Abstract
Nitric oxide (NO) is a second messenger with diverse roles in the cardiovascular system, such as inhibiting thrombosis and limiting vascular inflammation. One mechanism by which NO modulates such disparate physiological processes is by regulating protein trafficking within cells. NO inhibits exocytosis of endothelial granules which would otherwise trigger inflammation. NO also blocks platelet secretion of granules that would otherwise activate thrombosis. NO decreases granule trafficking from the Golgi to the plasma membrane by targeting a key component of the exocytic machinery, N-ethylmaleimide sensitive factor (NSF). In contrast to its inhibitory effects on exocytosis, NO accelerates endocytosis. S-nitrosylation of dynamin increases its ability to hydrolyze GTP, assemble in oligomers around a nascent vesicle, and cleave the endocytic vesicle free from the plasma membrane. NO regulation of vesicle trafficking is a molecular mechanism that explains some of the cardiovascular effects of NO, and may be of broad physiological significance.
1. Introduction
NO was originally identified as an endogenous vasodilator, and was later found to regulate every type of vascular cell. NO relaxes smooth muscle cells, inhibits platelet aggregation, promotes angiogenesis, recruits endothelial progenitor cells, and decreases inflammation. Although some of these effects of NO are mediated by its ability to stimulate guanylyl cyclase and elevate cGMP, other pathways targeted by NO were initially elusive. Subsequent research revealed that NO controls inflammation and thrombosis in part by regulating vesicle trafficking. NO moderates the antegrade transport of proteins from the Golgi apparatus to the plasma membrane, and NO modulates the retrograde transport of proteins from the plasma membrane to endosome. S-nitrosylation of critical molecular motors is a fundamental mechanism by which NO controls the rate of protein trafficking.
2. NO Inhibits Vascular Inflammation
NO is a potent anti-inflammatory agent. Exogenous NO donors decrease leukocyte adherence to the vessel wall; conversely, NOS inhibitors increase leukocyte rolling along the endothelium [1]. NO derived from each of the NOS isoforms inhibits leukocyte interactions with the vessel wall. Leukocyte adherence to the vessel wall is elevated 10-fold in eNOS knockout mice and 4-fold in nNOS knockout mice compared to wild-type mice [2]. LPS induces a 3-fold higher number of leukocytes rolling on venules in iNOS knockout mice than in wild-type mice [3]. NO also inhibits inflammation in diverse murine models of vascular disease, including myocardial infarction, glomerulonephritis, lung injury, and stroke [4-6]. Thus NO derived from each of the NOS isoforms inhibits vascular inflammation. How?
Clues to the anti-inflammatory mechanism of NO came from studies of leukocyte interactions with the vessel wall. Leukocyte trafficking involves 5 discrete stages: (1) Rolling: loose attachment of leukocytes to endothelial cells is mediated by selectins and their glycoprotein ligands. (2) Activation: chemokines and other pro-inflammatory signals activate leukocytes and endothelial cells, inducing a variety of rapid responses including integrin surface expression and conformational changes. (3) Adherence: leukocytes stop rolling and become fixed to endothelial cells, mediated by intercellular adhesion molecules (ICAM) and their integrin receptors. (4) Diapedesis: leukocytes travel between endothelial cells into the vascular wall. (5) Migration: leukocytes follow a chemotactic gradient to the site of tissue injury.
NO inhibits the first stage of leukocyte trafficking, leukocyte rolling. Leukocyte rolling is mediated in part by P-selectin, and NO decreases expression of P-selectin on the endothelial surface [7]. P-selectin is a transmembrane protein normally stored within resting endothelial cells in granules called Weibel-Palade bodies (WPB) [8-10]. However, inflammatory signals such as histamine, hypoxia, or oxidized LDL activate endothelial exocytosis of these granules [11]. During exocytosis, the membranes of the WPB rapidly fuse with the endothelial plasma membrane, translocating P-selectin to the exterior. On the outer surface of the endothelial cell, P-selectin interacts with its ligand P-selectin glycoprotein ligand-1 (PSGL-1) on the surface of leukocytes, causing transient leukocyte rolling along the vessel wall [12, 13]. Since exocytosis of WPB triggers the initial phase of leukocyte rolling, NO inhibition of exocytosis limits vascular inflammation.
NO also inhibits other stages of leukocyte trafficking. NO decreases expression of integrins and intercellular adhesion molecules necessary for the third stage of leukocyte rolling, leukocyte adherence [14-17]. One possible mechanism may be through S-nitrosylation of nuclear factor kappaB, a transcription factor that drives expression of many pro-inflammatory molecules [18]. Furthermore, NO blunts the second sage of leukocyte trafficking, leukocyte and endothelial activation. For example, NO limits cellular activation by acting as an anti-oxidant. Superoxide is an innate immune effector produced by NADPH oxidases in neutrophils and macrophages. Superoxide can injure cells in part by oxidizing proteins, lipids, and nucleic acids. However, NO can react rapidly with superoxide, and diminish superoxide toxicity [19-21]. Furthermore, NO can directly inhibit the NADPH oxidase that produces superoxide [22]. NO also limits inflammation induced by other oxygen radicals such as hydrogen peroxide [23]. Thus NO limits leukocyte trafficking at multiple stages.
3. NO Inhibits Exocytosis
3.1 The Stages of Vesicle Trafficking
In order to understand how NO inhibits exocytosis, a brief overview of vesicle trafficking is necessary. Proteins within the secretory pathway are transported in vesicles from the endoplasmic reticulum (ER) to the Golgi apparatus into granules before reaching their ultimate destination. Vesicle trafficking consists of several discrete stages: cargo loading, budding, translocation, priming, triggering, membrane fusion, and recycling [24-27]. A Weibel-Palade body inside an endothelial cell follows this cycle of vesicle trafficking. A nascent granule is loaded with VWF and P-selectin, and then buds off from the Golgi apparatus. The granule is directed by kinesin along a network of microtubules to the plasma membrane [28]. The granule docks with the plasma membrane, and is primed for fusion. When the endothelial cell is stimulated by inflammation or injury, intracellular calcium levels rise and trigger fusion of the granule and plasma membranes. VWF is expelled from the cell into the blood stream, and P-selectin is translocated from the interior to the exterior of the endothelial cell. After exocytosis, the empty granule recycles back to the Golgi.
3.2 Trafficking Machinery
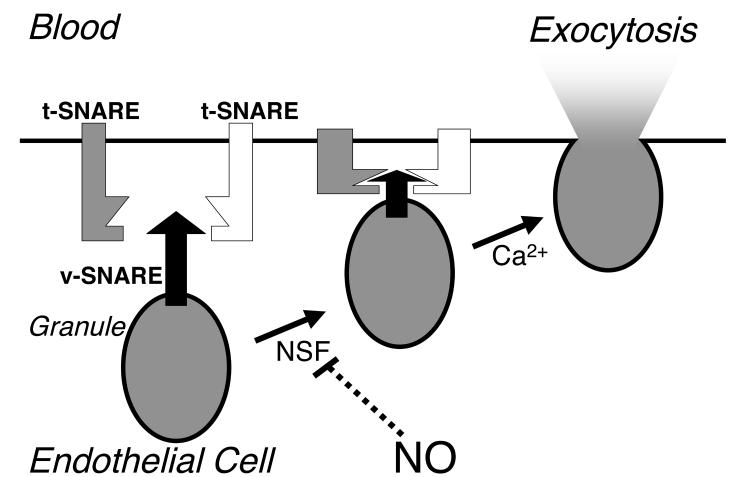
Distinct sets of proteins regulate the cycle of vesicle trafficking, including Rab family members, N-ethylmaleimide sensitive factor (NSF), soluble NSF attachment receptor proteins (SNAREs), and the Sec/Munc family [24-27]. Rab proteins are small GTPases that are involved in every stage of vesicle trafficking, from budding to targeting [29]. The SNAREs are a group of transmembrane proteins that specify fusion partners [30]. One SNARE on a vesicle can interact with two SNAREs on a target membrane; this interaction brings the vesicle close to its fusion partner (Fig. 1). The complex of three unique SNARE molecules specifies which vesicle will fuse with a particular membrane. For example, VAMP-3 on an endothelial granule might interact with syntaxin-4 and SNAP-23 on a plasma membrane, targeting the granule for fusion with the plasma membrane [31].
Figure 1.
NO inhibits exocytosis by targeting NSF. During exocytosis, a SNARE on a vesicle (v-SNARE) interacts with two target SNAREs (t-SNARE) on the plasma membrane, forming a metastable SNARE complex. When intracellular calcium levels rise, the membranes of the vesicle and the cell fuse, expelling the contents of the vesicle. NSF is required either to prime the vesicle for fusion or to recycle the vesicle. NO blocks exocytosis by S-nitrosylation of NSF.
Vesicle trafficking is regulated by enzymes that stimulate formation of the ternary SNARE complex, and by other enzymes that separate the SNARE complex. Sec/Munc proteins accelerate the assembly of the SNARE complex [32]. In contrast, NSF disassembles the three part SNARE complex. NSF interacts with the SNARE complex via an accessory protein, and then converts the chemical energy of ATP hydrolysis into mechanical energy to disassemble the SNARE complex [33]. The precise stage of trafficking at which NSF functions is unclear: NSF may play a role in priming the vesicle before membrane fusion (Fig. 1), or NSF may play a role in preparing the empty vesicle for recycling after fusion.
Calcium triggers rapid exocytosis of granules that have been primed by assembly of the SNARE complex. Although assembly of the SNARE complex can drive membrane fusion forward, the SNARE complex is clamped in a metastable state by complexins [34, 35]. When calcium levels rise, calcium binds to synaptotagmin which displaces complexin from the SNARE complex. The SNARE complex rapidly completes its conformational change, and drives membrane fusion.
3.3 NO Regulates Trafficking by S-Nitrosylating NSF
Out of the many proteins that regulate vesicle trafficking, NSF has been identified as a major target of NO. NSF is a critical part of the exocytic machinery [36, 37]. Inhibition of NSF by antibodies or peptides decreases exocytosis in neurons and in endothelial cells [31, 38]. Mutations in NSF cause paralysis in Drosophila, accompanied by a decrease in neurotransmitter release and an accumulation in docked neurovesicles [39, 40].
NSF is a plausible target of NO: NSF was purified due to its sensitivity to N-ethylmaleimide [36, 37]. Sensitivity to N-ethylmaleimide suggested that NSF contains catalytic cysteine residues, since N-ethylmaleimide alkylates cysteine residues. However, N-ethylmaleimide is an artificial compound, and alkylation of cysteine residues is not physiological. In contrast, NO can reversibly modify cysteine residues, a process called S-nitrosylation, and NO is a physiological messenger molecule [41]. Thus the method by which NSF was discovered (and named) suggested that it was a potential target of NO.
Several experiments supported the hypothesis that NSF is a target of NO [31]. (1) NO can S-nitrosylate NSF on 3 of its 9 cysteine residues. (2) Exogenous NO S-nitrosylates NSF; and endogenous NO can S-nitrosylate NSF, both in endothelial cells ex vivo and in mice in vivo. (3) NO derived from eNOS can inhibit NSF, since NSF is not S-nitrosylated in eNOS knockout mice. Thus NSF is a target of NO.
How does NO inhibit NSF? NSF has three domains [33]. The N-terminal domain of NSF interacts with the SNARE complex via an accessory protein; the D1 domain of NSF hydrolyzes ATP and generates mechanical force to separate the SNARE complex; and the D2 domain binds ATP and mediates oligomerization of NSF into hexamers. Although NO modifies cysteine residues C21 and C91 within the amino terminal domain of NSF that interacts with SNAREs, NO does not affect NSF binding to SNAREs [31]. However, NO also S-nitrosylates C264 within the D1 domain of NSF that hydrolyzes ATP and disassembles the SNARE complex. NO does not affect the ATPase activity of NSF. However, NO blocks NSF disassembly activity [31]. In support of this concept, a C264A mutation of this cysteine residue to alanine also blocks NSF separation of the SNARE complex [42]. These data suggest that NO S-nitrosylates NSF on C264, blocking NSF disassembly of SNARE complexes, and thereby blocking exocytosis (Fig. 1).
Does NO regulate vesicle trafficking by modifying other proteins in addition to NSF? Clearly NSF is a major target of NSF, since adding recombinant NSF back to cells treated with NO can restore exocytosis. But NO can stabilize a SNARE complex of recombinant proteins [43]. NO can also regulate small G-proteins such as ras that may lie upstream of the exocytic machinery [44]. Finally, by S-nitrosylating the ryanodine receptor, NO can modulate intracellular calcium levels that are necessary for exocytosis [45]. Therefore NO may regulate exocytosis by S-nitrosylating not only NSF but other targets as well.
4. NO Regulates Trafficking of Platelet Granules
NO inhibits platelet aggregation: platelet aggregation is decreased by exogenous NO gas, by endogenous EDRF, by NO donors ex vivo, and by endogenous NO in vivo [46-49]. Many of these inhibitory effects of NO are mediated through a cGMP dependent pathway. NO elevation of cGMP decreased calcium transients, eicosanoid formation, thromboxane receptor expression, and gpIIb/IIIa conformational changes. However, NO also blocks platelet activation through a cGMP independent pathway [50-53].
4.1 Platelet Activation
Platelet activation involves three stages—adherence, secretion, and aggregation. Following injury to the vessel wall, platelets adhere to the extracellular matrix via membrane receptor glycoprotein Ib/IX/V that interacts with VWF, and via GPVI that binds to collagen. Adherence activates the platelet, triggering intraplatelet pathways that lead to arachidonic acid metabolism and exocytosis of granules. Messengers released from platelet granules activate adjacent platelets, alter the conformation of the gpIIb/IIIa receptor, and ultimately lead to aggregation.
The secretory phase of platelet activation amplifies the initial platelet response to ligands. During this secretory phase, agonists trigger platelet release of alpha-granules, dense granules, and lysosomal granules. Platelet exocytosis of alpha-granules releases adhesive molecules such as VWF and P-selectin, coagulation factors such as Factor V and Factor VIII, and chemokines such as PF4, RANTES, and IL-8. Exocytosis of dense granules releases pro-thrombotic messengers including ATP, ADP, and serotonin. Secretion of these messengers play a critical role in activating platelets and stimulating aggregation. Emphasizing the important role of exocytosis in platelet activation, impaired formation of platelet dense granules in human diseases such as Hermansky-Pudlak syndrome or Chediak-Higashi syndrome is characterized by bleeding disorders.
4.2 NO Inhibits Exocytosis of Platelet Granules
NO inhibits platelet activation in part by blocking platelet exocytosis. Exogenous NO in doses as low as 1 – 10 nM inhibits P-selectin translocation from alpha-granules to the platelet surface [54, 55]. Endogenous NO also inhibits platelet exocytosis [49, 55]. NO inhibits the secretion of all three platelet granules, although the granules appear to be differentially sensitive to NO: lysosomal granules appear to be most sensitive to NO inhibition with an EC50 for NO of about 1 nM; alpha-granules are of intermediate sensitivity with an EC50 of about 10 nM; and dense granules least responsive with an EC50 of 1 μM [55].
NO inhibition of platelet exocytosis alters thrombosis in mice. Platelet rolling along vessels can be mediated by P-selectin stored within platelet alpha-granules. P-selectin surface expression in platelets is increased in eNOS knockout mice. Platelets from eNOS knockout mice have increased rolling along vessels, compared to wild-type platelets [55]. Finally, the bleeding time and the time to thrombosis is greatly diminished in eNOS knockout mice [55, 56]. The use of eNOS knockout mice has revealed that eNOS within platelets influences platelet exocytosis more than eNOS within endothelial cells [56]. These data support the idea that NO regulates platelet exocytosis in vivo.
As discussed above, NO inhibits specific aspects of platelet activation through cGMP pathways. However, NO inhibition of platelet exocytosis is independent of cGMP. The guanylyl cyclase inhibitor ODQ has no effect on the ability of NO to block exocytosis in platelets [55]. Instead, DTT blunts the effects of NO upon exocytosis, suggesting that NO regulates platelet granule exocytosis by S-nitrosylation.
As in endothelial cells, NSF is the target of NO inside platelets. NO S-nitrosylates NSF inside platelets [55]. After treating platelets with NO, adding recombinant NSF back to permeabilized platelets restores exocytosis. Thus NO inhibits platelet granule secretion in part by interfering with the exocytic machinery.
4.3 Conflicting Evidence on the Role of NO in Platelets
Contrary to the hypothesis that NO inhibits platelet degranulation, some evidence shows that NO stimulates platelet exocytosis. Insulin stimulates platelet secretion in an NO dependent manner [57]. Furthermore, insulin fails to trigger granule release from platelets of eNOS knockout mice. Why are there discrepancies between this study and others? One possible explanation is a difference between thrombin signaling and insulin signaling within platelets. Furthermore, the insulin-NO stimulatory pathway depends upon cGMP, whereas the NO inhibitory pathway involves S-nitrosylation. In fact, some data suggest that cGMP and PKG may activate platelets in certain circumstances [58].
5. NO Regulates Constitutive Vesicle Trafficking from Golgi to Plasma Membrane
5.1 NO Regulates Antegrade Trafficking in Endothelial Cells
NO also regulates trafficking of proteins that are constitutively routed to the plasma membrane. For example, NO decreases the transport of a GFP-fusion protein that travels from endoplasmic reticulum to Golgi to the plasma membrane [59]. This effect may also be due to NO modification of NSF. However, NO can only regulate protein trafficking if its source is adjacent to the trafficking machinery. When eNOS is localized in the nucleus, NO has no effect on cytosolic protein trafficking. In contrast, when eNOS is directed to the Golgi, trafficking from Golgi to plasma membrane is disrupted [59]. If NO inhibits protein trafficking in intracellular domains close to eNOS, then high local concentrations might be necessary to affect the transport machinery. Taken together, these findings suggest that NO can regulate protein transport, as long as the source of NO is adjacent to the transport machinery.
5.2 NO Regulates Trafficking of Neuronal Receptors
NO also regulates protein trafficking in neurons. Glutamate receptors mediate excitatory synaptic transmission in the central nervous system. Changes in synaptic density of the AMPA subtype of glutamate receptor may serve as a mechanism to store information in the central nervous system. NSF serves as a chaperone that escorts the AMPA receptor to the surface of neurons, regulating synaptic plasticity [60]. NO derived from nNOS promotes the surface expression of the AMPA receptor by S-nitrosylating NSF, augmenting NSF binding to the AMPA receptor, and inserting the receptor into the membrane surface [61]. Since modulation of AMPAR expression is related to long term potentiation, this process may serve as a mechanism by which NO regulates synaptic plasticity.
6. NO Regulates Constitutive Vesicle Trafficking from Golgi to Plasma Membrane
6.1 Receptor Mediated Endocytosis
While exocytosis is the process by which vesicles deliver compounds to the cell surface, endocytosis involves the cell engulfing extracellular material and transporting it in vesicles into the cell. The three major forms of endocytosis are phagocytosis, macropinocytosis and receptor mediated uptake [24]. Receptor mediated endocytosis involves surface receptors that interact with soluble ligands and cluster at clathrin-coated pits [24]. After a coated vesicle forms at the cell surface, the GTPase dynamin assembles into multimers, forming a collar around the neck of the nascent vesicle, and facilitates scission of the vesicle from the membrane [61].
6.2 NO Regulates Endocytosis by S-Nitrosylating Dynamin
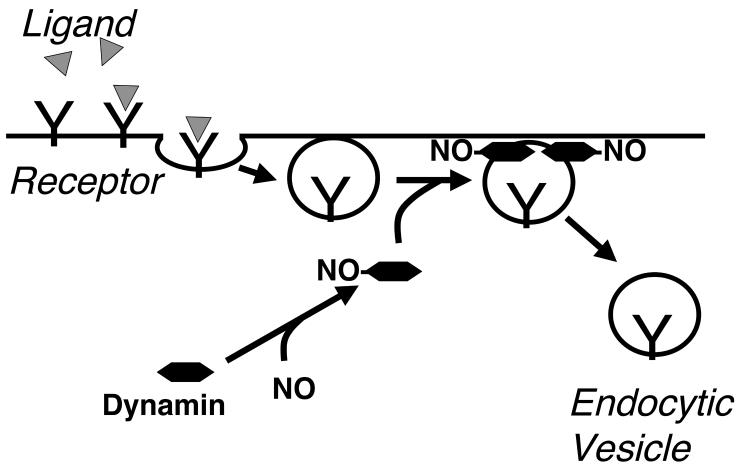
NO accelerates receptor mediated endocytosis by targeting dynamin (Fig. 2). NO S-nitrosylates dynamin, increases dynamin oligomerization and GTPase activity, thereby increasing endocytosis [62]. In both transformed cell lines and in endothelial cells, NO modification of dynamin increases receptor mediated endocytosis [62, 63]. Furthermore, NO production is localized to sites of endocytosis by an interaction between dynamin and eNOS. This interaction not only localizes NO production, but also increases eNOS activity [64].
Figure 2.
NO accelerates endocytosis by S-nitrosylation of dynamin. In receptor mediated endocytosis, ligands bind to receptor, recruiting an endocytic complex that includes clathrin (not shown) and dynamin. Multiple dynamin units assemble around the neck of the endocytic vesicle, hydrolyze GTP, and cleave the vesicle free from the membrane. By S-nitrosylation of dynamin, NO increases the rate of dynamin assembly, GTPase activity, and membrane scission.
What is the structural basis by which NO increases dynamin activity? Dynamin has an amino terminal proline rich domain (PRD) which increases self-assembly, and a pleckstrin homology (PH) domain which decreases self-assembly and GTPase activity [65, 66]. NO nitrosylates dynamin within its PH domain on C607 [62]. S-nitrosylation of dynamin C607 modulates the inhibitory role of this domain, increasing both the GTPase activity and the assembly of recombinant dynamin. Mutation of this critical C607 residue blocks the effects of NO [62]. Taken together, these data support a role for NO in the regulation of endocytosis.
7. Summary
NO regulates cardiovascular physiology in part by controlling protein trafficking. S-nitrosylation of dynamin accelerates endocytosis; the physiological consequences of this modulation are not yet clear, but may be of major importance in regulating the responsiveness of cells to adrenergic signals.
By S-nitrosylating NSF, NO retards exocytosis. Exocytosis is an important form of communication employed by diverse cells within the cardiovascular system: platelets activate neighboring platelets, cardiac myocytes release atrial natriuretic factor, endothelial cells attract leukocytes, and leukocytes kill target cells—all relying upon the process of exocytosis of unique granules. Exocytosis also plays a major role in signaling outside of the cardiovascular system. For example, exocytosis plays a critical role in neurotransmission, insulin secretion, and fertilization. NO regulation of vesicle trafficking may be a widespread phenomenon of broad physiological significance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, et al. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol. 1999;276:H1943–50. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 3.Hickey MJ, Sharkey KA, Sihota EG, Reinhardt PH, Macmicking JD, Nathan C, et al. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. Faseb J. 1997;11:955–64. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- 4.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd'Heuil D, et al. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–73. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 5.Heeringa P, van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, et al. Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2000;156:879–88. doi: 10.1016/S0002-9440(10)64957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminski A, Pohl CB, Sponholz C, Ma N, Stamm C, Vollmar B, et al. Up-regulation of endothelial nitric oxide synthase inhibits pulmonary leukocyte migration following lung ischemia-reperfusion in mice. Am J Pathol. 2004;164:2241–9. doi: 10.1016/S0002-9440(10)63780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107:1050–8. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 8.Weibel ER, Palade GE. New Cytoplasmic Components In Arterial Endothelia. J Cell Biol. 1964;23:101–12. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989;56:1033–44. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- 10.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–12. [PubMed] [Google Scholar]
- 11.Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–8. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–103. [PubMed] [Google Scholar]
- 13.Hartwell DW, Wagner DD. New discoveries with mice mutant in endothelial and platelet selectins. Thromb Haemost. 1999;82:850–7. [PubMed] [Google Scholar]
- 14.Kubes P, Kurose I, Granger DN. NO donors prevent integrin-induced leukocyte adhesion but not P-selectin-dependent rolling in postischemic venules. Am J Physiol. 1994;267:H931–7. doi: 10.1152/ajpheart.1994.267.3.H931. [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr., et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–8. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao PS, Buitrago R, Chan JR, Cooke JP. Fluid flow inhibits endothelial adhesiveness. Nitric oxide and transcriptional regulation of VCAM-1. Circulation. 1996;94:1682–9. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- 17.Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci U S A. 1996;93:9114–9. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry. 2001;40:1688–93. doi: 10.1021/bi002239y. [DOI] [PubMed] [Google Scholar]
- 19.Rubanyi GM, Ho EH, Cantor EH, Lumma WC, Botelho LH. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991;181:1392–7. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- 20.Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993;265:H862–7. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- 21.Wink DA, Hanbauer I, Krishna MC, DeGraff W, Gamson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993;90:9813–7. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–21. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston B, Kanwar S, Kubes P. Hydrogen peroxide induces leukocyte rolling: modulation by endogenous antioxidant mechanisms including NO. Am J Physiol. 1996;271:H614–21. doi: 10.1152/ajpheart.1996.271.2.H614. [DOI] [PubMed] [Google Scholar]
- 24.Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- 25.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 26.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 27.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–33. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 28.Rondaij MG, Bierings R, Kragt A, Gijzen KA, Sellink E, van Mourik JA, et al. Dynein-dynactin complex mediates protein kinase A-dependent clustering of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:49–55. doi: 10.1161/01.ATV.0000191639.08082.04. [DOI] [PubMed] [Google Scholar]
- 29.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 30.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–43. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–50. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–95. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 33.May AP, Whiteheart SW, Weis WI. Unraveling the mechanism of the vesicle transport ATPase NSF, the N-ethylmaleimide-sensitive factor. J Biol Chem. 2001;276:21991–4. doi: 10.1074/jbc.R100013200. [DOI] [PubMed] [Google Scholar]
- 34.Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–87. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–80. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra V, Orci L, Glick BS, Block MR, Rothman JE. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988;54:221–7. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- 37.Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988;85:7852–6. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweizer FE, Dresbach T, DeBello WM, O'Connor V, Augustine GJ, Betz H. Regulation of neurotransmitter release kinetics by NSF. Science. 1998;279:1203–6. doi: 10.1126/science.279.5354.1203. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1976;73:3253–7. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki F, Mattiuz AM, Ordway RW. Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J Neurosci. 1998;18:10241–9. doi: 10.1523/JNEUROSCI.18-24-10241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita K, Morrell CN, Mason RJ, Yamakuchi M, Khanday FA, Irani K, et al. Hydrogen peroxide regulation of endothelial exocytosis by inhibition of N-ethylmaleimide sensitive factor. J Cell Biol. 2005;170:73–9. doi: 10.1083/jcb.200502031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meffert MK, Calakos NC, Scheller RH, Schulman H. Nitric oxide modulates synaptic vesicle docking fusion reactions. Neuron. 1996;16:1229–36. doi: 10.1016/s0896-6273(00)80149-x. [DOI] [PubMed] [Google Scholar]
- 44.Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, et al. Redox regulation of cell signalling. Nature. 1996;381:380–1. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 45.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 46.Mellion BT, Ignarro LJ, Ohlstein EH, Pontecorvo EG, Hyman AL, Kadowitz PJ. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981;57:946–55. [PubMed] [Google Scholar]
- 47.Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986;88:411–5. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 49.Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, Michelson AD. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest. 1997;100:350–6. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsikas D, Ikic M, Tewes KS, Raida M, Frolich JC. Inhibition of platelet aggregation by S-nitroso-cysteine via cGMP-independent mechanisms: evidence of inhibition of thromboxane A2 synthesis in human blood platelets. FEBS Lett. 1999;442:162–6. doi: 10.1016/s0014-5793(98)01633-0. [DOI] [PubMed] [Google Scholar]
- 51.Sogo N, Magid KS, Shaw CA, Webb DJ, Megson IL. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem Biophys Res Commun. 2000;279:412–9. doi: 10.1006/bbrc.2000.3976. [DOI] [PubMed] [Google Scholar]
- 52.Beghetti M, Sparling C, Cox PN, Stephens D, Adatia I. Inhaled NO inhibits platelet aggregation and elevates plasma but not intraplatelet cGMP in healthy human volunteers. Am J Physiol Heart Circ Physiol. 2003;285:H637–42. doi: 10.1152/ajpheart.00622.2002. [DOI] [PubMed] [Google Scholar]
- 53.Crane MS, Rossi AG, Megson IL. A potential role for extracellular nitric oxide generation in cGMP-independent inhibition of human platelet aggregation: biochemical and pharmacological considerations. Br J Pharmacol. 2005;144:849–59. doi: 10.1038/sj.bjp.0706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michelson AD, Benoit SE, Furman MI, Breckwoldt WL, Rohrer MJ, Barnard MR, et al. Effects of nitric oxide/EDRF on platelet surface glycoproteins. Am J Physiol. 1996;270:H1640–8. doi: 10.1152/ajpheart.1996.270.5.H1640. [DOI] [PubMed] [Google Scholar]
- 55.Morrell CN, Matsushita K, Chiles K, Scharpf RB, Yamakuchi M, Mason RJ, et al. Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci U S A. 2005;102:3782–7. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84:1416–21. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 57.Randriamboavonjy V, Schrader J, Busse R, Fleming I. Insulin induces the release of vasodilator compounds from platelets by a nitric oxide-G kinase-VAMP-3-dependent pathway. J Exp Med. 2004;199:347–56. doi: 10.1084/jem.20030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, et al. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell. 2003;112:77–86. doi: 10.1016/s0092-8674(02)01254-0. [DOI] [PubMed] [Google Scholar]
- 59.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, et al. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci U S A. 2006;103:19777–82. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 61.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, et al. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–40. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc Natl Acad Sci U S A. 2006;103:1295–300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang-Decker N, Cao S, Chatterjee S, Yao J, Egan LJ, Semela D, et al. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- 64.Cao S, Yao J, McCabe TJ, Yao Q, Katusic ZS, Sessa WC, et al. Direct interaction between endothelial nitric-oxide synthase and dynamin-2. Implications for nitric-oxide synthase function. J Biol Chem. 2001;276:14249–56. doi: 10.1074/jbc.M006258200. [DOI] [PubMed] [Google Scholar]
- 65.Muhlberg AB, Warnock DE, Schmid SL. Domain structure and intramolecular regulation of dynamin GTPase. Embo J. 1997;16:6676–83. doi: 10.1093/emboj/16.22.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]