial laser coagulation. Such treatments can be carried out in an ambulatory care environment under simple analgesia or sedation with minimal anaesthesia, but they generally require a prolonged period of bladder catheterisation. Most of these treatments are used in men with smaller prostate volumes (between 30 ml and 100 ml, and levels of prostatic specific antigen <4.0 ng/ml) and no history of urinary retention or previous prostate surgery. Improvement in symptoms and urinary flow rate is slightly poorer with minimally invasive treatments than with transurethral resection of the prostate but better than with α blocker therapy.16,17,18 Adverse effects are less common than with transurethral resection of the prostate, but repeat treatment or more invasive treatment is needed for about 30% of men.2 3 19,20,21,22,23
Tissue ablative procedures use similar transurethral instrumentation as transurethral resection of the prostate but differing energy sources that can remove tissue efficiently by vaporisation or resection but cause less bleeding. They include laser resection or vaporisation, monopolar diathermy vaporisation, and bipolar diathermy vaporisation/resection. Procedures such as monopolar diathermy vaporisation and holmium laser enucleation of the prostate give similar improvement in symptoms and quality of life as transurethral resection of the prostate, with less risk of major blood loss. The improved haemostatic properties of these procedures also allow earlier discharge from hospital, saving about one bed-day compared with transurethral resection of the prostate. These procedures may offer particular advantages for men taking anticoagulants and those with cardiac or renal disease as the requirement for irrigation of the bladder during and after surgery is much reduced and haemoglobin concentrations are maintained.
Adverse effects of surgery
Sexual side effects, particularly loss of ejaculation and erectile dysfunction, are of concern to men having prostate surgery. The risk of retrograde ejaculation is significantly lower for minimally invasive procedures. For ablative procedures, the risk is similar to transurethral resection of the prostate.16,17,18 Reassuringly, the occurrence of ejaculatory dysfunction does not seem to lower quality of life much after prostate surgery. Rates of erectile dysfunction are similar across all procedures, although lack of baseline data and spontaneous development of erectile dysfunction in this older age group are likely sources of bias.2 3 19 21,22,23
Incontinence is similar across all interventions with the exception of transurethral needle ablation of the prostate and laser coagulation (where reported rates were lower), although comparative analysis is hampered by variability in definition. The other most pertinent long term adverse effect is the need for further treatment owing to stricture formation, urinary retention, or disease relapse. Unfortunately, long term follow-up data from randomised controlled trials are not available. The best estimates concern transurethral resection of the prostate, for which long term observational studies suggest a 1% annual risk of requiring further treatment. Shorter term studies suggest further treatment is more frequently required when minimally invasive options such as transurethral microwave thermotherapy are used, which probably reflects the smaller amount of tissue destroyed by these procedures.
Transurethral resection of the prostate remains widely used throughout the world. In communities with ageing populations and access to newer technologies the lower risk of bleeding during laser resection or vaporisation techniques may be advantageous for men with extensive comorbidity or long term anticoagulation. For most healthcare providers, however, the benefits of widespread introduction of new technologies are insufficient to justify the start-up and consumable costs currently associated with these procedures. For the UK NHS minimally invasive options such as transurethral microwave thermotherapy are not approved for use, and the availability of newer methods of ablation such as laser vaporisation or holmium laser enucleation of the prostate currently depends on local enthusiasm and investment.
When is referral to a urologist indicated?
The vast majority of men can receive appropriate treatment by their primary care provider. In our earlier article we said that referral to a urologist may be indicated on the basis of patient preference or for further assessment of men presenting with atypical lower urinary tract signs or symptoms including new onset urinary incontinence, haematuria, dysuria, recurrent urinary tract infections, urinary obstruction, or raised levels of prostate specific antigen.1 Referral for potential surgical or minimally invasive interventions is appropriate for men with moderate to severe bothersome symptoms who might prefer surgery to medical treatment or for those in whom medical treatment has not provided adequate symptom improvement or is not well tolerated.
Data sources
We searched Medline and the Cochrane Library up to November 2007 for randomised trials, systematic reviews, evidence reports, and recent evidence based guidelines from the American Urological Association, European Urological Association, and the National Institute for Health and Clinical Excellence.
Additional educational resources
PatientPlus (www.patient.co.uk/showdoc/40002437/)—Comprehensive, free, up to date health information as provided by general practitioners to patients during consultations
Foundation for Informed Medical Decision Making (www.fimdm.org)—Provides information to help patients make sound decisions affecting their health and wellbeing
Health Dialog (www.healthdialog.com)—Provides care management and analytical services
Cochrane Library (www.cochrane.org)—Publishes systematic reviews of the effects of healthcare interventions
Ongoing research
Discover and test new drugs to prevent or reduce benign prostatic hyperplasia
Determine whether phytotherapies including saw palmetto and Pygeum africanum used alone or in combination improve urinary symptoms and/or prevent progression
Determine whether antimuscarinics plus α blockers are safe and more effective for men with urinary symptoms than monotherapy alone
Determine whether medical treatments (5α-reductase inhibitors) can reduce incidence and mortality of prostate cancer
Determine whether drugs typically used to treat erectile dysfunction are also effective for treatment of urinary symptoms
Determine factors limiting use and outcomes of minimally invasive surgery
Unanswered questions
Given the chronic nature of benign prostatic hyperplasia, are escalating treatment strategies cost effective compared with one-off treatments (for example, is a single surgical intervention more cost effective than many years of drug treatment)?
Should surgical ablation be used as a primary treatment or only after failure of drug therapy?
Do phytotherapeutic compounds improve urinary symptoms and prevent progression?
Contributors: Both authors conceived the idea; contributed intellectual content; acquired and analysed evidence; and wrote, reviewed, and edited the manuscript.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Wilt TJ, N’Dow J. Benign prostatic hyperplasia. Part 1—Diagnosis. BMJ 2008;336:PAGES - BMJ TO COMPLETE. [DOI] [PMC free article] [PubMed]
- 2.American Urological Association. Guideline on the management of benign prostatic hyperplasia (BPH) www.auanet.org/guidelines/bph.cfm (updated 2006).
- 3.European Association of Urology. Guidelines on benign prostatic hyperplasia. www.uroweb.org/fileadmin/user_upload/Guidelines/11%20BPH.pdf (updated 2004).
- 4.Helfand M, Muzyk T, Garzatto M. Benign prostatic hyperplasia (BPH). Management in primary care—screening and therapy Department of Veterans Affairs Health Services Research and Development. 2007. www1.va.gov/hsrd/publications/esp/BPH-2007.pdf [PubMed]
- 5.Brown CT, Yap T, Cromwell DA, Rixon L, Steed L, Mulligan K, et al. Self management for men with lower urinary tract symptoms: randomised controlled trial. BMJ 2007;334:25-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speakman MJ, Kirby RS, Joyce A, Abrams P, Pocock R. Guideline for the primary care management of male lower urinary tract symptoms. BJU Int 2004;93:985-90. [DOI] [PubMed] [Google Scholar]
- 7.Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995;154:1770-4. [DOI] [PubMed] [Google Scholar]
- 8.Wilt T, MacDonald R, Rutks I. Tamsulosin for benign prostatic hyperplasia. Cochrane Database Syst Rev 2002;(4):CD002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilt T, Howe RW, Rutks I, MacDonald R. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev 2000;(1):CD003851. [DOI] [PubMed] [Google Scholar]
- 10.McConnell JD, Roehrborn CB, Bautistia OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387-98. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215-24. [DOI] [PubMed] [Google Scholar]
- 12.Wilt TJ, MacDonald R, Hagerty K, Schellhammer P, Kramer BS. 5-α-reductase inhibitors for prostate cancer chemoprevention. Cochrane Library (in press). [DOI] [PMC free article] [PubMed]
- 13.Wilt T, Ishani A, MacDonald R, Rutks I, Stark G. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev 1998;(1):CD001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev 2002;(3):CD001423. [DOI] [PubMed] [Google Scholar]
- 15.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med 2006;354:557-66. [DOI] [PubMed] [Google Scholar]
- 16.Chapple CR. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol 2006;49:651-8. [DOI] [PubMed] [Google Scholar]
- 17.Ruggieri MR, Braverman AS, Pontarivol MA. Combined use of alpha-adrenergic and muscarinic antagonists for the treatment of voiding dysfunction. J Urol 2005;174:1743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapple C, Khullarb V, Gabriel Z, Dooley JA. The effects of antimuscarinictreatments in overactive bladder: a systematic review and meta-analysis. Eur Urol 2005;48:5-26. [DOI] [PubMed] [Google Scholar]
- 19.Lourenco T, Armstrong N, Nabi G, Deverill M, Pickard R, Vale L, et al. Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement. Health Technology Assessment (in press). (www.hta.ac.uk/project/1468.asp) [DOI] [PubMed]
- 20.Emberton M, Neal DE, Black N, Harrison M, Fordham M, McBrien MP, et al. The national prostatectomy audit: the clinical management of patients during hospital admission. Br J Urol 1995;75:301-16. [DOI] [PubMed] [Google Scholar]
- 21.Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol 2006;49:970-8. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman RM, MacDonald R, Wilt TJ. Laser prostatectomy for benign prostatic obstruction. Cochrane Database Syst Rev 2000;(1):CD001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman RM, Monga M, Elliot SP, MacDonald R, Wilt TJ. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database Syst Rev 2007;(4):CD004135. [DOI] [PubMed] [Google Scholar]