Abstract
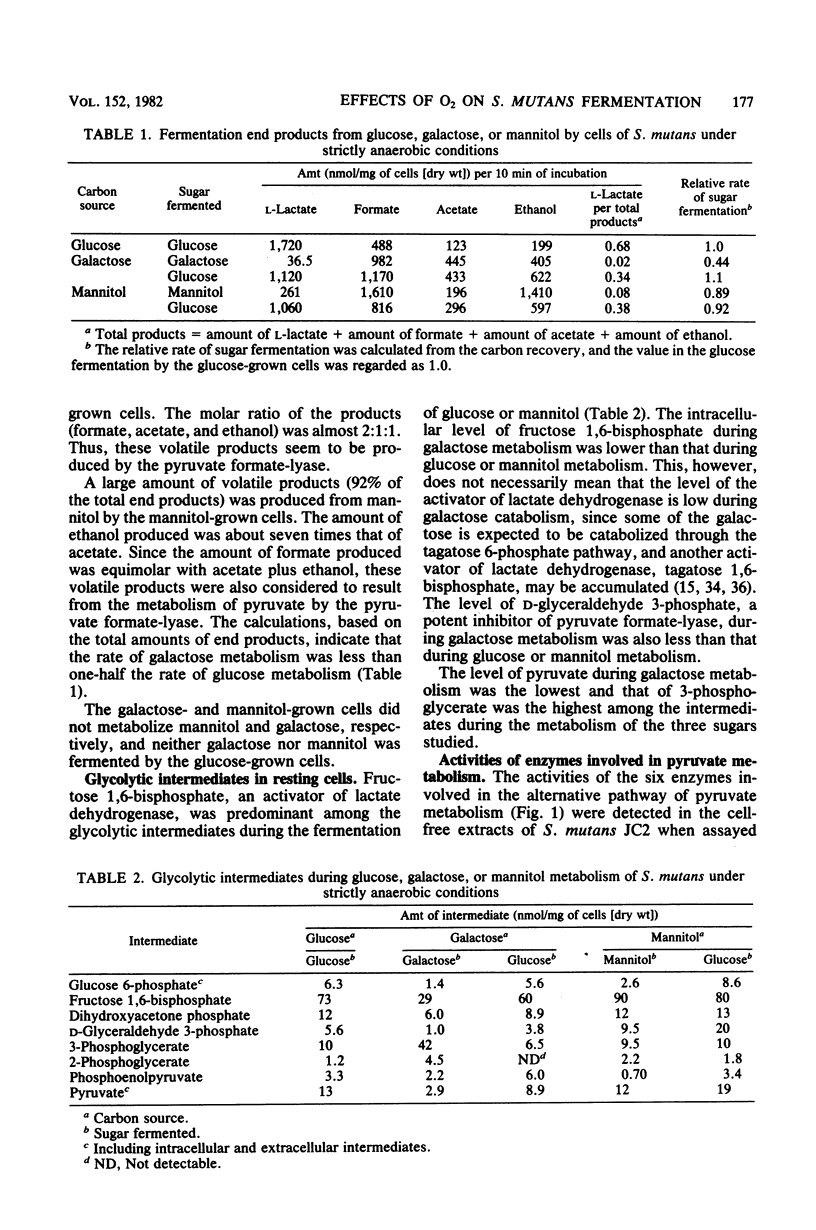
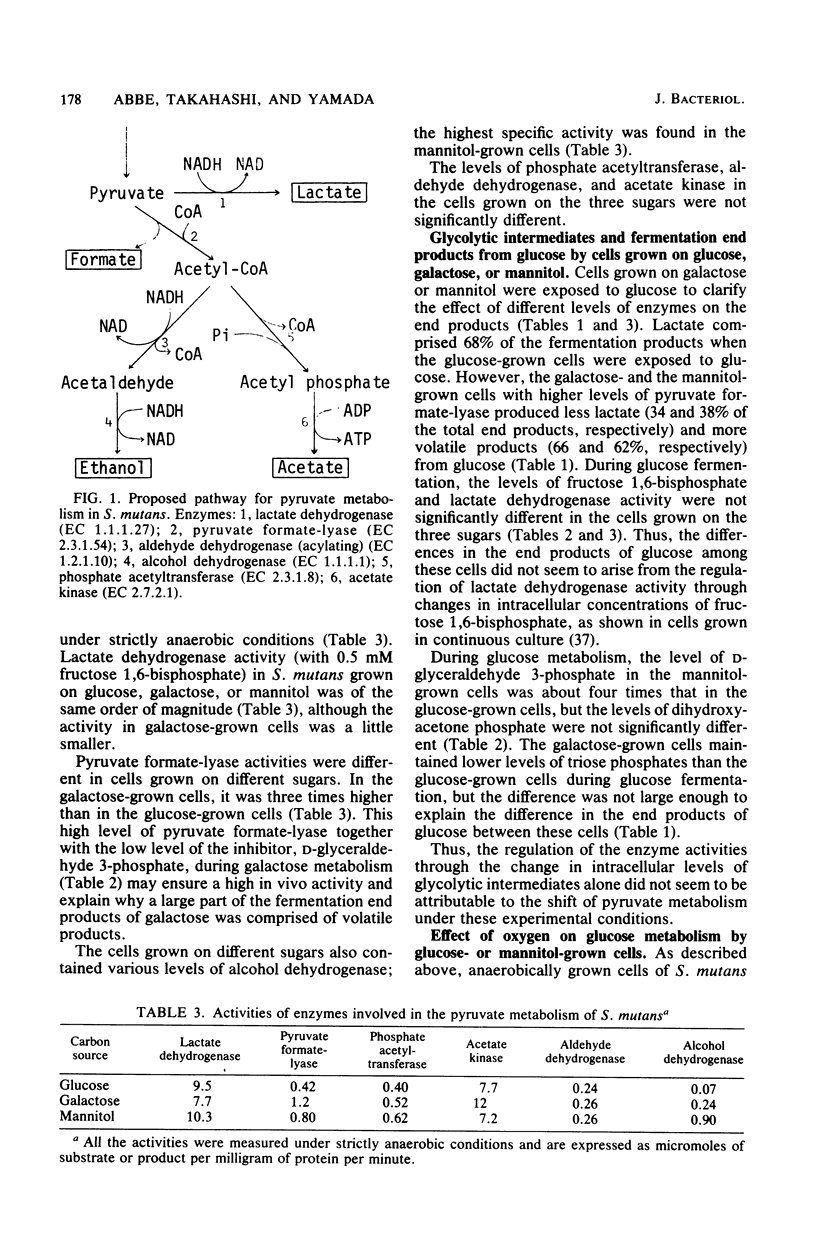
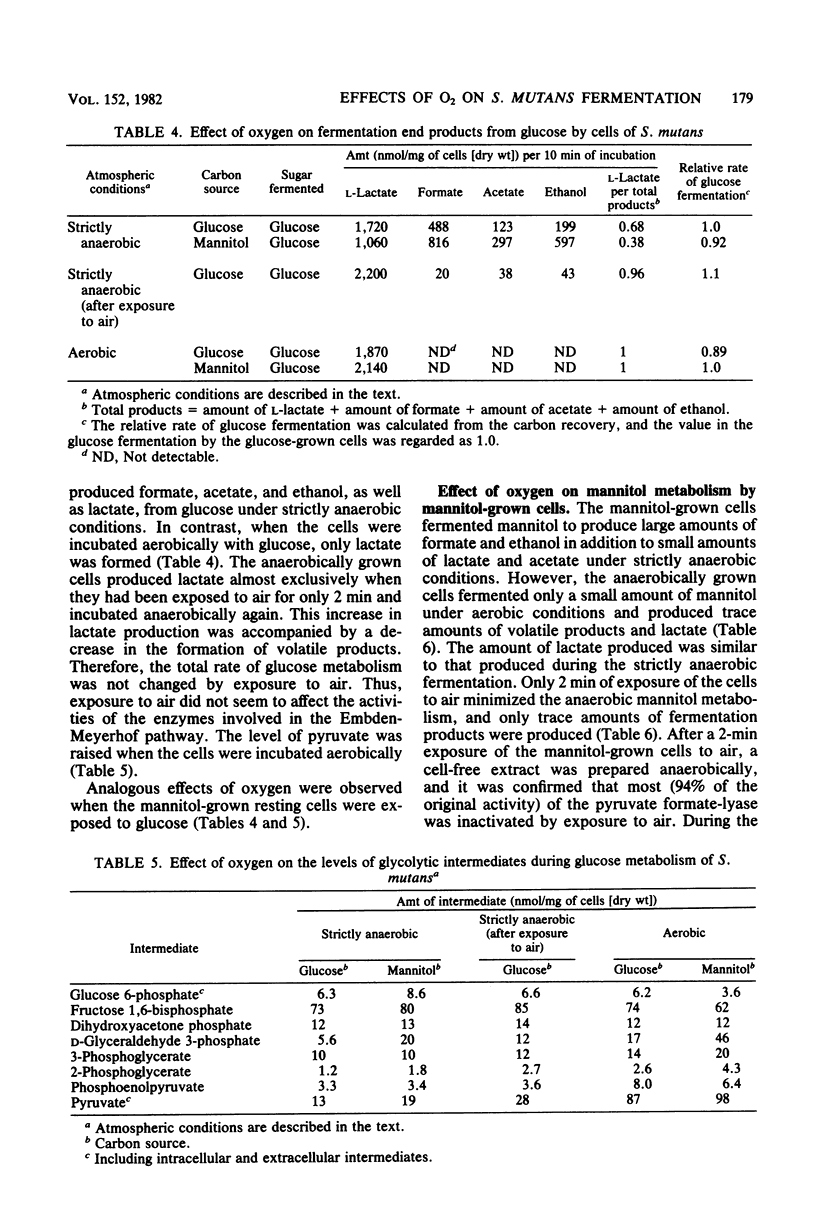
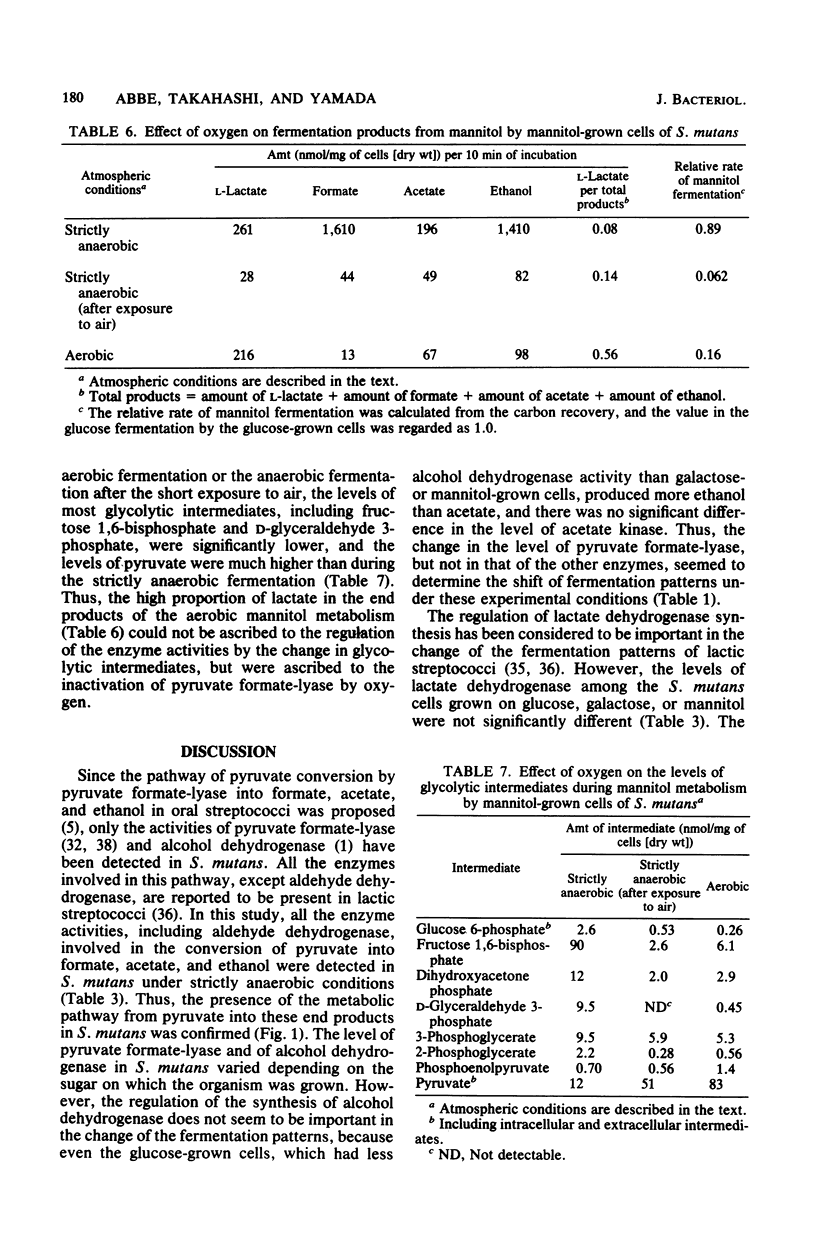
Streptococcus mutans JC2 produced formate, acetate, ethanol, and lactate when suspensions were incubated with an excess of galactose or mannitol under strictly anaerobic conditions. The galactose- or mannitol-grown cell suspensions produced more formate, acetate, and ethanol than the glucose-grown cells even when incubated with glucose. The levels of lactate dehydrogenase and fructose 1,6-bisphosphate were not significantly different in these cells, but the level of pyruvate formate-lyase was higher in the galactose- or mannitol-grown cells, and that of triose phosphate was lower in the galactose-grown cells. This suggests that the regulation of pyruvate formate-lyase may play a major role in the change of the fermentation patterns. The cells of S. mutans grown on glucose produced a significant amount of volatile products even in the presence of excess glucose under strictly anaerobic conditions. However, when the anaerobically grown cells were exposed to air, only lactate was produced from glucose. When cells were anaerobically grown on mannitol and then exposed to air for 2 min, only trace amounts of fermentation products were formed from mannitol under anaerobic conditions. It was found that the pyruvate formate-lyase in the cells was inactivated by exposure of the cells to air.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Patterson C. E. Ethanol production and alcohol dehydrogenase activity in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):127–131. doi: 10.1016/0003-9969(73)90027-7. [DOI] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Fructose-1,6-diphosphate-dependent lactate dehydrogenase from a cariogenic streptococcus: purification and regulatory properties. J Bacteriol. 1972 May;110(2):604–615. doi: 10.1128/jb.110.2.604-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T., Wittenberger C. L. Mannitol and sorbitol catabolism in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):117–126. doi: 10.1016/0003-9969(73)90026-5. [DOI] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Cole J. A. A biochemical approach to the control of dental caries. Biochem Soc Trans. 1977;5(4):1232–1239. doi: 10.1042/bst0051232. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Hunter J. R., Longyear V. M. Growth of Streptococcus mutans in a chemostat. Arch Oral Biol. 1974 Aug;19(8):659–664. doi: 10.1016/0003-9969(74)90134-4. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lebtag H. Lactose metabolism by Streptococcus mutans: evidence for induction of the tagatose 6-phosphate pathway. J Bacteriol. 1979 Dec;140(3):1102–1104. doi: 10.1128/jb.140.3.1102-1104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami Y., Yamada T., Araya S. Glycolytic intermediates in Streptococcus mutans PK 1. Arch Oral Biol. 1975 Oct;20(10):695–697. doi: 10.1016/0003-9969(75)90140-5. [DOI] [PubMed] [Google Scholar]
- Iwami Y., Yamada T. Rate-limiting steps of the glycolytic pathway in the oral bacteria Streptococcus mutans and Streptococcus sanguis and the influence of acidic pH on the glucose metabolism. Arch Oral Biol. 1980;25(3):163–169. doi: 10.1016/0003-9969(80)90015-1. [DOI] [PubMed] [Google Scholar]
- Jordan H. V. Bacteriological aspects of experimental dental caries. Ann N Y Acad Sci. 1965 Sep 30;131(2):905–912. doi: 10.1111/j.1749-6632.1965.tb34856.x. [DOI] [PubMed] [Google Scholar]
- Katayama T., Suzuki T., Okada S. Clinical observation of dental plaque maturation. Application of oxidation-reduction indicator dyes. J Periodontol. 1975 Oct;46(10):610–613. doi: 10.1902/jop.1975.46.10.610. [DOI] [PubMed] [Google Scholar]
- Kenney E. B., Ash M. M., Jr Oxidation reduction potential of developing plaque, periodontal pockets and gingival sulci. J Periodontol. 1969 Nov;40(11):630–633. doi: 10.1902/jop.1969.40.11.630. [DOI] [PubMed] [Google Scholar]
- Krasse B., Jordan H. V., Edwardsson S., Svensson I., Trell L. The occurrence of certain "caries-inducing" streptococci in human dental plaque material with special reference to frequency and activity of caries. Arch Oral Biol. 1968 Aug;13(8):911–918. doi: 10.1016/0003-9969(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., Van der Hoeven J. S. Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch Oral Biol. 1975 Jul;20(7):407–410. doi: 10.1016/0003-9969(75)90224-1. [DOI] [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- Ritz H. L. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967 Dec;12(12):1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Abbe K., Yamada T. Purification of pyruvate formate-lyase from Streptococcus mutans and its regulatory properties. J Bacteriol. 1982 Mar;149(3):1034–1040. doi: 10.1128/jb.149.3.1034-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Krichevsky M. I., Keyes P. H. The metabolic fate of glucose catabolized by a washed stationary phase caries-conducive streptococcus. Caries Res. 1969;3(2):167–177. doi: 10.1159/000259580. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Tagatose-1, 6-diphosphate activation of lactate dehydrogenase from Streptococcus cremoris. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1035–1042. doi: 10.1016/0006-291x(75)90673-7. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



