Abstract
The evolution of alpha blocker therapy for benign prostatic hyperplasia (BPH) has focused on improving convenience and tolerability. Indications for treating BPH include reversing signs and symptoms or preventing progression of the disease. The indication that most commonly drives the need for intervention is relief of lower urinary tract symptoms (LUTS) with the intent of improving quality of life. Alpha blockers are the most effective, least costly, and best tolerated of the drugs for relieving LUTS. Four long-acting alpha 1 blockers are approved by the Food and Drug Administration for treatment of symptomatic LUTS/BPH: terazosin, doxazosin, tamsulosin, and alfuzosin. All are well tolerated and have comparable dose-dependent effectiveness. Tamsulosin and alfuzosin SR do not require dose titration. Alfuzosin, terazosin, and doxazosin have all been shown to be effective in relieving LUTS/BPH independent of prostate size.
Key words: Benign prostatic hyperplasia, Lower urinary tract symptoms, Quality of life, Alpha blockers, Terazosin, Doxazosin, Tamsulosin, Alfuzosin
Benign prostatic hyperplasia (BPH) describes a proliferative process of both stomal and epithelial elements of the prostate.1 Its prevalence is age dependent.2 Histological evidence of BPH is rarely observed in men under 50 years of age, but by age 80 virtually all men will have some histological evidence of the process. It is unclear what specific factors regulate the degree of hyperplasia, which ultimately dictates the size of the prostate gland, nor is there any consensus regarding the prostate size that qualifies for the diagnosis of benign prostatic enlargement (BPE).
As men age, the caliber of the urinary stream diminishes.3 The diminution of the urinary stream was assumed to be attributable to bladder outlet obstruction (BOO) arising directly from the BPE.4 It was also assumed that BOO resulted in bladder dysfunction leading to lower urinary tract symptoms (LUTS), impaired bladder emptying (post void residual urine), and urinary tract infection. In the most severe and relatively rare cases of benign prostatic enlargement, acute urinary retention, urosepsis, chronic renal insufficiency, and death developed secondary to BPH. Hematuria may also be attributed to BPH, but only as a diagnosis of exclusion. Therefore, the clinical manifestations attributed to BPH include LUTS, incomplete bladder emptying, urinary tract infection, acute and chronic urinary retention, urosepsis, chronic renal insufficiency, and hematuria (Table 1).5
Table 1.
Clinical Manifestations of Benign Prostatic Hyperplasia
|
The indications for treating BPH include reversing existing signs and symptoms of the disease or preventing the progression of the disease (Table 2). In the Medical Therapy of Prostatatic Symptoms (MTOPS) trial, only 14% of men with BPH developed symptom progression, 2% developed acute urinary retention, and < 1% each developed incontinence or urinary tract infection/urosepsis over a follow-up interval of 4 years.6 Therefore, the indication that most commonly drives the need for intervention is the relief of LUTS with the intent of improving quality of life. The combination arm (alpha blocker plus 5-alpha reductase inhibitor) in the MTOPS trial achieved the greatest risk reduction for BPH progression.6 Men with larger prostates in the MTOPS trial were at greatest risk for developing acute urinary retention.7 Therefore, in men with “large” prostates, combination therapy may be recommended as the most effective regimen to treat LUTS and prevent BPH progression. Interestingly, in a recent randomized, placebo-controlled study of 1522 men at high risk for BPH progression (PSA levels between 1.4–10.0 g/dL, prostate volume > 30 cm3, IPSS > 13, and PVR > 350 mL) the alpha blocker alfuzosin alone was also very effective at preventing BPH progression.8
Table 2.
Indications for Treating Benign Prostatic Hyperplasia
|
Assessment of Lower Urinary Tract Symptoms
LUTS includes urinary storage (irritative) and voiding (obstructive) symptoms. The storage and voiding symptoms are best captured and quantified using self-administered symptom questionnaires that assess the individual patient’s symptoms. The American Urological Association Symptoms Index (AUASI) and the International Prostate Symptom Index Score (IPSS) are the most widely used instruments to capture severity of LUTS.9 Both instruments capture 7 symptoms: emptying the bladder, urinary frequency, interrupted urinary stream, postponing of urination, weak stream, straining to initiate urination, and nocturia. The total score for both ranges between 0 and 35. Scores of 0–7, 8–18, and ≥ 19, respectively, designate mild, moderate, and severe symptoms.10 The IPSS has an additional question that assesses quality of life and is scored separately on a scale of 0 to 6, with 6 representing the poorest quality.
Pathophysiology of Lower Urinary Tract Symptoms
A fundamental question is whether severity of LUTS depends on prostate size. Several studies have demonstrated only a weak correlation between the 2, whether in men diagnosed with BPH11 or men in the general community over the age of 50 years.12 It is, therefore, not surprising that 5 alpha reductase inhibitors, which reduce prostate volume, have virtually no benefit at relieving LUTS in men with BPH.13 The classes of drugs that relieve LUTS include the alpha blockers,14 phosphodiesterase inhibitors,15 and anticholinergics.16 None of these drugs has any impact on prostate volume. The most effective, least costly, and best tolerated of these drugs for relieving LUTS are the alpha blockers. The clinical benefit of alpha blockers has consistently been shown to be independent of baseline prostate volume,17,18 which is further evidence for the limited relevance of prostate volume to LUTS.
Another fundamental question is whether the severity of LUTS depends on BOO. The observation that urinary flow rate decreases and LUTS increases with age provides the rationale for linking LUTS and BOO.4 The fact that alpha blockers and transurethral resection of the prostate relieve both BOO and LUTS supported the assumption that LUTS was caused by the BOO. The first indication that the severity of LUTS is not related to BOO was derived from the previously discussed epidemiological studies of men with BPH and in the general community. These studies showed that the severity of BOO and LUTS were only weakly correlated.11,12 If the mechanism for improving LUTS in men receiving alpha blockers and undergoing transurethral resection of the prostate is due to the relief of BOO, then there should be a strong correlation between the changes in urinary flow rate and the changes in LUTS following intervention. The fact that these relationships were not observed provides compelling evidence that neither the pathophysiology nor the mechanism for symptom improvement following these interventions is primarily related to relief of BOO.17,19 The recent observation that phosphodiesterase inhibitors improve LUTS without having any effect on urinary flow rate is further evidence that symptom improvement is not dependent upon BOO.15
Rationale for Alpha Blockers and BPH
The relative degree of stromal and epithelial hyperplasia is highly variable. Overall, approximately 80% and 20% of the hyperplastic volume is composed of stromal and epithelial elements, respectively.20 Half of the stromal hyperplasia is composed of smooth-muscle elements.21 For decades, it was assumed that the enlarged hyperplastic prostate caused BOO via both dynamic and static mechanisms.22 The dynamic obstruction was thought to be the result of smooth-muscle hyperplasia causing a functional obstruction and static obstruction arising from the bulk enlargement of the hyperplastic process encroaching upon the prostatic urethra.
Marco Caine demonstrated in 1975 that strips of human prostate contracted in response to norepinephrine.23 The norepinephrine-induced contractions were inhibited by pretreatment with phenoxybenzamine, a non-selective inhibitor of alpha adrenoceptor. These studies implicated the alpha adrenoceptor as the mediator of prostate smooth-muscle contraction. Lepor and Shapiro were the first investigators to characterize both alpha 1 and 2 adrenoceptors in the human prostate using radioligand binding studies.24,25 An abundance of both alpha 1 and alpha 2 adrenoceptors were identified. Functional studies suggested that it was the alpha 1 adrenoceptor subtype that mediated prostate muscle contraction.26
Rationale for Developing Alpha 1 Subtype Selective Blockers for BPH
Three subtypes of the alpha 1 adrenoceptor (alpha 1a, alpha 1b, alpha 1d) have been cloned and pharmacologically characterized. Lepor and associates demonstrated that the predominant alpha 1 adrenoceptor subtype in the human prostate was the alpha 1a subtype.27 Subsequent studies by the same group using both autoradiography28 and immunohistochemistry29 localized the alpha 1a subtype to the prostatic stroma. Functional studies demonstrated that the alpha 1a subtype mediated human prostate contraction.30 (Note: Alpha 1a was originally called alpha 1c.)
If one assumes that the efficacy of alpha blockers is mediated by prostate smooth-muscle relaxation, then there would be a compelling reason to develop alpha 1a subtype selective inhibitors for the treatment of BPH. Presumably, some of the adverse events associated with non-selective alpha 1 blockade are mediated by the alpha 1b and alpha 1d adrenoceptor subtypes. These adverse events would be minimized or eliminated by administrating an alpha 1a selective antagonist. There is now abundant evidence that alpha 1 blockers relieve LUTS via mechanisms unrelated to prostate smooth-muscle relaxation.4 Therefore, it is conceivable that an alpha 1a subtype selective antagonist would retain its modest impact on relieving BOO while failing to relieve LUTS if the therapeutic effect on LUTS is mediated by alpha 1b or alpha 1d subtypes. The developments of several alpha 1a subtype selective antagonists were terminated due to lack of any clinical advantage.
Development of Alpha Blockers for the Treatment of BPH
Nonselective Alpha Antagonists
In 1976, phenoxybenzamine was the first alpha blocker reported to be effective for the treatment of BPH.31 Two years later, the therapeutic benefit of phenoxybenzamine was confirmed by a randomized, placebo-controlled study.32 Marco Caine and associates are to be commended for recognizing the importance of conducting randomized clinical trials in order to demonstrate a treatment-related effect in BPH. Validated symptom indices for quantifying changes in LUTS had not been developed at the time Caine reported this early study. Phenoxybenzamine was found to be superior to placebo at relieving LUTS and increasing peak flow rate. The primary limitation of phenoxybenzamine was its side effects, which included tiredness, dizziness, impaired ejaculation, nasal stuffiness, and hypotension.
Selective Short-Acting Alpha 1 Blockers
Prazosin was the first selective alpha 1 antagonist investigated for BPH. Several small, randomized, placebo-controlled trials suggested that prazosin exhibited comparable efficacy and better tolerability relative to phenoxybenzamine.33,34 Prazosin requires multiple daily dosing, and adverse events related to its blood pressure lowering properties remained problematic. Larger, multicenter, randomized clinical trials were never performed with prazosin, presumably due to the availability of generic prazosin and the general notion at the time that medical therapy would not be widely accepted by urologists for the treatment of BPH.
Long-Acting Selective Alpha 1 Blockers
Four long-acting alpha 1 blockers are approved by the Food and Drug Administration (FDA) for the treatment of symptomatic LUTS/BPH (Table 3). It is imperative when comparing different alpha 1 blockers to recognize that both efficacy and tolerability are dose dependent. Therefore, observed differences in both efficacy and toxicity may simply be due to different levels of alpha 1 blockade achieved and not to inherent advantages of the specific drug. It is, therefore, important to compare both efficacy and tolerability at various doses of drugs.
Table 3.
Long-Acting Alpha Blockers Approved for the Treatment of Benign Prostatic Hyperplasia in the United States
|
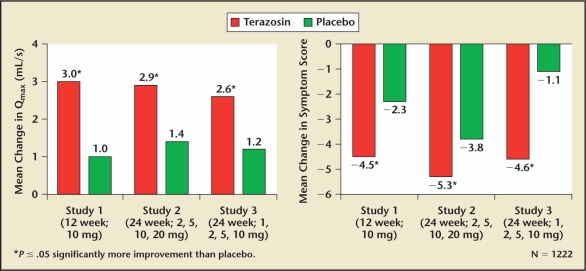
Terazosin was the first selective long-acting alpha 1 blocker investigated for the treatment of BPH. Lepor and colleagues35 reported the first multicenter, randomized, placebo-controlled trial of any alpha blocker that was properly powered to show that statistically significant changes in LUTS were also clinically significant. LUTS at baseline and throughout the study were ascertained using a quantitative symptom questionnaire. Statistically significant improvements were observed relative to placebo for both symptom scores and peak flow rate. Terazosin doses of 2 mg, 5 mg, or 10 mg were given once daily. Only 4% and 7% of the participants randomized to placebo and terazosin, respectively, withdrew from the 3-month study due to an adverse event. Two additional studies were part of the new drug application (NDA) submitted for terazosin’s FDA approval for the treatment of symptomatic BPH.36,37 All of the terazosin studies included a dose titration study design beginning at 1 mg in order to avoid the first-dose effect. The treatment-related efficacy and adverse events of terazosin are shown in Figure 1 and Table 4, respectively.
Figure 1.
The effect of terazosin on lower urinary tract symptoms and peak flow rate relative to placebo.38 Qmax, peak urinary flow.
Table 4.
Terazosin: Adverse Effects38
| Terazosin | Placebo | |
|---|---|---|
| (n = 636) | (n = 360) | |
| Asthenia/fatigue | 7.4% | 3.3% |
| Postural hypotension | 3.9% | 0.8% |
| Dizziness | 9.1% | 4.2% |
| Somnolence | 3.6% | 1.9% |
| Nasal congestion/rhinitis | 1.9% | 0.0% |
| Impotence | 1.6% | 0.6% |
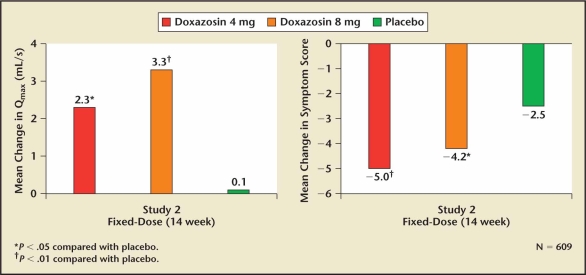
Doxazosin was the second alpha 1 blocker approved by the FDA for the treatment of symptomatic BPH. Two pivotal multicenter, randomized clinical trials were performed comparing various doses of doxazosin with placebo.39,40 The potential advantage of doxazosin was its longer half-life tolerability. The treatment-related efficacy and side effects are shown in Figure 2 and Table 5. Both doxazosin studies included a dose titration design in order to avoid the first-dose effect related to efficacy or tolerability. Based on its efficacy and tolerability comparable with terazosin, doxazosin’s longer half-life did not appear to confirm any clinical advantage.
Figure 2.
The effect of doxazosin on lower urinary tract symptoms and peak flow rate relative to placebo.41 Qmax, peak urinary flow.
Table 5.
Doxazosin: Adverse Effects41
| Doxazosin | Placebo | |
|---|---|---|
| (n = 665) | (n = 300) | |
| Dizziness (includes vertigo) | 15.6% | 9.0% |
| Fatigue | 8.0% | 1.7% |
| Hypotension | 1.7% | 0.0% |
| Edema | 2.7% | 0.7% |
| Dyspnea | 2.6% | 0.3% |
Both terazosin and doxazosin exhibited lowering of blood pressure only in those men who were hypertensive at baseline.41–43 The effect on blood pressure was interpreted as a desirable outcome because 2 common conditions (BPH and hypertension) in the aging male could be treated effectively with a single agent. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) subsequently demonstrated that alpha blockers were inferior to other classes of drugs as first-line therapy for the treatment of hypertension.44 This led many to conclude that in men with both BPH and hypertension, the 2 disease entities should be treated independently with the best available agents.
Tamsulosin was the third alpha 1 blocker to be approved for the treatment of BPH. Tamsulosin was brought to market as the first subtype selective alpha 1 antagonist for the treatment of BPH. Tamsulosin alpha 1 subtype selective was supported by binding studies, which showed that tamsulosin was approximately tenfold more selective for the alpha 1a versus alpha 1b subtype.45,46 There was no demonstrable subtype selectivity of tamsulosin for the alpha 1a versus alpha 1d subtypes. The modest receptor selectivity of tamsulosin is not sufficient to result in a clinically meaningful advantage. Typically, clinical advantages attributed to pharmacological selectivity require a receptor selectivity well beyond the tenfold difference observed with tamsulosin.
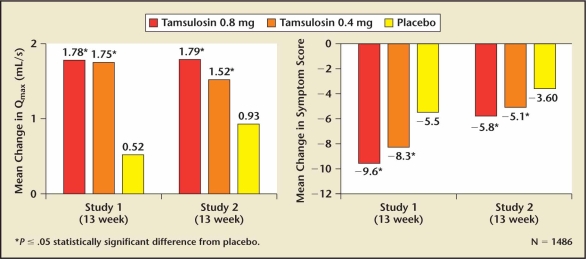
Two pivotal trials of tamsulosin supported the NDA for the treatment of symptomatic BPH.47,48 Both 0.4 and 0.8 mg of tamsulosin achieved clinically significant improvements in symptom scores and peak flow rate (Figure 3, Table 6). The ability of the 0.4 mg tamsulosin dose to achieve a clinically significant effect without the requirement for dose titration represented a unique advantage over the other approved alpha blockers. Although a 0.8 mg daily dose was more effective than 0.4 mg, it did not gain popularity because it required both dose titration and taking 2 tablets of 0.4 mg. (A 0.8 mg tablet was not commercially available.) The primary reason tamsulosin was prescribed over terazosin and doxazosin was not due to greater efficacy or better tolerability, but simply the lack of dose titration. The prescribing community placed a greater value on eliminating the dose response at the expense of increasing the incidence of ejaculatory dysfunction, which was thought to be retrograde ejaculation as a result of relaxation of the bladder neck. Recent studies have demonstrated that tamsulosin causes anejaculation rather than retrograde ejaculation.50 The mechanism for the increased incidence of ejaculatory dysfunction associated with tamsulosin has been attributed to its affinity for dopaminergic and other central nervous system receptors.50,51
Figure 3.
The effect of tamsulosin on lower urinary tract symptoms and peak flow rate relative to placebo.49 Qmax, peak urinary flow.
Table 6.
Tamsulosin: Adverse Effects49
| 0.4 mg | 0.8 mg | Placebo | |
|---|---|---|---|
| (n = 501) | (n = 492) | (n = 493) | |
| Dizziness | 14.9% | 17.1% | 10.1% |
| Abnormal ejaculation | 8.4% | 18.1% | 0.2% |
| Asthenia/fatigue | 7.8% | 8.5% | 5.5% |
| Libido decreased | 1.0% | 2..0% | 1.2% |
| Amblyopia | 0.2% | 2.0% | 0.4% |
Selective alpha blockers like prazosin offered the advantage of improved tolerability over nonselective alpha blockers like phenoxybenzamine. The long-acting selective alpha 1 blockers like terazosin and doxazosin offered the convenience of once a day dosing with better tolerability compared with the shorter-acting agents like prazosin. Tamsulosin achieved a therapeutic effect without the need for dose titration and with minimal effects on blood pressure. The convenience of eliminating the dose titration came at the expense of ejaculatory dysfunction. Therefore, there was a need for a long-acting selective alpha 1 blocker that did not require dose titration, had minimal side effects on blood pressure, and had no effects on ejaculatory function (retrograde ejaculation or anejaculation).
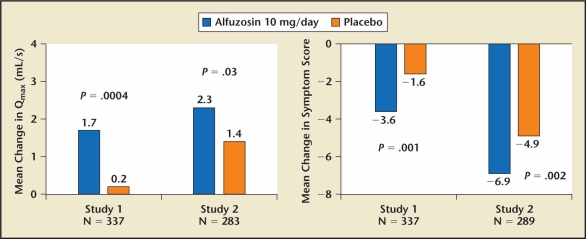
Alfuzosin 10 mg OD is the fourth alpha 1 selective blocker approved by the FDA for the treatment of symptomatic BPH. Radioligand binding studies failed to show any receptor selectivity of alfuzosin for the alpha 1 subtypes.45,46 The excellent tolerance has been attributed to its slow release formulation. Two randomized, placebo-controlled trials were conducted as part of the NDA for the approval of alfuzosin in the United States for the treatment of symptomatic BPH (Figure 4, Table 7).53,54 Alfuzosin 10 mg achieved a clinically significant improvement in LUTS without dose titration. The AUA Guidelines Committee concluded that alfuzosin has comparable clinical efficacy with tamsulosin and the other approved alpha blockers but does not cause ejaculatory dysfunction.55
Figure 4.
The effect of alfuzosin on lower urinary tract symptoms and peak flow rate relative to placebo.52 Qmax, peak urinary flow.
Table 7.
Alfuzosin: Adverse Effects52
| Alfuzosin | Placebo | |
|---|---|---|
| (n = 473) | (n = 678) | |
| Upper respiratory tract infection | 3.0% | 0.6% |
| Dizziness | 5.7% | 2.8% |
| Headache | 3.0% | 1.8% |
| Fatigue | 2.7% | 1.8% |
Alpha Blockers for Treating Acute Urinary Retention
Acute urinary retention is a potentially life-threatening consequence of BPH. The initial management of acute urinary retention is to temporarily insert an indwelling urinary catheter. The catheter is typically removed in a few days in order to attempt a trial of voiding without a catheter. A randomized, double-blind, placebo-controlled study has shown that alfuzosin increases the likelihood of successfully removing the catheter while also decreasing the risk of a subsequent episode of acute urinary retention.56 An episode of acute urinary retention is no longer an absolute indication for surgical intervention. Alpha blockers are a very reasonable initial option for managing acute urinary retention.
Summary
All the alpha blockers evaluated for the treatment of symptomatic BPH have comparable effectiveness. Over the past 30 years, the evolution of alpha blocker therapy for BPH has focused primarily on improving convenience and tolerability. All of the long-acting alpha 1 blockers are well tolerated, but only tamsulosin and alfuzosin SR are administered without the requirement for dose titration. The advantage of alfuzosin over tamsulosin is the lower incidence of ejaculatory dysfunction. Without a doubt, minimizing the effect on ejaculatory dysfunction represents a step forward in the development of alpha blockers for the treatment of LUTS/BPH.
Many consider alfuzosin 10 mg to be the superior alpha blocker currently available for treating BPH because it achieves clinically significant improvements in LUTS and has no significant effects on dizziness, asthenia, and ejaculatory dysfunction.
Alpha 1a Subtype Selective Agents
As previously discussed, there is increasing evidence that alpha blockers relieve LUTS through mechanisms other than prostate smooth-muscle relaxation.4 It is universally agreed that prostate smooth-muscle contraction is mediated by the alpha 1a subtype,30 but the specific alpha 1 subtype mediating LUTS is unknown. There is also increasing evidence that adverse events associated with alpha blocker therapy, including asthenia and dizziness, are not due to blood pressure lowering.57 Therefore, developing a drug that preferentially relaxes prostate smooth muscle without affecting vascular smooth muscle will not necessarily yield a more effective drug with fewer side effects.
Several alpha 1a subtype selective drugs entered the developmental phase at different pharmaceutical companies. All of these drug development programs were discontinued because of failure to relieve LUTS despite increasing peak urinary flow rate. The clinical outcomes with alpha 1a subtype selective drugs provide compelling evidence that the improvement in LUTS is not related to relief of BOO. Understandably, there are presently few active development programs to bring subtype selective alpha blocker drugs to market.
It is unlikely that an alpha 1 blocker more effective or better tolerated than alfuzosin will be developed in the future. Efforts should be directed toward unraveling the pathophysiology of LUTS instead of pursuing subtype selective alpha 1 blockers. This knowledge will pave the way for the development of novel pharmacologic strategies for improving LUTS, which will substantively advance the field. It is likely that maximal reduction of LUTS will ultimately be achieved by combining different classes of drugs such as alpha blockers, phosphodiesterase inhibitors, and anticholinergic agents. The challenge will be to define criteria for prescribing the most effective pharmacological regimen.
Overlapping Syndromes With LUTS/BPH
Recent epidemiologic and demographic evidence has demonstrated the overlap among LUTS/BPH and other symptom complexes such as erectile impotence, ejaculatory dysfunction, and overactive bladder.57 New data presented at the annual AUA meeting in 2007 suggest an overlap between LUTS/BPH and abdominal obesity, diabetes, and the metabolic syndrome (Table 8).58,59 The biological plausibility of these associations is currently being explored. Primary care physicians treating diabetes, obesity, and other metabolic conditions should recognize that these patients may have co-existing and latent symptoms of LUTS/BPH.60 The primary care physician is in the ideal position to identify and treat these symptoms, if clinically indicated.
Table 8.
Overlapping Syndromes With Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia
|
Main Points.
The indications for treating benign prostatic hyperplasia (BPH) include reversing signs and symptoms (lower urinary tract symptoms [LUTS], incomplete bladder emptying, urinary tract infections, urinary retention, urosepsis, chronic renal insufficiency, and hematuria) or preventing progression of the disease.
Four long-acting alpha 1 blockers are approved by the Food and Drug Administration for treatment of symptomatic LUTS/BPH: terazosin, doxazosin, tamsulosin, and alfuzosin. When comparing them, it is imperative to recognize that both efficacy and tolerability are dose dependent.
Both terazosin and doxazosin lower blood pressure, but other classes of drugs are superior to alpha blockers as first-line therapy for hypertension, leading many to conclude that it should be treated independently in men with BPH.
The ability of the 0.4 mg tamsulosin dose to achieve a clinically significant effect without the requirement for dose titration represented a unique advantage over the other approved alpha blockers, but this convenience came at the expense of ejaculatory dysfunction.
The American Urological Association Guidelines Committee concluded that alfuzosin has comparable clinical efficacy to tamsulosin and the other approved alpha blockers but does not cause ejaculatory dysfunction.
An episode of acute urinary retention is no longer an absolute indication for surgical intervention. Alpha blockers are a very reasonable initial option for managing acute urinary retention.
References
- 1.McNeal JG. The prostate gland: morphology and pathobiology. Monogr Urol. 1983;4:3–33. [Google Scholar]
- 2.Berry SJ, Coffey DS, Walsh PC, et al. The development of human prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 3.Girman CJ, Jacobsen SJ, Guess HA, et al. Natural history of prostatism: relationship among symptoms, prostate volume and peak urinary flow rate. J Urol. 1995;153:1510–1515. doi: 10.1016/s0022-5347(01)67448-2. [DOI] [PubMed] [Google Scholar]
- 4.Lepor H. The pathophysiology of lower urinary tract symptoms in the aging male population. In: Lepor H, editor. Prostatic Diseases. Philadelphia, PA: WB Saunders; 2000. pp. 163–196. [Google Scholar]
- 5.Jepsen JB, Bruskewitz RC. Clinical manifestations and indications for treatment. In: Lepor H, editor. Prostatic Diseases. Philadelphia, PA: WB Saunders; 2000. pp. 127–142. [Google Scholar]
- 6.McConnell JD, Roehrborn CG, Bautista OM, et al. for the Medical Therapy of Prostatic Symptoms (MTOPS) Research Group, authors. The long-term effects of doxazosin, finasteride and the combination on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SA, McConnell JD, Roehrborn CG, et al. for the MTOPS Research Group, authors. Relationship between prostate size and the effect of combination therapy with doxazosin and finasteride versus either drug alone on the clinical progression of benign prostatic hyperplasia. J Urol. 2006;75:217–221. doi: 10.1016/S0022-5347(05)00041-8. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG. Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006;97:734–741. doi: 10.1111/j.1464-410X.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ, Fowler FJ, Jr., O’Leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 10.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association Symptom Index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 11.Barry MJ, Cockett ATK, Holtgrewe HL, et al. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150:351–358. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 12.Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85–89. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 13.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 14.Lepor H. a-Adrenergic blockers for the treatment of benign prostatic hyperplasia. In: Lepor H, editor. Prostatic Diseases. Philadelphia, PA: WB Saunders; 2000. pp. 297–307. [Google Scholar]
- 15.Kaplan SA, Gonzalez RR. Phosphodiesterase type 5 inhibitors for the treatment of male lower urinary tract symptoms. Rev Urol. 2007;9:73–77. [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder. JAMA. 2006;296:2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 17.Lepor H, Williford WO, Barry MJ, et al. The impact of medical therapy on bother due to symptom, quality of life and global outcome, and factors predicting response. J Urol. 1998;160:1358–1367. [PubMed] [Google Scholar]
- 18.Roehrborn CG, Van Kerrebroeck P, Nordling J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int. 2003;92:257–261. doi: 10.1046/j.1464-410x.2003.04309.x. [DOI] [PubMed] [Google Scholar]
- 19.Lepor H, Rigaud G. The efficacy of transurethral resection of the prostate in men with moderate symptoms of prostatism. J Urol. 1990;143:533–537. doi: 10.1016/s0022-5347(17)40012-7. [DOI] [PubMed] [Google Scholar]
- 20.Bartsch G, Muller HR, Oberholzer M, et al. Light microscopic stereological analysis of the normal human prostate and of benign prostatic hyperplasia. J Urol. 1979;122:487–489. doi: 10.1016/s0022-5347(17)56476-9. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro E, Becich MJ, Hartanto V, Lepor H. The relative proportion of stromal and epithelial hyperplasia as related to the development of clinical BPH. J Urol. 1992;147:1293–1297. doi: 10.1016/s0022-5347(17)37546-8. [DOI] [PubMed] [Google Scholar]
- 22.Lepor H. Nonoperative management of benign prostatic hyperplasia. J Urol. 1989;141:1283–1289. doi: 10.1016/s0022-5347(17)41282-1. [DOI] [PubMed] [Google Scholar]
- 23.Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 1975;27:193–202. doi: 10.1111/j.1464-410x.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- 24.Lepor H, Shapiro E. Characterization of the alpha1 adrenergic receptor in human benign prostatic hyperplasia. J Urol. 1984;132:1226–1229. doi: 10.1016/s0022-5347(17)50110-x. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro E, Lepor H. Alpha2 adrenergic receptors in hyperplastic human prostate: identification and characterization using [3H] rauwolscine. J Urol. 1986;135:1038–1042. doi: 10.1016/s0022-5347(17)45971-4. [DOI] [PubMed] [Google Scholar]
- 26.Lepor H, Gup DI, Baumann M, Shapiro E. Laboratory assessment of terazosin and alpha1 blockade in prostatic hyperplasia. Urology. 1988;32:21–26. [PubMed] [Google Scholar]
- 27.Lepor H, Tang R, Meretyk S, Shapiro E. Alpha 1 adrenoceptor subtypes in the human prostate. J Urol. 1993;149:640–642. doi: 10.1016/s0022-5347(17)36170-0. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol. 1993;150:2002–2006. doi: 10.1016/s0022-5347(17)35954-2. [DOI] [PubMed] [Google Scholar]
- 29.Walden P, Gerardi C, Lepor H. Localization and expression of the alpha1A-1, alpha1B and alpha1D-adrenoceptors in hyperplastic and non-hyperplastic human prostate. J Urol. 1999;161(2):635–640. [PubMed] [Google Scholar]
- 30.Forray C, Bard JA, Wetzel JM, et al. The alpha1 adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacologic properties of the cloned human alpha 1c subtype. Mol Pharmacol. 1994;45:703–708. [PubMed] [Google Scholar]
- 31.Caine M, Pfau A, Perlberg S. The use of alpha adrenergic blockers in benign prostatic obstruction. Br J Urol. 1976;48:255–263. doi: 10.1111/j.1464-410x.1976.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 32.Caine M, Perlberg S, Meretyk S. A placebo-controlled double-blind study of the effect of phenoxybenzamine in benign prostatic obstruction. Br J Urol. 1978;50:551–554. doi: 10.1111/j.1464-410x.1978.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirby RS, Coppinger SWC, Corcoran MO, et al. Prazosin in the treatment of prostatic obstruction: A placebo-controlled study. Br J Urol. 1987;60:136–142. doi: 10.1111/j.1464-410x.1987.tb04950.x. [DOI] [PubMed] [Google Scholar]
- 34.Martorana G, Giberti C, Damonte P, et al. The effect of prazosin in benign prostatic hypertrophy, a placebo controlled double-blind study. IRCS Med Sci. 1984;12:11–12. [Google Scholar]
- 35.Lepor H, Auerbach S, Puras-Baez A, et al. A multicenter fixed-dose study of the safety and efficacy of terazosin in the treatment of the symptoms of benign prostatic hyperplasia. J Urol. 1992;148:1467–1474. doi: 10.1016/s0022-5347(17)36941-0. [DOI] [PubMed] [Google Scholar]
- 36.Brawer MK, Adams G, Epstein H, et al. Terazosin in the treatment of benign prostatic hyperplasic. Arch Fam Med. 1993;2:929–935. doi: 10.1001/archfami.2.9.929. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd SN, Buckley JF, Chilton CP, et al. Terazosin in the treatment of benign prostatic hyperplasia: A multicentre, placebo-controlled trial. Br J Urol. 1992;70(Suppl 1):17–21. doi: 10.1111/j.1464-410x.1992.tb15862.x. [DOI] [PubMed] [Google Scholar]
- 38.Terazosin [package insert] North Chicago, IL: Abbott Laboratories; 2003. [Google Scholar]
- 39.Fawzy A, Braun K, Lewis GP, et al. Doxazosin in the treatment of benign prostatic hyperplasia in normotensive patients: a multicenter study. J Urol. 1995;154:105–109. [PubMed] [Google Scholar]
- 40.Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: A double-blind, placebo-controlled dose response multicenter study. J Urol. 1995;154:110–115. [PubMed] [Google Scholar]
- 41.Doxazosin [package insert] New York, NY: Pfizer Inc; 2003. [Google Scholar]
- 42.Kirby RS. Terazosin in benign prostatic hyperplasia: effect on blood pressure in normotensive and hypertensive men. Br J Urol. 1998;82:373–379. doi: 10.1046/j.1464-410x.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 43.Kirby RS. Doxazosin in benign prostatic hyperplasia: effects on blood pressure and urinary flow in normotensive and hypertensive men. Urology. 1995;46:182–186. doi: 10.1016/s0090-4295(99)80191-5. [DOI] [PubMed] [Google Scholar]
- 44.ALLHAT Collaborative Research Group, authors. Major cardiovascular events in hypertensive patients randomized to doxazosin versus chlorthalidone. The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 45.Kenny BA, Miller AM, Williamson IJ, et al. Evaluation of the pharmacological selectivity profile of alpha 1 adrenoceptor antagonists at prostatic alpha 1 adrenoceptors: binding, functional and in vivo studies. Br J Pharmacol. 1996;118:871–878. doi: 10.1111/j.1476-5381.1996.tb15480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forray C, Bard JA, Wetzel JM, et al. The alpha 1-adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacological properties of the cloned human alpha 1c subtype. Mol Pharmacol. 1994;45:703–708. [PubMed] [Google Scholar]
- 47.Lepor H for the Tamsulosin Investigator Group, authors. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Urology. 1998;51:892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 48.Narayan P, Tewari A. The United States 93-01 Study Group: A second phase III multicenter placebo controlled study of 2 doses of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. J Urol. 1998;160:1701–1706. [PubMed] [Google Scholar]
- 49.Tamsulosin [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2003. [Google Scholar]
- 50.Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176(4):1529–1533. doi: 10.1016/j.juro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Wolters JP, Hellstrom WJ. Current concepts in ejaculatory dysfunction. Rev Urol. 2006;8(4 suppl):S18–S25. [PMC free article] [PubMed] [Google Scholar]
- 52.Alfuzosin [package insert] Bridgewater, NJ: Sanofi-Synthelabo, Inc; 2004. [Google Scholar]
- 53.Van Kerrebroeck P, Jardin A, Laval KU, van Cangh P ALFORTI Study Group, authors. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2000;37:306–313. doi: 10.1159/000052361. [DOI] [PubMed] [Google Scholar]
- 54.Roehrborn CG. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia; a randomized, placebo-controlled trial. Urology. 2001;58:953–959. doi: 10.1016/s0090-4295(01)01448-0. [DOI] [PubMed] [Google Scholar]
- 55.Roehrborn CG, McConnell JD, Barry MJ the AUA BPH Guideline Update Panel, authors. [Accessed October 1, 2007];Guideline on the management of benign prostatic hyperplasia (BPH) 2003 chapters 1–23. Available at: http://www.auanet.org/guidelines/bph.cfm.
- 56.McNeill SA, Hargreave TB, Roehrborn CG the ALAUR Study Group, authors. Alfuzosin 10 mg once daily in the management of acute urinary retention: results of a double-blind placebo-controlled study. Urology. 2005;65:83–89. doi: 10.1016/j.urology.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 57.Rosen RC, Catania JA, Althof SE, et al. Development and validation of four-item version of male sexual health questionnaire to assess ejaculatory dysfunction. Urology. 2007;69:805–809. doi: 10.1016/j.urology.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan S, Fisch H, Berriman SJ, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms, sexual dysfunction, and components of the metabolic syndrome [abstract 1508] J Urol. 2007;177(4 suppl):497–498. [Google Scholar]
- 59.Kaplan S, Wilson TW. Association between BPH and the metabolic syndrome in the REDUCE population [abstract 1548] J Urol. 2007;177(4 suppl):511. [Google Scholar]
- 60.Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol. 2007;178:395–401. doi: 10.1016/j.juro.2007.03.103. [DOI] [PubMed] [Google Scholar]