Abstract
RhoA is a critical signaling molecule regulating a variety of cellular processes, such as cytoskeletal organization, adhesion, and apoptosis. It is recently considered responsive to reactive oxygen species (ROS). Nevertheless, how RhoA regulates anoikis, a detachment-initiated apoptosis, and how this regulation is affected by ROS are not clear. The present study investigated the role of RhoA in apoptosis/anoikis in gastric cancer cells and the changes of RhoA and anoikis under oxidative stress. Immunohistochemistry showed that RhoA expression was upregulated in the primary gastric carcinoma compared with normal gastric mucosa. Overactivation of RhoA by transfection with the V14RhoA mutant prevented gastric cancer line SGC-7901 cells from arsenic-induced apoptosis and conferred anoikis resistance through, at least in part, promoting formations of F-actin fibers and focal adhesion. Oxidative stress caused by emodin, an ROS producer, in combination with arsenic trioxide (ATO) led to RhoA inactivation that triggered structural disruption of focal adhesion complex and eventually resulted in anoikis, and these effects could be partially reversed by antioxidant N-acetylcysteine (NAC). In conclusion, activation of RhoA is required for the maintenance of anoikis resistance phenotype of gastric cancer cells, and oxidative stress might be a therapeutic strategy for the inhibition of RhoA in cancer cells.
Introduction
Rho family GTPases serve as critical molecular coordinators in regulating various cellular behaviors. In their active GTP-bound form, Rho proteins bind to effector proteins, thereby triggering numerous cellular events, from cell adhesion and migration to cell survival, death, and transcriptional regulation. Each of these events is of importance for the development and progression of cancer [1–5].
Although Rho genes have never been found mutated in human tumors [2], the high incidence of overexpression of various Rho family members in a spectrum of human cancers suggests that these proteins are involved in cancer [2,6]. There are three Rho isoforms (RhoA, RhoB, and RhoC) in mammals [5]. RhoA has been found overexpressed or overactivated in breast cancer, bladder cancer, ovarian cancer, and other cancers [7–10]. Active RhoA promotes transcriptional activation of AP-1 and NFκB and regulates the activation of cyclin D1, p21cip1, and p27kip1 [11–13]. Thus, the role of RhoA in cancer is likely attributed to prosurvival and antiapoptosis.
Paradoxically, RhoA is also a signaling mediator in the morphologic changes associated with apoptosis through its effects on actin polymerization and actomyosin contractility. Introduction of a constitutively activated form of RhoA is sufficient for cell contraction and membrane blebbing that are the necessary events of apoptosis [3]. The RhoA effector protein ROCK acts in plasma membrane blebbing of apoptotic cells through activated myosin light chain which promotes actin filaments polymerization and regulates the actomyosin contractility [14,15]. Therefore, the role of RhoA in apoptosis remains to be clarified.
Loss of cell-extracellular matrix (ECM) contacts results in cell death by apoptosis, a phenomenon known as anoikis, or detachmentinduced apoptosis [16]. All the features that characterize apoptosis are also observed during anoikis, such as caspases' activation and nuclear fragmentation [17]. The interest in understanding the molecular mechanisms of anoikis has increased significantly during the last few years as it becomes evident that resistance to anoikis is a critical requirement for survival, invasion, and metastasis of cancers derived from epithelial cells [18]. Despite the increasing interest in the study of anoikis, the basic mechanisms of this phenomenon are still poorly understood [16,19]. A better understanding of anoikis could have an impact on the development of novel therapies for cancer, because it has been demonstrated that the reversion of anoikis resistance inhibits tumor progression [20]. Focal adhesion sites are specific areas on the cell membrane where cells attach to ECM. They are complexes of structural and signaling proteins, anchoring actin filaments and microtubules to the plasma membrane where integrins locate [21]. Most integrin β-subunits interact with proteins, such as paxillin, talin, vinculin, and other focal adhesion proteins, which act as linkers between integrins and the actin cytoskeleton [22–24]. It has been known that key players in integrin-mediated signal transduction are a group of integrin-associated nonreceptor kinases, two of them being focal adhesion kinase (FAK) and integrin-linked kinase (ILK), which may render influence on anoikis [21,25–28]. Because integrity of the focal adhesion complex depends on the actin cytoskeleton and their associated proteins, we may wonder the plausible role of RhoA in regulating anoikis. However, there has been little documentation in this regard.
Arsenic trioxide (As2O3 or ATO), a novel chemotherapeutic agent for treating acute promyelocytic leukemia, induces apoptosis of tumor cells with dependence on the cellular reactive oxygen species (ROS) [29,30]. In our previous studies, we have found a dramatic actin cytoskeleton change in gastric cancer cells exposed to ATO (data unpublished), and that the generation of ROS by emodin (6-methyl-1,3,8-trihydroxyanthraquinone) can sensitize some solid tumor cells to ATO-induced apoptosis [31–33]. Recently, RhoA was considered responsive to ROS and redox state [34,35]. These have raised questions on how RhoA functions in cancer cells apoptosis/anoikis and how this function is regulated by oxidation/reduction (redox) state of cells.
We herein report that RhoA plays an antiapoptotic role in gastric cancer cells through promoting the resistance of anoikis.Moreover, oxidative stress caused by emodin in combination with ATO can inhibit RhoA activation to sensitize human gastric carcinoma SGC-7901 cells to anoikis by disordered F-actin assembly and vinculin distribution.
Materials and Methods
Cell Culture, Reagents, and Treatments
Human gastric cancer line SGC-7901 cells [36,37] were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Gaitherburg, MD), supplemented with 100 U/ml penicillin, 100 mg/l streptomycin, and 10% fetal bovine serum, and were maintained at 37°C in a humidified incubator with 5% CO2. Arsenic trioxide (ATO or As2O3), emodin, and N-acetylcysteine (NAC) were purchased from Sigma (St. Louis, MO). Cells were exposed to various treatments for indicated times. ATO was used alone at 5 µM or in combination with emodin. To achieve a synergistic cytotoxic effect with arsenic, emodin was added at 10 µM, at which dose emodin alone had no cytotoxicity, according to our previous studies [31]. To assess the role of ROS, cells were pretreated with antioxidant NAC at 10 mM 4 hours before the above treatments, whenever it was used.
Immunohistochemistry
The human tissue samples were collected using institutionally approved protocols, in which 10 normal gastric tissues were derived from pathologic autopsy and 60 gastric carcinoma tissues were archived pathologic specimens in Ren Ji and Rui Jin Hospitals and were paraformaldehyde-fixed and paraffin-embedded specimens. A polyclonal mouse anti-human RhoA antibody (Ab) (Santa Cruz Biotechnology, Santa Cruz, CA) was incubated with the sections overnight at 4°C followed by biotinylated secondary Ab. Immunohistochemical reactions were visualized by the peroxidase-conjugated streptavidin, for which DAB was used as a chromagen. The results were analyzed using an image analysis software (QWin Plus; Leica Microsystems, Wetzler, Germany). The positivity rate was scored based on the percentage of the cells positive for RhoA in total cancer cells [38,39]. (-) indicates that the positive cells were <10%; (+), 10% ∼ 50%; and (++), >50%.
RhoA Constructs' Transfection
SGC-7901 cells were transfected with the wild-typed and mutated RhoA to determine the impact of RhoA on cell behaviors. Actively growing cells were transiently transfected with the wild-typed and site-mutated RhoA constructs, V14RhoA, the constitutively activated, and N19RhoA, the dominant-negative mutants (kindly provided by Dr. Richard D. Ye, University of Illinois at Chicago) [40,41]. Transfection was performed using a reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA) in accordance with the manufacturer¡¯s recommendation. Cells were then assayed for various purposes at 40 hours posttransfection. For all assays involving transfected cells, transfection efficiency was first checked and confirmed at 30% ∼ 40%.
Soft Agar Clonogenesis
Anchorage-independent growth as a characteristic of in vitro tumorigenicity was assessed by soft agar clonogenesis assay. Briefly, SGC-7901 cells were transiently transfected for 40 hours and were then trypsinized and mixed with DMEM containing 0.3% agar. Cell-agar mixture was plated on a 0.5% agar underlay (1 x 103 per well in six-well plates) and allowed to grow for 2 weeks. When cells needed drug treatment, they were exposed to various treatments for 9 hours and rinsed before being seeded. The assay was performed in triplicate for each group. Colony was identified when more than 50 cells grew within it. Calculation was based on the colony number of the whole well.
Apoptosis Assay
In the early apoptosis, phosphatidylserine, normally located in the inner leaflet of the plasma membrane, translocates to the outer membrane. In the present study, cells were treated with the indicated drugs for 48 hours. After washing once with ice-cold PBS, cells were collected and stained using an Annexin V-fluorescein isothiocyarate (FITC)/propidium iodide (PI) kit (BD Pharmingen, San Diego, CA), in which Annexin V bound to exposed phosphatidylserine of the early apoptotic cells, whereas PI stained the cells that had an increased membrane permeability, i.e., the late apoptotic cells. Samples were prepared according to the manufacturer's instruction and analyzed by flow cytometry on a FACS Calibur (Becton Dickinson, San Diego, CA).
ROS Detection
2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma) was used as ROS capture in the cells. It is cleaved intracellularly by nonspecific esterases to form 2,7-dichlorodihydrofluorescein (DCFH), which is further oxidized by ROS and becomes a highly fluorescent compound 2,7-dichlorofluorescein (DCF). In the present study, SGC-7901 cells were transiently transfected for 40 hours and were then exposed to various drugs for the indicated times. DCFH-DA at 10 µM was coincubated with cells for 20 minutes. After washing once with ice-cold PBS, cells were harvested and kept on ice for an immediate detection by flow cytometry. The average intensity of DCF stands for intracellular ROS levels.
Western Blot
SGC-7901 cells were lysed in a lysis buffer. Proteins were separated on 12% polyacrylamide gels and were transferred to nitrocellulose membranes. The blots were then incubated with first Ab, mouse anti-human Ab, followed by a peroxidase-conjugated second anti-mouse Ab (Promega, Madison, WI). Enhanced chemiluminescence (Amersham, Freiburg, Germany) was used for detection.
Immunofluorescence for RhoA and Vinculin, and Fluorescence for F-Actin
Cell monolayers on cover slides were fixed by 4% paraformaldehyde, permeated in 0.2% Triton X-100 at 4°C, and blocked with 5% BSA before double labeling for RhoA/vinculin, RhoA/F-actin, or F-actin/vinculin. Cell monolayers were incubated with the mouse anti-human vinculin (1:50; NeoMarkers, Fremont, CA) or mouse anti-human RhoA (1:50; Santa Cruz Biotechnology, CA) Ab at 4°C for overnight. Subsequently, the cells were incubated with rhodamine- or FITC-conjugated anti-mouse Ab (1:200; SouthernBiotech, Birmingham, AL) for 2 hours at 37°C separately and, when needed, coincubated with rhodamine-phalloidin (Sigma) for 40 minutes. Then the slides were examined under a laser confocal microscope (LSM510; Zeiss, Oberkochen, Germany).
RhoA Pull-Down Assay
Recombinant protein for Rhotekin Rho binding domain (RBD) can specifically bind to and precipitate GTP-, not GDP-formed Rho from cell lysates. RBD is linked to GST-coated agarose beads to form GST-RBD (Upstate, Lake placid, NY). Cells were treated with the indicated drugs for 9 hours before being lysed with buffers and methods as the manufacturer recommended. The lysate was incubated with 40 µg of GST-RBD for 1 hour. After binding, the samples were washed with lysis buffer three times. Pulled-down proteins that are activated Rho were fractionated on 12% SDS-PAGE and immunoblotted with polyclonal Ab against RhoA (Santa Cruz Biotechnology). The total cell lysates were also blotted with Ab for RhoA as a loading control. The level of activated RhoA was determined after normalization with the total RhoA present in the same cell lysates.
Caspase-3 Activity Assay
Caspase-3 activity was determined using the caspase-3 assay kit (BD Pharmingen) according to the manufacturer's instructions. This assay depends on the activity of cleavage of a specific caspase-3 substrate N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC) to liberate fluorescent AMC. After various treatments, cells were collected by scraping in cold PBS, centrifuged (at 800g for 5 minutes), and lysed in the cell lysis buffer provided in the kit on ice for 30 minutes. Extracts were mixed with an equal volume of 2x reaction buffer containing the Ac-DEVD-AMC and left for reaction in a water bath at 37°C for 60 minutes. The fluorescence intensity of liberated AMC, positively proportional to the caspase-3 activity, was measured using a plate reader with an excitation wavelength of 380 nm and an emission wavelength range of 420 to 460 nm.
Statistics
SPSS 13.0 software package (SPSS Inc., IL) was used for statistical analysis. Chi-square test was applied for enumeration data. Analysis of variance (ANOVA) was applied for comparison of the means of two or multiple groups of measurement data, in which Student-Newman-Keuls (SNK) test was used for further comparison of each group. For all of the value differences, P < .05 was considered significant.
Results
RhoA Was Overexpressed in Gastric Carcinoma Tissues, and the Level of Expression Was Positively Related to Malignancy
RhoA expression was examined in human normal gastric tissues and gastric carcinoma tissues by immunohistochemistry. In general, RhoA was undetectable in normal gastric mucosa, only showing positive in a few of cells mainly in the gastric pits in 20% specimens of nontumor tissues and 10% ones of normal mucosa adjacent to tumors. RhoA expression was largely positive in gastric carcinoma cells (85%). The value difference was considered significant between gastric carcinoma and normal gastric mucosa/benign tissue adjacent to the tumor (P < .05). In addition, the expression was more predominant in lowly differentiated carcinomas. The values for the strong positivity (++) were significantly different between lowly and highly differentiated gastric carcinoma, as well as between moderately and highly differentiated gastric carcinoma (P < .05) (Figure 1 and Table 1).
Figure 1.
RhoA was overexpressed in gastric carcinoma tissues, and the level of expression was related to malignancy (peroxidase-DAB immunohistochemistry): (A) normal gastric mucosa, (B) highly differentiated gastric carcinoma, (C) moderately differentiated gastric carcinoma, and (D) lowly differentiated gastric carcinoma (original magnification, x200).
Table 1.
RhoA Was Overexpressed in Gastric Carcinoma Tissues, and the Degree of the Positive Expression Was Related to Malignancy.
| Specimens | (-) | (+) | (++) |
| Nontumor tissue (n = 10) | 8 (80%) | 2 (20%) | 0 |
| Normal tissue adjacent to the tumor (n = 40) | 36 (90%) | 4 (10%) | 0 |
| Highly differentiated gastric carcinoma (n = 20) | 4 (20%) | 14 (70%) | 2 (10%) |
| Moderately differentiated gastric carcinoma (n = 25) | 3 (12%) | 14 (56%) | 8 (32%) |
| Lowly differentiated gastric carcinoma (n = 15) | 2 (13%) | 7 (47%) | 6 (40%) |
The positivity rate was scored based on the percentage of the cells positive for RhoA in total cancer cells. (-) indicates that the positive cells were <10%; (+), 10% ∼ 50%; (++), >50%. Chi-square analysis was applied. The value difference was considered significant between gastric carcinoma and normal gastric mucosa/benign tissue adjacent to the tumor when P < .05. The values for strong positivity (++) were significantly different between lowly and highly differentiated gastric carcinoma, as well as between moderately and highly differentiated gastric carcinoma, when P < .05.
Overexpression or Overactivation of RhoA in SGC-7901 Cells Antagonized Apoptosis
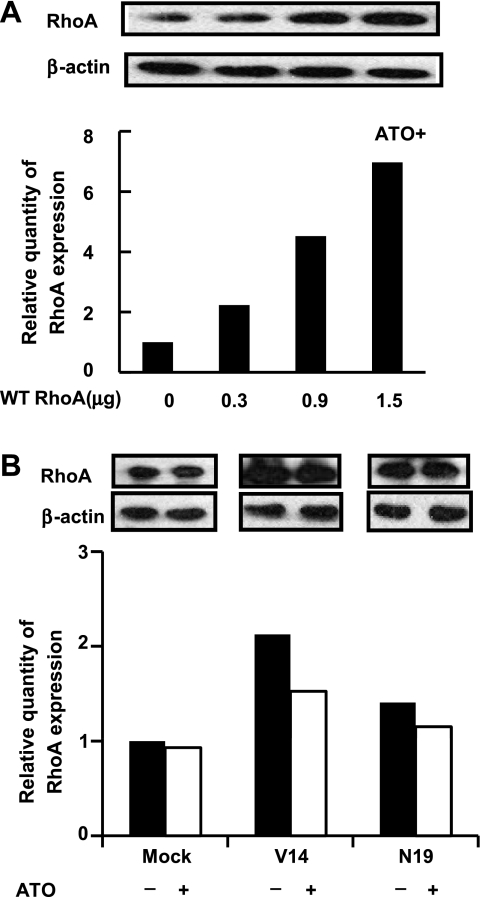
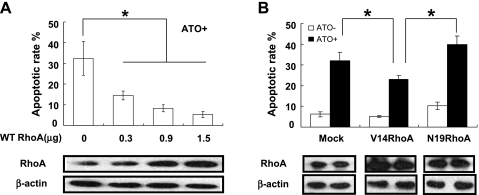
After SGC-7901 cells were transfected with different doses of wild-typed RhoA, the expression of RhoA was increased in a dose-dependent manner. RhoA obviously rescued ATO-induced apoptosis in a dose-dependent manner (Figure 2A and Figure W1). Likewise, in SGC-7901 cells transfected with the vector, the constitutively activated mutant V14RhoA, and the dominant negative one N19RhoA, the activated RhoA was capable of antagonizing apoptosis induced by ATO treatment, compared to the normal and inactivated RhoA, although the antiapoptosis function of RhoA was not apparent before ATO treatment (Figure 2B and Figure W1).
Figure 2.
Overexpression or overactivation of RhoA in SGC-7901 cells antagonized ATO-induced apoptosis (Annexin V/PI flow cytometry). (A) Cells were transfected with wild-typed (WT) RhoA at increased doses and were exposed to ATO (5 µM) for 48 hours. (B) Cells were transfected with mock DNA, V14RhoA, and N19RhoA at 40 hours before treatment with or without ATO (5 µM) for 48 hours. The quantity of RhoA expression was controlled by Western blot analysis. Values were mean ± SD of three different experiments. ANOVA was used for comparison of each group. *P < .05.
RhoA Activation Rendered SGC-7901 Cells' Anoikis Resistance
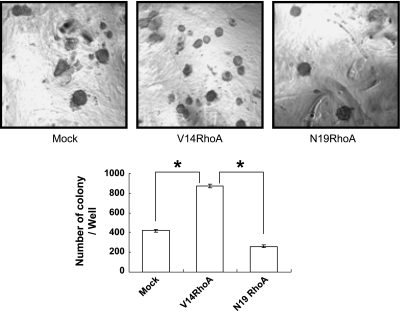
To determine whether RhoA overactivation rescued SGC-7901 cells through inhibiting anoikis, a classic assay, colony formation in soft agar, was performed. A more potent capacity of colony formation derived from single cell in soft agar represents an increased resistance to anoikis [42]. Results showed that the colonies in the V14RhoA-transfected cells were obviously more numerous than in the mockand N19RhoA-transfected cells (Figure 3). This result suggested that RhoA activation rendered cells' anoikis resistance, which might account for, at least partially, the capability of antiapoptosis in SGC-7901 cells.
Figure 3.
RhoA activation promoted anoikis resistance of SGC-7901 cells (colony formation in soft agar). Cells were seeded in the agar at 40 hours posttransfection and remained growing for 2 weeks before colony calculation (original magnification, x50). Values were mean ± SD of three different experiments. ANOVA was used for comparison of each group. *P < .05.
RhoA Activation Altered Assembly of F-Actin and Distribution of Vinculin
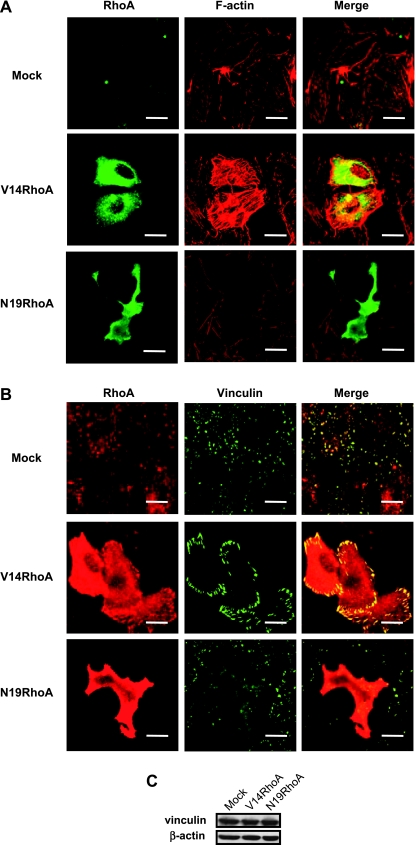
In the V14RhoA- and N19RhoA-transfected SGC-7901 cells, immunofluorescence was performed for visualizing the expression and distribution of RhoA and vinculin, and rhodamine-phalloidin staining was performed for visualizing F-actin. In the V14RhoA-transfected cells where RhoA was overexpressed and overactivated, F-actin was shown with a tremendously high intensity and was in concentrated bundles. In contrast, F-actin was hardly detectable in the N19RhoA-transfected cells where RhoA was overexpressed but inactivated (Figure 4A). Obviously, owing to reorganization of the actin fibers, the V14RhoA-transfected cells appeared more spread and thus larger, whereas the shape of N19RhoA-transfected cells was shrunk and highly irregular. Normally, vinculin was evenly distributed over the whole cytoplasm, but spottily concentrated to the plasmic membrane where the focal adhesion sites formed, as seen in cells transfected with mock DNA. However, in cells expressing RhoA mutants, the distribution of vinculin was changed. Compared with the mock DNA-transfected cells, the fluorescence of vinculin in V14RhoA cells aggregated into coarser plaques at the periphery of the cells, indicating that the focal adhesion was abnormally strengthened, whereas in N19RhoA cells, it was dispersed and much weaker, and the adhesive spots were nearly disappeared (Figure 4B). Notably, Western blot analysis showed that the quantities of vinculin and actin were not changed in cells, whether RhoA was overexpressed and activated or not (Figure 4C). These data indicated that overactivation of RhoA in SGC-7901 cells could enhance assembly of the actin filaments, and meanwhile enhance the cell attachment by simultaneously changing the distribution of vinculin, which could explain RhoA-mediated resistance to anoikis.
Figure 4.
RhoA activation changed the assembly of actin and distribution of vinculin (confocal microscopy and Western blot analysis). Cells were assayed at 40 hours posttransfection with the vector DNA or the RhoA mutants. (A) RhoA immunofluorescence (green) and F-actin staining with rhodamine-phalloidin (red). (B) RhoA and vinculin double immunofluorescence (red for RhoA and green for vinculin). (C) Western blot analysis for vinculin and actin. Scale bar, 10 µm.
Oxidative Stress Caused by Emodin in Combination with Arsenic Enhanced Apoptosis, By Suppressing the Activation of RhoA, but not Downregulating the Expression of Total RhoA
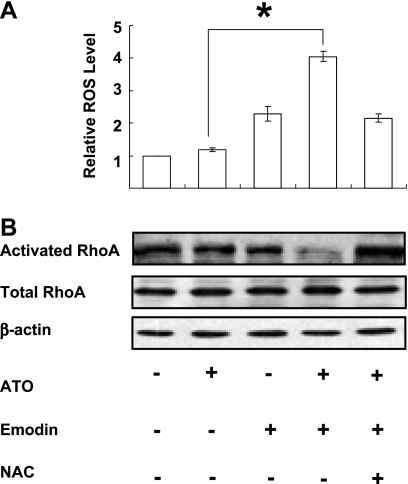
According to our previous studies, emodin, an ROS producer, can enhance cytotoxicity of the various drugs by inducing a high oxidative stress [31–33]. We therefore examined the effect on relative ROS level and RhoA activation under oxidative stress caused by emodin in combination with ATO in native SGC-7901 cells. The quantity of the activated form of RhoA was determined by GST-RBD pull-down assay in which activated RhoA was isolated. The results showed that the ROS generation was rapidly and obviously increased in cells exposed to the combinative treatment (Figure 5A). In parallel, activation of RhoA is remarkably suppressed a bit later by this oxidative stress, whereas the expression of total RhoA remained stable (Figure 5B). These effects could be completely or partially reversed by the antioxidant NAC (Figure 5).
Figure 5.
Oxidative stress caused by emodin in combination with arsenic suppressed the activation of RhoA, but did not downregulate the expression of total RhoA (DCF flow cytometry, GST-RBD pull-down, and Western blot). (A) Relative cellular ROS level of native SGC-7901 cells after 1-hour treatments. ATO, 5 µM; Emodin, 10 µM; NAC, 10 mM. Values were mean ± SD of three different experiments. ANOVA was applied for comparison of the means of two groups. *P < .05. (B) Western blot for RhoA of native SGC-7901 cells after 9-hour treatments. ATO, 5 µM; Emodin, 10 µM; NAC, 10 mM. Blots were representative of two experiments.
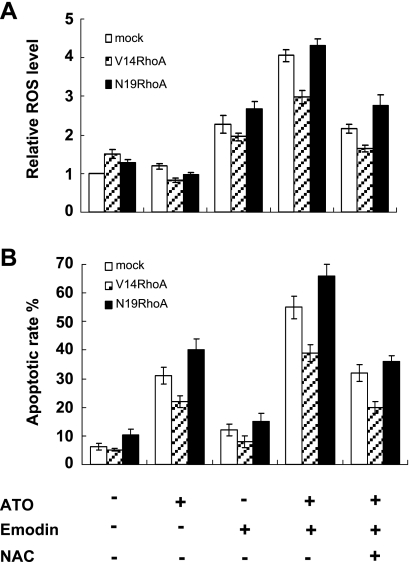
We then examined if the combinative treatment caused similar effects in cells with enforced expression of RhoA. After treating the transfected cells with emodin in combination with ATO for 1 hour, the level of relative ROS was increased in all three transfection groups. Also in parallel, after treatment for 48 hours, the apoptotic rate was significantly increased in cells exposed to the combinative treatment in all three transfection groups. Notably, apoptosis in V14RhoA-transfected cells was similarly enhanced, although to a modest extent. These effects could be partially reversed by the antioxidant NAC (Figure 6). To validate the redox role of emodin/arsenic combination, we also used staurosporine in combination with H2O2; however, the effect remained the same (data not shown). These results suggested that the combinative treatment caused oxidative stress in SGC-7901 cells and enhanced apoptosis, during which RhoA activation was inhibited in an ROS-dependent manner in the early phase. These also implied that oxidative stress could overcome the force of antiapoptosis rendered by activation of RhoA, as in V14-transfected cells.
Figure 6.
Oxidative stress caused by emodin in combination with arsenic-enhanced apoptosis, and these effects could be partially reversed by NAC. (A) Relative ROS level after 1-hour treatments in SGC-7901 cells 40 hours posttransfection with DNA for mock and RhoA mutants: ATO, 5 µM; Emodin, 10 µM; NAC, 10 mM. ATO versus ATO + Emodin: P < .05. (B) Apoptotic rates after 48-hour treatments in cells with transfections and treatments similar to panel A. ATO versus ATO + Emodin: P < .05; ATO + Emodin versus ATO + Emodin + NAC: P < .05. Values were mean ± SD of three different experiments. ANOVA was used for comparison of each group.
Oxidative Stress Caused by Emodin in Combination with Arsenic Could Overcome Anoikis Resistance of SGC-7901 Cells Transfected with V14RhoA
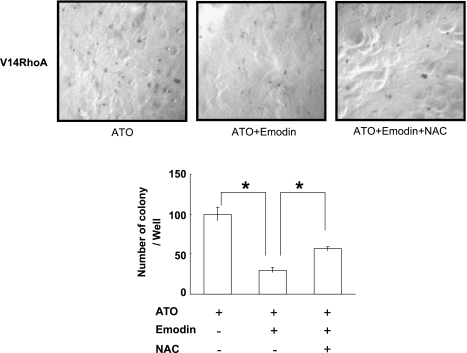
Because overactivation of RhoA promoted anoikis resistance in V14RhoA-transfected SGC-7901 cells, we checked colony formation of V14RhoA cells exposed to oxidative stress. Drugs or reagents were administered for a short period (9 hours) and were rinsed off before cells were seeded into agar and allowed to grow for 2 weeks. The number and size of colonies were significantly decreased, compared with those under nondrug-treated condition as in Figure 3. More importantly, in the wells exposed to the combinative treatment, the number of colonies was dramatically decreased, compared with ATO alone treatment. This effect could be partially reversed by the antioxidant NAC (Figure 7). Therefore, it was implied that anoikis resistance mediated by overactivation of RhoA could be reversed by oxidative stress.
Figure 7.
Oxidative stress caused by emodin in combination with arsenic inhibited anoikis resistance of SGC-7901 cells transfected with V14RhoA (colony formation in soft agar). Cells were seeded in the agar after drug treatments for 9 hours at 40 hours posttransfection of V14RhoA, and remained growing for 2 weeks before calculation (original magnification, x50). ATO, 5µM; Emodin, 10µM; NAC, 10 mM. Values were mean ± SD of three different experiments. ANOVA was applied for comparison of each group. *P < .05.
Oxidative Stress Caused by Emodin in Combination with Arsenic Altered Assembly of Actin and Distribution of Vinculin
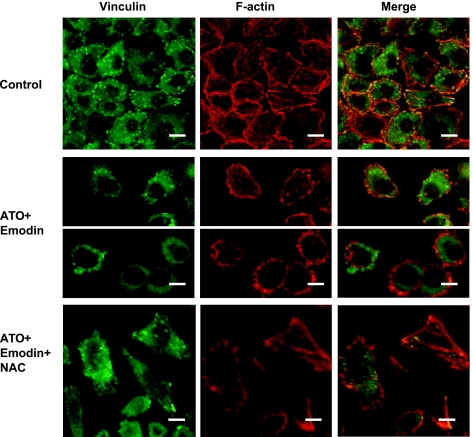
How two-drug-caused oxidative stress changed actin filaments and cell attachment was observed in the native SGC-7901 cells. In untreated cells, the bundles of the stress fiber were assembled across the cytoplasm, and the vinculin was distributed over the whole cytoplasm, but spottily concentrated at the focal adhesion sites where the fibers terminated and actin/vinculin were well colocalized (Figure 8, upper panel). In the cells exposed to emodin combined with arsenic for 12 hours (Figure 8, middle panel), the cells became detached and finally round up in which F-actin was not assembled into the elongated stress fibers, but rather, concentrated beneath the plasmic membranes to form cortical rings. Meanwhile, the vinculin was dispersed, no longer focused at the adhesive foci. Moreover, actin and vinculin were not colocalized anymore, especially in round up cells that might represent apoptotic cells (lower parts of the middle panel). These effects of cotreatment were abolished by NAC (Figure 8, lower panel).
Figure 8.
Oxidative stress caused by emodin in combination with arsenic-altered assembly of F-actin and distribution of vinculin, especially in apoptotic cells, whereas NAC could antagonize this effect (confocal microscopy). Native SGC-7901 cells were exposed to drugs for 12 hours. ATO, 5 µM; Emodin, 10 µM; NAC, 10 mM. Scale bar, 10 µm. Two parts of the middle panel showed cells separated in the field with two typical morphologies of cell detachment. Cells in the upper parts of the middle panel started to detach and the lower parts of the middle panel were round up with cortex actin rings.
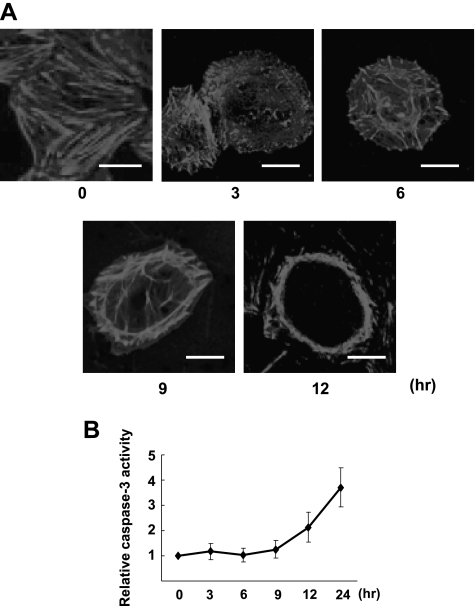
Oxidative Stress Caused by Emodin in Combination with Arsenic Induced Disassembly of F-Actin That Preceded Caspase-3 Activation
To determine the temporal association of disassembly of F-actin and apoptosis, we observed the change of assembly of F-actin and caspase-3 activation on oxidative stress. Figure 9 illustrates the time courses of change in caspase-3 protease activity and concomitant actin assembly pattern in SGC-7901 cells exposed to the combinative treatment. As early as 3 hours on the combinative treatment, the bundles of the stress fiber started to disassemble; the fibers gradually shortened and accumulated to the cortex of the cells. At 12 hours of treatment, the cells rounded up and actin formed cortex rings when caspase-3 began to be activated. Caspase-3 activity had substantial elevation after 12 hours. These results demonstrated that oxidative stress inhibited RhoA activation and induced F-actin disassembly, which was followed by apoptosis.
Figure 9.
Oxidative stress caused by emodin in combination with arsenic-induced disassembly of F-actin that preceded caspase-3 activation. (A) Confocal microscopy showing time course of Factin disassembly of native SGC-7901 cells after the combinative treatment (ATO + Emodin: ATO, 5 µM; Emodin, 10 µM; scale bar, 10 µm). (B) Time course for relative caspase-3 activities of native SGC-7901 cells after the same treatment as in A. Values were mean ± SD of three different experiments.
Discussion
RhoA and Gastric Cancer
RhoA has been intensively studied for its functions in cell signaling that regulates cytoskeleton-dependent responses, including cell phagocytosis, attachment, and migration [3,5,43]. In recent years, RhoA has been found overexpressed or overactivated in breast cancer, bladder cancer, ovarian cancer, and other cancers [7–10]. Hence, it is important to understand how RhoA plays a role in cancer biology through aberrant function in regulating assembly of cytoskeleton, i.e. actin, and cell/ECM adhesion. Here we have demonstrated in 10 normal human gastric tissues and 60 human gastric carcinoma tissues by immunohistochemistry that the expression level of RhoA protein is significantly higher in gastric carcinoma cells, especially in lowly differentiated carcinoma, than in normal gastric mucosa cells. RhoA expression is associated with the differentiation grade, suggesting that the expression level of RhoA correlated with the progressiveness of gastric cancer. To further explore the contribution of RhoA to gastric cancer cells, we interfere with RhoA function in cultured SGC-7901 cells, a cell line derived from a metastatic gastric cancer, by transfection with the wild-typed RhoA, the constitutively active RhoA and the dominant negative RhoA. The results reveal that both of overexpressed and overactivated RhoA prevent gastric cancer cells from apoptosis induced by ATO, or in fact, confer them resistance to anoikis. These in vitro data likely reflect the biologic function of RhoA in the primary gastric cancers.
RhoA and Anoikis
The mechanisms of anoikis involve a multitude of signal pathways, therefore anoikis resistance phenotype of transformed cells is endowed by various factors. Phosphorylation regulation of some focal adhesion proteins such as FAK and paxillin are known to be of utmost importance in the control of focal adhesion structure turnover and anoikis [21,44]. Normally, FAK is stimulated by transmembrane integrin proteins that bind to fibronectin extracellularly and (indirectly) to actin filaments intracellularly. FAK, when activated by integrins, can suppress anoikis [45]. Many oncogenic growth factors, kinases, and prosurvival transcription factors, for instance, NFκB, inhibit anoikis by activation of FAK, independently of integrins [46,47]. Because the actin filaments terminate at focal adhesion, and the integrity of focal adhesion complex requires correct organization of ECM, integrins, actin, and a series of cytoskeletal proteins, anoikis is readily affected by the reorganization of actin that processes RhoA modulation. However, so far, the mechanism that RhoA regulates anoikis has not drawn adequate study, although other two major members of the Rho family, i.e., Rac1 and Cdc42, are supposed to inhibit anoikis [48]. Recently, it has been reported that RhoA is activated in the ethanol-induced anoikis in astrocytes [49].
Based on our findings that RhoA is upregulated in the gastric cancer cells, and that overactivation of RhoA makes the gastric cancer cells resistant to anoikis, we hypothesize that, in these cells, RhoA confers anoikis resistance through, at least in part, promoting F-actin assembly and focal adhesion formation. It is known that active RhoA can initiate the assembly of a new actin filament from actin monomers [50], and vinculin is a key protein in focal adhesion linking actin filament to integrin [23]. We found in cells where endogenous RhoA activation is suppressed by the introduction of dominant negative mutant that actin fails to organize to the fibers and that vinculin could not localize to focal adhesion sites. Moreover, these cells are sensitive to the autonomous and ATO-induced apoptosis, as well as anoikis, compared to their parental cells. In contrast, in cells bearing the constitutively activated RhoA, focal adhesion is strengthened and cells are better spreading in culture, and in addition, cells are remarkably resistant to apoptosis and anoikis. Therefore, our study has verified for the first time that RhoA activation is necessary for the maintenance of anoikis resistance phenotype in cancer cells in vitro, simultaneously suggesting that RhoA could be a useful therapeutic target for gastric cancer. Despite that RhoA activation may result in anoikis resistance parallel that of a non-cytoskeletal pathway, as we also have found that NFκB activation is involved (data not shown), the striking morphologic difference presented in the two types of RhoA mutant-transfected cells is evidence of the predominant contribution by the cytoskeletal pathway.
It is interesting to evaluate the function of RhoA-related actin assembly in mediating two opposite behaviors: anoikis resistance and apoptotic morphologic changes. A typical morphology of apoptosis consists of two phases: first, contraction and blebbing; and second, breakdown of actin filaments and formation of apoptotic body. RhoA activation is responsible for both phases of actin reorganization [3]. In our study, by counting the apoptotic rate and colony formation in RhoA constitutively activated cells and repressed cells, respectively, we have revealed that RhoA activation is of antiapoptosis. It suggests that whether RhoA is transiently activated or not during apoptosis, its constitutive activation rescues apoptosis. It seems that the former is the issue of form of death, and the latter is the issue of living or dead. We then speculate that these two behaviors are controlled by entirely different upstream signals. The former is only triggered by irreversible apoptotic events, for instance, activation of the executive caspases, but the latter is concomitantly stimulated by the molecules that induce cell transformation or by various insults that initiate apoptosis. Overactivation of RhoA is thus one of the cancerous phenotypes.
ROS, RhoA, and Anoikis
As anoikis resistance is a hallmark of the transformed cells, it has been well recognized that the reversion of anoikis resistance can be a strategy to block tumor progression [20]. Meanwhile, Rho GTPases are considered potential candidates for anticancer therapy [6,10,51]. Hence, it is demanded to develop novel drugs and approaches to repress Rho members that confer anoikis resistance.
Chemotherapy is the most commonly used therapeutic approach in addition to operation for gastric cancers. Cellular redox state affects the cytotoxicity of a number of chemotherapeutic agents [52–54]. We previously demonstrated that an ROS producer emodin could strengthen ATO-induced apoptosis in a variety of cancer cells both in culture and in tumor-bearing mice [32,33]. Here we show that after treatment with ATO/emodin combination, the higher level of oxidative stress triggers apoptosis of gastric cancer cells, during which the activation of RhoA is markedly repressed at the early phase. The decreased or diminished cell colonies formed in soft agar have indicated cell detachment and recovery of anoikis by oxidative stress. Further investigation for time course has demonstrated that oxidative stress-caused disassembly of actin fibers is not a late event secondary to apoptosis initiation, rather it precedes caspase-3 activation, providing more evidence of anoikis.
It has long been noticed that coincident with endothelial cell detachment, there is a dramatic rise in the intracellular ROS level, and reattachment to a solid surface rapidly attenuates the ROS level. Thus, ROS are suggested to serve as regulators of anoikis [55], although the signaling pathway is not clear. In contrast with the case that ROS elevation follows detachment of endothelial cells, in out study, ROS likely initiates detachment through suppressing RhoA to abrogate anoikis resistance of gastric cancer cells.
As a key modulator of cell response to various stimuli, RhoA may be activated by a modest oxidative stress [35]. Conversely, it is inactivated by a severe oxidative stress due to oxidative modification of the specific cysteine residues [34], despite that transit activation may be observed during the progress, which makes researchers draw the controversial claims in respect to ROS-RhoA and RhoA-apoptosis associations [49,56]. In our previous studies, we have found that multiple proteins, including NFκB and caspase-9, undergo oxidative modification in the emodin-caused redox stress, resulting in differential switch of molecular activity [33]. Whether RhoA is oxidatively modified and what the responsible cysteine site(s) are under our experimental conditions are issues for future investigation. In addition to the proposal that RhoA is a novel target regulated by oxidative stress, the present study has suggested that emodin might be an inhibitor of RhoA with therapeutic benefit, especially when applied in synergy with other anticancer drugs.
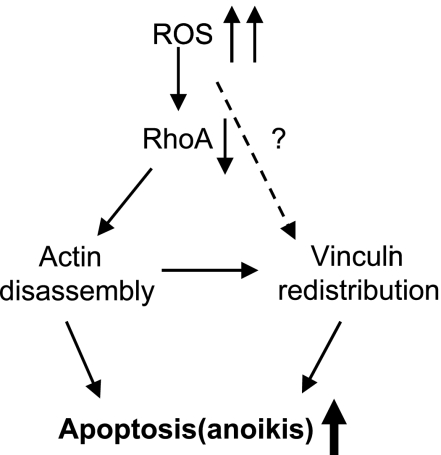
In conclusion, we provide evidence that the inhibition of RhoA by a high oxidative stress induces anoikis; that is, apoptosis caused by lack of correct cell/ECM attachment. As proposed in our model (Figure 10), RhoA inactivation by ROS leads to the actin filaments' disruption, and consequently followed by vinculin mislocalization, which triggers structural changes in focal adhesion and eventually results in anoikis. As the basis of this viewpoint, active RhoA is required for antianoikis. The regulatory role of other proteins of focal adhesion that are probably involved in RhoA-mediated anoikis resistance, for instance, vinculin, is worth exploring.
Figure 10.
Hypothetical model for the role of RhoA in ROS-induced apoptosis.
Supplementary Material
Acknowledgments
We thank Richard D. Ye (Department of Pharmacology, University of Illinois at Chicago) for the RhoA plasmid gifts.
Abbreviations
- Ab
antibody
- ATO
arsenic trioxide
- DCFH-DA
dichlorodihydrofluorescein diacetate
- ECM
extracellular matrix
- Emodin
6-methyl-1,3,8-trihydroxyanthraquinone
- FAK
focal adhesion kinase
- ILK
integrin-linked kinase
- NAC
N-acetylcysteine
- RBD
Rho binding domain
- ROS
reactive oxygen species
Footnotes
This work was supported by grants from the National Natural Science Foundation of China (no. 30570965) and Shanghai Science and Technology Committee (no. 05JC14033).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Hall A. RhoA GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ. Rho family proteins: coordinating cell responses. Cancer Metastasis Rev. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 3.Coleman ML, Olson MF. Rho GTPase signaling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 5.Vega FM, Ridley AJ. SnapShot: Rho Family GTPases. Cell. 2007;129:1430–1431. doi: 10.1016/j.cell.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Aznar S, Fernandez-Valeron P, Espina C, Lacal JC. Rho GTPases: potential candidates for anticancer therapy. Cancer Lett. 2004;206:181–191. doi: 10.1016/j.canlet.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumors: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–644. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamai T, Tsujii T, Arai K, Takagi K, Asami H, Ito Y, Oshima H. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder. Clin Cancer Res. 2003;9:2632–2641. [PubMed] [Google Scholar]
- 9.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–870. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 10.Ridley AJ. Rho proteins and cancer. Breast Cancer Res Treat. 2004;84:13–19. doi: 10.1023/B:BREA.0000018423.47497.c6. [DOI] [PubMed] [Google Scholar]
- 11.Liberto M, Cobrinik D, Minden A. Rho regulates p21(CIP1), cyclin D, and checkpoint control in mammary epithelial cells. Oncogene. 2002;21:1590–1599. doi: 10.1038/sj.onc.1205242. [DOI] [PubMed] [Google Scholar]
- 12.Vidal A, Millard SS, Miller JP, Koff A. Rho activity can alter the translation of p27 mRNA and is important for RasV122 induced transformation in a manner dependent on p27 status. J Biol Chem. 2002;277:16433–16440. doi: 10.1074/jbc.M112090200. [DOI] [PubMed] [Google Scholar]
- 13.Liu CA, Wang MJ, Chi CW, Wu CW, Chen JY. Rho/Rhotekin-mediated NF-kappaB activation confers resistance to apoptosis. Oncogene. 2004;23:8731–8742. doi: 10.1038/sj.onc.1208106. [DOI] [PubMed] [Google Scholar]
- 14.Hagmann J, Burger MM, Dagan D. Regulation of plasma membrane blebbing by the cytoskeleton. J Cell Biochem. 1999;73:488–499. [PubMed] [Google Scholar]
- 15.Riento K, Ridley AJ. ROCKS: multifunctional kinases in cell behaviour. Nature Rev Mol Biol Cell. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 16.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 17.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 18.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 19.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen K, Rak J, Leung T, Dean NM, Kerbel RS, Filmus J. ROCKS: multifunctional kinases in cell behaviour. J Cell Biol. 2000;149:447–456. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 22.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore AP. Anoikis. Cell Death Differ. 2005;12:1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- 26.Parson JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 27.Legate KR, Montañez E, Kudlacek O, Fässler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 28.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Chen GQ, Shen ZX, Chen SJ, Wang ZY. Treatment of acute promyelocytic leukemia with arsenic compounds: in vitro and in vivo studies. Semin Hematol. 2001;38:26–36. doi: 10.1053/shem.2001.20863. [DOI] [PubMed] [Google Scholar]
- 30.Maeda H, Hori S, Ohizumi H, Segawa T, Kakehi Y, Ogawa O, Kakizuka A. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and l-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 31.Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res. 2004;64:108–116. doi: 10.1158/0008-5472.can-2820-2. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Li H, Chen YY, Wang XJ, Shi GY, Hu QS, Kang XL, Lu Y, Tang XM, Guo QS, et al. Anthraquinones sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo via reactive oxygen species mediated dual regulation of apoptosis. Free Radic Biol Med. 2004;37:2027–2041. doi: 10.1016/j.freeradbiomed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Jing Y, Yang J, Wang Y, Li H, Chen Y, Hu Q, Shi G, Tang X, Yi J. Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor κB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med. 2006;40:2183–2197. doi: 10.1016/j.freeradbiomed.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Heo J, Raines KW, Mocanu V, Campbell SL. Redox regulation of RhoA. Biochemistry. 2006;45:14481–14489. doi: 10.1021/bi0610101. [DOI] [PubMed] [Google Scholar]
- 35.Kajimoto H, Hashimoto K, Bonnet SN, Haromy A, Harry G, Moudgil R, Nakanishi T, Rebeyka I, Thebaud B, Michelakis ED, et al. Oxygen activates the Rho/Rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondriaderived reactive oxygen species: a newly recognized mechanism for sustaining ductal constriction. Circulation. 2007;115:1777–1788. doi: 10.1161/CIRCULATIONAHA.106.649566. [DOI] [PubMed] [Google Scholar]
- 36.Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, Chen Y, Wang X, Liu J, Guo X, et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cells SGC7901 and AGS. FASEB J. 2007;21:2247–2256. doi: 10.1096/fj.06-7799com. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Huang Y, Ni Y, Wang H, Hou Y. siRNA targeting midkine inhibits gastric cancer cells growth and induces apoptosis involved caspase-3,8,9 activation and mitochondrial depolarization. J Biomed Sci. 2007;14:783–795. doi: 10.1007/s11373-007-9192-0. [DOI] [PubMed] [Google Scholar]
- 38.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–870. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 39.Aida J, Izumiyama-Shimomura N, Nakamura K, Ishii A, Ishikawa N, Honma N, Kurabayashi R, Kammori M, Poon SS, Arai T, et al. Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol. 2007;38:1192–1200. doi: 10.1016/j.humpath.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Shepard LW, Yang M, Xie P, Browning DD, Voyno-Yasenetskaya T, Kozasa T, Ye RD. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J Biol Chem. 2001;276:45979–45987. doi: 10.1074/jbc.M104783200. [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Chen LY, Zuraw BL, Ye RD, Pan ZK. Chemoattractant-stimulated NF-κB activation is dependent on the low molecular weight GTPase RhoA. J Biol Chem. 2001;276:40977–40981. doi: 10.1074/jbc.M105242200. [DOI] [PubMed] [Google Scholar]
- 42.Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH. E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chimini G, Chavrier P. Function of Rho family proteins in actin dynamics during phagocytosis and engulfment. Nat Cell Biol. 2000;2:E191–E196. doi: 10.1038/35036454. [DOI] [PubMed] [Google Scholar]
- 44.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 45.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 46.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toruner M, Fernandez-Zapico M, Sha JJ, Pham L, Urrutia R, Egan LJ. Antianoikis effect of nuclear factor-kappaB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J Biol Chem. 2006;281:8686–8696. doi: 10.1074/jbc.M512178200. [DOI] [PubMed] [Google Scholar]
- 48.Cheng TL, Symons M, Jou TS. Regulation of anoikis by Cdc42 and Rac1. Exp Cell Res. 2004;295:497–511. doi: 10.1016/j.yexcr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Miñambres R, Guasch RM, Perez-Aragó A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006;119:271–282. doi: 10.1242/jcs.02723. [DOI] [PubMed] [Google Scholar]
- 50.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Fritz G, Kaina B. Rho GTPases: promising cellular targets for novel anticancer drugs. Curr Cancer Drug Targets. 2006;6:1–14. [PubMed] [Google Scholar]
- 52.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- 53.Yi J, Gao F, Shi G, Li H, Wang Z, Shi X, Tang X. The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis. 2002;7:209–215. doi: 10.1023/a:1015331229263. [DOI] [PubMed] [Google Scholar]
- 54.Friesen C, Kiess Y, Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 55.Li AE, Ito H, Rovira II, Kim KS, Takeda K, Yu ZY, Ferrans VJ, Finkel T. A role for reactive oxygen species in endothelial cell anoikis. Circ Res. 1999;85:304–310. doi: 10.1161/01.res.85.4.304. [DOI] [PubMed] [Google Scholar]
- 56.Kang WK, Lee I, Ko U, Park C. Differential effects of RhoA signaling on anticancer agent-induced cell death. Oncol Rep. 2005;13:299–304. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.