Abstract
Flavin-containing monooxygenase 3 (FMO3) is a hepatic microsomal enzyme that oxidizes a host of drugs, xenobiotics and other chemicals. Numerous variants in the gene encoding FMO3 have been identified, some of which result in altered enzymatic activity and, consequently, altered substrate metabolism. Studies also implicate individual and ethnic differences in the frequency of FMO3 polymorphisms. In addition, new variants continue to be identified with potentially important clinical implications. For example, the role of FMO3 variants in the pathophysiology of gastrointestinal diseases is an evolving area of research. Two commonly occurring polymorphisms of FMO3, E158K and E308G, have been associated with a reduction in polyp burden in patients with familial adenomatous polyposis who were treated with sulindac sulfide, an FMO3 substrate. These findings suggest a potential role for prospective genotyping of common FMO3 polymorphisms in the treatment of disease states that involve the use of drugs metabolized by FMO3. This review summarizes the current state of research on the genetic polymorphisms of FMO3, with a focus on their clinical implications in gastrointestinal diseases.
Keywords: chemoprevention, familial adenomatous polyposis, flavin-containing monooxygenase 3, gastrointestinal diseases, SNP, sulindac sulfide, trimethylaminuria
Humans metabolize a vast array of endogenous and exogenous compounds, including drugs and xenobiotics. It has been observed that different individuals, genders and ethnicities respond differently to the same compounds. This has been attributed to multiple factors, one of which is SNPs. With the completion of the human genome sequence and the discovery of existing SNPs in the genome, interest has focused on the role of genetic polymorphisms of enzyme-encoding genes involved in the metabolism of both endogenous and exogenous substances.
The flavin-containing monooxygenases (FMOs) belong to a family of flavoprotein enzymes that catalyze the oxidation of a broad array of nucleophilic heteroatom-containing drugs, pesticides and chemicals [1–4]. There are six human isoforms of flavin-containing monooxygenases, of which five are functional. An additional gene cluster containing five pseudogenes has also been identified in both the human and mouse genome [5–10]. Koukouritaki and colleagues confirmed that FMO1 is the major fetal isoform, while FMO3 is the major adult isoform [11]. They further showed that FMO1 suppression occurs within the first few days of life in a process coupled with birth, not gestational age, and FMO3 expression becomes detectable in most individuals by the age of 1–2 years [11]. Also noted was a variation of 2- to 20-fold in expression of FMO1 and FMO3 among individuals, depending on the age bracket [11]. FMO3 is the most abundantly expressed FMO in the adult human liver [12]. Its structure and function and the implications of its polymorphisms have been widely studied [8,12,13]. This enzyme has a wide substrate specificity, including the dietary-derived tertiary amines trimethylamine, tyramine and nicotine; commonly used drugs including cimetidine, ranitidine, clozapine, methimazole, itopride, ketoconazole, tamoxifen and sulindac sulfide; and agrichemicals, such as organophosphates and carbamates [14–22].
The genes encoding five of the FMOs (1, 2, 3, 4 and 6) are located around a 250 kb cluster at chromosome locus 1q23–24 [6,8]. The FMO5 gene is separately mapped to chromosome 1q21 [5]. The FMO3 gene consists of eight coding and one noncoding exons that translate into a protein with 531 amino acids [23,24]. As mentioned earlier, the genes encoding the various flavin-containing monooxygenases have distinct tissue- and temporal-specific patterns of expression, with FMO3 being most widely expressed in the human adult liver [8,12,23]. The molecular basis for the regulation of the postnatal expression of FMO3 is not defined at this time.
Effects of FMO3 polymorphisms on substrate metabolism in vitro
Numerous variants in the FMO3 gene have been reported, with new ones being identified every year that have phenotypic ramifications. Mutations vary from missense, nonsense and deletion to truncation. Several databases are available for review, including GenBank [101], The Human Genome Mutation Database [102], the human mutation database [25,103] and The Swiss Prot Protein Knowledge Database [104]. The mutations occur in the coding region, promoter sequences, intronic sequences, or can cause splice variants. Both in vitro and in vivo studies have shown that mutations affect the structure and function of the enzyme by interfering with protein folding or disrupting the β-pleated sheet structure [26].
In vitro studies have validated the genotype–phenotype relationship of several FMO3 mutations. Cashman and colleagues showed substantial differences in the N-oxygenation kinetics for the biogenic amine substrate tyramine in in vitro studies of FMO3 V257M and E158K cDNA [27]. Similarly, the effect of a homozygous polymorphism at E158K has been shown to reduce N-oxygenation of benzydamine [28]. Zschocke and colleagues showed a markedly reduced FMO3 enzymatic activity in individuals with a variant allele that comprises the two common amino-acid polymorphisms E158K and E308G [29]. The D132H polymorphism has also been shown to decrease methimazole and phenothiazine metabolism [30]. By contrast, the L360 P polymorphism has been shown to increase the catalytic efficiency of FMO3 [30,31]. In addition, Sachse and colleagues, using a population-based analysis model, did not establish a clear link between certain FMO3 polymorphisms and the metabolism of clozapine or caffeine, suggesting that the mutations examined have only minor functional effects, or that substrate affinity may be too low to be clinically relevant [17]. Of the several variants, the E158K, E308G and V257M polymorphisms have been shown to have a relatively high population distribution [27]. Interestingly, studies have shown incongruence between effects of similar variants in different subgroups in a given population [32]. Also, a substrate-dependent variation in catalytic activity has been observed for the E158K and E308G polymorphisms [30,32].
In vivo effects of FMO3 polymorphisms
New FMO3 variants continue to be reported in the literature [7,33,34]. Although the physiological role of FMO3 is still unclear, knowledge of its role in the metabolism of drugs, xenobiotics and other chemicals is accumulating. FMO3 metabolizes nucleophilic heteroatom-containing compounds, exemplified by oxygenating primary amines to hydroxylamines and then to oximes. In contrast to mammals where metabolism of amines by FMO3 leads to their detoxification, a similar process in plants confers growth-enhancing properties [35].
Numerous drugs are metabolized by FMO3. Some have a narrow therapeutic index, and increased drug levels may cause significant toxicity while low levels may lead to resistance. Although relatively few studies have examined the in vivo effects of FMO3 polymorphisms on drug metabolism, investigators have used certain drugs in individuals as probes to characterize the effect of FMO3 polymorphisms. The use of trimethylamine (TMA) as a substrate probe is relatively common since TMA is solely metabolized by FMO3. Other drug substrates have been used for both in vitro and in vivo analyses. Zschocke and colleagues investigated the metabolism of benzydamine in controls and patients with severe FMO3 deficiency, and found evidence for markedly reduced N-oxygenation capacity in both serum and urine samples [36]. Ryu and colleagues showed that overproduction of nitric oxide in the liver may suppress FMO3 activity directly via reversible S-nitrosylation [37]. N-oxygenation of nicotine [38], clozapine [17] and the histamine 2 receptor (H2) blockers, cimetidine [39] and ranitidine [40], have all been used to phenotype patient populations with FMO3 variants.
FMO3 mutations & trimethylaminuria
The clinical significance of the polymorphisms of human FMO3 is most clearly reflected by the condition of trimethylaminuria. In fact, the identification of new mutations in FMO3 is partly driven by genotyping efforts based on self-reporting by patients with this condition [33]. Also known as the fish odor syndrome, trimethylaminuria may have a population-based carrier rate of up to 1%, with a possible over-representation in women [41]. Trimethylaminuria is characterized by the inability to metabolize the simple tertiary aliphatic amine trimethylamine, a compound derived from several dietary sources including choline- and lecithin-containing compounds present in marine fish, eggs, liver, kidney, and certain vegetables such as beetroot, legumes and other foods. To date, 16 mutations of the FMO3 gene have been linked to trimethylaminuria [25]. Individuals with this condition have the impaired ability to N-oxygenate trimethylamine, which leads to its accumulation in the body and excretion of malodorous free amines in the urine, sweat, breath and other body secretions. This has the clinical effect of the individual manifesting a peculiar smell. Although not fatal, the condition is sometimes associated with hypertension and adverse reactions to tyramine. Also, the peculiar odor in this syndrome sometimes leads to social isolation, depression and suicidal tendencies [42]. Diagnosis can be made by measuring the trimethylamine/trimethylamine N-oxide ratio in the urine after an oral challenge [43]. With the advent of genetic testing, the primary genetic varieties can now be easily identified, although determining the resulting phenotype continues to remain a challenge [42]. The management of this condition involves avoidance of trimethylamine overloading in the diet, empiric trial of antibiotics, counseling and the use of TMA sequestering agents such as copper chlorophillin [43,44]. Trimethylaminuria is an excellent illustration of how diet, environmental exposure and genetic makeup can interact and affect each other. As specific FMO3 mutations are now well-established in patients suffering from this disorder, the application of genotyping in diagnosing this condition may become feasible in the future.
Clinical significance of interindividual and interethnic differences in FMO3
In a population-based analysis, Cashman and colleagues demonstrated statistically significant differences in the frequency of single and multiple alleles and genotypes of the human FMO3 among ethnically diverse populations [45]. For instance, they demonstrated a relative frequency of the E158K polymorphism among non-Hispanic Caucasians of 0.39, versus 0.15 for Asians in the USA. Similarly, the relative frequency of the V257M polymorphism among African–Americans was 0.07 versus a frequency of 0.20 for Asians, and the relative frequency of the E308G polymorphism among African–Americans was 0.04, as opposed to 0.12 for Hispanics [45]. A recent study compared the allele and genotype frequencies of the same polymorphisms among Han Chinese and African–American subjects, and found a significant difference between the two groups. In Han Chinese, the minor allele frequencies were 0.229 (E158K), 0.203 (V257M) and 0.148 (E308G). In African–Americans, the minor allele frequencies were 0.48 (E158K), 0.05 (V257M) and 0 (E308G) [46]. Similarly, Lattard and colleagues demonstrated a higher frequency of the V257M and E308G polymorphisms in Caucasians than in African–Americans [30].
In validating the presence of individual differences in human FMO3 activity, many studies have demonstrated the effects of FMO3 polymorphisms in altering the metabolism of its substrates, including the drugs sulindac, ranitidine, methimazole and benzydamine [40,43,47–50]. These drugs have been used as in vivo probes to demonstrate interindividual differences in their metabolic rates due to different FMO3 polymorphisms. As noted previously, individual differences have also been demonstrated in the metabolism of compounds such as trimethylamine. The prevalence of trimethylaminuria in some parts of the population and not in others is valid proof of the individual differences in the metabolism of compounds by FMO3. As a further example, Koukouritaki and colleagues demonstrated that two novel structural variants of FMO3, E24D and K416N caused modest changes in catalytic efficiency when expressed recombinant enzymes were tested with methimazole, trimethylamine, sulindac and ethylene-thiourea, while the N61K variant showed an almost total loss of activity in metabolizing these compounds [50]. The frequency of these polymorphisms was noted to be different among African–Americans, Hispanics and non-Latino whites [50]. Similarly, Mayatepek and colleagues demonstrated markedly impaired metabolism of benzydamine, manifested as decreased levels of the N-oxide metabolite in the serum and urine, in subjects with severe FMO3 deficiency due to polymorphisms [49]. These and other studies establish that FMO3 mutations have a role in the interindividual and interethnic metabolic differences noted in the population. This conclusion is supported by in vitro, in vivo and population-based studies. Whether this could lead to guidelines alluding to individualized and ethnic-based dosing regimens remains to be seen.
Gastrointestinal implications of FMO3 polymorphisms
The study of genetic polymorphisms has been gaining pace in the practice of gastroenterology. For example, study of polymorphisms in the thiopurine methyl transferase (TPMT) gene led to the application of TPMT genotyping as a marker for TPMT activity, and therefore dosing decisions for azathioprine and 6-mercaptopurine, two known substrates of TPMT, in the treatment of inflammatory bowel diseases [51–54]. In another case, mutations in the dihydropyridine dehydrogenase (DPD) gene affect enzymatic activity and lead to clinically significant differences in the metabolism of 5-fluorouracil, an antimetabolite used in cancer therapy, causing variation in the effects and toxicities associated with this medication [54,55].
The role of FMO3 mutations in diseases of the gastrointestinal tract has been studied with regard to the nonsteroidal anti-inflammatory drug (NSAID) sulindac and the histamine 2 receptor (H2) blockers, ranitidine and cimetidine. Kang and colleagues found a direct correlation between FMO3 genotypes and the corresponding phenotypes in a group of 210 volunteers who were administered ranitidine, which is primarily metabolized by FMO3. Using HPLC measurements of urine ranitidine and ranitidine N-oxide levels, the study showed that subjects who were homozygous or heterozygous for the E158K and/or the E308G polymorphisms had lower in vivo FMO3 activity than volunteers with a wild-type FMO3 genotype [40].
Interest has focused on the role of certain pharmacological agents in the chemoprevention of colorectal cancer. NSAIDs have received the most attention, since epidemiological evidence indicates that they effectively reduce the risk of colorectal adenoma and cancer [56,57]. Sandler and colleagues demonstrated, in a randomized, double-blinded placebo-controlled trial of 635 patients with a history of colorectal cancer, that daily use of aspirin was associated with a significant reduction of subsequent colorectal adenoma [58]. Similarly, Baron and colleagues demonstrated a moderate chemopreventive effect of aspirin in patients with a recent history of colorectal adenoma [58]. Sulindac has also been shown to reduce polyp burden in randomized control trials of patients with familial adenomatous polyposis (FAP) [59–61].
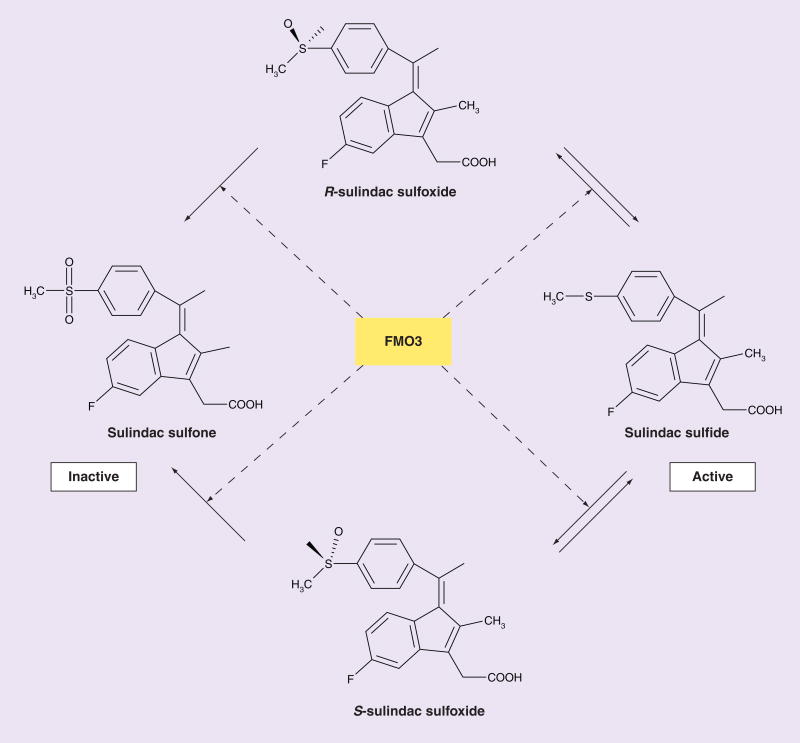
Sulindac is a prodrug that is biotransformed by two pathways, one by reduction to its active sulfide form and the other by oxygenation to the sulfone form [62] (Figure 1). The reduction of sulindac to its active sulfide form is catalyzed by the aldehyde oxidase system in monkey liver [63] and the methionine sulfoxide reductase system in Escherichia coli [63]. Once absorbed, the oxidative inactivation of the sulfide form to its sulfoxide and sulfone forms is catalyzed by FMO3 [14] (Figure 1). An important phenomenon in the metabolism of sulindac is the enterohepatic circulation of the three redox forms with rate of biliary secretion of sulindac, sulfoxide > sulfone > sulfide [62]. Since the gastrointestinal toxicity of NSAIDs is partly determined by the exposure of the gut mucosa to the active form, this disproportionate secretion of prodrug relative to active drug provides a theoretical basis for the relatively low toxicity of sulindac [64].
Figure 1. Metabolism of sulindac by flavin-containing monooxygenase 3.
The scheme depicts the enzymatic metabolism of sulindac by FMO3. Sulindac is ingested as a prodrug, sulindac sulfoxide, which exists as the R- and S-enantiomeric forms. Both forms are converted by the gut bacteria to its reduced sulfide form, which is then absorbed. It is the sulfide form that is metabolically active. The sulfide form is then inactivated in the body by FMO3 back to the sulfoxide and then to the inactive sulfone form.
FMO3: Flavin-containing monooxygenase 3 gene.
Adapted with permission from [54].
Two studies, one performed in a primary prevention model [47] and the other in a regression model [48] of chemoprevention, demonstrated that polymorphisms in FMO3, particularly at the E158K and E308G loci, were associated with a protective effect on adenoma formation in FAP patients who were treated with sulindac. In the first study, the authors examined the correlation between the presence of several polymorphisms in the FMO3 gene and the de novo development of adenomas in genotypically positive but phenotypically negative FAP patients at baseline who received sulindac for the subsequent 4 years [65]. A significant number of patients who remained adenoma-free at the end of the treatment period exhibited homozygous FMO3 polymorphisms at the E158K or E308G loci. By contrast, none of the patients who developed adenomas exhibited homozygosity in either of the variant alleles. Furthermore, polymorphisms in the E158K or E308G allele were associated with a significant decrease in mucosal prostanoid levels in patients treated with sulindac. It was concluded that mutations in FMO3, particularly at the E158K and E308G loci, may reduce the ability of FMO3 to catabolize sulindac and result in an increased efficacy of sulindac to prevent polyposis in FAP [47].
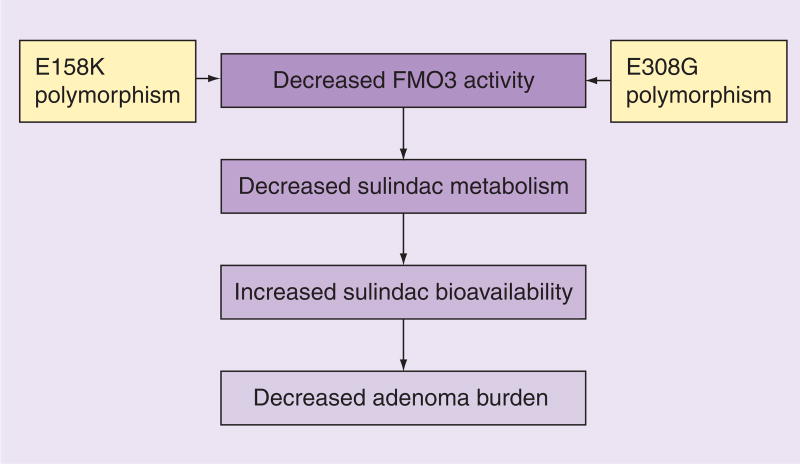
In another study [48], 19 genotypically and phenotypically positive FAP patients were treated with sulindac for 6 months. Six patients exhibiting polymorphisms in both E158K and E308G loci were designated as genotype combination 1. The remaining patients were designated as genotype combination 2. Over the course of treatment, patients with genotype combination 1 had a greater reduction in both the size and number of adenomas than those with genotype combination 2. These results suggest that combined polymorphic changes in the E158K and E308G alleles may protect against polyposis in patients with FAP treated with sulindac [48]. These studies demonstrated that FMO3 polymorphisms at the E158K and E308G alleles, which have been shown to decrease FMO3 activity, are protective of polyposis in FAP patients treated with sulindac. This outcome is presumably due to increased bioavailability of sulindac due to decreased metabolism, as suggested by the significant decrease of mucosal prostanoid levels in patients with the variant FMO3 alleles in the first study (Figure 2).
Figure 2. Effect of flavin-containing monooxygenase 3 gene polymorphisms on sulindac metabolism in familial adenomatous polyposis patients.
The scheme depicts the sequential effect of two common polymorphisms of FMO3, E158K and E308G, on reducing polyp burden in patients with FAP being treated with sulindac [47,48].
FAP: Familial adenomatous polyposis; FMO3: Flavin-containing monooxygenase 3 gene.
Expert commentary
Although the study of FMO3 pharmacogenetics is still in its infancy, the fact that so much remains to be learned makes this a rich field of research. This is of particular interest to clinicians, pharmacologists, policy makers and patients because of the potential for practical application. Recent studies of the genotype–phenotype relationship between FMO3 mutations and their effects on enzyme activity range from in vitro models [31,34] to translational studies examining the direct impact of these mutations in humans [40,47,48]. Trimethylaminuria is a good example; this disorder has been a cornerstone for the developing field of FMO3 polymorphisms. Self-reporting patients with this condition, along with population-based studies, have led to the discovery of novel variants [25,33,66]. Expanded knowledge of the effects of FMO3 polymorphisms will open up new avenues of research and, ultimately, clinical applications. In the field of gastroenterology, the effect of FMO3 polymorphisms has been demonstrated in the metabolism of sulindac and H2 blockers. Both medications have important roles in the management of common gastrointestinal diseases including colorectal adenoma and peptic ulcer disease [60,61,67]. Translational data is accumulating on the role of certain polymorphisms in in vivo human clinical settings [40,47,48,67], and the process of discovery and clinical utilization needs to be pursued.
Conclusion
Exciting research opportunities with direct application to diseases of the gastrointestinal tract exist in the field of pharmacogenetics. Data from recent studies point toward the role of genetic polymorphisms in modulating the phenotype with effects on drug metabolism. With rapid advances in high-throughput sequencing and gene-chip technology, individualized medicine may soon become a reality. Even today clinicians are utilizing genotyping to prescribe medications such as azathioprine and 6-mercaptopurine, the metabolism of which is dependent on highly polymorphic TPMT enzyme systems.
The flavin-containing monooxygenases are a major drug-metabolizing enzyme system in humans, of which FMO3 is the primary form in the liver. Numerous variants have been reported in the FMO3 gene. These variants have been shown in both in vitro and in vivo systems to affect enzyme activity. Several FMO3 variants have also been shown to have clinical implications, as illustrated by the disease trimethylaminuria, the metabolism of several commonly used medications, and more recently, in the chemoprevention of FAP using sulindac.
Despite advances in understanding the role of FMO3 polymorphisms in human diseases, significant challenges lie ahead for this field. For example, certain FMO3 polymorphisms are not associated with common diseases such as colorectal cancer and hypertension [68,69]. This is one gray area illustrative of the amount of additional research needed to establish the significance of FMO3 polymorphisms in human diseases and their management. Furthermore, clinical application of existing information is still in its infancy, the ethical and financial implications of genetic testing need to be addressed, and the vast number of polymorphisms makes the task of sorting the relevant ones formidable. With the conclusion of the Human Genome Project and the ongoing HapMap project, a great deal of information on the location of SNPs, their link to diseases and their impact on drug metabolism will become available and this will unquestionably aid this research.
Executive summary.
Flavin-containing monooxygenase 3 metabolizes numerous drugs, including those used in gastrointestinal diseases.
Many mutations have been identified in FMO3.
A genotype–phenotype correlation has been suggested in both in vitro and in vivo models.
Alterations in enzyme kinetics because of FMO3 polymorphisms have been highlighted by several studies.
Trimethylaminuria, a rare genetic disorder of FMO3 dysfunction, validates the role of FMO3 polymorphisms in determining FMO3 enzyme activity and, importantly, provides a clinical model for FMO3 genotyping and its application.
Sulindac and H2 blockers are examples of the drugs involved in the gastrointestinal system that are affected by FMO3 polymorphisms.
Certain FMO3 polymorphisms have been shown to have a protective effect on polyposis in FAP patients treated with sulindac.
More research is needed to validate the principal of applying pharmacogenetics to disease management, although clear and tangible benefits will result from such work and may usher in an era of individualized medicine with great benefits for patient care.
Acknowledgments
In memoriam
The authors would like to acknowledge the immense contributions of Dr Daniel Ziegler to the study of the flavin-containing monooxygenases. Dr Ziegler's group was the first to purify a member of this class of enzyme and subsequent studies in his laboratory demonstrated the importance of FMOs in the metabolism of a large number of medicinal amines and sulfur-containing drug. Through elegant experimental design and careful chemical analysis, Dr Ziegler's research group was eventually able to elucidate the mechanism of action of the FMOs. Dr Ziegler's impact extended beyond his work with FMO, as he provided fresh insights in enzymology, intermediary metabolism and organic reaction mechanisms. Dr Ziegler passed away in November, 2005, at the age of 78 in Austin,TX, USA. He is survived by his wife of 53 years, Mary Alice, four children and eight grandchildren.
This work was in part supported by grants from the National Institutes of Health (DK52230 and CA84197). VWY was the recipient of a Georgia Cancer Coalition Distinguished Cancer Clinician Scientist Award.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Eswaramoorthy S, Bonanno JB, Burley SK, Swaminathan S. Mechanism of action of a flavin-containing monooxygenase. Proc Natl Acad Sci USA. 2006;103:9832–9837. doi: 10.1073/pnas.0602398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 2006;46:65–100. doi: 10.1146/annurev.pharmtox.46.120604.141043. [DOI] [PubMed] [Google Scholar]; •• This review article summarizes recent information on the pharmacological and toxicological significance of human flavin-containing monooxygeanses (FMOs).
- 3.Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen GP, Ziegler DM. Liver microsome and flavin-containing monooxygenase catalyzed oxidation of organic selenium compounds. Arch Biochem Biophys. 1994;312:566–572. doi: 10.1006/abbi.1994.1346. [DOI] [PubMed] [Google Scholar]
- 5.McCombie RR, Dolphin CT, Povey S, Phillips IR, Shephard EA. Localization of human flavin-containing monooxygenase genes FMO2 and FMO5 to chromosome 1q. Genomics. 1996;34:426–429. doi: 10.1006/geno.1996.0308. [DOI] [PubMed] [Google Scholar]
- 6.Shephard EA, Dolphin CT, Fox MF, Povey S, Smith R, Phillips IR. Localization of genes encoding three distinct flavin-containing monooxygenases to human chromosome 1q. Genomics. 1993;16:85–89. doi: 10.1006/geno.1993.1144. [DOI] [PubMed] [Google Scholar]
- 7.Koukouritaki SB, Poch MT, Cabacungan ET, McCarver DG, Hines RN. Discovery of novel flavin-containing monooxygenase 3 (FMO3) single nucleotide polymorphisms and functional analysis of upstream haplotype variants. Mol Pharmacol. 2005;68:383–392. doi: 10.1124/mol.105.012062. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez D, Janmohamed A, Chandan P, Phillips IR, Shephard EA. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: identification of novel gene and pseudogene clusters. Pharmacogenetics. 2004;14:117–130. doi: 10.1097/00008571-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hines RN, Hopp KA, Franco J, Saeian K, Begun FP. Alternative processing of the human FMO6 gene renders transcripts incapable of encoding a functional flavin-containing monooxygenase. Mol Pharmacol. 2002;62:320–325. doi: 10.1124/mol.62.2.320. [DOI] [PubMed] [Google Scholar]
- 10.Dolphin C, Shephard EA, Povey S, et al. Cloning, primary sequence, and chromosomal mapping of a human flavin-containing monooxygenase (FMO1) J Biol Chem. 1991;266:12379–12385. [PubMed] [Google Scholar]
- 11.Koukouritaki SB, Simpson P, Yeung CK, Rettie AE, Hines RN. Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr Res. 2002;51:236–243. doi: 10.1203/00006450-200202000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Overby LH, Carver GC, Philpot RM. Quantitation and kinetic properties of hepatic microsomal and recombinant flavin-containing monooxygenases 3 and 5 from humans. Chem Biol Interact. 1997;106:29–45. doi: 10.1016/s0009-2797(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 13.Dolphin CT, Cullingford TE, Shephard EA, Smith RL, Phillips IR. Differential developmental and tissue-specific regulation of expression of the genes encoding three members of the flavin-containing monooxygenase family of man, FMO1, FMO3 and FM04. Eur J Biochem. 1996;235:683–689. doi: 10.1111/j.1432-1033.1996.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamman MA, Haehner-Daniels BD, Wrighton SA, Rettie AE, Hall SD. Stereoselective sulfoxidation of sulindac sulfide by flavin-containing monooxygenases. Comparison of human liver and kidney microsomes and mammalian enzymes. Biochem Pharmacol. 2000;60:7–17. doi: 10.1016/s0006-2952(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 15.Cashman JR, Yang Z, Yang L, Wrighton SA. Stereo- and regioselective N- and S-oxidation of tertiary amines and sulfides in the presence of adult human liver microsomes. Drug Metab Dispos. 1993;21:492–501. [PubMed] [Google Scholar]
- 16.Chung WG, Park CS, Roh HK, Lee WK, Cha YN. Oxidation of ranitidine by isozymes of flavin-containing monooxygenase and cytochrome P450. Jpn J Pharmacol. 2000;84:213–220. doi: 10.1254/jjp.84.213. [DOI] [PubMed] [Google Scholar]
- 17.Sachse C, Ruschen S, Dettling M, et al. Flavin monooxygenase 3 (FMO3) polymorphism in a white population: allele frequencies, mutation linkage, and functional effects on clozapine and caffeine metabolism. Clin Pharmacol Ther. 1999;66:431–438. doi: 10.1053/cp.1999.v66.a102203. [DOI] [PubMed] [Google Scholar]
- 18.Lattard V, Zhang J, Cashman JR. Alternative processing events in human FMO genes. Mol Pharmacol. 2004;65:1517–1525. doi: 10.1124/mol.65.6.1517. [DOI] [PubMed] [Google Scholar]
- 19.Mushiroda T, Douya R, Takahara E, Nagata O. The involvement of flavin-containing monooxygenase but not CYP3A4 in metabolism of itopride hydrochloride, a gastroprokinetic agent: comparison with cisapride and mosapride citrate. Drug Metab Dispos. 2000;28:1231–1237. [PubMed] [Google Scholar]
- 20.Fang J. Metabolism of clozapine by rat brain: the role of flavin-containing monooxygenase (FMO) and cytochrome P450 enzymes. Eur J Drug Metab Pharmacokinet. 2000;25:109–114. doi: 10.1007/BF03190076. [DOI] [PubMed] [Google Scholar]
- 21.Parte P, Kupfer D. Oxidation of tamoxifen by human flavin-containing monooxygenase (FMO) 1 and FMO3 to tamoxifen-N-oxide and its novel reduction back to tamoxifen by human cytochromes P450 and hemoglobin. Drug Metab Dispos. 2005;33:1446–1452. doi: 10.1124/dmd.104.000802. [DOI] [PubMed] [Google Scholar]
- 22.Furnes B, Schlenk D. Extrahepatic metabolism of carbamate and organophosphate thioether compounds by the flavin-containing monooxygenase and cytochrome P450 systems. Drug Metab Dispos. 2005;33:214–218. doi: 10.1124/dmd.104.000984. [DOI] [PubMed] [Google Scholar]
- 23.Phillips IR, Dolphin CT, Clair P, et al. The molecular biology of the flavin-containing monooxygenases of man. Chem Biol Interact. 1995;96:17–32. doi: 10.1016/0009-2797(94)03580-2. [DOI] [PubMed] [Google Scholar]
- 24.Dolphin CT, Riley JH, Smith RL, Shephard EA, Phillips IR. Structural organization of the human flavin-containing monooxygenase 3 gene (FMO3), the favored candidate for fish-odor syndrome, determined directly from genomic DNA. Genomics. 1997;46:260–267. doi: 10.1006/geno.1997.5031. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez D, Addou S, Lee D, Orengo C, Shephard EA, Phillips IR. Trimethylaminuria and a human FMO3 mutation database. Hum Mutat. 2003;22:209–213. doi: 10.1002/humu.10252. [DOI] [PubMed] [Google Scholar]; •• Article describes a mutation database of FMO3 and its relationship to trimethylaminuria.
- 26.Koukouritaki SB, Hines RN. Flavin-containing monooxygenase genetic polymorphism: impact on chemical metabolism and drug development. Pharmacogenomics. 2005;6:807–822. doi: 10.2217/14622416.6.8.807. [DOI] [PubMed] [Google Scholar]; • Study reviewed the clinical and therapeutic implications of genetic polymorphisms of FMO3.
- 27.Cashman JR, Akerman BR, Forrest SM, Treacy EP. Population-specific polymorphisms of the human FMO3 gene: significance for detoxication. Drug Metab Dispos. 2000;28:169–173. [PubMed] [Google Scholar]; • Study examined two prevalent polymorphisms of FMO3 in the Canadian and Australian white populations on substrate metabolism.
- 28.Stormer E, Roots I, Brockmoller J. Benzydamine N-oxidation as an index reaction reflecting FMO activity in human liver microsomes and impact of FMO3 polymorphisms on enzyme activity. Br J Clin Pharmacol. 2000;50:553–561. doi: 10.1046/j.1365-2125.2000.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E. Mild trimethylaminuria caused by common variants in FMO3 gene. Lancet. 1999;354:834–835. doi: 10.1016/s0140-6736(99)80019-1. [DOI] [PubMed] [Google Scholar]
- 30.Lattard V, Zhang J, Tran Q, Furnes B, Schlenk D, Cashman JR. Two new polymorphisms of the FMO3 gene in Caucasian and African–American populations: comparative genetic and functional studies. Drug Metab Dispos. 2003;31:854–860. doi: 10.1124/dmd.31.7.854. [DOI] [PubMed] [Google Scholar]
- 31.Borbas T, Zhang J, Cerny M, Liko I, Cashman JR. Investigation of structure and function of a catalytically efficient variant of the human flavin-containing monooxygenase form 3 (FMO3) Drug Metab Dispos. 2006;34:1995–2002. doi: 10.1124/dmd.106.010827. [DOI] [PubMed] [Google Scholar]
- 32.Park CS, Kang JH, Chung WG, et al. Ethnic differences in allelic frequency of two flavin-containing monooxygenase 3 (FMO3) polymorphisms: linkage and effects on in vivo and in vitro FMO activities. Pharmacogenetics. 2002;12:77–80. doi: 10.1097/00008571-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki H, Fujita H, Gunji T, et al. Stop codon mutations in the flavin-containing monooxygenase 3 (FMO3) gene responsible for trimethylaminuria in a Japanese population. Mol Genet Metab. 2007;90:58–63. doi: 10.1016/j.ymgme.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu M, Fujita H, Aoyama T, Yamazaki H. Three novel single nucleotide polymorphisms of the FMO3 gene in a Japanese population. Drug Metab Pharmacokinet. 2006;21:245–247. doi: 10.2133/dmpk.21.245. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Christensen SK, Fankhauser C, et al. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 36.Zschocke J, Mayatepek E. Biochemical and molecular studies in mild flavin monooxygenase 3 deficiency. J Inherit Metab Dis. 2000;23:378–382. doi: 10.1023/a:1005647701321. [DOI] [PubMed] [Google Scholar]
- 37.Ryu SD, Yi HG, Cha YN, et al. Flavin-containing monooxygenase activity can be inhibited by nitric oxide-mediated S-nitrosylation. Life Sci. 2004;75:2559–2572. doi: 10.1016/j.lfs.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Cashman JR, Zhang J. Interindividual differences of human flavin-containing monooxygenase 3: genetic polymorphisms and functional variation. Drug Metab Dispos. 2002;30:1043–1052. doi: 10.1124/dmd.30.10.1043. [DOI] [PubMed] [Google Scholar]; • • Article reviewed the role of FMO3 polymorphisms in substrate metabolism and disease states.
- 39.Cashman JR. Human flavin-containing monooxygenase: substrate specificity and role in drug metabolism. Curr Drug Metab. 2000;1:181–191. doi: 10.2174/1389200003339135. [DOI] [PubMed] [Google Scholar]
- 40.Kang JH, Chung WG, Lee KH, et al. Phenotypes of flavin-containing monooxygenase activity determined by ranitidine N-oxidation are positively correlated with genotypes of linked FM03 gene mutations in a Korean population. Pharmacogenetics. 2000;10:67–78. doi: 10.1097/00008571-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Zhang AQ, Mitchell SC, Smith RL. Discontinuous distribution of N-oxidation of dietary-derived trimethylamine in a British population. Xenobiotica. 1996;26:957–961. doi: 10.3109/00498259609052497. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell SC, Smith RL. Trimethylaminuria: the fish malodor syndrome. Drug Metab Dispos. 2001;29:517–521. [PubMed] [Google Scholar]; • Study summarizes the clinical presentations of trimethylaminuria
- 43.Chalmers RA, Bain MD, Michelakakis H, Zschocke J, Iles RA. Diagnosis and management of trimethylaminuria (FMO3 deficiency) in children. J Inherit Metab Dis. 2006;29:162–172. doi: 10.1007/s10545-006-0158-6. [DOI] [PubMed] [Google Scholar]
- 44.Busby MG, Fischer L, da Costa KA, Thompson D, Mar MH, Zeisel SH. Choline- and betaine-defined diets for use in clinical research and for the management of trimethylaminuria. J Am Diet Assoc. 2004;104:1836–1845. doi: 10.1016/j.jada.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 45.Cashman JR, Zhang J, Leushner J, Braun A. Population distribution of human flavin-containing monooxygenase form 3: gene polymorphisms. Drug Metab Dispos. 2001;29:1629–1637. [PubMed] [Google Scholar]; • • Article describes a population-based study of the distribution of polymorphisms in FMO3.
- 46.Hao D, Sun J, Furnes B, et al. Allele and genotype frequencies of polymorphic FMO3 gene in two genetically distinct populations. Cell Biochem Funct. 2006 doi: 10.1002/cbf.1326. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Hisamuddin IM, Wehbi MA, Chao A, et al. Genetic polymorphisms of human flavin monooxygenase 3 in sulindac-mediated primary chemoprevention of familial adenomatous polyposis. Clin Cancer Res. 2004;10:8357–8362. doi: 10.1158/1078-0432.CCR-04-1073. [DOI] [PubMed] [Google Scholar]; • • The work examined the role of FMO3 polymorphisms in primary chemoprevention of adenomas in familial adenomatous polyposis patients being treated with sulindac.
- 48.Hisamuddin IM, Wehbi MA, Schmotzer B, et al. Genetic polymorphisms of flavin monooxygenase 3 in sulindac-induced regression of colorectal adenomas in familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2005;14:2366–2369. doi: 10.1158/1055-9965.EPI-05-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]; • • The work examined the role of FMO3 polymorphisms in a regression model of adenoma chemoprevention in FAP patients treated with sulindac.
- 49.Mayatepek E, Flock B, Zschocke J. Benzydamine metabolism in vivo is impaired in patients with deficiency of flavin-containing monooxygenase 3. Pharmacogenetics. 2004;14:775–777. doi: 10.1097/00008571-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Koukouritaki SB, Poch MT, Henderson MC, et al. Identification and functional analysis of common human flavin-containing monooxygenase 3 genetic variants. J Pharmacol Exp Ther. 2007;320:266–273. doi: 10.1124/jpet.106.112268. [DOI] [PubMed] [Google Scholar]; • • Study examined the distribution of FMO3 variants in different ethnic populations.
- 51.Krynetski EY, Schuetz JD, Galpin AJ, Pui CH, Relling MV, Evans WE. A single point mutation leading to loss of catalytic activity in human thiopurine S-methyltransferase. Proc Natl Acad Sci USA. 1995;92:949–953. doi: 10.1073/pnas.92.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai HL, Krynetski EY, Yates CR, et al. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet. 1996;58:694–702. [PMC free article] [PubMed] [Google Scholar]
- 53.Coulthard SA, Hall AG. Recent advances in the pharmacogenomics of thiopurine methyltransferase. Pharmacogenomics J. 2001;1:254–261. doi: 10.1038/sj.tpj.6500066. [DOI] [PubMed] [Google Scholar]
- 54.Hisamuddin IM, Wehbi MA, Yang VW. Pharmacogenetics and diseases of the colon. Curr Opin Gastroenterol. 2007;23:60–66. doi: 10.1097/MOG.0b013e32801145c2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Article reviews the effects of genetic polymorphisms of several drug-metabolizing genes in the therapy of a number of gastrointestinal diseases.
- 55.van Kuilenburg AB, Haasjes J, Richel DJ, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res. 2000;6:4705–4712. [PubMed] [Google Scholar]
- 56.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 57.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121:241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 58.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 60.Giardiello FM, Offerhaus JA, Tersmette AC, et al. Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut. 1996;38:578–581. doi: 10.1136/gut.38.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 62.Duggan DE, Hare LE, Ditzler CA, Lei BW, Kwan KC. The disposition of sulindac. Clin Pharmacol Ther. 1977;21:326–335. doi: 10.1002/cpt1977213326. [DOI] [PubMed] [Google Scholar]
- 63.Kitamura S, Ohashi KNK, Sugihara K, Hosokawa R, Akagawa Y, Ohta S. Extremely high drug-reductase activity based on aldehyde oxidase in monkey liver. Biol Pharm Bull. 2001;24:856–859. doi: 10.1248/bpb.24.856. [DOI] [PubMed] [Google Scholar]
- 64.Dujovne CA, Pitterman A, Vincek WC, Dobrinska MR, Davies RO, Duggan DE. Enterohepatic circulation of sulindac and metabolites. Clin Pharmacol Ther. 1983;33:172–177. doi: 10.1038/clpt.1983.26. [DOI] [PubMed] [Google Scholar]
- 65.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study examined the effect of sulindac in preventing onset of polyposis in a primary chemoprevention trial in FAP patients.
- 66.Teresa E, Lonardo F, Fiumara A, et al. A spectrum of molecular variation in a cohort of Italian families with trimethylaminuria: identification of three novel mutations of the FM03 gene. Mol Genet Metab. 2006;88:192–195. doi: 10.1016/j.ymgme.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Koizumi W, Tanabe S, Imaizumi H, et al. Inhibition of peptic ulcer relapse by ranitidine and ecabet independently of eradication of Helicobacter pylori: a prospective, controlled study versus ranitidine. Hepatogastroenterology. 2003;50:577–581. [PubMed] [Google Scholar]
- 68.Bae SY, Choi SK, Kim KR, et al. Effects of genetic polymorphisms of MDR1, FMO3 and CYP1A2 on susceptibility to colorectal cancer in Koreans. Cancer Sci. 2006;97:774–779. doi: 10.1111/j.1349-7006.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dolan C, Shields DC, Stanton A, et al. Polymorphisms of the flavin containing monooxygenase 3 (FMO3) gene do not predispose to essential hypertension in Caucasians. BMC Med Genet. 2005;6:41. doi: 10.1186/1471-2350-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suh JK, Poulsen LL, Ziegler DM, Robertus JD. Molecular cloning and kinetic characterization of a flavin-containing monooxygenase from Saccharomyces cerevisiae. Arch Biochem Biophys. 1996;336:268–274. doi: 10.1006/abbi.1996.0557. [DOI] [PubMed] [Google Scholar]
Websites
- 101.National Center for Biotechnology Information. www.ncbi.nlm.nih.gov.
- 102.The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff. www.hgmd.cf.ac.uk/ac/index.php.
- 103.Allelic Variant Database, University College Lodon, UK. http://human-fmo3.biochem.ucl.ac.uk/Human_FMO3/
- 104.UniProt Knowledgebase. http://ca.expasy.org/sprot/