Abstract
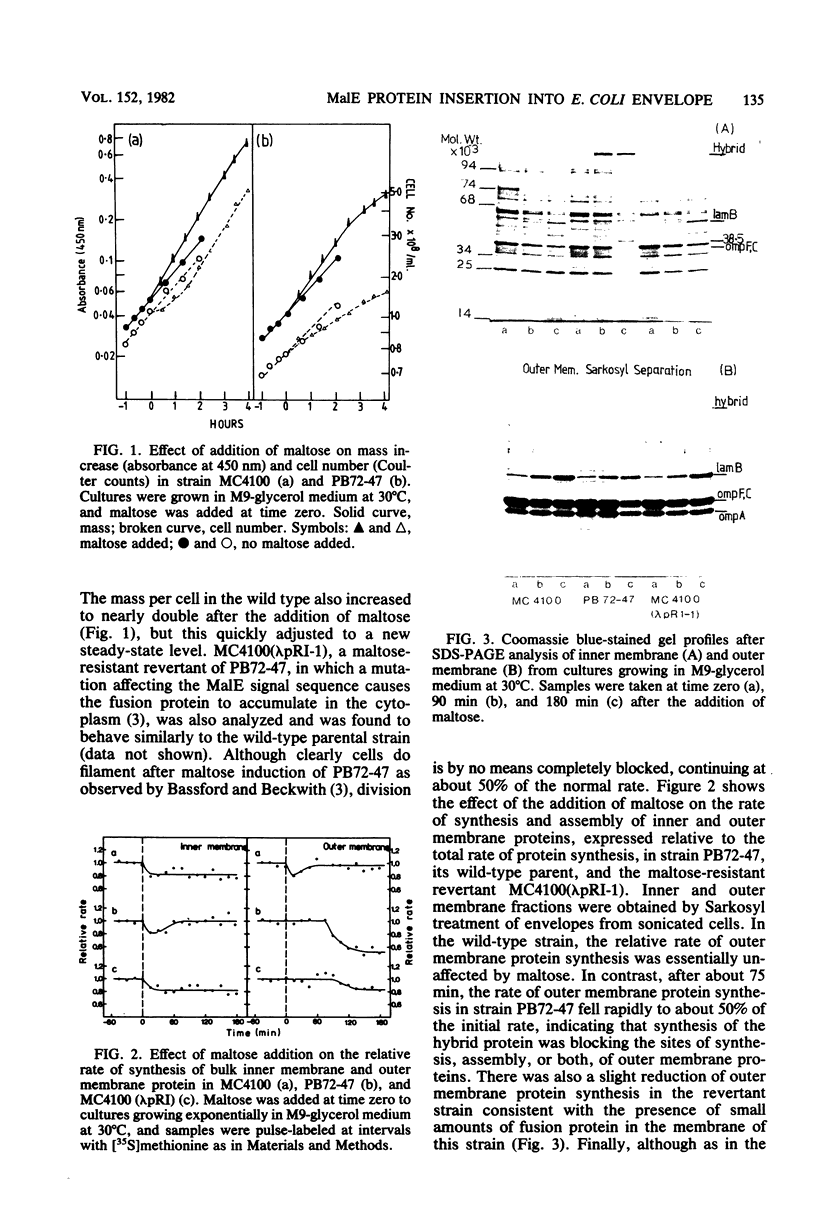
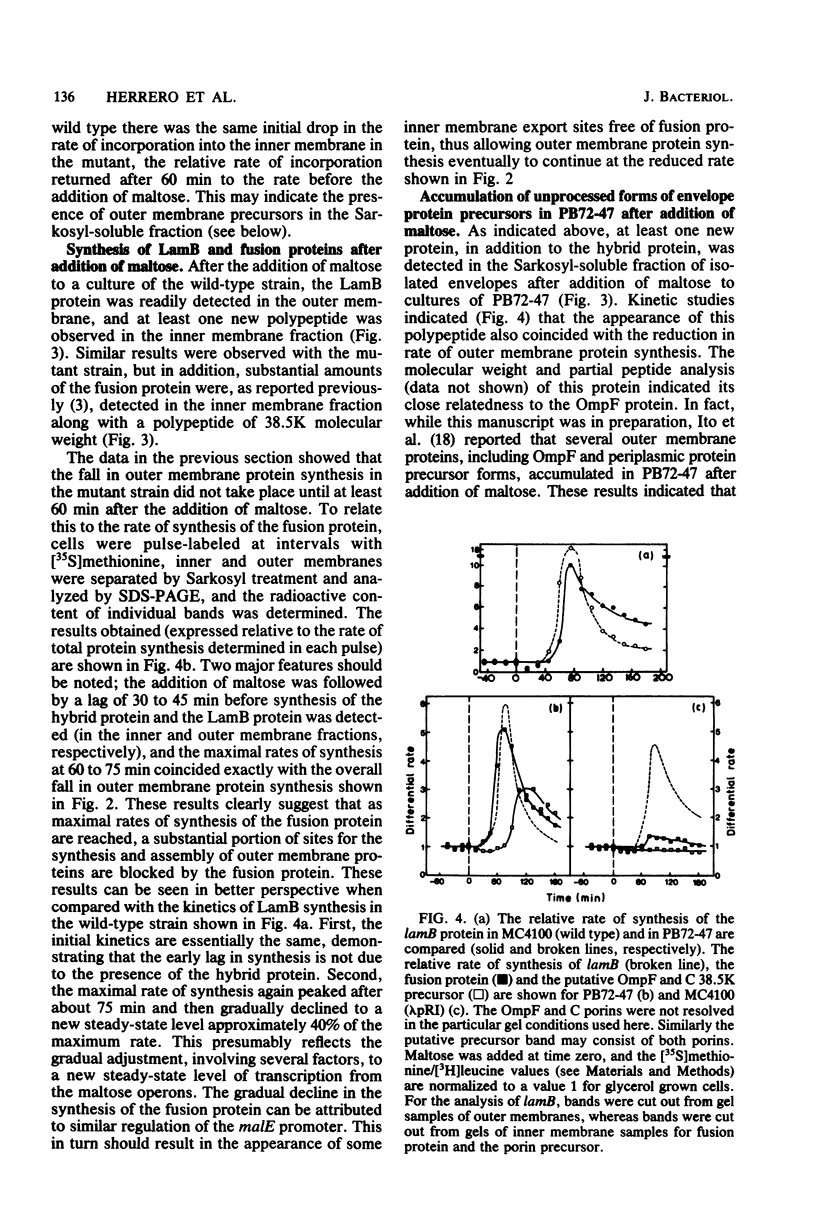
The synthesis of a membrane-bound MalE β-galactosidase hybrid protein, when induced by growth of Escherichia coli on maltose, leads to inhibition of cell division and eventually a reduced rate of mass increase. In addition, the relative rate of synthesis of outer membrane proteins, but not that of inner membrane proteins, was reduced by about 50%. Kinetic experiments demonstrated that this reduction coincided with the period of maximum synthesis of the hybrid protein (and another maltose-inducible protein, LamB). The accumulation of this abnormal protein in the envelope therefore appeared specifically to inhibit the synthesis, the assembly of outer membrane proteins, or both, indicating that the hybrid protein blocks some export site or causes the sequestration of some limiting factor(s) involved in the export process. Since the MalE protein is normally located in the periplasm, the results also suggest that the synthesis of periplasmic and outer membrane proteins may involve some steps in common. The reduced rate of synthesis of outer membrane proteins was also accompanied by the accumulation in the envelope of at least one outer membrane protein and at least two inner membrane proteins as higher-molecular-weight forms, indicating that processing (removal of the N-terminal signal sequence) was also disrupted by the presence of the hybrid protein. These results may indicate that the assembly of these membrane proteins is blocked at a relatively late step rather than at the level of primary recognition of some site by the signal sequence. In addition, the results suggest that some step common to the biogenesis of quite different kinds of envelope protein is blocked by the presence of the hybrid protein.
Full text
PDF

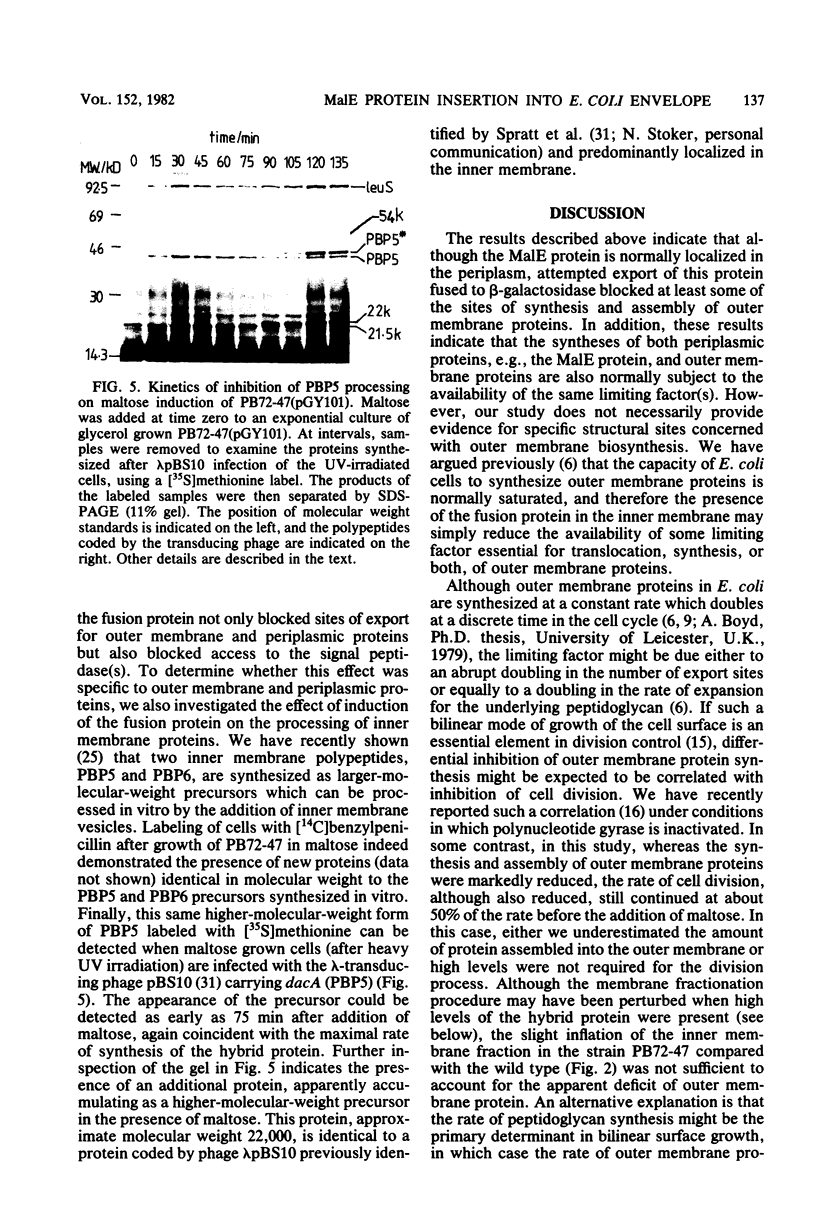


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Herrero E., Esteve M. I., Guerrero R. Surface density of major outer membrane proteins in Salmonella typhimurium in different growth conditions. J Gen Microbiol. 1980 Oct;120(2):355–367. doi: 10.1099/00221287-120-2-355. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Intermediate location in the assembly of the matrix protein or porin into the outer membrane of Escherichia coli. J Bacteriol. 1980 Sep;143(3):1538–1541. doi: 10.1128/jb.143.3.1538-1541.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Holland I. B. Regulation of the synthesis of surface protein in the cell cycle of E. coli B/r. Cell. 1979 Oct;18(2):287–296. doi: 10.1016/0092-8674(79)90048-5. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Model P., Blobel G. Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1251–1255. doi: 10.1073/pnas.76.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G. G., Holland I. B. Envelope synthesis during the cell cycle in Escherichia coli B/r. J Mol Biol. 1976 Aug 5;105(2):245–261. doi: 10.1016/0022-2836(76)90110-8. [DOI] [PubMed] [Google Scholar]
- Crowlesmith I., Gamon K., Henning U. Precursor proteins are intermediates in vivo in the synthesis of two major outer membrane proteins, the OmpA and OmpF proteins, of Escherichia coli K12. Eur J Biochem. 1981 Jan;113(2):375–380. doi: 10.1111/j.1432-1033.1981.tb05076.x. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Inouye M. Translocation and assembly of outer membrance proteins of Escherichia coli. Selective accumulation of precursors and novel assembly intermediates caused by phenethyl alcohol. J Mol Biol. 1979 May 5;130(1):39–61. doi: 10.1016/0022-2836(79)90551-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Herrero E., Fairweather N. F., Holland I. B. Envelope protein synthesis and inhibition of cell division in Escherichia coli during inactivation of the B subunit of DNA gyrase. J Gen Microbiol. 1982 Feb;128(2):361–369. doi: 10.1099/00221287-128-2-361. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wang S., Inouye M. Cell-free synthesis of a specific lipoprotein of the Escherichia coli outer membrane directed by purified messenger RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4149–4153. doi: 10.1073/pnas.71.10.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Levine A., Bailone A., Devoret R. Cellular levels of the prophage lambda and 434 repressors. J Mol Biol. 1979 Jul 5;131(3):655–661. doi: 10.1016/0022-2836(79)90014-7. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Plastow G. S., Holland I. B. Identification of an Escherichia coli inner membrane polypeptide specified by a lambda-tonB transducing. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1007–1014. doi: 10.1016/0006-291x(79)91927-2. [DOI] [PubMed] [Google Scholar]
- Pratt J. M., Holland I. B., Spratt B. G. Precursor forms of penicillin-binding proteins 5 and 6 of E. coli cytoplasmic membrane. Nature. 1981 Sep 24;293(5830):307–309. doi: 10.1038/293307a0. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Sekizawa J., Inouye S., Halegoua S., Inouye M. Precursors of major outer membrane proteins of Escherichia coli. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1126–1133. doi: 10.1016/s0006-291x(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Boyd A., Stoker N. Defective and plaque-forming lambda transducing bacteriophage carrying penicillin-binding protein-cell shape genes: genetic and physical mapping and identification of gene products from the lip-dacA-rodA-pbpA-leuS region of the Escherichia coli chromosome. J Bacteriol. 1980 Aug;143(2):569–581. doi: 10.1128/jb.143.2.569-581.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Insertion of newly synthesized proteins into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1978 Sep 22;512(2):365–376. doi: 10.1016/0005-2736(78)90260-2. [DOI] [PubMed] [Google Scholar]