Abstract
Meniscal tears are attributed to either trauma or degeneration processes. Clinical data suggest that meniscal degeneration (MD) is associated with knee osteoarthritis; however, the molecular events underpinning the pathogenesis of MD in humans remain elusive. Here we immunohistochemically examined the expression of p38 MAPK, its phosphorylated/activated form (p-p38), its target NF-κB (p50–p65 dimer), and COX-2 in ruptured menisci and investigated their involvement in MD development. Our findings demonstrate increased expression of the p38–NF-κB axis elements and COX-2 in disintegrated fibrocartilage, suggesting a role of these molecules in the pathobiochemistry of MD and consequential rupture.
INTRODUCTION
Menisci are wedge-shaped semilunar structures that lie on the superior tibial surface, improving its congruency with the femoral condyles. They are composed of fibrocartilaginous tissue that contains primarily water (72%), collagen (22%), and glycosaminoglycans (0.8%) (1). Collagen type I accounts for 90% of the total meniscal collagen, whereas types II, III, and IV are present only in small quantities (2). Meniscus occupies primarily two cell types: fibroblast-like cells at the periphery and chondrocyte-like cells at the middle and inner part. The latter, which are called fibrochondrocytes, are the hallmark of meniscal cytology (3).
Because of their multiple functions and central anatomic position, menisci are exposed to a complex and diverse array of mechanical stresses leading to injury and tears. Menisci can fail because of biomechanical and biochemical cues (4,5). The latter situation is frequently attributed to the presence of osteoarthritis (OA) of the knee (6,7).
Experimental evidence suggests the involvement of the p38 mitogen-activated protein kinase (MAPK) signal transduction pathway in the pathogenesis of numerous stress and inflammatory conditions including chronic joint inflammation (8,9). The p38 family comprises four distinct isoforms (α, β, γ, and δ), all of which are serine-threonine protein kinases that share the conserved Thr-Gly-Tyr (TGY) phosphorylation motif within their activation loop (10). The p38α isoform is the best characterized and is functional in many cell types including chondrocytes and synovial cells (11,12). Hormones, G protein–coupled receptors, and inflammatory mediators can trigger the p38 cascades (11). The phosphorylated, hence activated, form of p38, p-p38, regulates the activity of several factors including nuclear factor (NF)-κB (13,14).
NF-κB is a collective name for dimeric transcriptional modulators comprising the Rel family of proteins that include RelA (p65), c-Rel, RelB, NF-κB1 (p50), and NF-κB2 (p52). The most abundant form in stimulated cells is the NF-κB1–RelA (p50–p65) heterodimer (often called a “classic” NF-κB) that interacts with the consensus DNA motif 5′-GGGRNNYYCC-3′ (15). In quiescent cells, NF-κB resides in the cytoplasm in a latent form and must translocate to the nucleus to function. NF-κB is induced by a plethora of stimuli (antigens, viruses, bacteria, inflammatory cytokines, phorbol esters) leading to transcriptional activation of diverse sets of genes engaged in immune, inflammatory, and cell proliferation responses (16). The function of NF-κB is regulated to a large extent by a family of proteins named inhibitors of NF-κB (IκBs). In the cytoplasm of quiescent cells, IκBs are bound to NF-κB, ensuring its inactive state. Activation of the NF-κB pathway triggers a cascade of events that lead to stimulation of the IκB kinases, which finally phosphorylate IκBs. Notably, p38 MAPK is one of the kinases implicated in IκB phosphorylation (14). Once phosphorylated, IκBs undergo poly-ubiquitination and ultimately proteosomic degradation, allowing NF-κB to enter the nucleus and promote the transcription of inflammatory genes, e.g., TNF-α, IL-1β, IL-6, IL-8, COX (17).
The two isoforms of COX (cyclooxygenase), COX-1 and COX-2, are membrane-bound proteins that share homology at the 60% level and catalyze the rate-limiting step in the conversion of arachidonic acid to prostaglandins (PGs) and thromboxane A2 (18). Within the cell, both enzymes are associated with endoplasmic reticulum and nuclear envelope (19). COX-1 is expressed constitutively in most tissues and appears to regulate the production of PGs that control physiological functions (18). In contrast, COX-2 is expressed in minute amounts in most normal tissues; however, its expression is rapidly elevated in response to inflammatory and mitogenic stimuli (20).
In articular cartilage, conditions such as trauma, inflammation, and mechanical compression can induce the expression of COX-2 and PGE2, two of the major osteoarthritis pathogens (21). The involvement of COX-2 and its upstream effectors NF-κB and p38 MAPK has been studied extensively in diseases of synovium and hyaline cartilage such as chronic synovitis, rheumatoid arthritis, and OA (2,9). Nevertheless, the expression and function of these factors in the meniscal fibrocartilage and their participation in the pathogenesis of meniscal lesions have not been thoroughly investigated.
We undertook this study to explore and immunohistochemically characterize the expression and/or activation profile of the p38 MAPK–NF-κB signaling pathway constituents and COX-2 in the fibrochondrocytes of human torn menisci. Furthermore, we correlated the expression levels of the examined proteins with pathologic and clinical parameters, such as the presence of fibrocartilaginous degeneration and the coexistence of clinically identified OA.
MATERIALS AND METHODS
Patients
We used 57 human menisci obtained from patients treated at the KAT Hospital of Athens, Greece. Informed consent was obtained in all cases. The study protocol was approved by the Ethics Committee of the KAT Hospital of Athens. Among the patients 43 (75.4%) were male and 14 (24.6%) female. Their mean age was 32.6 years (SD 11.19, range 17–60). The mean duration of knee pain before the surgery was 17.36 months (SD 24.15 months, range 1–120 months). The medial meniscus was ruptured in 43 patients (75.4%) and the lateral in 14 (24.6%). Synchronous anterior cruciate ligament (ACL) failure was observed in 11 cases (19.3%). In 39 of the patients (68.4%), meniscal tearing was attributed to trauma and in 18 (31.6%) to a background of clinically diagnosed OA. The histopathologic identification of meniscal degeneration (MD) was based on established microscopy criteria (6,22). More specifically, the presence of inflammation, calcification, increased cellularity, and meniscal cell clustering, development of myxoid changes, and existence of stimulated perimeniscal layer composed of activated synovial cells were evaluated. MD was observed in 34 (59.4%) of the examined menisci.
Immunohistochemistry
The classic biotin-streptavidin-peroxidase assay was performed on 4-μm-thick, formalin-fixed, paraffin-embedded sections, as described (23). The following commercially available antibodies were employed (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA): anti-p38 (polyclonal, sc-7149; dilution 1:70), anti–p-p38 (activated form of p38) (monoclonal, sc-7972; dilution 1:100), anti–NF-κB p50 (polyclonal, sc-114; dilution 1:100), anti–NF-κB p65 (polyclonal, sc-109; dilution 1:100), and anti–COX-2 (polyclonal, sc-1746; dilution 1:70).
Stain intensity and proportion of immunopositive meniscal fibrochondrocytes was assessed by light microscopy and evaluated independently by two investigators (D.J.P. and A.G.P.). Because fibrocartilage is a relatively acellular tissue, a minimum of about 300 meniscal cells were evaluated in each specimen. Immunohistochemical staining was graded on a scale of 0 to 3 (0, no immunoreactivity; 1, mild immunoreactivity, 1%–33% positive cells; 2, moderate immunoreactivity, 33%–66% positive cells; 3, strong immunoreactivity, 67%–100% positive cells) (24).
Statistical Analysis
Mann-Whitney tests were used to compare nonparametric variable scores between the patients with and without MD and OA. The strength of association between categorical variables was assessed by Kendall’s τ test. For some correlations, protein expression levels were recoded from the four level (0–3) into a two-level scale (low/high expression). All statistical analyses were performed using SPSS for Windows (version 13.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical Correlations
Statistical analysis revealed significant correlation between the age of the patients and the presence of MD (Kendall’s τ –0.384, P = 0.0001). MD was observed in 28 of 34 (~82%) patients older than 25 years and only 6 of 34 (~18%) patients 25 or younger. In addition, patients’ age was strongly associated with clinical OA (Kendall’s τ 0.295, P = 0.008). Indeed, among the 18 patients who had clinically diagnosed OA, 15 (~84%) were older than 25 years and only 3 (~16%) were 25 or younger. Another interesting clinical finding was that the duration of the patient’s symptoms before the arthroscopy exhibited substantial association with the development of knee OA (Kendall’s τ 0.379, P = 0.001). Not surprisingly, the identification of MD was robustly correlated with the presence of clinical OA (Kendall’s τ 0.405, P = 0.002).
All the other clinical variables did not display any significant correlations at the level of P < 0.05.
Expression and Activation Profile of the p38 MAPK Signaling Pathway Elements
p38 was expressed in 47 of 57 (82.5%) torn menisci examined. Its localization was both cytoplasmic and nuclear (Figure 1A, B). Its expression levels were significantly higher in degenerated compared with nondegenerated menisci (Mann-Whitney test, P = 0.001). More specifically, 53.0% of the disrupted menisci displayed high (2 or 3) p38 immunoexpression. By contrast, only one of the 23 (4.3%) nondisrupted menisci showed enhanced p38 immunopositivity (Table 1). In line with this finding, menisci from patients with preexisting knee OA showed significantly increased p38 expression levels compared with those without OA (Mann-Whitney test, P = 0.001). In fact, 66.7% of the OA and 15.4% of the non-OA menisci revealed enhanced p38 immunoreactivity (Table 1).
Figure 1.
(A) Nondegenerated torn meniscus displaying weak cytoplasmic immunoreactivity for p38 MAPK (20×). (B) Degenerated meniscus exhibiting strong p38 MAPK immunoexpression. Note the formation of fibrochondrocytic clusters, a characteristic finding in disintegrated menisci (40×). (C) Weak immunopositivity for p-p38 in a patient with no evidence of meniscal degeneration or clinical history of OA (20×). (D) Intense nuclear and cytoplasmic p-p38 immunoreactivity in a patient with meniscal degeneration (40×).
Table 1.
Immunohistochemical expression of p38 and its phosphorylated/activated form, p-p38
| p38
|
p-p38
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Immunohistochemistry | Meniscal degeneration | No meniscal degeneration | Osteoarthritis | No osteoarthritis | Meniscal degeneration | No meniscal degeneration | Osteoarthritis | No osteoarthritis |
| 0 | 3 (8.8) | 7 (30.4) | 0 (0.0) | 10 (25.6) | 0 (0.0) | 4 (17.4) | 0 (0.0) | 1 (2.6) |
| 1 | 13 (38.2) | 15 (65.2) | 5 (27.8) | 23 (59.0) | 9 (26.5) | 16 (69.6) | 4 (22.2) | 21 (53.8) |
| 2 | 14 (41.2) | 1 (4.3) | 10 (55.5) | 5 (12.8) | 21 (61.8) | 6 (26.1) | 12 (66.7) | 15 (38.5) |
| 3 | 4 (11.8) | 0 (0.0) | 2 (11.1) | 1 (2.5) | 3 (8.8) | 0 (0.0) | 1 (5.5) | 2 (5.1) |
| Low expression (0, 1) | 16 (47.0) | 22 (95.7) | 5 (27.8) | 33 (84.6) | 9 (26.5) | 20 (87.0)) | 4 (22.2) | 22 (56.4) |
| High expression (2, 3) | 18 (53.0) | 1 (4.3) | 12 (66.7) | 6 (15.4) | 24 (70.6) | 6 (26.1) | 13 (78.2) | 17 (43.6) |
| Total | 34 | 23 | 18 | 39 | 34 | 23 | 18 | 39 |
Data are n (%).
The immunohistochemical profile of the phosphorylated, hence activated, form of p38, p-p38, was both nuclear and cytoplasmic, albeit primarily nuclear (Figure 1C, D). In concert with its quiescent species, p-p38 expression was considerably elevated in degenerated compared with nondegenerated and in OA compared with non-OA ruptured menisci (Mann-Whitney test, P < 0.001). Phosphorylated p38 displayed positive immunostaining in the vast majority of the cases (98.2%). Augmented p-p38 expression levels were observed in 70.6% of the degenerative but only in 26.1% of the nondegenerated menisci (Table 1). OA fibrocartilage displayed increased p-p38 reactivity in 72.28% of the cases. Menisci from patients lacking OA revealed p-p38 immunopositivity in 43.6% of the cases (Table 1). The cellular levels of p-p38 and p38 were positively and significantly correlated to each other (Kendall’s τ = 0.574, P = 0.0001).
The downstream effectors of p38, NF-κB subunits p50 and p65, were expressed in 91.2% and 98.2% of the assessed menisci, respectively. Their immunolocalization was both cytoplasmic and nuclear (Figure 2A–D). High p50 expression was detected in 76.5% of the degenerated and 83.3% of the OA menisci (Table 2). Its binding partner, p65, exhibited high protein expression in 83.3% of the OA and 64.1% of the non-OA patients (Table 2). The cellular levels of NF-κB species were significantly higher in degenerated and OA fibrocartilage (Mann-Whitney test, P = 0.001). Additionally, p50 and p65 expression levels were positively and significantly correlated to each other (Kendall’s τ = 0.850, P < 0.0001) and to the cellular levels of p38 and p-p38 (Kendall’s τ = 0.295 to 0.519, P = 0.0001 to 0.027). Normal fibrochondrocytes did not reveal immunoreactivity for any of the aforementioned proteins (Figure 3D).
Figure 2.
(A) Traumatic, nondegenerated meniscal tear showing faint immunoreactivity for NF-κB p50 (20×). (B) Intense nuclear and cytoplasmic immunostaining of NF-κB p50 in degenerated meniscus. There are areas with myxoid change and fibrocartilage tear in the upper part of the field (40×). (C) Weak immunopositivity for the NF-κB species p65 (40×). (D) Strong, primarily cytoplasmic staining for NF-κB p65 in fibrochondrocytic clusters in degenerated meniscus. Myxoid changes and tear formation can be easily appreciated (40×).
Table 2.
Immunohistochemical expression of the NF-κB p50 and p65 subunits.
| NF-κB p50
|
NF-κB p65
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Immunohistochemistry | Meniscal degeneration | No meniscal degeneration | Osteoarthritis | No osteoarthritis | Meniscal degeneration | No meniscal degeneration | Osteoarthritis | No osteoarthritis |
| 0 | 1 (2.9) | 4 (17.4) | 1 (5.5) | 4 (10.3) | 0 (0.0) | 1 (4.3) | 0 (0.0) | 1 (2.6) |
| 1 | 7 (20.6) | 16 (69.6) | 2 (11.1) | 21 (53.8) | 5 (14.7) | 11 (47.8) | 3 (16.7) | 13 (33.3) |
| 2 | 23 (67.6) | 2 (8.7) | 14 (77.8) | 12 (30.8) | 22 (64.7) | 10 (43.5) | 13 (72.2) | 19 (48.7) |
| 3 | 3 (8.8) | 0 (0.0) | 1 (5.5) | 2 (5.1) | 7 (20.6) | 1 (4.3) | 2 (11.1) | 6 (15.4) |
| Low expression (0, 1) | 8 (23.5) | 20 (87.0) | 3 (16.7) | 25 (64.1) | 5 (14.7) | 12 (52.2) | 3 (16.7) | 14 (35.9) |
| High expression (2, 3) | 26 (76.5) | 2 (8.7) | 15 (83.3) | 14 (35.9) | 29 (85.3) | 11 (47.8) | 15 (83.3) | 25 (64.1) |
| Total | 34 | 23 | 18 | 39 | 34 | 23 | 18 | 39 |
Data are n (%).
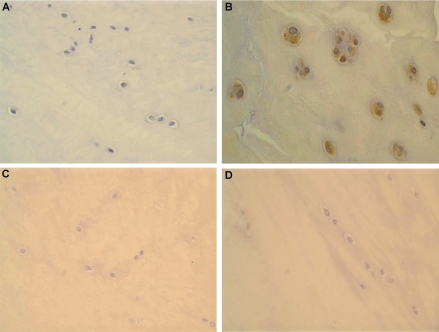
Figure 3.
(A) Weak COX-2 expression in traumatic meniscus with no degenerative lesions (20×). (B) A case of meniscus displaying intense, principally cytoplasmic COX-2 immunoexpression. Cell clusters, myxoid changes and disrupted fibrocartilaginous tissue are evident (40×). (C, D) Fibrocartilage of normal menisci does not display immunoreactivity for COX-2 and p38 under the conditions employed (20×).
Expression of COX-2
COX-2 immunoreactivity was observed in 96% of the examined menisci and was both nuclear and cytoplasmic (Figure 3A, B). Increased COX-2 expression levels were detected in 61.8% of the degenerated and in only one case (4.3%) of histologically blunt menisci. Normal fibrochondrocytes did not display COX-2 immunoreactivity (Figure 3C). Augmented COX-2 expression levels were identified in 77.8% of the OA but only in 20.5% of the non-OA knee joints (Table 3). There were significant differences in the expression levels of COX-2 between degenerated and nondegenerated fibrocartilage, as well as between OA and non-OA joints (Mann-Whitney test, P = 0.001 for both). Moreover, statistical analysis revealed significant and positive correlation between COX-2 and its upstream modulators, p38 and NF-κB (Kendall’s τ = 0.645 to 0.688, P = 0.002 to 0.0001).
Table 3.
Immunohistochemical expression of the pro-inflammatory factor COX-2.
| COX-2
|
||||
|---|---|---|---|---|
| Immunohistochemistry | Meniscal degeneration | No meniscal degeneration | Osteoarthritis | No osteoarthritis |
| 0 | 3 (8.8%) | 5 (21.7%) | 2 (11.1%) | 6 (15.4%) |
| 1 | 10 (29.4%) | 17 (74.0%) | 2 (11.1%) | 25 (64.1%) |
| 2 | 18 (53.0%) | 1 (4.3%) | 13 (72.2%) | 6 (15.4%) |
| 3 | 3 (8.8%) | 0 (0.0%) | 1 (5.5%) | 2 (5.1%) |
| Low expression (0, 1) | 13 (38.2%) | 22 (95.7%) | 4 (22.2%) | 31 (79.5%) |
| High expression (2, 3) | 21 (61.8%) | 1 (4.3%) | 14 (77.8%) | 8 (20.5%) |
| Total | 34 | 23 | 18 | 39 |
Data are n (%).
DISCUSSION
Meniscal rupture is one of the most common conditions in orthopedic surgery. It is presumed to be associated with traumatic events as well as with fibrocartilaginous degeneration processes (6). During the past decades, the pivotal and comprehensive role of meniscus in knee biomechanics has been established (25). Injury or removal of menisci generates knee instability, articular cartilage destruction, and eventually OA (26). Notably, a strong association between cartilage lesions and meniscal disintegration/rupture has been documented (7). Nonetheless, the molecular mechanisms that underlie these clinical events have not been studied extensively.
In the present study, we observed a significant correlation between the patients’ age and MD development. This could be attributed to multiple genetic and mechanical factors. Experiments in rabbits have shown that mature menisci have increased mRNA quotient of factors engaged in cartilage degradation (matrix metalloproteinase-1 [MMP-1], MMP-3, aggrecanase, COX-2) (5,27). In addition, animal studies have demonstrated significant upregulation of the pro-apoptotic genes caspase-8, Fas, and Fas-ligand in aged meniscus, indicating a role of programmed cell death in the age-related MD (28). Several reports support the notion that knee joint functions as an “organ”; hence, humoral mediators targeting one of its parts are expected to target other components of the joint as well. In accordance with this hypothesis, in the present study we observed that disintegrated menisci exhibited a significant association with the presence of knee OA. Not surprisingly, OA was significantly more common in older than in younger individuals. This is in agreement with data from a recent MRI study, which revealed that tear development is strongly associated with knee OA, especially in older women (7). A possible explanation could be that age-related reduction of physical activity and increased static loading induce depletion of proteoglycans and damage the collagen network, resulting in impairment of cartilage synthesis and ultimately joint damage. Moreover, the process of ageing facilitates the expression of matrix degradation and apoptosis-related genes that arbitrate OA (28,29).
The three mammalian MAPKs (Jun N-terminal kinase [JNK], extracellular signal-regulated kinase [ERK], p38) are well-conserved serine/threonine kinases that convert extracellular stimuli into specific cellular responses. They are connected to vital cell processes such as development, differentiation, proliferation, and apoptosis (30,31). The p38 MAPK signaling pathway is also strongly involved in the regulation of inflammatory responses. A variety of physical and chemical cues (oxidative stress, hypoxia, UV light, cytokines) activate the MAPK kinase kinases (MAPKKKs), which in turn phosphorylate/potentiate the MEK3 and MEK6 kinases. Activated MEK3/6 (along with MEK4 and the non-MAPK, TAB1) targets the TGY phosphorylation motif of p38 vertebrate isoforms (α, β, γ, δ) (11,12). In joints, p38 MAPK cascade is potentiated by divergent autocrine, paracrine, and endocrine factors, which induce synovium and/or cartilage damage leading to synovitis, rheumatoid arthritis, and OA. In an effort to investigate the role of this pathway in the pathogenesis of MD, we studied immunohistochemically the expression and/or activation profile of p38 MAPK and its targets, NF-κB p50/NF-κB p65 in tears from degenerated and nondegenerated human menisci.
Several clinical and experimental studies have documented that NF-κB is up-regulated in a plethora of inflammatory diseases, namely rheumatoid arthritis, asthma, inflammatory bowel disease, and OA (32). In concert with these reports, we observed overexpression of NF-κB and p38 species in the majority of degenerated but only in a small fraction of nondegenerated menisci. Normal fibrocartilage was void of these immunoreactivities under the conditions employed. Furthermore, our study revealed that the expression levels of these proteins were substantially higher in MD compared with non-MD samples. These data imply that the p38–NF-κB signaling cascade may participate in MD development in humans. Interestingly, the cellular levels of the examined proteins were significantly increased in the menisci of patients suffering from OA, in comparison with menisci from non-OA patients. Because the detection of MD was strongly connected to the presence of OA, one can postulate that the p38–NF-κB axis may also be implicated in the progression of OA to MD and eventually to meniscus rupture.
Notably, the expression and/or activation status of the constituents of the p38–NF-κB axis were found to be strongly and positively associated to each other. Moreover, in our study the NF-κB family members, p50 and p65, were shown to be coimmunolocalized in the analogous cellular compartments of menisci. Their expression levels displayed robust correlation to each other and were considerably augmented in patients with MD and OA. The parallel upregulation of p50 and p65 species indicates that these proteins most likely serve as the functional NF-κB complex linked to MD pathophysiology. Interestingly, the tested proteins exhibited both nuclear and cytoplasmic immunolocalization, indicating that the p38–NF-κB cascade is possibly implicated in the pathology of MD in a coordinated fashion. Indeed, under the impact of inflammatory stimuli, activated p38 MAPK either translocates to the nucleus or endorses the release of NF-κB, which also enters the nucleus to modulate gene transcription.
COX-2 regulates PG and thromboxane production in inflammatory diseases. Several lines of evidence pinpoint the critical role of COX-2 in the pathogenesis of human OA (33,34). Specifically, experiments on human articular cartilage and meniscus have demonstrated that COX-2 modulates cartilage proteoglycan degradation resulting in OA and MD (5,34). Following IL-β stimulation, the investigators observed enhanced COX-2 immunoexpression in the fibrochondrocytes of human degraded menisci. In agreement with these data, our study revealed enhanced COX-2 expression in the majority of torn menisci, whereas normal chondrocyte-like cells were immunonegative. Furthermore, the expression levels of this factor were significantly higher in degenerated compared with nondegenerated menisci and in OA compared with non-OA knees. These findings highlight the possibility that COX-2 might be involved in the pathobiochemistry of MD. Even though the paramount importance of COX-2 in knee inflammation has been well recognized, little is known about the precise signaling networks that control its expression. From a historical perspective, Gram-negative bacterial polysaccharides are the first reported COX-2 activators (35). However, nowadays it is clear that COX-2 is induced by a broad spectrum of pro-inflammatory mediators. Sequence analysis of the human COX-2 gene promoter has uncovered two cAMP-response elements (CREs), a sterol-response element (SRE), two activator protein-1 (AP-1) binding sites, and two NF-κB binding sites (19). With regard to transcriptional regulation, in vitro experiments have documented COX-2 upregulation in response to several signaling networks, including JNK/p38 and NF-κB (19,20). Mechanical strain and UV irradiation are also implicated in COX-2 activation (20,33). In symphony with these data, we found that COX-2 immunolocalization parallels that of its upstream effectors, p38 and NF-κB. Further statistical analysis revealed that the expression levels of the aforementioned proteins were significantly associated with each other. These results suggest that two distinct transcription effectors, p38 MAPK and NF-κB, might be involved in the pathobiochemistry of MD, either synergistically as constituents of the same signaling cascade or separately via upregulation of COX-2 gene. Additional biochemical studies are necessary to substantiate this hypothesis.
Collectively, the present study introduces a model concerning the molecular pathogenesis of meniscal degeneration and consequential rupture. More specifically, the combination of age and OA that leads to reduced mobility and increased static load on the knee joint might result in potentiation of the p38–NF-κB signaling cascade and the stimulation of its downstream effector, COX-2. Alternatively, p38 may independently or through a NF-κB–independent pathway regulate COX-2 expression. These molecular events could eventually augment fibrocartilage disintegration, resulting in meniscal tears.
ACKNOWLEDGMENTS
We thank Drs. V. Gorgoulis and D. Corradi for helpful comments and suggestions.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Proctor CS, Schmidt MB, Whipple RR, Kelly MA, Mow VC. Material properties of the normal medial meniscus. J Orthop Res. 1989;7:771–82. doi: 10.1002/jor.1100070602. [DOI] [PubMed] [Google Scholar]
- 2.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Rel Res. 1990;252:8–18. [PubMed] [Google Scholar]
- 3.Ghadially F, Lalonde J, Wedge J. Ultrastructure of normal and torn menisci of the human knee joint. J Anat. 1983;136:773–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Radin EL. Factors influencing the progression of osteoarthritis. In: Ewing JW, editor. Articular Cartilage and Knee Joint Function. Raven Press; New York: 1990. pp. 301–9. [Google Scholar]
- 5.Hellio Le Gravetand M, Vigon E, Otterness IG, Hart DA. Early changes in lapine menisci during osteoarthritis development. Part II: molecular alterations. Osteoarthritis Cart. 2001;9:65–72. doi: 10.1053/joca.2000.0351. [DOI] [PubMed] [Google Scholar]
- 6.Hough AJ, Webber RJ. Pathology of the meniscus. Clin Orthop Rel Res. 1989;252:32–40. [PubMed] [Google Scholar]
- 7.Lange AK, Fiatarone Singh MA, Smith RM, Foroughi N, Baker MK, Shnier R, Vanwanseele B. Degenerative meniscus tears and mobility impairment in women with knee osteoarthritis. Osteoarthritis Cart. 2007;15:701–8. doi: 10.1016/j.joca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Berenbaum F. Signaling transduction: target in osteoarthritis. Curr Opin Rheumatol. 2004;16:616–22. doi: 10.1097/01.bor.0000133663.37352.4a. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney SE, Firestein GS. Signal transduction in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16:231–7. doi: 10.1097/00002281-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Bohem J, Lee JC. p38 MAP kinases: key signaling molecules as therapeutic targets for inflammatory disease. Nat Rev Drug Discov. 2003;2:717–26. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 11.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK and p38 protein kinases. Science. 2002;289:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 12.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem Sci. 2000;25:257–60. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 13.Pomerantz JL, Baltimore D. Two pathways to NF-κB. Mol Cell. 2002;10:693–701. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 14.Tergaonkar V. NF-κB pathway: a good signaling paradigm for therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–53. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Grilli M, Chiu JJ, Lenardo MJ. NF-κB and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 18.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y-L, Mbonye UR, DeLong CL, Wada M, Smith WL. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipids Res. 2007;46:108–25. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signaling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–61. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Harris E. Rheumatoid arthritis: pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 22.Mesiha M, Zurakovski D, Soriano J, Nielson JH, Zarins B, Murray M. Pathologic characteristics of the torn human meniscus. Am J Sports Med. 2007;35:103–12. doi: 10.1177/0363546506293700. [DOI] [PubMed] [Google Scholar]
- 23.Papachristou DJ, Papachristou GI, Papaefthimiou OA, Agnantis NJ, Basdra EK, Papavassiliou AG. The MAPK-AP-1/Runx-2 signaling axes are implicated in chondrosarcoma pathobiology either independently or via up-regulation of VEGF. Histopathology. 2005;47:565–74. doi: 10.1111/j.1365-2559.2005.02266.x. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulou AK, Papachristou DJ, Chatzopoulos SA, Pirttiniemi P, Papavassiliou AG, Basdra EK. Load application induces changes in the expression of Sox-9 and FGFR-3 and VEGF in condylar chondrocytes. FEBS Lett. 2007;581:2041–6. doi: 10.1016/j.febslet.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Noble J, Hamblen DL. The pathology of degenerated meniscus lesion. J Bone Joint Surg Br. 1975;57:180–6. [PubMed] [Google Scholar]
- 26.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–60. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellio Le Gravetand M, Reno C, Hart DA. Gene expression in menisci from knees of skeletally mature female rabbits. J Orthop Res. 1999;17:738–44. doi: 10.1002/jor.1100170518. [DOI] [PubMed] [Google Scholar]
- 28.Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum. 2007;56:1529–36. doi: 10.1002/art.22523. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Suzuki M, Kushida K, Hoshino H, Innue T. The effect of ageing and osteoarthritis on the mature and senescent cross-links of collagen in human meniscus. Arthroscopy. 1998;14:366–72. doi: 10.1016/s0749-8063(98)70003-9. [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 31.Cuardo A, Lafarga V, Cheung PCF, Donaldo I, Llanos S, Cohen P, Nebreda AR. A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO J. 2007;26:2115–26. doi: 10.1038/sj.emboj.7601657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tak PP, Firestein GS. NF-κB: a key role in inflammatory disease. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cart. 2002;10:792–8. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 34.Hardy MM, Seibert KS, Manning PT, Currie MG, Woerner M, Edwards DE, Koki A, Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–8. [PubMed] [Google Scholar]